Abstract
Electrochemiluminescence (ECL) has been a useful analytical tool for fields such as molecular biology, food analysis, and analytical chemistry. In situ generation of reactive oxygen species (ROS) by surface mediated catalysis works collaboratively with the luminol based ECL system. This work describes an ECL strategy involving in situ ROS generation from the surface of gold nanoparticles (AuNP). We reported on a novel condition to induce AuNP catalysis, and examined the application of this phenomenon to an immunosensing platform based on AuNP catalysis and ECL.
1. Introduction
Electrochemiluminescence (ECL) does not require a light source, and allows higher sensitivity measurements compared to colorimetric and fluorescence methods. These characteristics make ECL a promising tool for various biosensing applications [1,2,3,4,5]. In this work, to achieve highly sensitive biosensing based on ECL (Figure 1 and Figure 2), we focused on gold nanoparticle (AuNP) catalysis. AuNPs are widely known to catalyze various reactions such as the oxidation of carbon monoxide to carbon dioxide [6]. Our purposes are to use AuNP catalysis for the high efficiency generation of ROS, and to measure the generated ROS by ECL. To this end, we investigated the mechanism of AuNP catalyzed ROS generation. Also, a sandwich-type immunoassay for the detection of IgA using AuNP catalysis, magnetic beads (MB) and measurement by ECL was proposed.
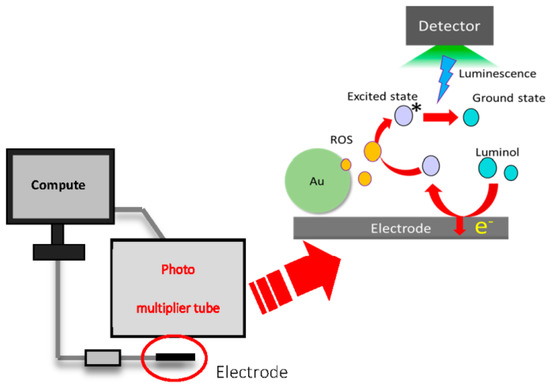
Figure 1.
Device and measurement principle of ECL.
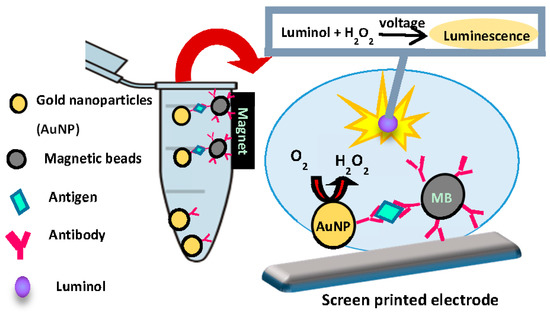
Figure 2.
Schematic of biosensing application of AuNP catalysis.
2. Materials and Methods
2.1. Reagents and Chemicals
All materials used are of analytical grade. Luminol and was purchased from Sigma-Aldrich (Saint Louis, MO, USA). NHS activated magnetic beads were kindly provided by Tamagawa Seiki (Nagano, Japan). Tris-HCl buffer was adjusted to pH 8.0 with HCl. Luminol was prepared by dissolving in 0.1 M NaOH solution and stirred until completely dissolved and stored at −20 °C until use. Gold nanoparticles was purchased from TANAKA Precious Metals (Tokyo, Japan). IgA antigen and antibody were purchased from Bethyl Laboratories, Inc. (Montgomery, TX, USA).
2.2. Instruments
The ECL monitoring system (BDTeCLP100, Bio Device Technology Ltd., Ishikawa, Japan) comprises a photon detection unit (C969212; Hamamatsu Photonics K.K., Hamamatsu, Japan) and an USB powered hand-held potentiostat (Bio Device Technology Ltd., Ishikawa, Japan). The potentiostat has a system that sends a trigger signal to the photon detection unit to coordinate the electrochemical and the ECL measurements. Disposable screen-printed electrode (SPE) chips were used for the electrochemical measurements. These electrode chips consist of carbon working and counter electrodes, and an Ag/AgCl reference electrode. The electrode chip (type EP-P) used with the ECL monitoring system has dimensions of 12.5 × 4 mm and the working electrode area was 2.64 mm2. For the following experiments, a new chip was used for every measurement.
2.3. ECL Measurement Procedure for ROS Generation from AuNPs
For the evaluation of ROS generation from AuNPs using the ECL system, AuNPs were dispersed in various solvents, and left for several minutes. 20 μL of this sample was then pipetted on an SPE after mixing with 0.1 mM luminol, and luminescence intensity was measured during linear sweep voltammetry (LSV) of the sample (0~700 mV, scan rate 50 mV/s).
2.4. Antibody Modification on Magnetic Beads
MBs were modified with anti-IgA antibodies according to a protocol from previous research [6]. In brief, MBs activated with NHS (200 nm, Tamagawa Seiki) were washed with methanol by centrifugation for 5 min at 15,000 rpm and 4 °C. After discarding the supernatant, antibodies were allowed to react with the NHS groups on the MBs for 1 day at 4 °C. The modified MBs were collected and dispersed in aminoethanol overnight to mask unreacted NHS groups. After this step, the MBs were washed three times with buffer containing 200 mM PBS-NaOH (pH 7.40), re-dispersed in the same buffer and stored at 4 °C until use.
2.5. Antibody Modification on AuNP
AuNPs were also modified with anti-IgA antibodies according to a protocol from previous research [6]. 100 μL anti-IgA was added to 10 mL of AuNPs dispersed in PBS under stirring for 24 h at 4 °C. The resulting anti-IgA/AuNP composites were obtained by centrifuging (15,000 rpm, 30 min) and washing with 200 mM PBS (pH 7.40) three times. Afterwards, the anti-IgA/AuNP composites were dispersed in PBS (pH 7.40) and stored at 4 °C until use.
2.6. ECL Immunoassay for IgA
90 μL of the anti-IgA/MB composites was mixed with 10 μL of PBS spiked with various concentrations of IgA. The mixture was then incubated under mild agitation at room temperature for about 1.5 h until reaction equilibrium between the IgA and the anti-IgA/MB composites was reached. Afterwards, these mixtures containing different IgA concentrations were incubated with 100 μL of anti-IgA/AuNP composites for 30 min. As a result, magnetic sandwich-type conjugates formed between the IgA and the antibodies coated on the two nanomaterials respectively, through antigen-antibody recognition. Finally, the conjugates were collected by a magnet, and used for ECL detection as described earlier.
3. Results and Discussion
3.1. Characterization of AuNP Catalysis
ECL was used to measure the changes in AuNP catalyzed ROS generation by varying the concentration and size of AuNPs and the type of solvent. The ECL intensity was observed to increase with both decreasing AuNP size and increasing AuNP concentration. In addition, luminescence was observed only from AuNPs dispersed in buffers that contain species with both hydroxyl and amino groups (Figure 3). Based on these results, it is likely that solvent structure is related to ROS generation from AuNPs.
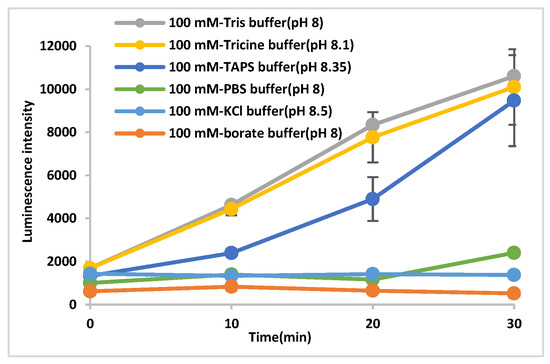
Figure 3.
Luminescence from AuNP (15 nm) dispersed in various buffers.
Next the effect of dissolved oxygen on ROS generation from AuNPs was investigated. AuNPs were first dispersed in Tris buffer, and ECL was measured both without and after bubbling the AuNP solution with nitrogen gas. Luminescence was observed only for the buffer that was not bubbled with nitrogen gas. This result indicates that ROS is generated from the reaction of oxygen dissolved in solution on the surface of AuNPs.
To determine whether ROS generation was occurring on or away from the surface of AuNPs, BSA was used to coat AuNP surfaces prior to ECL measurements. AuNPs were coated by mixing with BSA (concentration of 0, 10 nM, 1 μM, 100 μM) for 1 h. The ECL intensity was observed to decrease with increasing concentrations of BSA (Figure 4). This suggests that ROS generation from oxygen and water at the AuNP surface is enabled by the binding of Tris to the AuNP surface.
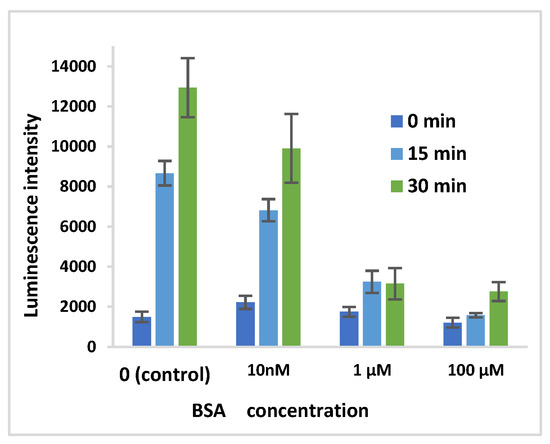
Figure 4.
Luminescence from AuNP after coating with BSA.
3.2. Analytical Performance of the ECL Immunoassay for IgA Using AuNP and MB
The sensitivity and quantitative range of the ECL immunoassay were studied by measuring samples with different IgA concentrations using the procedure. As seen in Figure 5, the luminescence intensity increases with increasing sample IgA concentrationin in the range of 0~100 μg/mL. This result points to the potential applicability of this immunoassay for various biosensing applications. It is expected that its sensitivity could be improved further by reducing the luminescence from blank samples (no IgA added).
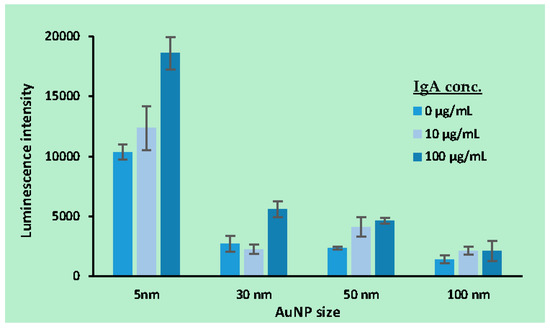
Figure 5.
Luminescence from AuNPs (5 nm, 30 nm, 50 nm, 100 nm) after immunoassay for IgA.
4. Conclusions
In this work, the AuNP-catalyzed in situ generation of ROS was studied using ECL and applied to an immunoassay for the detection of IgA. It was found that ROS was generated only from AuNPs dissolved in buffers that contain species with both hydroxyl and amino groups. The obtained results indicate that ROS generation from oxygen and water at the AuNP surface is enabled by the binding of Tris to the AuNP surface.
Furthermore, the luminescence intensity was found to increase with increasing IgA concentration in the range of 0~100 μg/mL. It is expected that further improvement of this immunoassay could enable its use in other biosensing applications.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuki, I.; Masato, S. Quenched Electrochemiluminescence Imaging using Electro-Generated Substrate for Sensitive Detection of Catalase as Potential Enzyme Reporter System. Electrochem. Acta 2017, 240, 447–455. [Google Scholar] [CrossRef]
- Parajuli, S.; Miao, W. Sensitive determination of triacetone triperoxide explosives using electrogenerated chemiluminescence. Anal. Chem. 2013, 85, 8008–8014. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.S.; Le, Q.H.; Hasan, Q.; Yoshikawa, H.; Saito, M.; Tamiya, E. Enhanced Electrochemiluminescence of N-(aminobutyl)-N-(ethylisoluminol) Functionalized Gold Nanoparticles by Graphene Oxide Nanoribbons. Electrochim. Acta 2015, 180, 409–418. [Google Scholar] [CrossRef]
- Nagatani, N.; Inoue, Y.; Araki, A.; Ushijima, H.; Hattori, G.; Sakurai, Y.; Ogidou, Y.; Saito, M.; Tamiya, E. Rapid sensing of antioxidant capacity based on electrochemiluminescence induced by electrochemically generated reactive oxygen species. Electrochim. Acta 2016. [Google Scholar] [CrossRef]
- Ismail, N.S.; Le, Q.H.; Huong, V.T.; Inoue, Y.; Yoshikawa, H.; Saito, M.; Tamiya, E. Electrochemiluminescence based enzymatic urea sensor using nanohybrid of isoluminol-gold nanoparticle-graphene oxide nanoribbons. Electroanalysis 2016, 2, 28. [Google Scholar] [CrossRef]
- Huang, X.P.; Li, Y.Q. A novel reverse fluorescent immunoassay approach for sensing human chorionic gonadotropin based on silver-gold nano-alloy and magnetic nanoparticles. Anal. Bioanal. Chem. 2016, 408, 619–627. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).