Abstract
Leptospirosis is one of the most widespread zoonotic diseases and can infect both humans and animals worldwide. The role of the cat as a susceptible host and potential environmental reservoir of Leptospira is still not well understood, due to the lack of obvious clinical signs associated with Leptospira spp. infection in this species. This study aims to describe the first European detection of Leptospira interrogans serogroup Australis ST 24 in a young outdoor cat with a severe comorbidity (feline panleukopenia virus). In addition, the results of a preliminary study conducted in 2014–2016 are presented (RC IZSVE 16/12), which reports an investigation of Leptospira exposure of outdoor cats in Northeast Italy by means of serological investigation and molecular evaluation of urine. The animals included in the survey are part of samples collected during active and passive surveillance (diagnostic samples). The study reported a seroprevalence of 10.5% among outdoor cats and the serogroups identified were Grippotyphosa, Icterohaemorrhagiae, Bratislava, Canicola and Ballum. Symptomatic cats reported high MAT titres (ranging from 1:800 to 1:1600) towards antigens belonging to the serovars Grippotyphosa (1:800), Bratislava (1:1600), Icterohaemorrhagiae (1:200) and Copenhageni (1:200–1:800). In one subject, urine tested positive for Leptospira PCR. Cats with high antibody titres for Leptospira and/or positivity on molecular test suffered from immunosuppressive comorbidities (feline immunodeficiency virus and feline leukaemia virus; feline herpesvirus and lymphoma; hyperthyroidism). The overall prevalence of serum antibodies against Leptospira found in free-ranging cats (10.53%, 95% CI: 4.35–16.70%) and the identification of L. interrogans ST 24 in a young cat with immunosuppressive disease (feline panleukopenia virus) suggest the possibility of natural resistance to clinical leptospirosis in healthy cats. In a One Health perspective, further studies are needed to better define the pathogenesis of leptospirosis in cats and their epidemiological role as environmental sentinels or possible carriers of pathogenic Leptospira.
1. Introduction
Leptospirosis is caused by spirochaetal bacteria of the genus Leptospira and is an almost endemic disease worldwide. Leptospira spp. potentially affects all mammals that can act either as primary/defective hosts, which develop the acute disease, or as carrier hosts, which are primarily responsible for spreading the disease. The genus Leptospira is currently divided into three phylogenetic clusters, which supposedly correlate with the virulence of the bacteria [1,2]. To date, at least 64 Leptospira species have been described, and these have been classified into four subclades (S1, S2, S3 and S4), including more than 300 serovars [3,4,5]. Recently, 43 novel species of Leptospira were isolated from tropical soils, suggesting a highly unexplored biodiversity in the genus [4,6,7]. Leptospirosis occurrence is related to specific climatic conditions (i.e., moist soils with neutral pH and warm temperature around 25 °C) that could allow leptospires to persist and remain infectious for several months, leading to potentially relevant environmental contamination [1,4,8,9].
The serogroups Australis, Autumnalis, Ballum, Canicola, Grippotyphosa, Icterohaemorrhagiae, Pomona and Sejroe are the most frequently reported among cats in Europe, although with significant regional variation, both in terms of serovars and hosts distribution [9,10]. The overall prevalence reported worldwide by microscopic agglutination tests (MAT) ranges from 4% to 33.3%, whereas molecular assays have reported widely different prevalence values, probably influenced by the molecular techniques employed (i.e., PCR-primers) and the characteristics of the geographical area [9]. Antibodies against Leptospira spp. are mostly reported in older, outdoor, urban cats with hunting behaviour [9,11,12,13]. In addition, a recent serologic and urinary survey in Canada reported that exposure to Leptospira was unexpectedly relevant in feral cats [14].
Cats are now considered to represent a possible zoonotic risk, although the clinical course of the disease related to the pathogenesis of leptospirosis in this species is still not fully elucidated [8,9,10]. Previous literature reported that the cat could be a potential chronic reservoir host, since, in urine, Leptospira can be isolated and its DNA can be identified for more than 8 months, even when the infected cat presents detectable specific serum antibodies [15]. Concerning the cat’s natural exposure to pathogenic Leptospira, the most recent literature reported that in Europe, the serological prevalence of anti-Leptospira antibodies varies geographically: 14% in Andalusia [16], 12.8% in Estonia [17], 4.1% in Spain [18] and 9.2% in the Czech Republic [19]. Previous studies reported a Leptospira serological positivity of 18.2% in Austria (Tyrol’s districts) [20] and 9.2% in Scotland [11]. Recently, a seroprevalence of 17.9% was described in Germany in outdoor cats, with titres ranging from 1:100 to 1:6400. The most common serovars were Australis (8.7%, MAT titres range 1:100–1:6400), Bratislava (7.2%, range 1:100–1:400) and Grippotyphosa (5.6%, range 1:100–1:400) [21].
Reports of clinical leptospirosis in cats are rare: the clinical presentation is characterised by a plethora of signs ranging from asymptomatic to fulminant disease, making the diagnostic process challenging. Specifically, the acute clinical disease mainly involves the young incidental host infected by haemolysin-producing Leptospira species, but in the majority of cases clinical signs are likely to be mild [10,22]. Experimentally described clinical signs include polyuria, polydipsia, haematuria, uveitis, lethargy, anorexia, weight loss, ascites, vomiting, diarrhoea, pain on handling, inflammatory lesions on the skin and digits, cavity effusions, and nephritis, although infection in cats is not always associated with suggestive symptoms [10,23,24]. Clinicopathologic changes are a fluctuating leukocytes count, azotaemia, hypokalaemia, hyperphosphatemia, hyposthenuria, haematuria and proteinuria. Therefore, while the interstitial nephritis appears a common clinical manifestation, the liver dysfunction is not reported as frequently as in dogs [9,24,25].
Outdoor behaviour and predatory activity seem to be the most relevant risk factors. In addition, living in rural areas in close contact with livestock farms, contact with synanthropic or wildlife species and the presence of other cats in the household are also associated with an increased risk of leptospirosis [9,12]. Further environmental risk factors have been identified, such as living in flooded areas where agricultural activities use water flowing in streams or backwaters [26]. Accordingly, in tropical countries, the frequency of clinical manifestations of leptospirosis in people is strongly correlated with rainy seasons: flooding contributes strongly to disease transmission, since a high degree of flooding leads to more infected individuals [27,28].
The presence of diseases associated with immunosuppression or immune system deficiency can lead to decreased resistance to infection, debilitation and other complications of illness. Varieties of infectious and non-infectious diseases, stress, poor nutrition, drugs, toxins and medical procedures have been associated with immunosuppression in dogs and cats [29]. Specifically, the viral agents that could commonly affect outdoor and shelter cats are feline panleukopenia virus (FPV), feline herpesvirus, feline caliciviruses (FCV), feline infectious peritonitis virus (FIPV), feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) [30]. FIP is caused by a feline coronavirus (FCoV) in which the immune system is known to play a crucial, but complex, role in the pathogenesis. This role is still not fully understood, with involvement of both host and viral factors [31]. Young cats of less than 2 years of age seem especially vulnerable, and it has been estimated that 0.3% to 1.4% of feline deaths at veterinary institutions are caused by FIP [32]. FeLV and FIV infections are reported in cats worldwide. Both infections are associated with a variety of clinical signs and can impact quality of life and affect longevity [33]. The major route of FIV transmission is through bite wounds that introduce saliva containing virus and FIV-infected white blood cells. Following the primary phase of the infection, cats enter a long asymptomatic stage that can last for many years. During this chronic stage, progressive dysfunction of the immune system can occur. Thus, FIV-infected cats are predisposed to chronic and recurrent infections. Moreover, neoplasia is about five times more common than in uninfected cats [34]. Infection with FeLV is transmitted through close contact among cats. Commonly, it is spread vertically and horizontally from infected queens to their kittens and horizontally among cats that live together or that fight [34]. The prevalence in individually kept cats is usually less than 1%; differently, in multi-cat households, this figure may be 20%: the high density of cat populations represents a facilitating factor [35]. Immune suppression in FeLV infections is more complex and severe than the more selective effects caused by FIV. Whether showing clinical signs or not, every FeLV-viraemic cat is immune suppressed, with retarded and decreased primary and secondary antibody responses [36]. FPV may affect cats of all ages, but kittens are most susceptible. FPV has become less frequent in the domestic cat population over the last decades because of vaccination. However, outbreaks in shelter cats are commonly reported and often associated with a high number of fatalities [37]. Signs of disease include diarrhoea, lymphopenia and neutropenia, followed by thrombocytopenia and anaemia, immunosuppression (transient in adult cats), cerebellar ataxia (in kittens only) and abortion [38]. Feline panleukopenia mortality is 25–90% in cats with the acute form of the disease and up to 100% in hyperacute infections [39].
To the best of the author’s knowledge, there are no recent studies about the possible association between immunosuppressive infectious disease and clinical leptospirosis. A proportion of the cat population in Italy has outdoor access, where they have the opportunity to hunt prey [40]. Therefore, a large population of outdoor cats could be exposed to Leptospira spp. and might play a role in the complex epidemiology of the disease. Only a few recent studies have described the most commonly circulating strains of Leptospira spp. among dogs in Northeast Italy [41], whereas no recent reports of serological investigation are known in cats in these regions.
The present study reports the first identification of L. interrogans serogroup Australis ST 24 in an immunocompromised young cat and describes four suspected clinical cases of feline leptospirosis. Furthermore, the results of a preliminary serological and epidemiological survey of Leptospira in free-roaming cats in Northeast Italy are described.
2. Materials and Methods
2.1. Diagnostic in a Young Cat Referred for Sudden Death
A 4-month-old cat was referred from the veterinarian practitioner to the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVE) laboratory for post-mortem examination. The cat was a young free-roaming male domestic short hair from a shelter. The cat had been receiving itraconazole (Itrafungol®, Virbac, Milan, Italy) for dermatomycosis, which was previously diagnosed by the veterinary surgeon. The cat presented severe and acute gastrointestinal syndrome with vomiting, haemorrhagic diarrhoea, anorexia and dehydration, which rapidly deteriorated, leading to his death within hours.
2.1.1. Microbiological Analysis
Based on the necropsy findings, selected organs were sampled for further diagnostic investigations. Specifically, swabs from faeces, liver and lung were submitted to laboratory routine aerobic and anaerobic culture; faeces and a pool of visceral organs (liver, gall bladder and spleen) were analysed for Salmonella spp. isolation, according to the World Organization for Animal Health (WOAH), Chapter 3.10.07 [42]. Bacterial identification was phenotypically performed using a routine test.
Leptospira isolation was attempted on liver, kidney and lung tissue. A sample of 1 cm3 of these organs was homogenized with a pestle and mortar and added to 9 mL of liquid Ellinghausen–McCullough–Johnson–Harris (EMJH) medium, according to WOAH, Chapter 3.1.12 [3].
2.1.2. Molecular Analysis and Genome Sequencing
The liver, lung and kidney tissue samples were assessed via a pathogen-specific Leptospira TaqMan real-time polymerase chain reaction (real-time PCR) kit. The tissue samples were homogenized at a 1:10 dilution in 600 µL of PBS, with TissueLyser II (QIAGEN, Hilden, Germany), and DNA isolation from 100 µL of tissue homogenate was performed after a prelysis treatment with 2.5 μL of lysozyme (10 mg/mL in 10 mM of Tris-HCl, pH 8.0) and an incubation period of 15 min at +37 °C. The DNA extraction was performed on the KingFisher™ Flex Purification System (Life Technologies, Carlsbad, CA, USA) platform using the ID Gene® Mag Universal Extraction Kit (IDvet, Grabels, France), in accordance with the manufacturer’s instructions. Every DNA extraction included a negative process or extraction control (water). To detect the presence of pathogenic species of Leptospira, a screening real-time PCR targeting a 87 bp fragment that corresponds to a portion of the gene encoding the 16S rDNA was applied [43]. The real-time PCR was performed in a 25 µL final volume, containing 3 µL of extracted DNA, 12.5 µL of 2× Master Mix TaqMan Universal 2× (Thermo Fisher Scientific, Waltham, MA, USA), 300 nM of each primer, and 100 nM of a 5′ 6-carboxyfluorescein (FAM)–3′-tetramethylrhodamine (TAMRA) probe. The amplification assay included a negative control (water), a negative bacterial genomic control (DNA of Leptospira biflexa sv Patoc), the negative extraction control and a positive control (DNA of L. interrogans sv Icterohaemorrhagiae). The assay was performed with the following thermal conditions: a holding step at 95 °C for 10 min, and 45 cycles of 95 °C for 15 s and 60 °C for 60 s. Samples with cycle threshold (Ct) < 38 were considered positive. Samples with Ct values within the 38–40 range were considered doubtful, whereas samples with no FAM fluorescence signal or with Ct ≥ 40 were considered negative.
The positive samples were referred to the Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna (IZSLER) National Reference centre for Leptospirosis for the genomic characterization with the multi locus sequence typing (MLST) technique. The genotyping was performed using the 7-loci scheme proposed by Boonsilp in 2013 [44], which is based on the housekeeping genes glmU, pntA, sucA, tpiA, pfkB, mreA and caiB, as previously described [41].
For allelic number and ST identification, the assembled and trimmed sequences were queried against the Bacterial Isolate Genome Sequence Database (BIGSdb) available on the Leptospira MLST website (https://pubmlst.org/leptospira/, accessed on 1 March 2022), sited at the University of Oxford [45]. Comparisons between the STs found and those present in BIGSdb as reference isolates were used to deduce the species of the Leptospira being tested. To perform comparisons among historical serological studies (where serovars and serogroups were defined) and genotyping data (where species and genomic profiles were defined), we chose to assign to each identified ST a classification at the serogroup and serovar levels obtained from BIGSdb, knowing that this information was deduced and did not result from active serological typing.
A pool of tissue samples from the stomach and small intestine were submitted to quantitative molecular testing for FPV [46]. The tissue samples were homogenized at a 1:10 dilution in 800 µL of PBS supplemented with antibiotics (PBS-A: 10,000 IU/mL of penicillin G, 10 mg/mL of streptomycin, 5000 IU/mL of nystatin and 0.25 mg/mL of gentamicin sulphate), with TissueLyser II (QIAGEN, Hilden, Germany) at 30 Hz for 3 min. The DNA extraction was performed on the KingFisher™ Flex Purification System (Life Technologies, Carlsbad, CA, USA) platform using the ID Gene® Mag Universal Extraction Kit (IDvet, Grabels, France), in accordance with the manufacturer’s instructions, adding a pre-treatment with 20 µL of Proteinase K(QIAGEN, Hilden, Germany) for 10 min at 70 °C before the extraction. Every DNA extraction included a negative control (water). A real-time PCR on a conserved region of gene VP2 of FPV [46] was carried out in a final volume of 25 µL, consisting of 5 µL of eluted DNA, 5 µL of Quantifast Pathogen Master Mix 5× (QIAGEN, Hilden, Germany), 2.5 µL of Internal Control Assay 10× (QIAGEN, Hilden, Germany), 600 nM of each primer and 200 nM of probe. The assay was performed with the following thermal conditions: a holding step at 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 s and 59 °C for 30 s. The real-time PCR was performed on a Biorad CFX96 instrument (Biorad, Hercules, CA, USA). Samples with cycle threshold (Ct) < 35 were considered positive, whereas samples with no FAM fluorescence signal or with Ct ≥ 35 were considered negative.
2.2. Ricerca Corrente IZSVE 16/12: Sample Collection from 2014 to 2016
The study design involved the collection of samples according to both active and passive epidemiological survey. Ninety-five samples were collected from free-roaming cats with no suspected Leptospira infection reported (active surveillance), while four cases were reported as cases of suspected leptospirosis (passive surveillance). Leptospira investigation of the free-roaming cats (n = 95) was conducted by retrospectively testing the cat samples collected for other purposes (health status control, pre-surgery investigation, other diagnostics investigations) and enrolled for the study. The test included Leptospira antibody detection by means of a microscopic agglutination test (MAT) on serum [3] and a real-time PCR on urine for the detection of pathogenic Leptospira, as previously described. The samples were collected from 2014 to 2016 by the veterinary service of the health authority in Northeast Italy (Veneto region) and by veterinary private practices: the samples were submitted to the laboratory of IZSVE and tested for Leptospira through serological and/or molecular methods, as reported above. Anamnestic data, including the clinical features, of the cats enrolled for the study were collected when available. The average age of these subjects was 5.95 years (standard deviation ± 3.75), accounting for 34 young cats (age from 1 to 3 y/o), 42 adult cats (age from 4 to 8 y/o) and 19 senior cats (age > 9 y/o). The study population enrolled 47 female cat (47/95, 49.67%, 95% CI: 36.42–59.53%), and 48 male cat (48/95, 50.53%, 95% CI: 40.47–60.58%).
Moreover, as part of the passive surveillance, samples from suspected clinical cases came from veterinary clinical practices. The clinical symptoms included polyuria, polydipsia, kidney disease and dehydration. The samples (EDTA-whole blood, serum and urine) were collected during routinely diagnostic procedures carried out by the veterinary surgeons. In this context, considering the specific case, it was not deemed necessary to submit a specific request to the Ethics Committee. All procedures complied with the ethical standards of the relevant national and European regulations on animal welfare. Data concerning the cat’s clinical features and the presence of co-morbidities were reported by the attending veterinarians.
Serological Analysis: Micro Agglutination Test (MAT)
All the collected serum samples (n = 99, active and passive surveillance) were submitted to MAT, according to the WOAH method (Chap 3.1.12) [3]. The antigen panel included 8 serogroups and 9 serovars distributed by the Italian Reference Centre for Animal Leptospirosis as antigens in the routine diagnostic MAT (L. interrogans serogroup (sg) Australis serovar Bratislava; L. interrogans sg Canicola serovar Canicola; L. kirschneri sg Grippotyphosa serovar Grippotyphosa; L. interrogans sg Icterohaemorrhagiae serovar Copenhageni; L. interrogans sg Icterohaemorrhagiae serovar Icterohaemorrhagiae; L. interrogans sg Pomona serovar Pomona; L. interrogans sg Sejroe serovar Hardjo; L. borgpetersenii sg Tarassovi serovar Tarassovi; L. borgpetersenii sg Ballum serovar Ballum) [47].
The serum samples were pretested at the final dilution of 1:100. Samples with 50% agglutination were retested to determine an endpoint using dilutions of serum beginning at 1:100 through to 1:6400. Serum samples with the widely accepted minimum significant titre of 1:100 (reciprocal of the final dilution of serum with 50% agglutination) were assessed as positive. In cases of clinically suspected Leptospira infection, urine samples were collected and analysed for bacterial culture and Leptospira isolation (n = 2). In addition, these urine samples were assessed via a pathogen-specific Leptospira TaqMan (real-time PCR) kit [43], as previously described [41].
3. Results
3.1. Leptospira Interrogans Serogroup Australis ST 24 in an Immunocompromised Cat
The gross pathology main findings included moderate, acute, catarrhal-haemorrhagic gastritis with areas of erosion in the pyloric region, as well as severe segmental catarrhal-haemorrhagic enteritis of the proximal enteric tracts (duodenum and digiunum). Moderate hepatomegaly was observed in association with mild and diffuse prominence of the parenchyma, as well as multifocal and irregular areas of greyish discoloration with ill-defined margins. The kidney showed multifocal areas of poorly defined cortico-medullary demarcation. The renal cortex was prominent with multifocal, rarely coalescing, bright red radial striations. A moderate quantity of sero-haemorrhagic pleural effusion was reported in both hemithorax, and the lungs were bilaterally characterized by multifocal to locally diffuse, dark-red, irregular and haemorrhagic areas associated with pulmonary edema. In addition, an extensive area of adhesion between the middle and caudal lobes of the right lung was described, associated with pleural fibrin and a moderate increase in parenchymal consistency (Figure 1). Finally, the mucous membranes appeared pale and the peripheral lymph nodes and tonsils were moderately increased in volume with prominent follicles on the cut surface. Unfortunately, histopathology was not performed, as reported in the study limitation paragraph.
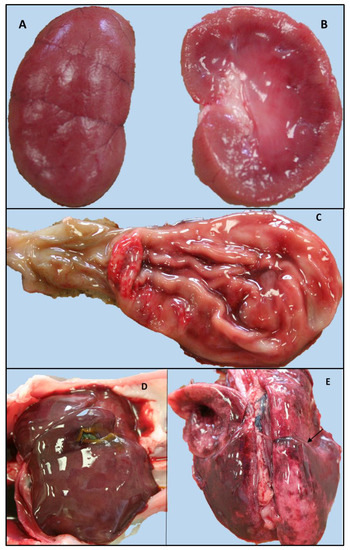
Figure 1.
Gross findings of the kitten positive for FPV and Leptospira interrogans serogroup Australis ST 24. Left kidney dorsal and longitudinal section: multifocal, ill-defined cortico-midline demarcation and a prominent renal cortex with multifocal, rarely coalescent, bright red radial striations (A,B). (B) Stomach and proximal intestine (duodenum): catarrhal-haemorrhagic gastritis, erosive alteration of the mucosa of the pyloric region. (C) Increased size of the liver with blurred margins and slight diffuse prominence of the parenchyma. (D) Severe pulmonary edema and haemorrhagic suffusions: area of adhesion between the middle and caudal lobes of the right lung (arrow) (E).
Bacteriological samples from intestine, liver and lung were positive for Escherichia coli, whereas Salmonella spp. analysis tested negative. Finally, gastrointestinal samples tested positive for the molecular detection of FPV (Ct 23.01) (stomach and small intestine tissue samples).
Bacterial culture tested negative for Leptospira spp. (kidney, lung and liver).
The molecular analysis reported positivity for Leptospira spp. only in the kidney tissues (Ct 35.95), while lung and liver resulted negative. The Leptospira DNA, submitted to an MLST analysis, was typed as Leptospira ST 24, which identifies L. interrogans serogroup Australis.
3.2. Survey on the Exposure to Leptospira spp. of Feline Populations in Northeast Italy (2014–2016): RC IZSVE 16/12
3.2.1. Leptospira Investigation in Free-Roaming Cats: Active Surveillance
Ninety-five free-roaming cats were tested for anti-Leptospira antibodies (MAT) and for pathogenic Leptospira detection in urine (real-time PCR). The MAT was positive in 10 out 95 cats (10.53%, 95% CI: 4.35–16.70%), and the most representative serogroups were Grippotyphosa (n = 6/95; 6.32%, 95% CI: 1.42–11.21%), Icterohaemorrhagiae (n = 2/95; 2.11%, 95% CI: 0.00–4.99%) and Bratislava (n = 3/95; 3.16%, 95% CI: 0.00–6.67%). None of these cats tested positive in urine and blood by real-time PCR. The animals reported low antibody titres (<1:200), both against L. kirschneri sg Grippotyphosa serovar Grippotyphosa and L. interrogans sg Icterohaemorrhagiae serovar Icterohaemorrhagiae. One cat reported antibodies against L. interrogans sg Australis serovar Bratislava (titre 1:200) and one cat showed positivity for L. interrogans Canicola serovar Canicola (titre 1:100). One young and pregnant queen showed antibody titres against L. kirschneri sg Grippotyphosa serovar Grippotyphosa (titre 1:100), L. interrogans sg Icterohaemorrhagiae serovar Icterohaemorrhagiae (titre 1:100) and L. interrogans sg Icterohaemorrhagiae serovar Copenhageni (titre 1:100). Finally, a pregnant adult queen reported low titres for L. borgpetersenii sg Ballum serovar Ballum (titre 1:100) (n = 1/95, 1.05%, 95%CI 0.00–3.10) (Table 1). The real-time PCR on the urine samples tested negative.

Table 1.
MAT titres detected during the survey in cats (study RC 16/12); the significant clinical features and concurrent diseases (active surveillance). Clinically symptomatic cats tested positive for Leptospira spp. MAT and/or real-time PCR, and the presence of clinical signs and concurrent diseases (passive surveillance).
3.2.2. Leptospira Investigation in Free-Roaming Cats with Clinical Symptoms: Passive Surveillance
Among the four cats selected by passive surveillance showing clinical symptoms suggestive of possible Leptospira infection, high MAT titres (>1:400) were recorded towards serogroup/serovars Grippotyphosa (n = 2/5; 40%, 95% CI: 0.00–82.9%) and Bratislava (n = 2/5; 40%, 95% CI: 0.00–82.9%). MAT titres of 1:200 were reported for Icterohaemorrhagiae (n = 1/5, 20%, 95% CI 0.00–55.06%) and Copenhageni (n = 2/5; 40%, 95% CI: 0.00–82.9%). Moreover, two cats reported a co-presence of different serovars (L. Grippotyphosa, L. Icterohaemorrhagiae and L. Copenhageni; L. Bratislava and L. Copenhageni, respectively). Interestingly, the cats that were positive for Leptospira (high antibody titres and/or molecular positivity) presented severe immunocompromising co-morbidities, such as FIV and FeLV, FHV-1 infection and hyperthyroidism, and lymphoma. (Table 1).
4. Discussion
Leptospirosis in cats still has several unclear aspects. Epidemiological studies and the identification of serovars of Leptospira circulating in this species help to fill the gaps in the definition of the eco-pathological picture of leptospirosis and the role of the cat. Although outdoor cats are potentially easily exposed to the pathogen (predatory behaviour, contact with reservoirs and contaminated environments), they appear to be less prone to the development of clinical disease than other susceptible animals. It is not completely clarified which serovars can cause incidental infections in cats. Based on previously published reports of acute leptospirosis in cats, serovars belonging to Autumnalis, Australis, Icterohaemorrhagiae, Grippotyphosa, Pomona and Sejroe serogroups seem to be mostly involved [9]. Interestingly, during an ongoing study conducted by these authors’ research team (Mazzotta E., et al.), L. interrogans Australis ST 24 was detected in hedgehogs, mice and foxes in Northeast Italian regions, suggesting a possible prey–predator epidemiological scenario (preliminary unpublished data).
The present study describes the identification of L. interrogans Australis ST 24 in a kitten with severe immunosuppressive co-morbidity (FPV). The clinical symptoms (haemorrhagic diarrhoea) and the gross pathology findings (haemorrhagic enteritis) were highly suggestive of FPV infection [48,49], as confirmed by the positive real-time PCR result. FPV is transmitted via the faecal–oral route, and the infected subject is able to shed high titres of virus, rapidly contaminating the environment. Feline panleukopenia represents a severe disease, common signs of which include lymphopenia and neutropenia, followed by thrombocytopenia and anaemia, immunosuppression (transient in adult cats), neurological and reproductive symptoms [50]. In addition, this cat reported both pulmonary severe diffuse haemorrhagic lesions and hepatic alterations: bacteriological identification revealed in both lung and liver samples showed a positive high load for E. coli. The identification may indicate an extra intestinal localization of this bacterium, likely related to the poor immunity of the kitten.
In this context, the shelter condition/household, the young age of the cat, the expected presence of immune dysfunction due to FPV and the possible presence of bacterial co/secondary infection, could likely have favoured the occurrence of Leptospira infection. As previously reported in the literature [9,12], this cat could be more likely considered as an incidental host rather than a chronic carrier for leptospirosis.
Concerning the epidemiological evaluation among free-roaming cats in Northeast Italy, these authors reported an apparently reassuring situation in clinically healthy cats, with sporadic seropositivity at low titres and no direct detection of Leptospira. Conversely, a different scenario appeared in four clinically suspected cases: these cats showed suggestive symptoms of clinical leptospirosis, high MAT antibody titres, and one animal tested positive in a real-time PCR analysis on the urine sample. All these cases presented severe systemic co-morbidities (i.e., FPV, FIV, FeLV and lymphoma).
To the best of the authors’ knowledge, a specific correlation between Leptospira ST and immunosuppressive co-morbidities has not been demonstrated, but significant associations with an inflammatory condition and stress response were reported in cats exposed to Leptospira spp. In Leptospira spp. antibody-positive cats, alterations in CBC (anaemia, neutrophilia, monocytosis and eosinopenia), in inflammation markers (i.e., hypoalbuminemia and hyperglobulinemia) and increased ALT activity have been reported [51]. Previous case reports described three confirmed, naturally infected clinical cases of feline leptospirosis, in which the major clinical findings were different stages of renal insufficiency without any liver involvement [24,52]. Dissimilar information is available about the correlation between chronic kidney disease and serological positivity for Leptospira [10,53]. Although further investigations are needed, it is possible that the lack of an adequate immune response in animals with immunosuppressive diseases may have favoured the development of systemic leptospirosis, in association with a clinically evident condition in the most severe cases.
The free-roaming cats found positive for Leptospira antibodies but without any suggestive clinical symptoms reported low serological titres (>1:100): these findings would be suggestive of exposure, possibly recurrent, to pathogenic Leptospira or subclinical and chronic infection, since it has been previously reported that cats can demonstrate positive serology of leptospirosis months after the suspected time of infection/exposure [9]. Thus, it is possible that cats may develop clinical signs after a longer period than what has been documented experimentally [23]. Unfortunately, it was not possible to evaluate the animals afterwards, so no follow-up data are available.
The most frequent serovars involved in feline leptospirosis in Europe, based on serological investigations and according to the European consensus statement on leptospirosis, belong to serogroups Australis, Autumnalis, Ballum, Canicola, Grippotyphosa, Icterohaemorrhagiae, Pomona and Sejroe [10]. According to this, our study showed the presence of antibodies against Grippotyphosa, Icterohaemorrhagiae, Canicola and Australis, despite these low serological titres being suggestive of exposure or subclinical infection. The comparison of the serological data available in the literature about anti-Leptospira antibodies in cats is likely biased by geographical origin, sampling method and the diagnostic technique applied [24,53,54]. Environmental factors, such as outdoor habits, the presence of livestock and farm animals that may shed Leptospira in the neighbourhood, wild animals’ Leptospira reservoirs, and seasonality, may result in different degrees of exposure to pathogenic Leptospira, thus, potentially justifying the broad ranges of antibody prevalence reported in the literature. A recent study conducted in cats in southern Italy reported antibodies against serovars Poi, Arborea and Mini, among others [51]. This study also described the spring season as the only risk factor for urinary Leptospira shedding, detected in 9% of urine samples. Moreover, laboratory variability determined by both the use of different methods and the application of different cut off values (≥1:100), and variations in host-specific humoral immune responses, can be a hindrance to the correct identification of positive results. Many different Leptospira antigens were tested in the immunoassay, but false-negative results occur when the infecting serogroups are not included. Furthermore, the significance and duration of Leptospira species antibodies, as detected by the MAT in cat sera, are largely unknown. It is even possible that seroconversion in cats is expressed at a lower titre compared to dogs [55]. However, the MAT is believed to be specific for Leptospira species antibodies, even if it is unknown whether antibodies against other spirochetes in feline sera can lead to falsely positive results. Although not completely elucidated, it has recently been experimentally assessed that antibodies produced following infection by other spirochetes in cats (i.e., Borrelia burgdorferi) are not detected in Leptospira MAT [55].
The evaluation of serological tests in cats is a challenge: cross-reactivity with non-vaccine serogroups has been demonstrated in dogs, as well as in cats, and antibody production after infection in cats appears to be serogroup-specific, although immune protection is not clearly understood [14]. The seroprevalence against Leptospira observed in our study was 10.53%, falling within the previous intervals (4% to 33.3%) described worldwide [18,26,55,56,57].
The cat’s role as a possible cause of Leptospira environmental contamination and source of exposure for people is not fully understood [9], whereas it is a susceptible host for Leptospira spp. and could potentially present a chronic leptospirosis infection with urinary shedding. Feline leptospirosis is likely to be underdiagnosed: it is common for leptospirosis not to be considered as a possible differential diagnosis, even in animals with clinical symptoms suggestive of acute Leptospira infection (i.e., acute renal failure). Moreover, the possible underestimation of leptospirosis in cats may be due to other factors, such as the challenging clinical diagnosis related to mild or atypical clinical signs, and difficulties in serological or molecular analysis. These circumstances may impact the scarcity of official reports and the perceived low prevalence of the infection within this species [58]. Therefore, the lack of a large serological survey and molecular detection among Leptospira spp.-positive cats does not adequately define the zoonotic risk factors nor the epidemiological role of this species.
5. Conclusions
The detection of new ST in cats highlights the need to consider the challenge of better understanding the effect of exposure to or infection with pathogenic Leptospira spp. in felines, and the need to define their epidemiological role as sentinel hosts or environmental reservoirs. Outdoor and shelter cats that are free-roaming and hunt prey would be a population worthy of special attention: although they may not explicitly manifest symptoms of leptospirosis, they may instead reveal circulating strains of Leptospira in a domestic–shelter (cat–prey) or domestic–domestic (cat–dog or other susceptible domestic mammals) context. To date, many questions remain to be clarified, particularly concerning the cat’s ability to be a chronic carrier rather than an environmental sentinel. In addition, as highlighted by the serological survey in outdoor cats, further studies are needed to increase knowledge about the host-immune response following infection or exposure to specific Leptospira serovars within the feline population. Furthermore, the evaluation of the possible aetiopathogenic link between clinical leptospirosis and immunosuppressive diseases, and the analysis of the immune pathways, would be useful to improve the diagnostic techniques.
Author Contributions
Conceptualization, E.M. and G.D.Z.; methodology, L.B. and L.C.; formal analysis, G.D.Z., M.C. and L.L.; investigation, E.M. and T.F.; resources, A.N., T.F., M.B.B., C.B. and L.C.; data curation, E.M., G.D.Z. and L.L.; writing—original draft preparation, E.M. and G.D.Z.; writing—review and editing, E.M., G.D.Z., L.B., T.F., C.B. and L.L.; visualization, A.N. and M.B.B.; supervision, A.N.; project administration, A.N.; funding acquisition, A.N. All authors have read and agreed to the published version of the manuscript.
Funding
Ministero della Salute, Ricerca Corrente IZSVE 16/12 grant number B28C13000510001.
Institutional Review Board Statement
The study received Ethics Committee IZSVE approval (CE_IZSVE 17/2022). Biological samples were collected by clinicians for diagnostic purposes and from stray cats undergoing spaying surgery sampled to perform pre-operative profile tests. No animals were sampled exclusively for the purposes of this study. Necropsy was performed on request of the legal manager of the shelter, as an official diagnostic surveillance of the veterinary service of the health authority. Animal care and procedures are in accordance with the Guide for the Care and Use of Laboratory Animals and Directive 2010/63/EU for animal experiments (National law: D.L. 26/2014).
Informed Consent Statement
The processing of biological material, data, anamnestic and epidemiological information of the animals were authorized as a part of the research project (RC IZSVE 16/12).
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This paper is dedicated to the memory of Carmelo Furnari. The authors would like to thank Ben Adler for his advice and scientific support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.N.; Amran, F.; Masuzawa, T.; et al. Revisiting the Taxonomy and Evolution of Pathogenicity of the Genus Leptospira through the Prism of Genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef] [PubMed]
- Goarant, C.; Adler, B.; de la Peña Moctezuma, A. Leptospira. In Pathogenesis of Bacterial Infections in Animals; Prescott, J.F., Rycroft, A.N., Boyce, J.D., MacInnes, J.I., Van Immerseel, F., Vázquez-Boland, J.A., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 502–527. [Google Scholar] [CrossRef]
- World Organization for Animal Health (WOAH). Leptospirosis. Man. Diagnostic Tests Vaccines Terr. Anim. OIE Terr. Man. 2021 2022, May 2022 (Chapter 3.1.12). Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.01.12_LEPTO.pdf (accessed on 27 December 2022).
- Casanovas-Massana, A.; Hamond, C.; Santos, L.A.; de Oliveira, D.; Hacker, K.P.; Balassiano, I.; Costa, F.; Medeiros, M.A.; Reis, M.G.; Ko, A.I.; et al. Leptospira Yasudae Sp. Nov. and Leptospira Stimsonii Sp. Nov., Two New Species of the Pathogenic Group Isolated from Environmental Sources. Int. J. Syst. Evol. Microbiol. 2020, 70, 1450–1456. [Google Scholar] [CrossRef]
- Caimi, K.; Ruybal, P. Leptospira spp., a Genus in the Stage of Diversity and Genomic Data Expansion. Infect. Genet. Evol. 2020, 81, 104241. [Google Scholar] [CrossRef] [PubMed]
- Jayasundara, D.; Senavirathna, I.; Warnasekara, J.; Gamage, C.; Siribaddana, S.; Kularatne, S.A.M.; Matthias, M.; Mariet, J.-F.; Picardeau, M.; Agampodi, S.; et al. 12 Novel Clonal Groups of Leptospira Infecting Humans in Multiple Contrasting Epidemiological Contexts in Sri Lanka. PLoS Negl. Trop. Dis. 2021, 15, e0009272. [Google Scholar] [CrossRef]
- Bierque, E.; Thibeaux, R.; Girault, D.; Soupé-Gilbert, M.-E.; Goarant, C. A Systematic Review of Leptospira in Water and Soil Environments. PLoS ONE 2020, 15, e0227055. [Google Scholar] [CrossRef]
- Dorsch, R.; Ojeda, J.; Salgado, M.; Monti, G.; Collado, B.; Tomckowiack, C.; Tejeda, C.; Müller, A.; Eberhard, T.; Klaasen, H.L.B.M.; et al. Cats Shedding Pathogenic Leptospira spp.—An Underestimated Zoonotic Risk? PLoS ONE 2020, 15, e0239991. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.; Goris, M.; Ahmed, A.; Cuenca, R.; Pastor, J. Leptospirosis in Cats: Current Literature Review to Guide Diagnosis and Management. J. Feline Med. Surg. 2020, 22, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European Consensus Statement on Leptospirosis in Dogs and Cats. J. Small Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef]
- Agunloye, C.A.; Nash, A.S. Investigation of Possible Leptospiral Infection in Cats in Scotland. J. Small Anim. Pract. 1996, 37, 126–129. [Google Scholar] [CrossRef]
- Hartmann, K.; Egberink, H.; Pennisi, M.G.; Lloret, A.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hosie, M.J.; et al. Leptospira Species Infection in Cats. J. Feline Med. Surg. 2013, 15, 576–581. [Google Scholar] [CrossRef]
- Fraga, T.R.; Carvalho, E.; Isaac, L.; Barbosa, A.S. Leptospira and Leptospirosis. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 1973–1990. [Google Scholar] [CrossRef]
- Bourassi, E.; Savidge, C.; Foley, P.; Hartwig, S. Serologic and Urinary Survey of Exposure to Leptospira Species in a Feral Cat Population of Prince Edward Island, Canada. J. Feline Med. Surg. 2021, 23, 1155–1161. [Google Scholar] [CrossRef]
- Shophet, R. A Serological Survey of Leptospirosis in Cats. N. Z. Vet. J. 1979, 27, 236–246. [Google Scholar] [CrossRef]
- Millán, J.; Candela, M.G.; López-Bao, J.V.; Pereira, M.; Jiménez, M.Á.; León-Vizcaíno, L. Leptospirosis in Wild and Domestic Carnivores in Natural Areas in Andalusia, Spain. Vector-Borne Zoonotic Dis. 2009, 9, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Lehtla, A.; Must, K.; Lassen, B.; Orro, T.; Jokelainen, P.; Viltrop, A. Leptospira Spp. in Cats in Estonia: Seroprevalence and Risk Factors for Seropositivity. Vector-Borne Zoonotic Dis. 2020, 20, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.; Cuenca, R.; Serrano, E.; Marga, G.; Ahmed, A.; Cervantes, S.; Caparrós, C.; Vieitez, V.; Ladina, A.; Pastor, J. Leptospira Detection in Cats in Spain by Serology and Molecular Techniques. Int. J. Environ. Res. Public Health 2020, 17, 1600. [Google Scholar] [CrossRef] [PubMed]
- Žákovská, A.; Schánilec, P.; Treml, F.; Dušková, M.; Agudelo, C.F. Seroprevalence of Antibodies against Borrelia Burgdorferi S. L.and Leptospira Interrogans S. L. in Cats in District of Brno and Its Environs, the Czech Republic. Ann. Agric. Environ. Med. 2020, 27, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Sebek, Z.; Wallner, H.; Sixl, W.; Kaaserer, G.; Valová, M. Leptospiral Antibodies in Domestic Animals in Tyrol. Folia Parasitol. 1976, 23, 15–23. [Google Scholar]
- Weis, S.; Rettinger, A.; Bergmann, M.; Llewellyn, J.R.; Pantchev, N.; Straubinger, R.K.; Hartmann, K. Detection of Leptospira DNA in Urine and Presence of Specific Antibodies in Outdoor Cats in Germany. J. Feline Med. Surg. 2017, 19, 470–476. [Google Scholar] [CrossRef]
- Bryson, D.G.; Ellis, W.A. Leptospirosis in a British Domestic Cat. J. Small Anim. Pract. 1976, 17, 459–465. [Google Scholar] [CrossRef]
- Larsson, C.E.; Santa Rosa, C.A.; Larsson, M.H.M.A.; Birgel, E.H.; Fernandes, W.R.; Paim, G.V. Laboratory and Clinical Features of Experimental Feline Leptospirosis. Int. J. Zoonoses 1985, 12, 111–119. [Google Scholar]
- Arbour, J.; Blais, M.-C.; Carioto, L.; Sylvestre, D. Clinical Leptospirosis in Three Cats (2001–2009). J. Am. Anim. Hosp. Assoc. 2012, 48, 256–260. [Google Scholar] [CrossRef]
- Sykes, J.E.E.; Hartmann, K.; Lunn, K.F.F.; Moore, G.E.E.; Stoddard, R.A.A.; Goldstein, R.E.E. 2010 ACVIM Small Animal Consensus Statement on Leptospirosis: Diagnosis, Epidemiology, Treatment, and Prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Azócar-Aedo, L.; Monti, G.; Jara, R. Leptospira Spp. in Domestic Cats from Different Environments: Prevalence of Antibodies and Risk Factors Associated with the Seropositivity. Animals 2014, 4, 612–626. [Google Scholar] [CrossRef]
- Rijks, J.M.; Cito, F.; Cunningham, A.A.; Rantsios, A.T.; Giovannini, A. Disease Risk Assessments Involving Companion Animals: An Overview for 15 Selected Pathogens Taking a European Perspective. J. Comp. Pathol. 2016, 155, S75–S97. [Google Scholar] [CrossRef]
- Chadsuthi, S.; Chalvet-Monfray, K.; Wiratsudakul, A.; Modchang, C. The Effects of Flooding and Weather Conditions on Leptospirosis Transmission in Thailand. Sci. Rep. 2021, 11, 1486. [Google Scholar] [CrossRef]
- Datz, C.A. Noninfectious Causes of Immunosuppression in Dogs and Cats. Veter-Clin. N. Am. Small Anim. Pr. 2010, 40, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, P.A.; Murphy, B.G. Common and Emerging Infectious Diseases in the Animal Shelter. Vet. Pathol. 2014, 51, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Malbon, A.J.; Russo, G.; Burgener, C.; Barker, E.N.; Meli, M.L.; Tasker, S.; Kipar, A. The Effect of Natural Feline Coronavirus Infection on the Host Immune Response: A Whole-Transcriptome Analysis of the Mesenteric Lymph Nodes in Cats with and without Feline Infectious Peritonitis. Pathogens 2020, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Thayer, V.; Gogolski, S.; Felten, S.; Hartmann, K.; Kennedy, M.; Olah, G.A. 2022 AAFP/EveryCat Feline Infectious Peritonitis Diagnosis Guidelines. J. Feline Med. Surg. 2022, 24, 905–933. [Google Scholar] [CrossRef]
- Liem, B.P.; Dhand, N.K.; Pepper, A.E.; Barrs, V.R.; Beatty, J.A. Clinical Findings and Survival in Cats Naturally Infected with Feline Immunodeficiency Virus. J. Vet. Intern. Med. 2013, 27, 798–805. [Google Scholar] [CrossRef]
- Little, S.; Levy, J.; Hartmann, K.; Hofmann-Lehmann, R.; Hosie, M.; Olah, G.; Denis, K.S. 2020 AAFP Feline Retrovirus Testing and Management Guidelines. J. Feline Med. Surg. 2020, 22, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline Leukaemia ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.K.; Tompkins, M.B.; Tompkins, W.A.F. Clinical and Immunologic Aspects of FeLV-Induced Immunosuppression. Vet. Microbiol. 1988, 17, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Rehme, T.; Hartmann, K.; Truyen, U.; Zablotski, Y.; Bergmann, M. Feline Panleukopenia Outbreaks and Risk Factors in Cats in Animal Shelters. Viruses 2022, 14, 1248. [Google Scholar] [CrossRef]
- Truyen, U.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline Panleukopenia ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 538–546. [Google Scholar] [CrossRef]
- Stuetzer, B.; Hartmann, K. Feline Parvovirus Infection and Associated Diseases. Vet. J. 2014, 201, 150–155. [Google Scholar] [CrossRef]
- Capello, K.; Bortolotti, L.; Lanari, M.; Baioni, E.; Mutinelli, F.; Vascellari, M. Estimate of the Size and Demographic Structure of the Owned Dog and Cat Population Living in Veneto Region (North-Eastern Italy). Prev. Vet. Med. 2015, 118, 142–147. [Google Scholar] [CrossRef]
- Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Bellinati, L.; Mazzucato, M.; Furlanello, T.; D’Incau, M.; Natale, A. Detection of New Leptospira Genotypes Infecting Symptomatic Dogs: Is a New Vaccine Formulation Needed? Pathogens 2020, 9, 484. [Google Scholar] [CrossRef]
- World Organization for Animal Health (WOAH). Salmonellosis. Man. Diagnostic Tests Vaccines Terr. Anim. OIE Terr. Man. 2021 2022, May 2022 (Chapter 3.10.07). Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.10.07_SALMONELLOSIS.pdf (accessed on 27 December 2022).
- Smythe, L.D.; Smith, I.L.; Smith, G.A.; Dohnt, M.F.; Symonds, M.L.; Barnett, L.J.; McKay, D.B. A Quantitative PCR (TaqMan) Assay for Pathogenic Leptospira Spp. BMC Infect. Dis. 2002, 2, 13. [Google Scholar] [CrossRef]
- Boonsilp, S.; Thaipadungpanit, J.; Amornchai, P.; Wuthiekanun, V.; Bailey, M.S.; Holden, M.T.G.; Zhang, C.; Jiang, X.; Koizumi, N.; Taylor, K.; et al. A Single Multilocus Sequence Typing (MLST) Scheme for Seven Pathogenic Leptospira Species. PLoS Negl. Trop. Dis. 2013, 7, e1954. [Google Scholar] [CrossRef]
- Jolley, K.A.; Maiden, M.C.J. BIGSdb: Scalable Analysis of Bacterial Genome Variation at the Population Level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Di Trani, L.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A Real-Time PCR Assay for Rapid Detection and Quantitation of Canine Parvovirus Type 2 in the Feces of Dogs. Vet. Microbiol. 2005, 105, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, S.; Figarolli, B.M.; D’Incau, M.; Foschi, G.; Gennero, M.S.; Giordani, R.; Natale, A.; Papa, P.; Ponti, N.; Scaltrito, D.; et al. Indagine Sierologica Sulla Presenza Di Leptospira Spp. in Italia: Dati Nazionali 2010–2011. Vet. Ital. 2016, 52, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sura, R.; Van Kruiningen, H.J.; Debroy, C.; Hinckley, L.S.; Greenberg, K.J.; Gordon, Z.; French, R.A. Extraintestinal Pathogenic Escherichia Coli-Induced Acute Necrotizing Pneumonia in Cats. Zoonoses Public Health 2007, 54, 307–313. [Google Scholar] [CrossRef]
- Highland, M.A.; Byrne, B.A.; DebRoy, C.; Samitz, E.M.; Peterson, T.S.; Oslund, K.L. Extraintestinal Pathogenic Escherichia Coli-Induced Pneumonia in Three Kittens and Fecal Prevalence in a Clinically Healthy Cohort Population. J. Vet. Diagn. Investig. 2009, 21, 609–615. [Google Scholar] [CrossRef]
- Barrs, V.R. Feline Panleukopenia: A Re-Emergent Disease. Vet. Clin. Small Anim. Pract. 2019, 49, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Donato, G.; Masucci, M.; Hartmann, K.; Goris, M.G.A.; Ahmed, A.A.; Archer, J.; Alibrandi, A.; Pennisi, M.G. Leptospira spp. Prevalence in Cats from Southern Italy with Evaluation of Risk Factors for Exposure and Clinical Findings in Infected Cats. Pathogens 2022, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- Beaudu-Lange, C.; Lange, E. Unusual Clinical Presentation of Leptospirosis in a Cat. Rev. Vet. Clin. 2014, 49, 115–122. [Google Scholar] [CrossRef]
- Rodriguez, J.; Blais, M.C.; Lapointe, C.; Arsenault, J.; Carioto, L.; Harel, J. Serologic and Urinary PCR Survey of Leptospirosis in Healthy Cats and in Cats with Kidney Disease. J. Vet. Intern. Med. 2014, 28, 284–293. [Google Scholar] [CrossRef]
- Larsson, C.E.; Santa Rosa, C.A.; Hagiwara, M.K.; Paim, G.V.; Guerra, J.L. Prevalence of Feline Leptospirosis: Serologic Survey and Attempts of Isolation and Demonstration of the Agent. Int. J. Zoonoses 1984, 11, 161–169. [Google Scholar]
- Shropshire, S.B.; Veir, J.K.; Morris, A.K.; Lappin, M.R. Evaluation of the Leptospira Species Microscopic Agglutination Test in Experimentally Vaccinated Cats and Leptospira Species Seropositivity in Aged Azotemic Client-Owned Cats. J. Feline Med. Surg. 2016, 18, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Sprißler, F.; Jongwattanapisan, P.; Luengyosluechakul, S.; Pusoonthornthum, R.; Prapasarakul, N.; Kurilung, A.; Goris, M.; Ahmed, A.; Reese, S.; Bergmann, M.; et al. Leptospira Infection and Shedding in Cats in Thailand. Transbound. Emerg. Dis. 2019, 66, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.E.; Bourtzi-Hatzopoulou, E.; Koutinas, A.F.; Petridou, E.; Saridomichelakis, M.N.; Leontides, L.; Siochu, A. Leptospiral Seroepidemiology in a Feline Hospital Population in Greece. Vet. Rec. 2005, 156, 615–616. [Google Scholar] [CrossRef] [PubMed]
- André-Fontaine, G. Canine Leptospirosis—Do We Have a Problem? Vet. Microbiol. 2006, 117, 19–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).