Abstract
Tuning the electrical properties of biocompatible materials with minimal amounts of nanofiller presents a significant challenge in neuroimplant development. This study investigates the influence of single-walled carbon nanotube (SWCNT) agglomerate size on the electrical conductivity of a bioinspired biopolymer composite. The composites were fabricated via photolithography. We analyzed the effect of ultrasonic homogenization on the size distribution of SWCNT bundles. Our results demonstrate that the degree of nanotube dispersion is critical for determining electrical conductivity. The highest conductivity was achieved with an average bundle size of 95 µm and a defect level of no more than 0.143, as measured by the ID/IG+ band ratio using Raman spectroscopy. We attribute this to the formation of an interconnected percolation network within the biopolymer matrix. These findings demonstrate a viable approach for controlling the conductive properties of bioinspired composites.
1. Introduction
The development of neuroimplantable devices is a key frontier in modern bioelectronics. Neuroimplants are devices integrated into the nervous system that enable stable, bidirectional communication between biological tissues and electronic systems. They primarily expand therapeutic possibilities by regenerating damaged nerves, restoring lost bodily functions, and blocking chronic pain signals [1].
Neuroimplants must meet biosafety requirements and ensure the long-term functionality of the device within the body [2]. Traditional electrode materials, such as platinum, gold, and titanium, are perceived by the body as foreign substances. Consequently, glial scarring occurs, greatly reducing electrical conductivity due to mechanical incompatibility [3,4,5]. Composite hydrogels mimic the mechanical and adhesive properties of the extracellular matrix [6]. Several options provide the hydrogel’s electrical conductivity: conductive polymers, nanoparticles, or ionogels [7].
This paper proposes a bio-inspired electrically conductive composite hydrogel based on bovine serum albumin (BSA), chitosan, eosin Y, and modified single-walled carbon nanotubes (SWCNTs). In contrast to the previously developed material [8], this work also includes type II collagen in the biopolymer matrix, which, when used with chitosan, allows control of another important property of the hydrogel, such as the degree of swelling [9], and also contributes to improved compatibility with native tissue [10,11].
The primary objective of this study is to investigate the influence of SWCNT bundle dimensions on the electrical conductivity of the material. The morphology of the SWCNTs network within the biopolymer matrix is the key parameter governing the composite’s electrical properties. Strong van der Waals interactions between individual nanotubes promote the formation of bundles and agglomerates of varying sizes and connectivity that can be regulated by ultrasonic processing [12]. During photopolymerization under laser irradiation, SWCNTs can weld together at defect sites, forming a percolating conductive network [13] throughout the polymer matrix. In this study, we regulate the hydrodynamic radius of SWCNT bundles to optimize the material’s electrical conductivity without increasing the nanofiller concentration.
2. Materials and Methods
2.1. Preparation of the Photocurable Hydrogel
Photocurable hydrogel composites were produced according to the method described in [8]. In this work, in addition to bovine serum albumin (BSA) (BioClot, Aidenbach, Germany), chitosan (Bioprogress LLC, Losino-Petrovsky, Russia), eosin Y (Agat-Med, Moscow, Russia), and single-walled carbon nanotubes (SWCNTs) (TUBALLTM, OCSiAl, Moscow, Russia), type II collagen (MacMedi LLC, Moscow, Russia) was also used.
SWCNTs were used, as in [8]. In this work, their bundle size was controlled, and aqueous nanotube dispersions were subjected to ultrasonic homogenization using a Q700 sonicator (Qsonica, Newtown, CT, USA). During sample processing, the duration and power of exposure were varied. As a result, four distinct dispersions were prepared with predominant hydrodynamic bundle radii of approximately 250 μm (5 min, 50 W), 95 μm (15 min, 110 W, filter with a pore size of 100 μm (Minimed, LTD, Bryansk, Russia)), 28 μm (30 min, 500 W, filter with a pore size of 100 μm), and 0.4 μm (45 min, 500 W, filter with a pore size of 10 μm (Minimed, LTD, Bryansk, Russia)). The sonication process was performed with cooling to prevent overheating. To evaluate the influence of SWCNT bundle size on electrical conductivity properties after filtration, all samples were aligned by optical density at a wavelength of 550 nm using a GENESYS UV-Vis-Nir spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The hydrodynamic radius of SWCNTs was characterized by Dynamic Light Scattering (DLS) using a Photocor Complex particle size analyzer (Photocor, Moscow, Russia). The final composition of all hydrogels was identical: 0.03 wt.% SWCNTs, 5 wt.% BSA, 10 wt.% chitosan, 2.5 wt.% collagen, and 0.1 wt.% eosin Y.
2.2. Electrical Conductivity and Swelling Rate of Composite Hydrogels Investigation
The specific electrical conductivity of the fabricated composite material structures was evaluated using the four-point probe method [8]. For hydrogels, a key property for ensuring tight contact between the electrode and nerve is the degree of swelling, given the nerve’s mobility. Vibra AJ-420CE scales (Shinko Denshi Co., Ltd., Tokyo, Japan) were used to determine mass. The degree of swelling was determined by the ratio S = mw/m0 × 100%, with mw wet sample weight and m0 dry sample weight.
3. Results and Discussion
3.1. Investigation of Hydrodynamic Radius
The change in the hydrodynamic radius distribution of SWCNT bundles as a function of ultrasonic homogenization time was analyzed using the DLS method (Figure 1). Increasing the processing time was found to reduce the size of the SWCNT bundles (Table 1). After 5 min of treatment, the dispersion contained large SWCNT agglomerates with a predominant radius of 250 µm (85%), while the smallest agglomerates were 2.5 µm. No individual SWCNTs were detected. This sample is characterized by an extremely high degree of nanotube agglomeration.
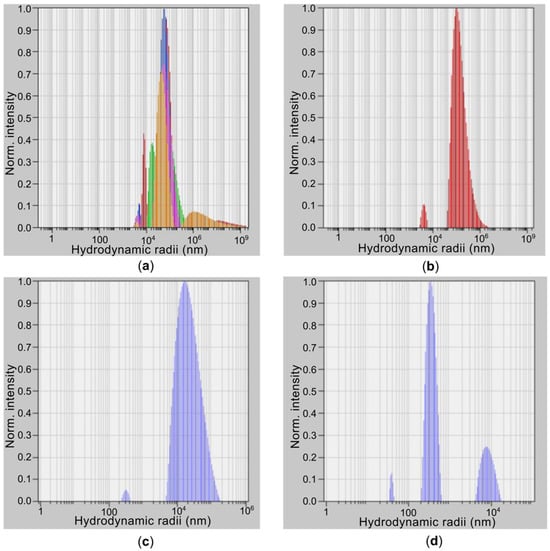
Figure 1.
Results of studies using the DLS for SWCNTs obtained under different ultrasonic treatment modes: (a) SWCNTs (250 µm), (b) SWCNTs (95 µm), (c) SWCNTs (28 µm) and (d) SWCNTs (0.4 µm).

Table 1.
Distribution analysis DLS for SWCNTs.
Increasing the ultrasonic treatment time to 15 min significantly reduced the size of the primary bundles to 95.0 µm, confirming the effectiveness of ultrasound in breaking down large structures. A weak signal (3%) from particles with a radius of 0.3 µm was also present, indicating the presence of individual single-walled carbon nanotubes (SWCNTs). After 30 min of treatment, the bundle size continued to decrease; however, the fraction of small particles (0.3 µm) remained insignificant.
The most substantial changes were observed after 45 min of ultrasonic treatment. The dispersion was dominated by particles with radii in the 0.1–0.4 µm range, comparable to the expected hydrodynamic radius of individual SWCNTs. The proportion of large bundles measuring 8.6 µm decreased to 24%.
Thus, the results clearly demonstrate that prolonged ultrasonic treatment is essential for achieving a high degree of SWCNT dispersion. It has been established that the hydrodynamic radius (R) decreases according to a power function of the processing time (t). R(t)~E−m, with a degree index equal to 0.5. This indicates a rupture caused by stress along the SWCNTs [12]. Sonication energy is defined by the expression E = t × P, with P the sonication power. The sample of SWCNTs (0.4 µm) deviates from this dependence because it is strongly influenced by filtering. In our previous work, we evaluated the defectiveness of SWCNTs using a sonication energy of 720 kJ. The ID/IG+ band ratio in Raman spectroscopy was 0.143, indicating the presence of defects, which may be due to carboxyl groups or arise from ultrasonic treatment. To further evaluate the influence of SWCNT bundle dimensions and defectiveness on electrical conductivity, studies were conducted on photocured composite hydrogels using the obtained nanotubes.
3.2. Electrical Conductivity and Swelling Rate of the Bioinspired Material
The electrical conductivity of the bioinspired material was investigated using the four-point probe method (Table 2).

Table 2.
Electrical conductivity of samples.
Sample 1, containing the largest bundles of SWCNTs (250 µm), exhibited the lowest conductivity. The SWCNTs are grouped into dense, isolated clumps, which explains this effect. A connected network of SWCNTs does not form within the dielectric biopolymer matrix. The maximum conductivity was achieved with SWCNTs (95 µm) that were subjected to 15 min of ultrasonic treatment. In this sample, large aggregates were broken down and individual SWCNTs were present, which promoted particle distribution within the matrix and the formation of multiple contacts. This leads to the formation of a branched percolation network and increased conductivity [13]. The defectiveness of these two samples’ SWCNTs was estimated in relation to the ID/IG+ bands at a level below 0.143 [8]. With continued ultrasonic treatment, the number of defects increases [12]. The defectiveness of the SWCNTs in the other two samples was already estimated to be above 0.143 in relation to the ID/IG+ bands. With further dispersion, the electrical conductivity of the samples decreases. This effect indicates that the short length and high degree of isolation of individual SWCNTs within the biopolymer matrix hinder the formation of stable contacts between them. Another possible reason is an increase in defects above 0.143 in relation to the strips [12]. Controlling such key factors as defectiveness and hydrodynamic radius simultaneously allows for adjustment of the composite’s electrical conductivity properties. All samples demonstrated sufficient swelling, with an average of 170 ± 20%. The impact of the ratio between collagen and chitosan was evident in this result, as previously reported in [9]. Notably, the variation in the hydrodynamic radii of the utilized SWCNTs did not influence this value.
4. Conclusions
This study demonstrates that the electrical conductivity of the bio-inspired composite is strongly governed by the size of SWCNT agglomerates and degree of defectiveness of their structure, which in turn is determined by the ultrasonic homogenization regime. Optimal performance is achieved at an average bundle radius of approximately 95 µm and defectiveness not exceeding 0.143 in relation to the strips ID/IG+, enabling the formation of a stable percolating network [13] and enhancing the material’s specific conductivity. In contrast, excessive dispersion leads to the presence of numerous isolated SWCNTs and an increase in their defect rate [12] that cannot form a continuous conductive pathway. Therefore, controlling the degree of SWCNT agglomeration and defectiveness represents an effective strategy for tailoring the electrical properties of biopolymer composites. These findings hold significant practical relevance for the design and optimization of neuroimplantable devices. At the same time, the use of a combination of collagen and chitosan made it possible to achieve a swelling rate of approximately 170%, which is important for ensuring close contact between the mobile nerve and the electrode.
Author Contributions
Conceptualization, A.Y.G. and M.S.S.; methodology, M.S.S. and E.P.O.; software, M.S.S. and P.N.V.; validation, V.V.S. and K.D.P.; formal analysis, A.Y.G. and S.V.S.; investigation, V.V.S., P.N.V. and E.P.O.; resources, A.Y.G. and M.S.S.; data curation, P.N.V., M.S.S. and S.V.S.; writing—original draft preparation, M.S.S. and E.P.O.; writing—review and editing, V.V.S. and K.D.P.; visualization, E.P.O.; supervision, A.Y.G., S.V.S. and M.S.S.; project administration, A.Y.G. and M.S.S.; funding acquisition, A.Y.G. and S.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Ministry of Education and Science of the Russian Federation (project FSMR-2024-0003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BSA | Bovine Serum Albumin |
| DLS | Dynamic Light Scattering |
| SWCNTs | Single-Walled Carbon Nanotubes |
References
- Gutruf, P. Monolithically defined wireless fully implantable nervous system interfaces. Acc. Chem. Res. 2024, 57, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Boufidis, D.; Garg, R.; Angelopoulos, E.; Cullen, D.K.; Vitale, F. Bio-inspired electronics: Soft, biohybrid, and “living” neural interfaces. Nat. Commun. 2025, 16, 1861. [Google Scholar] [CrossRef] [PubMed]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, V. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017, 1, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Park, S.; Lee, J.; Yu, K.J. Emerging materials and technologies with applications in flexible neural implants: A comprehensive review of current issues with neural devices. Adv. Mater. 2021, 33, 2005786. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.O.; Serruya, M.D.; Wolf, J.A.; Cullen, D.K. Bioactive neuroelectronic interfaces. Front. Neurosci. 2019, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Savelyev, M.S.; Kuksin, A.V.; Murashko, D.T.; Otsupko, E.P.; Suchkova, V.V.; Popovich, K.D.; Vasilevsky, P.N.; Vasilevskaya, Y.O.; Kurilova, U.E.; Eganova, E.M.; et al. Formation of Neurointerfaces Based on Electrically Conductive Biopolymers by Two-Photon Polymerization Method. Polymers 2025, 17, 1300. [Google Scholar] [CrossRef] [PubMed]
- Junzeng, S.; Yanhong, Y.; Xiaoling, X.; Feng, Y.; Peiyan, S. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. Polym. Degrad. Stab. 2019, 166, 73. [Google Scholar] [CrossRef]
- Di Lisa, D.; Muzzi, L.; Pepe, S.; Dellacasa, E.; Frega, M.; Fassio, A.; Martinoia, S.; Pastorino, L. On the Way Back from 3D to 2D: Chitosan Promotes Adhesion and Development of Neuronal Networks onto Culture Supports. Carbohydr. Polym. 2022, 297, 120049. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H. Collagen for neural tissue engineering: Materials, strategies, and challenges. Mater. Today Bio 2023, 20, 100639. [Google Scholar] [CrossRef] [PubMed]
- Rennhofer, H.; Zanghellini, B. Dispersion state and damage of carbon nanotubes and carbon nanofibers by ultrasonic dispersion: A review. Nanomaterials 2021, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Slepchenkov, M.M.; Barkov, P.V.; Glukhova, O.E. Hybrid Films Based on Bilayer Graphene and Single-Walled Carbon Nanotubes: Simulation of Atomic Structure and Study of Electrically Conductive Properties. Nanomaterials 2021, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).