Abstract
Decreasing global tuberculosis (TB) notifications indicate problems related to TB patient detection and treatment outcomes. Pharmaceutical care (PC) has potential roles in managing these issues. However, PC practices have not yet become widespread in the real world. This systematic scoping review aimed to identify and analyze the current literature on practical models of pharmaceutical care for improving tuberculosis patient detection and treatment outcomes. We then discussed the present challenges and future considerations for the successful implementation of PC services in TB. A systematic scoping review was performed to identify the practice models of PC in TB. Systematic searches and screening were used to identify relevant articles in the PubMed and Cochrane databases. We then discussed the challenges and recommendations for successful implementation using a framework to improve professional healthcare practice. Our analysis included 14 of 201 eligible articles. We identified that the focuses in the PC of TB are on increasing patient detection (four articles) and improving TB treatment outcomes (ten articles). Practices cover services in the community and hospital settings, such as screening and referring people with presumptive TB, tuberculin test services, collaborative practices for treatment completion, directly observed treatment, the solution of drug-related problems, reporting and managing adverse drug reactions, and medication adherence programs. Although PC services positively increase TB patient detection and treatment outcomes, hidden challenges in the actual practice are analyzed. Several factors should be comprehensively considered in successful implementation, such as guidelines, individual pharmacy personnel, patient, professional interaction, organizational capacity, regulation, incentive, and resource factors. Hence, a collaborative PC program that involves all related stakeholders should be considered to create successful and sustainable PC services in TB.
1. Introduction
Tuberculosis (TB) is a seriously threatening disease worldwide. A global report estimated that 10.6 million people contracted TB, and 1.4 million died in 2021 []. Multidrug-resistant TB (MDR-TB), an evolved pathogen that is resistant to two powerful anti-TB drugs (isoniazid and rifampicin), poses a major problem. The global treatment success rate of MDR-TB remains low, at 60% []. The spread of COVID-19 during the current pandemic and its global spread complicates the picture further. New notification TB cases worldwide significantly dropped from 7.1 million (2019) to 5.8 million (2020) [], indicating a number of potentially missed TB cases and poor treatment outcomes, leading to increased TB incidence and mortality in the coming years.
The updated global TB report described that the complexity of TB-related problems in countries with high TB prevalence leads to the largest contribution in decreasing global TB notification []. It highlights the potential problems of TB patient detection and treatment. Previous studies have demonstrated that restricted patient access to diagnosis and treatment and limited healthcare staff availability at community health centers are barriers to successful TB treatment in high-prevalence TB countries [,]. These works report that the current healthcare systems are unable to detect, treat, and report TB cases optimally. An improved engagement program for all resources is essentially needed to overcome problems related to TB patient detection and treatment outcomes.
Pharmacy personnel play a role in managing TB. Patient pathway studies show that pharmacies are primary facilities for first aid for people affected by TB [,] and can be a potential site for increased TB case detection. TB treatment complexity is greater in hospital environments due to various types of critically ill patients. Pharmacotherapeutic follow-up is essential for these patients since they may have more potential drug-related problems (DRPs), especially when they have other associated diseases in addition to TB [].
A joint statement by the International Pharmaceutical Federation (FIP) and World Health Organization (WHO) urged national TB programs and national pharmacy associations to jointly implement TB programs []. However, this has not seen widespread practical implementation []. Because improving the practice of health professionals involves complex determinants [], studies of practice models and factors of their successful implementation are needed. This will provide insight for developing modern pharmaceutical care (PC) to improve tuberculosis patient outcomes and minimize healthcare costs, especially in high-burden TB countries. Therefore, this study aimed to review various models of pharmaceutical services and their effects on improving TB patient detection and treatment outcomes. We then analyzed challenges and future considerations for the successful implementation of PC services in TB.
2. Materials and Methods
2.1. Study Design and Search Strategy
A systematic scoping review was performed to identify models of PC practice in TB. We then analyzed challenges and developed recommendations for successful implementation, observing the framework for improvements in professional healthcare practices developed by Fottorp et al., 2013 []. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidance for the transparent and systematic report [].
A study protocol was developed prior to the study. Since a scoping review protocol cannot be registered in the PROSPERO (a prospective international registration for systematic reviews), the unregistered protocol was internally used as a study guide for the research team. According to the study protocol developed, a comprehensive search strategy was used to retrieve relevant articles from two reputable medical databases. PubMed, a medical research database, was searched to identify relevant articles, and Cochrane Library, indexed articles in area intervention studies, was used to ensure a comprehensive search. Pharmaceutical services and TB terms combined medical subheadings (Mesh), and Boolean operators were also used. The search was conducted on texts published on 31 July 2022, without language or publication date restriction. The search strategies were developed involving several key terms, i.e., “Pharmacy”, “Pharmacies”, “Pharmacy Service”, “Hospital”, “Pharmacy Technicians”, “Community Pharmacy Services”, “Pharmacy Research”, “Pharmaceutical Services”, “Tuberculosis”, “Latent Tuberculosis”, “Multidrug-Resistant”. The full search strategies are given in Supplementary File S1.
2.2. Study Criteria
We defined the PECOS (population, exposure, comparison, outcomes, and study type) to obtain the relevant articles in this review. The population was presumptive, latent, and active tuberculosis subjects, while the exposure was PC intervention in community and hospital settings. The study comparator was a care without PC interventions or descriptive situations before the PC was applied in pre–post interventional studies. Improving TB case detection and treatment outcomes were defined as the study outcome in this review.
In terms of study type, we included original articles that analyzed the effects of PC interventions on TB. We excluded review articles, case reports, case series, commentaries/editorials, book chapters, study protocols, guidelines, and articles that only assessed baseline information for implementing PC.
2.3. Article Selection, Data Extraction, and Analysis
Selected articles from the databases were reviewed using the study criteria in two steps. The first step focused on the title and abstract, followed by full-text reviews in the second step. Three reviewers (E.O.Y., C.Y.N., and N.P.M.) independently performed title–abstract and full-text screening. Discrepancies were solved in intensive discussions with the fourth reviewer (I.S.P.). Since we conducted a scoping review that provided an overview of the existing evidence regardless of methodological quality, risk of bias assessments was not performed in our study [].
In the full-text review, we assessed article eligibility in detail regarding their population, intervention, comparator, outcome, and design. Essential information was extracted for articles fitting the criteria, i.e., the authors, objective, design, study period, location, target population, PC models, and outcomes. The effect measures for study outcome were assessed for each study. The effect measurements of the study outcomes were descriptive (percentage, number) or comparative (odds ratio or relative risk) analyses. Three reviewers (E.O.Y., C.Y.N., and N.P.M.) conducted the initial data extraction, and the fourth reviewer (I.S.P.) made the final decision. A qualitative synthesis was performed to map pharmaceutical models across studies, and the practical challenges and recommendations were described in the narrative analysis.
3. Results
During the search, we identified 199 records from the PubMed database and ten records from the Cochrane Library database. We found eight duplicate records. A total of 201 articles were screened for the title and abstract. This initial screening excluded 187 irrelevant articles with various reasons, i.e., incompatible intervention (108), outcome (1), population (18), and type of articles (i.e., case report/series (4), review (45), book chapter/report (2), protocol (3), commentary/editorial (5), and guideline (1)). We finally performed a full-text screening of 14 articles for the qualitative analysis. The heterogeneity in populations, interventions, and outcomes across the included studies prevented quantitative analysis. The flow diagram of the included articles is presented in Figure 1.
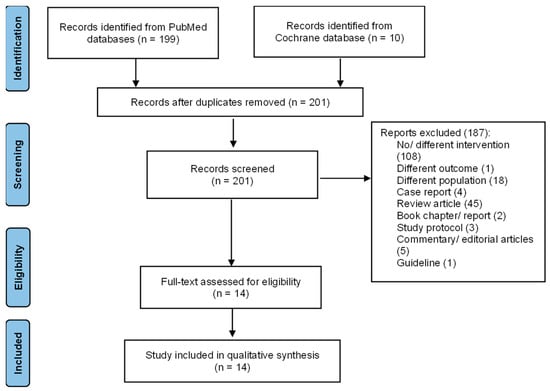
Figure 1.
Flow diagram of the included articles.
We obtained studies from high- and low-prevalence tuberculosis countries, namely Pakistan [], Vietnam [], Bolivia [], the United States [,,,,], Thailand [], Spain [], China [], Turkey [], Indonesia [], and Brazil []. PC interventions for latent TB infection (LTBI) and presumptive and active TB populations were studied. PC services were conducted in hospital (five studies) and community (nine studies) settings. We identified two main outcome orientations for PC intervention, i.e., increasing TB patient detection and improving TB treatment outcomes. Characteristics of the included studies are presented in Table 1.

Table 1.
Characteristics of the included studies.
3.1. PC in Increasing TB Patient Detection
Five studies presented means of increasing TB patient detection. Three focused on how pharmacy personnel can screen presumptive TB patients and refer them to appropriate facilities for further TB examination and diagnosis [,,], and two focused on pharmacists’ ability to administer tuberculin skin tests (TSTs) to support LTBI diagnosis [,]. PC models and their effects on increasing TB patient detection are presented in Table 2.

Table 2.
Pharmaceutical care models and the effects on increasing tuberculosis patient detection.
In screening and referring activities, pharmacy personnel identified presumptive TB patients among customers at their pharmacies. Pharmacy personnel directly communicated with customers in exploring TB signs and symptoms, such as persistent cough, weight loss, night sweats, high temperature, fatigue, loss of appetite, and neck swelling. Once a presumptive TB patient was identified, pharmacy personnel recommended further TB examination at a particular health facility.
The number of pharmacies included in these studies ranged between 70 and 100. The ability of pharmacy personnel to refer presumptive TB patients depended on the number of pharmacies engaged. A Pakistani study successfully referred 3025 visitors to health facilities from 500 pharmacies [], while studies in Vietnam and Bolivia only referred 310 and 41 individuals to health facilities from 150 and 70 pharmacies, respectively [,]. However, the data show that not all visitors followed pharmacy recommendations. We noted that the number of visitors following the pharmacy’s recommendation ranged from 11 to 1901. Furthermore, studies showed that a few visitors were then diagnosed with TB. Studies from Pakistan, Vietnam, and Bolivia showed successful diagnoses of TB in 547, 10, and 3 individuals, respectively.
Two studies from the US indicated that pharmacists were granted the authority to prescribe, administer, and read the TSTs [,]. This provides a wide access to TSTs, which can help support LTBI/TB diagnosis. Pharmacists should follow their training to maintain their TST license. Two studies reported that 43 and 2 pharmacists participated in each program; the total number of those tested in the two studies was 578 and 18, respectively. However, not all of those examined returned for the test reading. We noted that in those two studies that 536 and 17 people revisited the pharmacy for the reading test, with positivity rates of 3.1% and 0%, respectively. Most patients in studies with a zero positivity rate were from a low-risk LTBI/TB population, and the tests were generally needed for job requirements []. The average time for conducting the test in the pharmacy was less than 10 min [].
3.2. PC in Improving TB Treatment Outcomes
We identified nine studies with outcome orientations of improving TB treatment outcomes [,,,,,,,]. Of these, four focused on community pharmacy services and five on hospital services. Those focused on community pharmacy included three which specifically analyzed the role of pharmacy in improving LTBI treatment [,,], and one investigated community pharmacists as directly observed treatment (DOT) providers to improve the rational use of TB medication []. In the hospital pharmacy service, all studies analyzed PC activities in the hospital regarding the assessment, monitoring, and follow-up of patient DRPs, educational programs for medication adherence, direct/indirect scheduled meeting, and medication consultation [,,,,]. PC models and their effects on TB treatment outcomes are presented in Table 3.

Table 3.
Pharmaceutical care models and the effects on improving tuberculosis treatment outcomes.
In LTBI care, collaborative practices between community pharmacists and healthcare staff are described in three US studies. These studies highlighted the community pharmacist’s role in supporting treatment completion in LTBI patients. One study focused on a refugee population [], while the others examined the general LTBI population [,]. Generally, community pharmacists conducted regular meetings for assessing and reporting DRPs (including adverse drug reactions), drug monitoring, and counselling to support treatment completion in LTBI patients. Contributing community pharmacists produced a relatively high treatment completion of 59–91%. Most incomplete treatments were due to ADR, and the remainder were due to a lack of perception of treatment benefits or moving to another health facility. A study found that collaborative LTBI care between community pharmacists and public health departments reduced as much as 143 h of labor for the public health department [].
In active TB care, one study demonstrated a community pharmacy providing DOT services to improve medication adherence in high-risk non-adherent populations []. Collaborators included the pharmacist, a hospital pulmonologist, and a part-time social worker. The pharmacist provided several services during each patient visit, including reinforcing the importance of treatment adherence, inquiring about DRP events, reminding the patient of upcoming appointments at the pharmacy and hospital, and offering required sociosanitary support. That study reported that the community pharmacists successfully identified 108 DRPs. In a comparison between a PC intervention group and a self-administrated treatment (SAT) group, treatment completion was significantly higher in the PC intervention group (RR 3.07; 95%CI 2.13–4.41), and failed treatment was less likely in the PC group (RR 0.33; 95%CI 0.22–0.50) [].
In hospital settings, we found that hospital pharmacists contributed to the improvement of TB treatment outcomes through several services, including assessing, monitoring, following up, and reporting potential and actual DRPs, lab checks for identifying ADR, providing face-to-face or phone drug consultation, and administering educational programs for medication adherence with standardized written and oral counseling. An observational study reported that in a Thai hospital, PC intervention had the highest proportion of treatment success (94.90%; CI 91.57–95.63), better than home visits (93.60%; CI 91.57–95.63) and modified DOT (90.10%; CI 87.54–92.66) []. However, another study from China found no significant difference between PC intervention and usual care for TB treatment outcomes (i.e., treatment success, failure, default, transfer out, death, or sputum conversion time) [].
Furthermore, two studies have shown that the attendance of patient visits was significantly higher in the PC intervention group than in the PC intervention (p = 0.018 and p ≤ 0.005) [,]. Pharmacist involvement has driven DRP findings among patients with TB in hospitals [,,]. A range of 28–128 DRPs were identified. These consisted of inappropriate medication, additional medications needed, inappropriate doses, adverse drug reactions, and non-adherence to medication. Most DRPs were resolved by pharmacists. Two studies showed the benefits of pharmacist counselling in enhancing medication adherence [,]. Standardized counselling led by the pharmacist was significantly associated with patient attendance at the hospital, compared with the non-pharmacist intervention [], while a combination of pharmacist counseling and written material (leaflet) in improving medication adherence was superior to the other two groups, i.e., a group that only receives counseling and a group without counseling and leaflets [].
4. Discussion
Our review found that PC models focused on two outcomes, i.e., increasing TB patient detection and improving TB treatment outcomes. For increased TB patient detection, two practice models in the community setting screened the presumptive TB patients and referred them to the health facility for further diagnosis; TSTs are provided in community pharmacies for the general population to improve public accessibility and examine the status of LTBI. Concerning TB treatment outcomes, we identified PC practice in the community and hospital settings. In the community setting, PC activities described collaborative practices as improving LTBI medication completion rates and participation in the DOT program for improving medication adherence in a high-risk group of non-adherence to medication. In hospital settings, PC activities focused on improving TB treatment outcomes through several activities of the hospital pharmacist, such as assessing, monitoring, following up on potential and actual DRPs, and reporting on DRPs, including ADR. The other activities conducted in the hospital pharmacy are face-to-face or telephone drug consultations and educational programs for medication adherence with standardized written and oral counseling. Most PC services positively impact treatment success. However, another study showed no significant effect of PC on improving treatment success, highlighting various effects and hidden challenges in actual practice.
We identified different forms of care across the PC practice scenario. Prevention and timely treatment actions are more frequent in the community setting than in the hospital setting, while the management of serious patient conditions is part of pharmacy personnel’s actions to improve TB treatment outcomes in the hospital setting. These different forms of care underline the need for different approaches to engaging pharmacy personnel in TB care based on their practice setting. In the internal aspect, comprehensive knowledge and skill in managing severe TB conditions, patients with complications, and clinical data interpretation are mainly needed for hospital pharmacists in TB care []. Those are essential to developing a pharmaceutical care plan that can fully support the rational use of medicine by identifying and solving potential and actual DRPs among the complex problem of hospitalized patients with TB []. In the community setting, pharmacy personnel should have epidemiological and public health knowledge besides pharmacotherapy and pharmacovigilance knowledge. Importantly, the accessibility of pharmacy personnel to lab tests for identifying the potential ADR of TB medications is required in the community setting. It will support pharmacy personnel in managing medication adherence among patients with TB. However, the effects of PC practice are influenced by local contexts, involving several factors, such as individual pharmacy personnel, patient awareness, clear guidelines, professional interaction, incentives, resources, capacity for organizational change, and social, political, and legal factors [].
Among the individual aspects, the positive awareness of the importance of TB services from pharmacy personnel and the availability of guidance are essential. The willingness of pharmacy personnel to join a TB program has been reported as a challenge in the activities of TB patient detection []. Studies in Vietnam and Pakistan showed that personnel regarded it as lost pharmacy income if they referred a presumptive TB patient to a health facility [,]. Pharmacy personnel should have adequate knowledge and skill in managing TB in their facility [,]. A multicross-sectional study reported that TB training is associated with TB patient detection among community pharmacy personnel in Indonesia []. Previous studies showed that a lack of exposure to TB training might lead to a limited understanding of how to manage presumptive or active TB patients [,]. Challenges will arise if pharmacy personnel is not accompanied by guidance in TB practices. This guidance could be a supporting document providing standardized practices for managing TB patients []. Hence, TB awareness, knowledge, skills, and guidance are essential for the success of pharmacy personnel in managing TB.
We found that a lack of incentives, high workload due to limited staff [,], and an unavailability of an adequate facility (e.g., consultation room, tracing system, record system for DRPs, and transfer outpatient) were reported as challenges [,,,,]. Regarding new initiatives for PC practice, healthcare providers commonly consider the benefit and risks of providing the practices. Individual incentives are a consideration. An analysis of 19 case detection studies indicated that incentives should be considered in the recruitment of pharmacy personnel in TB patient detection programs []. The care taken for pharmacy income may worsen when profit is the primary goal of the pharmacy owner []. This can affect the capacity for organizational change to support TB.
In addition, workload, limited proper facilities, and drug availability were associated with limited patient case detection and improved treatment outcomes in TB care. Studies found that high workload was associated with TB patient detection in community pharmacies []. Moreover, a study in a hospital setting demonstrated that the limited activities performed by pharmacy personnel in managing TB patients were due to personnel limitations []. In terms of the facility, the availability of a consultation room for TB counselling, a tracing system for non-adherent/transfer-out patients, and integrating DRP reports with the medical record were beneficial for supporting PC in TB [,,]. Another challenge was the absence of an integrated system between pharmaceutical services with TB stakeholders and local/national TB programs. The lack of connectivity among pharmacies, laboratories, and NTPs was a barrier to successful TB patient detection programs in Bolivian community pharmacies []. The lack of preparation of related facilities for TB patient detection in the pharmacy programs (e.g., laboratories for sputum tests, clinics, and primary care) is a primary factor for the low positivity rate of presumptive TB patients. Many presumptive TB patients referred by pharmacy personnel were reluctant to visit laboratories, clinics, or primary health facilities due to long queues, limited facilities for sputum tests, and complex procedures required for medical examination []. In the hospital setting, professional interaction among TB healthcare teams should be intensified to follow up on DRPs and non-adherent patients [].
Following the principle of patient-centeredness, we identified several barriers in the community and hospital settings from the patient’s perspective. In TB patient detection programs at community pharmacies, services that patients should pay for out-of-pocket after receiving advice from pharmacy personnel for further TB examination may be a reason for not pursuing further TB examination [,]. Moreover, negative public perceptions of primary public health services were reported, which prevented presumptive TB patients from going to public health facilities []. This includes complex bureaucracy, long wait times, poor quality labs, and accessibility and privacy issues []. The lack of information on TB detection programs in community pharmacies affected the awareness of their recommendations among pharmacy personnel []. Furthermore, patient access to a health facility, worry about the potential drug side effects, adverse drug reactions, and complex and lengthy treatment regimens prevented successful pharmaceutical services [,,].
Several limitations of this review should be acknowledged for a cautious interpretation. First, other PC practice models may be found in other databases or unpublished articles. Second, the heterogeneity of the population, intervention, comparison, and outcomes across the studies examined may lead to difficulties in performing a meta-analysis. Third, potential bias may exist in the included studies. Fourth, there is no economic impact of the PC service in our included study. To ameliorate this, we searched two reputable databases without language or study period restrictions. We also used an independent team to select and screen articles based on the study criteria to minimize potential information bias in our study.
Innovative strategies that engage all resources should be adopted to stabilize TB management in the COVID-19 era, especially in countries with a high prevalence of TB. Our review demonstrated that pharmaceutical services could improve TB case detection and treatment outcomes. However, several pre-conditions should be considered for consistent and satisfactory effects of PC services. First, an integrated program involving not only pharmacy personnel as the central actor in the PC services, but also national TB programs, central/local government, professional organizations, and relevant stakeholders should be initiated. The integrated program should describe the professional interactions between pharmacy personnel and TB stakeholders, supported by the comprehensive practical guidelines for providing TB services. Regulations should be developed by local leaders, TB programmers, and professional organizations to build friendly working environments for program implementation. Program monitoring and intensive communication regarding barriers, conflicts of interest, and TB awareness can be led by local leaders or TB programmers. Second, attractive TB training, including structured programs, incentives, and certificates, can be developed for pharmacy personnel to attract and improve their awareness, knowledge, and skills in managing TB. The specific license can be given to pharmacy personnel, following comprehensive training in the particular TB practice. Professional organizations and academic institutions can develop the program as part of a continuing educational program for pharmacy personnel. Third, standardized, accessible facilities for diagnosis and treatment, such as pharmacies, laboratories, primary care, and referral hospitals, are critical for supporting the pharmaceutical program in improving TB detection and treatment outcomes. Fourth, program socialization regarding TB pharmaceutical services may enhance public awareness of the pharmacy’s role and benefits, and in particular, pharmaceutical services in TB. Fifth, digital technology can be developed to reduce a pharmacy personnel’s workload in managing TB patients [] and supporting activities related to TB recording and reporting. Finally, further studies on the clinical and economic impact of PC services are comprehensively needed.
5. Conclusions
Our review has identified PC models in increasing TB patient detection and improving treatment outcomes. The PC practices cover the area of community and hospital settings with several types of practice models, such as screening and referring people with presumptive TB, providing TSTs, collaborative practices for treatment completion, directly observed treatment, addressing DRPs, and reporting and managing adverse drug reactions and medication adherence programs.
This review indicates that the current pharmaceutical services could be beneficial for supporting TB control. Several factors should be comprehensively considered by pharmacy personnel and relevant stakeholders for successful implementation, such as: guidelines availability; sufficient individual knowledge and skill of pharmacy personnel; patient awareness for the pharmacy personnel roles; good professional interaction, incentives, and resources availability; strong capacity for organizational change; and existing regulation to support PC services. Hence, an integrative PC program for TB patient care should be considered for a successful program involving all related PC service stakeholders. Study on further implementation is needed to analyze the clinical and economic impacts of pharmaceutical services, considering the aforementioned comprehensive factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed8050287/s1, Supplementary File S1: The key terms for the search strategy in the Pubmed and Cochrane databases.
Author Contributions
Main idea: I.S.P.; conception and design of the work: I.S.P.; data collection and screening: E.O.Y., C.Y.N., N.P.M. and I.S.P.; data analysis and interpretation: I.S.P., E.O.Y., C.Y.N., N.P.M., D.P.D., L.S. and A.D.; preparing the first draft: I.S.P.; substantial revision of the manuscript: I.S.P., E.O.Y., C.Y.N., N.P.M., D.P.D., L.S. and A.D.; approval for the final manuscript: I.S.P., E.O.Y., C.Y.N., N.P.M., D.P.D., L.S. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the internal funding of Universitas Padjadjaran 2022. This funding source had no role in the concept development, study design, data analysis, or article preparation.
Institutional Review Board Statement
No ethical approval was needed as this is a review study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no competing interests.
References
- WHO. Global Tuberculosis Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Pradipta, I.S.; Idrus, L.R.; Probandari, A.; Puspitasari, I.M.; Santoso, P.; Alffenaar, J.-W.C.; Hak, E. Barriers to Optimal Tuberculosis Treatment Services at Community Health Centers: A Qualitative Study From a High Prevalent Tuberculosis Country. Front. Pharmacol. 2022, 13, 936. [Google Scholar] [CrossRef] [PubMed]
- Pradipta, I.S.; Idrus, L.R.; Probandari, A.; Lestari, B.W.; Diantini, A.; Alffenaar, J.-W.C.; Hak, E. Barriers and Strategies to Successful Tuberculosis Treatment in a High-Burden Tuberculosis Setting: A Qualitative Study from the Patient’s Perspective. BMC Public Health 2021, 21, 1903. [Google Scholar] [CrossRef] [PubMed]
- Lestari, B.W.; McAllister, S.; Hadisoemarto, P.F.; Afifah, N.; Jani, I.D.; Murray, M.; van Crevel, R.; Hill, P.C.; Alisjahbana, B. Patient Pathways and Delays to Diagnosis and Treatment of Tuberculosis in an Urban Setting in Indonesia. Lancet Reg. Health West. Pac. 2020, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Surya, A.; Setyaningsih, B.; Suryani Nasution, H.; Gita Parwati, C.; Yuzwar, Y.E.; Osberg, M.; Hanson, C.L.; Hymoff, A.; Mingkwan, P.; Makayova, J.; et al. Quality Tuberculosis Care in Indonesia: Using Patient Pathway Analysis to Optimize Public-Private Collaboration. J. Infect. Dis. 2017, 216, S724–S732. [Google Scholar] [CrossRef] [PubMed]
- De Resende, N.H.; de Miranda, S.S.; Ceccato, M.D.G.B.; Haddad, J.P.A.; Reis, A.M.M.; da Silva, D.I.; Carvalho, W.D.S. Drug Therapy Problems for Patients with Tuberculosis and HIV/AIDS at a Reference Hospital. Einstein 2019, 17, eAO4696. [Google Scholar] [CrossRef]
- Nakatani, H.; Buchmann, M. Working Together: WHO and FIP; FIP: Hyderabad, India, 2011. [Google Scholar]
- Flottorp, S.A.; Oxman, A.D.; Krause, J.; Musila, N.R.; Wensing, M.; Godycki-Cwirko, M.; Baker, R.; Eccles, M.P. A Checklist for Identifying Determinants of Practice: A Systematic Review and Synthesis of Frameworks and Taxonomies of Factors That Prevent or Enable Improvements in Healthcare Professional Practice. Implement. Sci. 2013, 8, 35. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Ullah, W.; Almansour, H.; Fatima, R.; Saini, B.; Khan, G.M. Engaging Community Pharmacies in Early Detection of Missing Tuberculosis Patients through Public-Private Mix Intervention in Pakistan. Am. J. Trop. Med. Hyg. 2020, 103, 221–230. [Google Scholar] [CrossRef]
- Lonnroth, K.; Karlsson, M.; Lan, N.T.N.; Buu, T.N.; Dieu, T.T.N. Referring TB Suspects from Private Pharmacies to the National Tuberculosis Programme: Experiences from Two Districts in Ho Chi Minh City, Vietnam. Int. J. Tuberc. Lung Dis. 2003, 7, 1147–1153. [Google Scholar]
- Lambert, M.L.; Delgado, R.; Michaux, G.; Vols, A.; Speybroeck, N.; Van Der Stuyft, P. Collaboration between Private Pharmacies and National Tuberculosis Programme: An Intervention in Bolivia. Trop. Med. Int. Health 2005, 10, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, B.; Logothetis, S.J.; Roberts, M.H.; Bachyrycz, A.; Fortune, D.; Borrego, M.E.; Ferreira, J.; Burgos, M. Addressing Latent Tuberculosis Infection Treatment through a Collaborative Care Model with Community Pharmacies and a Health Department. Prev. Chronic Dis. 2020, 17, E14. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, B.; Gross, B.; Fortune, D.; Babb, S.; Tinker, D.; Bachyrycz, A. Evaluation of a Pharmacist-Performed Tuberculosis Testing Initiative in New Mexico. J. Am. Pharm. Assoc. 2015, 55, 307–312. [Google Scholar] [CrossRef]
- Carter, K.L.; Gabrellas, A.D.; Shah, S.; Garland, J.M. Improved Latent Tuberculosis Therapy Completion Rates in Refugee Patients through Use of a Clinical Pharmacist. Int. J. Tuberc. Lung Dis. 2017, 21, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Hecox, N. Tuberculin Skin Testing by Pharmacists in a Grocery Store Setting. J. Am. Pharm. Assoc. 2008, 48, 86–91. [Google Scholar] [CrossRef]
- Hess, K.; Goad, J.; Wu, J.; Johnson, K. Isoniazid Completion Rates for Latent Tuberculosis Infection among College Students Managed by a Community Pharmacist. J. Am. Coll. Health 2009, 57, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Tanvejsilp, P.; Pullenayegum, E.; Loeb, M.; Dushoff, J.; Xie, F. Role of Pharmaceutical Care for Self-Administered Pulmonary Tuberculosis Treatment in Thailand. J. Clin. Pharm. Ther. 2017, 42, 337–344. [Google Scholar] [CrossRef]
- Juan, G.; Lloret, T.; Perez, C.; Lopez, P.; Navarro, R.; Ramón, M.; Cortijo, J.; Morcillo, E.J. Directly Observed Treatment for Tuberculosis in Pharmacies Compared with Self-Administered Therapy in Spain. Int. J. Tuberc. Lung Dis. 2006, 10, 215–221. [Google Scholar]
- Tang, Z.Q.; Jiang, R.H.; Xu, H. Bin Effectiveness of Pharmaceutical Care on Treatment Outcomes for Patients with First-Time Pulmonary Tuberculosis in China. J. Clin. Pharm. Ther. 2018, 43, 888–894. [Google Scholar] [CrossRef]
- Clark, P.M.; Karagoz, T.; Apikoglu-Rabus, S.; Izzettin, F.V. Effect of Pharmacist-Led Patient Education on Adherence to Tuberculosis Treatment. Am. J. Health-Syst. Pharm. 2007, 64, 497–506. [Google Scholar] [CrossRef]
- Karuniawati, H.; Putra, O.N.; Wikantyasning, E.R. Impact of Pharmacist Counseling and Leaflet on the Adherence of Pulmonary Tuberculosis Patients in Lungs Hospital in Indonesia. Indian J. Tuberc. 2019, 66, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.V.; De Miranda, S.S.; Ceccato, M.D.G.B.; Silveira, M.R.; de Resende, N.H.; Carvalho, W.S. Evaluation of the Impact of Pharmaceutical Care for Tuberculosis Patients in a Secondary Referral Outpatient Clinic, Minas Gerais, Brazil. An Acad. Bras. Cienc. 2017, 89, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Sánchez Pozo, A.; Rekkas, D.; Volmer, D.; Hirvonen, J.; Bozic, B.; Skowron, A.; Mircioiu, C.; Sandulovici, R.; Marcincal, A.; et al. Hospital and Community Pharmacists’ Perceptions of Which Competences Are Important for Their Practice. Pharmacy 2016, 4, 21. [Google Scholar] [CrossRef]
- Toni, J.; Lipovec, N.C.; Sorli, P.S.; Kosnik, M. Pharmacist’s Role in the Treatment of Patients with Tuberculosis ñ Our Positive Experience. Eur. J. Hosp. Pharm. 2012, 19, 250. [Google Scholar] [CrossRef]
- Pradipta, I.S.; Khairunnisa, K.; Bahar, M.A.; Kausar, M.N.; Fitriana, E.; Ruslami, R.; Aarnoutse, R.E. Knowledge, Attitude and Practice of Community Pharmacy Personnel in Tuberculosis Patient Detection: A Sectional Study in a Burden Tuberculosis Setting. BMJ Open 2022, 12, e060078. [Google Scholar] [CrossRef]
- Bigio, J.; Aquilera Vasquez, N.; Huria, L.; Pande, T.; Creswell, J.; Ananthakrishnan, R.; Bimba, J.S.; Cuevas, L.E.; Vo, L.; Bakker, M.I.; et al. Engaging Pharmacies in Tuberculosis Control: Operational Lessons from 19 Case Detection Interventions in High-Burden Countries. BMJ Glob. Health 2022, 7, e008661. [Google Scholar] [CrossRef]
- Ridho, A.; Alfian, S.D.; van Boven, J.F.M.; Levita, J.; Yalcin, E.A.; Le, L.; Alffenaar, J.W.; Hak, E.; Abdulah, R.; Pradipta, I.S. Digital Health Technologies to Improve Medication Adherence and Treatment Outcomes in Patients With Tuberculosis: Systematic Review of Randomized Controlled Trials. J. Med. Internet Res. 2022, 24, e33062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).