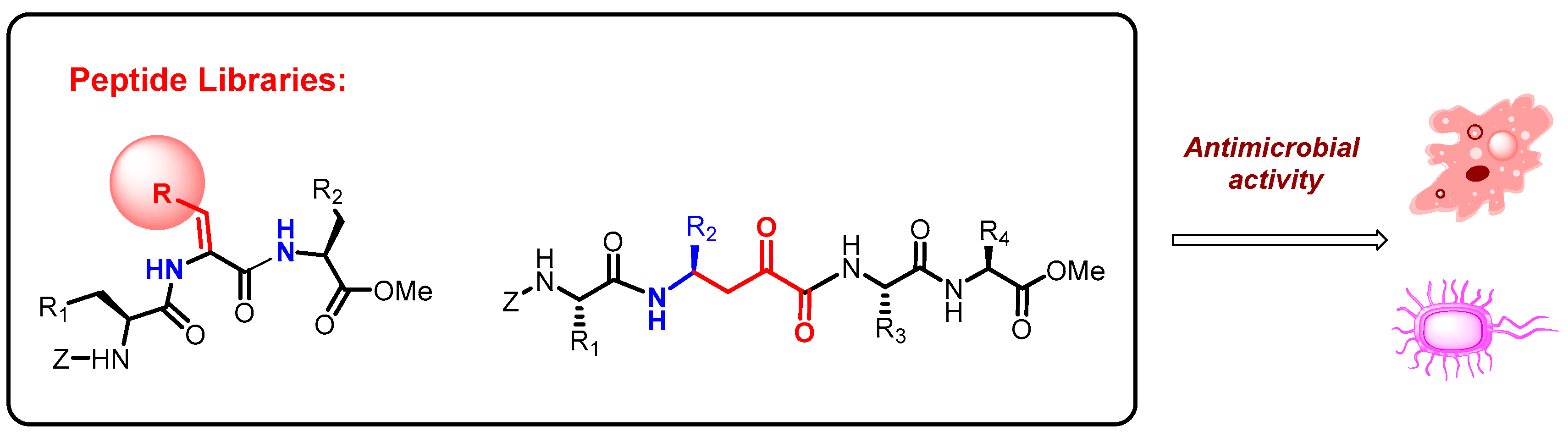
Libraries of compounds containing unnatural amino acids or amino acid analogues were prepared from inexpensive substrates using sustainable processes [1,2,3]. The compounds were evaluated for antimicrobial activity against different pathogens.
Author Contributions
D.H. and A.B. supervised the work carried out by D.H., C.S., A.B. and C.C.; J.M.P.d.L. supervised the antimicrobial screenings.
Acknowledgments
This work was supported by the Research Program SAF-2013-48399-R, Plan Estatal de I + D, Ministerio de Economía y Competitividad, Spain, and European Social Funds (FSE). D.H. thanks CSIC (Spanish Research Council) her JAE postdoctoral research contract, and also thanks her current contract financed by Cabildo de Tenerife, Program TF INNOVA 2016-21 (with MEDI & FDCAN Funds). C.S. thanks Gobierno de Canarias for his former research contract, and MINECO for his current Torres Quevedo contract (PTQ-15-07923) with BIOSIGMA SL. C.C. thanks MINECO for her Doctorados Industriales contract (D1406736) with BIOSIGMA SL.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saavedra, C.J.; Boto, A.; Hernández, R. “Customizable” Units in Di- and Tripeptides: Selective Conversion into Substituted Dehydroamino Acids. Org. Lett. 2012, 14, 3788–3791. [Google Scholar] [CrossRef] [PubMed]
- Boto, A.; Hernández, D.; Hernández, R. One-Pot Conversion of Proline Derivatives into Iodinated Iminosugar-Based Nucleosides, Useful Precursors of Highly Functionalized Nucleoside Analogues. Eur. J. Org. Chem. 2010, 6633–6642. [Google Scholar] [CrossRef]
- Hernández, D.; Boto, A.; Guzmán, D.; Alvarez, E. Metal-free, direct conversion of α-amino acids into α-keto γ-amino esters for the synthesis of α,γ-peptides. Org. Biomol. Chem. 2017, 15, 7736–7742. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).