Under-Reporting Cases and Deaths from Melioidosis: A Retrospective Finding in Songkhla and Phatthalung Province of Southern Thailand, 2014–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Sites, and Data Collection
2.2. Data Sets and Analyses
2.3. Statistical Analysis
2.4. Ethical Consideration
3. Results
3.1. Diagnostic Specimens
3.2. Demographic and Clinical Characteristics of Patients
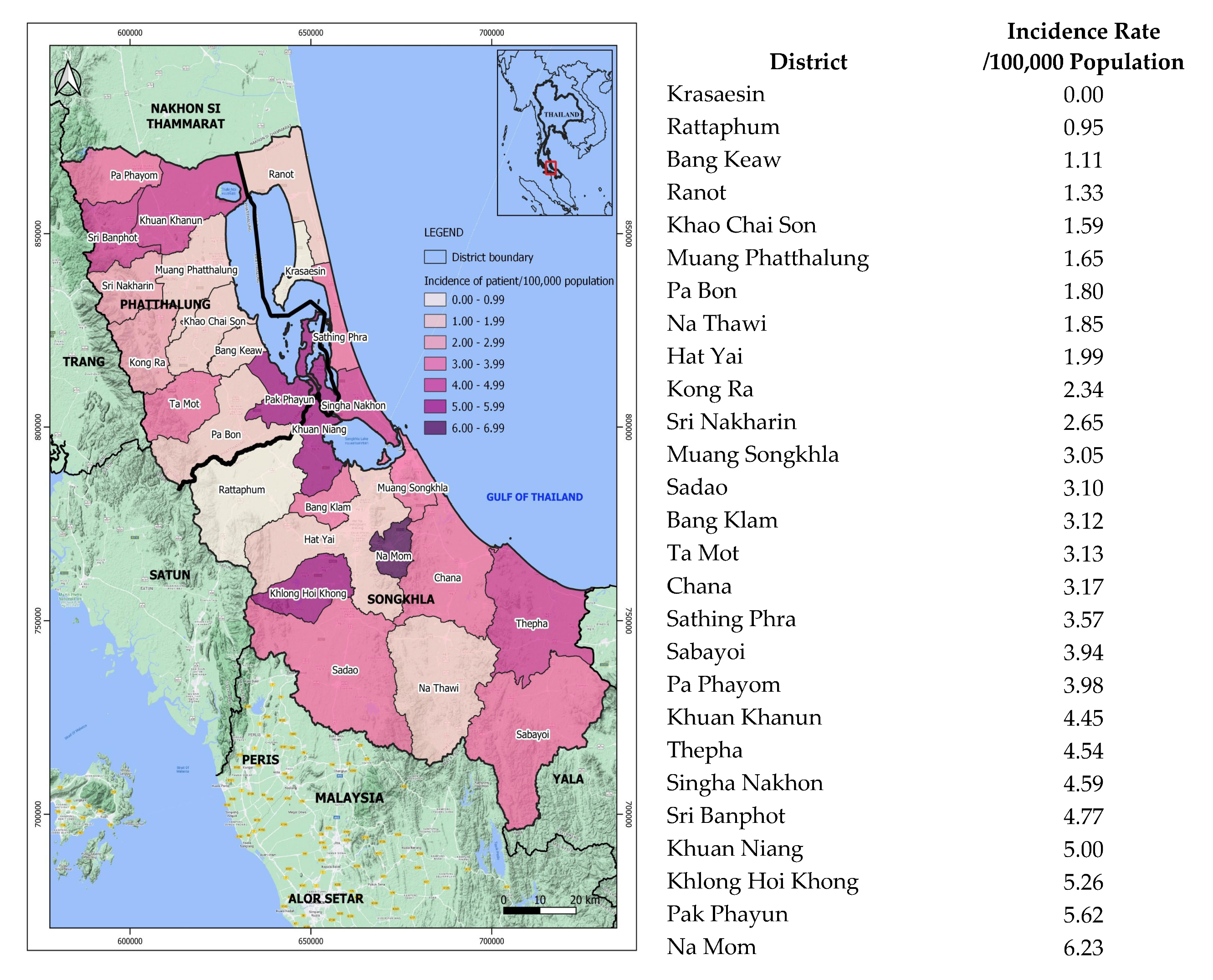
3.3. Geographic Distribution of the Patients
3.4. Melioidosis Cases Correlated with Rainfall
4. Discussion
4.1. Melioidosis in Southern Thailand Is Seasonal but Underreported
4.2. Death from Melioidosis Is a Pressing Issue Nationwide
4.3. Risks Associated with Preexisting Conditions
4.4. Bacteremia, a Common Clinical Manifestation Leading to Septicemic Melioidosis, and Death
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.J.; Peacock, S.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Melioidosis. Lancet 2003, 361, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Hantrakun, V.; Kongyu, S.; Klaytong, P.; Rongsumlee, S.; Day, N.P.J.; Peacock, S.J.; Hinjoy, S.; Limmathurotsakul, D. Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveillance System. Open Forum Infect. Dis. 2019, 6, ofz498. [Google Scholar] [CrossRef] [PubMed]
- Hinjoy, S.; Hantrakun, V.; Kongyu, S.; Kaewrakmuk, J.; Wangrangsimakul, T.; Jitsuronk, S.; Saengchun, W.; Bhengsri, S.; Akarachotpong, T.; Thamthitiwat, S.; et al. Melioidosis in Thailand: Present and Future. Trop. Med. Infect. Dis. 2018, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.A.; Atthasampunna, P.; Chulasamaya, M. Pseudomonas (Burkholderia) pseudomallei in Thailand, 1964–1967: Geographic distribution of the organism, attempts to identify cases of active infection, and presence of antibody in representative sera. Am. J. Trop. Med. Hyg. 2000, 62, 232–239. [Google Scholar] [CrossRef]
- Nachiangmai, N.; Patamasucon, P.; Tipayamonthein, B.; Kongpon, A.; Nakaviroj, S. Pseudomonas pseudomallei in southern Thailand. S. Asian J. Trop. Med. Public Health 1985, 16, 83–87. [Google Scholar]
- Vuddhakul, V.; Tharavichitkul, P.; Na-Ngam, N.; Jitsurong, S.; Kunthawa, B.; Noimay, P.; Noimay, P.; Binla, A.; Thamlikitkul, V. Epidemiology of Burkholderia pseudomallei in Thailand. Am. J. Trop. Med. Hyg. 1999, 60, 458–461. [Google Scholar] [CrossRef]
- Chierakul, W.; Winothai, W.; Wattanawaitunechai, C.; Wuthiekanun, V.; Rugtaengan, T.; Rattanalertnavee, J.; Jitpratoom, P.; Chaowagul, W.; Singhasivanon, P.; White, N.J.; et al. Melioidosis in 6 tsunami survivors in southern Thailand. Clin. Infect. Dis. 2005, 41, 982–990. [Google Scholar] [CrossRef]
- Svensson, E.; Welinder-Olsson, C.; Claesson, B.A.; Studahl, M. Cutaneous melioidosis in a Swedish tourist after the tsunami in 2004. Scand. J. Infect. Dis. 2006, 38, 71–74. [Google Scholar] [CrossRef]
- Ciervo, A.; Mattei, R.; Cassone, A. Melioidosis in an Italian Tourist Injured by the Tsunami in Thailand. J. Chemother. 2006, 18, 443–444. [Google Scholar] [CrossRef]
- Thaipadungpanit, J.; Chierakul, W.; Pattanaporkrattana, W.; Phoodaeng, A.; Wongsuvan, G.; Huntrakun, V.; Amornchai, P.; Chatchen, S.; Kitphati, R.; Wuthiekanun, V.; et al. Burkholderia pseudomallei in water supplies, southern Thailand. Emerg. Infect. Dis. 2014, 20, 1947–1949. [Google Scholar] [CrossRef]
- Silpapojakul, K. Melioidosis in southern Thai children. Songkla Med. J. 2004, 22, 363–369. [Google Scholar]
- Churuangsuk, C.; Chusri, S.; Hortiwakul, T.; Charernmak, B.; Silpapojakul, K. Characteristics, clinical outcomes and factors influencing mortality of patients with melioidosis in southern Thailand: A 10-year retrospective study. Asian Pac. J. Trop. Med. 2016, 9, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, H.P.; Limmathurotsakul, D.; Peacock, S.J. New insights from the 7th World Melioidosis Congress 2013. Emerg. Infect. Dis. 2014, 20, 131737. [Google Scholar] [CrossRef]
- Novak, R.T.; Glass, M.B.; Gee, J.E.; Gal, D.; Mayo, M.J.; Currie, B.J.; Wilkins, P.P. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J. Clin. Microbiol. 2006, 44, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tuanyok, A.; Auerbach, R.K.; Brettin, T.S.; Bruce, D.C.; Munk, A.C.; Detter, J.C.; Pearson, T.; Hornstra, H.; Sermswan, R.W.; Wuthiekanun, V.; et al. A Horizontal Gene Transfer Event Defines Two Distinct Groups within Burkholderia pseudomallei That Have Dissimilar Geographic Distributions. J. Bacteriol. 2007, 189, 9044–9049. [Google Scholar] [CrossRef] [PubMed]
- Number of Cases and Deaths by Month and Province. 2023. Available online: http://doe.moph.go.th/surdata/disease.php?dcontent=old&ds=72 (accessed on 29 March 2023).
- Hassan, M.R.; Pani, S.P.; Peng, N.P.; Voralu, K.; Vijayalakshmi, N.; Mehanderkar, R.; Aziz, N.A.; Michael, E. Incidence, risk factors and clinical epidemiology of melioidosis: A complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect. Dis. 2010, 10, 302. [Google Scholar] [CrossRef]
- Cheng, A.C.; Hanna, J.N.; Norton, R.; Hills, S.L.; Davis, J.; Krause, V.L.; Dowse, G.; Inglis, T.J.; Currie, B.J. Melioidosis in northern Australia, 2001–2002. Commun. Dis. Intell. 2003, 27, 272–277. [Google Scholar]
- Suputtamongkol, Y.; Hall, A.J.; Dance, D.A.; Chaowagul, W.; Rajchanuvong, A.; Smith, M.D.; White, N.J. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int. J. Epidemiol. 1994, 23, 1082–1090. [Google Scholar] [CrossRef]
- Currie, B.J.; Jacups, S.P. Intensity of rainfall and severity of melioidosis, Australia. Emerg. Infect. Dis. 2003, 9, 1538–1542. [Google Scholar] [CrossRef]
- Abu Hassan, M.R.; Aziz, N.; Ismail, N.; Shafie, Z.; Mayala, B.; Donohue, R.E.; Pani, S.P.; Michael, E. Socio-epidemiological and land cover risk factors for melioidosis in Kedah, Northern Malaysia. PLoS Negl. Trop. Dis. 2019, 13, e0007243. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Harris, P.N.A.; Seiler, R.L.; Ooi, P.L.; Cutter, J.; Goh, K.T.; Cook, A.R.; Fisher, D.; Chai, L.Y.A. Melioidosis, Singapore, 2003–2014. Emerg. Infect. Dis. 2018, 24, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat. Rev. Dis. Prim. 2018, 4, 17107. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J. Melioidosis: Evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. 2015, 36, 111–125. [Google Scholar] [CrossRef]
- Walsh, A.L.; Smith, M.D.; Wuthiekanun, V.; Suputtamongkol, Y.; Chaowagul, W.; Dance, D.A.; Angus, B.; White, N.J. Prognostic significance of quantitative bacteremia in septicemic melioidosis. Clin. Infect. Dis. 1995, 21, 1498–1500. [Google Scholar] [CrossRef]

| Positive Specimens | Number of Patients (%) | ||
|---|---|---|---|
| Inpatients | Outpatients (n = 18) | ||
| (n = 455) | CFR | ||
| Hemoculture | 324 (71.2) | 45.4% | 9 (50.0) |
| Pus/wound swab | 120 (26.4) | 19.2% | 7 (38.9) |
| Sputum | 104 (22.9) | 59.6% | 1 (5.6) |
| Urine | 32 (7.0) | 46.9% | 1 (5.6) |
| Synovial fluid | 20 (4.4) | 20.0% | 0 (0.0) |
| Parotid gland abscess | 18 (4.0) | 5.6% | 1 (5.6) |
| Plural fluid | 6 (1.3) | 16.7% | 0 (0.0) |
| Spleen/liver abscess | 6 (1.3) | 33.3% | 0 (0.0) |
| CSF | 2 (0.4) | 0.0% | 0 (0.0) |
| Positive > 1 | 117 (25.7) | 44.4% | 0 (0.0) |
| Province | Hospital Name | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Songkhla | Songklanakarind | 12 | 19 | 17 | 23 | 24 | 13 | 12 | 120 |
| Hatyai | 21 | 22 | 17 | 43 | 15 | 20 | 26 | 164 | |
| Songkhla Provincial | 17 | 14 | 15 | 28 | 14 | 15 | 14 | 117 | |
| Phatthalung | Phatthalung Provincial | 14 | 10 | 11 | 19 | 13 | 11 | 21 | 99 |
| Total | 64 | 65 | 60 | 113 | 66 | 59 | 73 | 500 |
| Characteristics of Patients | Number of Cases | % |
|---|---|---|
| Inpatients | 455 | 96.2 |
| Outpatients | 18 | 3.8 |
| Sex | ||
| Male | 337 | 71.2 |
| Female | 136 | 28.8 |
| Age (years) | ||
| 0–4 | 12 | 2.5 |
| 5–9 | 9 | 1.9 |
| 10–14 | 6 | 1.3 |
| 15–19 | 3 | 0.6 |
| 20–29 | 26 | 5.5 |
| 30–39 | 47 | 10.0 |
| 40–49 | 86 | 18.2 |
| 50–59 | 125 | 26.4 |
| 60–69 | 89 | 18.8 |
| 70–79 | 44 | 9.3 |
| >80 | 26 | 5.5 |
| Median age: 54 years (IQR 41.5–64) | ||
| Underlining medical conditions | ||
| Diabetes mellitus | 188 | 41.3 |
| Cancer/Malignancy | 35 | 7.7 |
| Chronic renal diseases | 26 | 5.7 |
| Immunosuppressant usage | 24 | 5.3 |
| Thalassemia | 22 | 4.8 |
| Excessive alcohol consumption | 14 | 3.1 |
| Chronic lung diseases (e.g., TB) | 14 | 3.1 |
| HBV/HIV | 13 | 2.9 |
| At least one of above | 272 | 59.8 |
| Clinical Manifestations | Number of episodes (%) | CFR 1 |
| Bacteremia | 274 (58.1%) | 45.8% |
| Pneumonia | 166 (35.2%) | 56.7% |
| Skin/wound infection | 101 (21.4%) | 13.3% |
| Urinary tract infection | 46 (9.7%) | 46.7% |
| Hepatosplenic abscesses | 33 (7.0%) | 21.9% |
| Parotid gland infection | 18 (3.8%) | 5.6% |
| Septic arthritis | 18 (3.8%) | 16.7% |
| CNS infection | 2 (0.4%) | 0.0% |
| Mortality Rate 1 | 181 (39.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewrakmuk, J.; Chusri, S.; Hortiwakul, T.; Kawila, S.; Patungkaro, W.; Jariyapradub, B.; Limvorapan, P.; Chiewchanyont, B.; Thananchai, H.; Duangsonk, K.; et al. Under-Reporting Cases and Deaths from Melioidosis: A Retrospective Finding in Songkhla and Phatthalung Province of Southern Thailand, 2014–2020. Trop. Med. Infect. Dis. 2023, 8, 286. https://doi.org/10.3390/tropicalmed8050286
Kaewrakmuk J, Chusri S, Hortiwakul T, Kawila S, Patungkaro W, Jariyapradub B, Limvorapan P, Chiewchanyont B, Thananchai H, Duangsonk K, et al. Under-Reporting Cases and Deaths from Melioidosis: A Retrospective Finding in Songkhla and Phatthalung Province of Southern Thailand, 2014–2020. Tropical Medicine and Infectious Disease. 2023; 8(5):286. https://doi.org/10.3390/tropicalmed8050286
Chicago/Turabian StyleKaewrakmuk, Jedsada, Sarunyou Chusri, Thanaporn Hortiwakul, Soontara Kawila, Wichien Patungkaro, Benjamas Jariyapradub, Pattamas Limvorapan, Bongkoch Chiewchanyont, Hathairat Thananchai, Kwanjit Duangsonk, and et al. 2023. "Under-Reporting Cases and Deaths from Melioidosis: A Retrospective Finding in Songkhla and Phatthalung Province of Southern Thailand, 2014–2020" Tropical Medicine and Infectious Disease 8, no. 5: 286. https://doi.org/10.3390/tropicalmed8050286
APA StyleKaewrakmuk, J., Chusri, S., Hortiwakul, T., Kawila, S., Patungkaro, W., Jariyapradub, B., Limvorapan, P., Chiewchanyont, B., Thananchai, H., Duangsonk, K., & Tuanyok, A. (2023). Under-Reporting Cases and Deaths from Melioidosis: A Retrospective Finding in Songkhla and Phatthalung Province of Southern Thailand, 2014–2020. Tropical Medicine and Infectious Disease, 8(5), 286. https://doi.org/10.3390/tropicalmed8050286







