Abstract
The development of selective and cheap metal oxide gas sensor at ambient temperature is still a challenging idea. In this study, SnO2 surface functionalization was performed in order to obtain sensitive and selective gas sensor operated at ambient temperature. 3-aminopropyltriethoxysilane (APTES) was used as an intermediate step, followed by functionalization with molecules having acyl chloride with different end functional groups molecules such as alkyl, acid and ester groups. Acid and ester modified sensors are sensitive to ammonia between 0.2 and 10 ppm at room temperature. However, ester modified SnO2 is more selective than acid modified sensor regarding ethanol and carbon monoxide gases.
Keywords:
SnO2; molecular modification; APTES; gas sensor; sensitivity; selectivity; ammonia; ambient temperature 1. Introduction
A simple and noninvasive tool for disease diagnosis is offered by the detection of gases in exhaled breath. Metal oxide chemical sensors are promising devices because they are small, easy to use and less expensive than other spectrometry techniques [1]. For such application, the gas sensor should be sensitive to low concentration of gas and selective to one gas in presence of other gases. Tin dioxide material allows the detection of many gaseous compounds. Nevertheless, the SnO2-based sensors suffer from two major drawbacks which constitute an obstacle to their development. SnO2 has lack of selectivity because it has high affinity with many gases. In addition, SnO2 has high operating temperature between 250 °C and 500 °C. One way to cover these drawbacks is the surface molecular functionalization of SnO2. Some work on silicon nanowire field effect transistors showed change in the working principle of the sensor. In this case, the interactions are between the gas and the grafted organic layer rather than the metal oxide substrate. The interactions of gases with the attached layer lead to change in the dipole moment that variate the electrical properties of substrate (e.g., SnO2) [2]. Such modified sensors work at ambient temperature which reduces the power consumption since these sensors will be used in portable devices.
In this work we are intended to achieve surface functionalization of SnO2 thick film. The functionalization of SnO2 was performed to cover these well-known disadvantages. The purpose of functionalization was to passivate the surface states on the SnO2 sensors by molecular layer aiming to optimize their interactions with ammonia gas. The first step of molecular modification is the attachment of APTES. The second step is to fix alkyl, acid and ester functional groups on APTES modified SnO2. These sensors are tested to ammonia gas at room temperature. The selectivity is examined versus ethanol, carbon monoxide and acetone gases.
2. Materials and Methods
In this study, SnO2 thick films are deposited on alumina substrate by screen-printing technique. A semi-automatic Aurel C890 machine was used. The procedures for preparing the SnO2 ink and sensor fabrication parameters have been described elsewhere [3]. The SnO2 ink is produced by mixing SnO2 powder (Prolabo Company) with a solvent and an organic binder. This ink was then screen printed on an alpha-alumina substrate (38 × 5 × 0.4 mm3) provided with sputtered gold electrodes. The deposited SnO2 material is finally annealed for 10 h at 700 °C in air to get SnO2 film with a thickness of 40 microns. The SnO2 particles sizes of the elaborated films were found to be around 75 nm.
The functionalization of SnO2 was carried out by two steps which lead to covalent attachment of alkyl, acid and ester end functional groups. The first step is the condensation reaction between the hydroxyl groups present on SnO2 surface and 3-aminopropyltriethoxysilane (APTES from ACROS Organics). This step generates a film terminated by amine groups (SnO2-APTES) which will act as a substrate for the second step of modifications. Silanization in liquid phase has been described elsewhere [4]. In a first step, SnO2 sensors were immersed in 50 mM APTES dissolved in 95% absolute ethanol and 5% of deionized water for 5 h under stirring at room temperature. After reaction, the sensors were rinsed with absolute ethanol and dried under N2 flow to remove the unbounded APTES molecules. As a second step, these terminal amine groups of APTES allow the coupling reaction with molecules bearing acyl chloride group. In the second step, alkyl, acid and ester end group grafting was achieved by immersing APTES modified SnO2 in a solution of 10 mM of hexanoyl chloride (98%, ALDRICH, alkyl: C6H11ClO), 1,4-butanedicarbonyl chloride (98%, Fluka, acid: C6H8Cl2O2) or methyl adipoyl chloride (96%, Alfa Aesar, ester: C7H11ClO3), with 5 μL of triethylamine (Fluka) in 5 mL of chloroform as solvent for 12 h under stirring. The sensors were then rinsed with chloroform and dried under N2 flow.
The elaborated sensors were tested under gases in a test bench at 25 °C to check the effects of functionalization on sensor performance. In this test bench, sensors are installed in an 80 cm3 glass chamber under constant gas flow of 15 L/h. It is provided with gas mass flow controllers which allow controlling the concentrations of different gases at same time. The sensitivity was tested to ammonia gas at 25 °C with air containing 5% of relative humidity (RH). It is defined as the slope of the tangent on the curve of response (GN-GA/GA, with GN: conductance after 20 min of ammonia injection, and GA: conductance under air with 5%RH) versus ammonia concentration. Sensors selectivity was tested versus ethanol, carbon monoxide and acetone gases.
3. Results and Discussion
The first part of the test under gases was to show the characteristic of the response of different sensors to ammonia gas. As shown in Figure 1, the sensors were tested to 100 ppm of ammonia balanced with 5%RH air at 25 °C. The conductance of pure SnO2 decreases upon exposure to ammonia gas. This type of response has been founded before by Kamalpreet Khun Khun et al. [5] at temperature between 25 to 200 °C. SnO2-APTES shows no change in conductance upon exposure to ammonia. This implies that no significant interactions occur between the grafted APTES and ammonia gas. In term of polarity and other chemical properties like acidity, the amine and ammonia groups are almost the same, since amine is one of the derivatives of ammonia. Hence, such response was expected for SnO2-APTES. In addition, this behavior indicates that the SnO2 surface is well covered by APTES molecules because the negative response observed on pure SnO2 is totally inhibited.
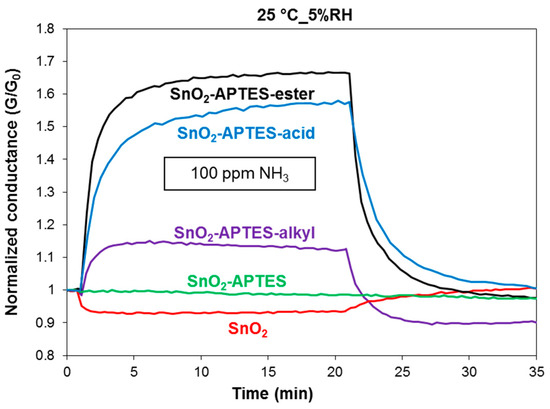
Figure 1.
The sensor response of SnO2, SnO2-APTES, SnO2-APTES-alkyl, SnO2-APTES-acid and SnO2-APTES-ester to 100 ppm of ammonia gas balanced with humid air (5%RH) at 25 °C. G is the conductance at any time and G0 is the conductance at the beginning of the test (t = 0).
SnO2-APTES-ester, SnO2-APTES-acid and SnO2-APTES-alkyl exhibit increase in conductance upon exposure to 100 ppm of ammonia gas (Figure 1). However, the response of SnO2-APTES-ester and SnO2-APTES-acid are more important than that of SnO2-APTES-alkyl. These responses could be related to the different polarities of the attached end functional groups. The interaction of ammonia with ester and acid modified SnO2 is dipole-dipole while it’s induced-dipole in the case of alkyl modified sensor. In addition, dipole-dipole interaction is always stronger than induced dipole interaction [2]. These interactions of ammonia molecule with the attached end functional groups lead to variation in the dipole moment of the molecular layer. The variation in the molecular layer’s dipole moment affects the electron mobility in SnO2 film which modifies the conductance [6].
Regarding the different sensors sensitivity against ammonia concentrations, Figure 2 shows the relative responses versus ammonia concentrations of SnO2-APTES-ester and SnO2-APTES-acid in comparison with pure SnO2, SnO2-APTES and SnO2-APTES-alkyl sensors. Pure SnO2 and SnO2-APTES sensors have almost no sensitivity to different ammonia concentrations. In addition, SnO2-APTES-alkyl gives no significant response between 0.2 ppm and 30 ppm. It can be noticed that SnO2-APTES-ester and SnO2-APTES-acid exhibit constant sensitivity between 0.2 ppm and 10 ppm, around 0.023 ppm−1.
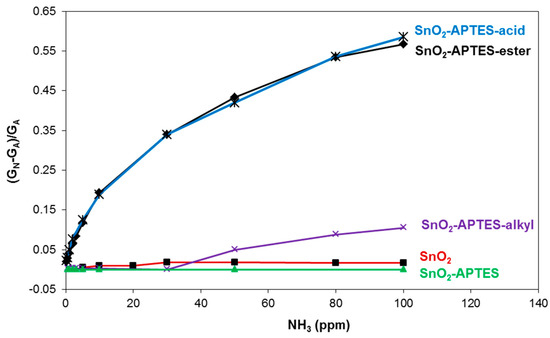
Figure 2.
Relative response of SnO2, SnO2-APTES, SnO2-APTES-alkyl, SnO2-APTES-acid and SnO2-APTES-ester sensors versus ammonia concentrations in 5%RH air at 25 °C.
Concerning the selectivity of ester and acid modified sensors, Figure 3 shows that the SnO2-APTES-ester sensor has almost no change in conductance upon exposure to 50 ppm of ethanol, carbon monoxide and acetone at 25 °C. This means that ester modified sensor is selective to ammonia, at least in regards of the three tested gases. However, acid modified SnO2 shows less selectivity than ester modified sensor versus ethanol and carbon monoxide. This particular selectivity derives from the unique interactions of the grafted layer on SnO2 with ammonia gas.
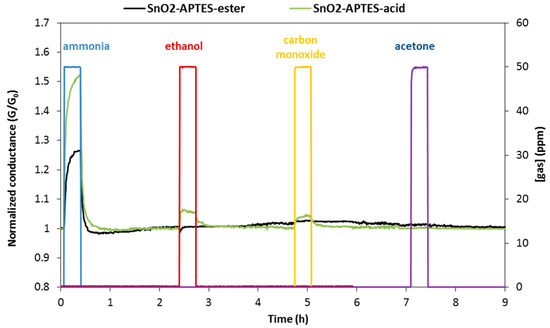
Figure 3.
The sensor response of SnO2-APTES-ester and SnO2-APTES-acid upon exposure to 50 ppm of ammonia, ethanol, carbon monoxide and acetone gases in humid air (5%RH) at 25 °C.
4. Conclusions
SnO2 thick films were produced by screen printing and molecularly modified by solution chemical processes. Pure SnO2 sensor and APTES modified SnO2 didn’t show any significant sensitivity to ammonia between 0.5–100 ppm under 5%RH at 25 °C. On the contrary, ester and acid modified sensors show constant sensitivity between 0.5 and 10 ppm to ammonia (0.023 ppm−1) at 25 °C. Moreover, ester modified SnO2-APTES sensor is more selective to ammonia gas with respect to reducing gases like ethanol, carbon monoxide and acetone than the acid modified one. As a conclusion, ester modified sensor is a good candidate for breath analysis applications for the diagnosis of diseases related to ammonia gas biomarker. Furthermore, working at ambient temperature is an important challenge to fulfill in view of flexible sensing substrate. Here, a potential molecular modification of SnO2 for smart devices and portable applications has been put in evidence by our results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kahn, N.; Lavie, O.; Paz, M.; Segev, Y.; Haick, H. Dynamic Nanoparticle-Based Flexible Sensors: Diagnosis of Ovarian Carcinoma from Exhaled Breath. Nano Lett. 2015, 15, 7023–7028. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Haick, H. Effect of Functional Groups on the Sensing Properties of Silicon Nanowires toward Volatile Compounds. ACS Appl. Mater. Interfaces 2013, 5, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Tournier, G.; Pijolat, C. Selective filter for SnO2-based gas sensor: Application to hydrogen trace detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Le, M.; Jimenez, C.; Chainet, E.; Stambouli, V. A Label-Free Impedimetric DNA Sensor Based on a Nanoporous SnO2 Film: Fabrication and Detection Performance. Sensors 2015, 15, 10686–10704. [Google Scholar] [CrossRef] [PubMed]
- Khun Khun, K.; Mahajan, A.; Bedi, R.K. SnO2 thick films for room temperature gas sensing applications. J. Appl. Phys. 2009, 106. [Google Scholar] [CrossRef]
- Hoft, R.C.; Ford, M.J.; Cortie, M.B. Effect of dipole moment on current-voltage characteristics of single molecules. In Proceedings of the International Conference on Nanoscience and Nanotechnology (ICONN), Brisbane, Australia, 3–7 July 2006. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).