Abstract
Due to the rapid evolution of the monkeypox virus, the means by which the monkeypox virus is spread is subject to change. Therefore, the present study aims to analyze the detection of the monkeypox virus according to the collection site of samples from confirmed monkeypox cases. A systematic literature review was performed using PubMed, Scopus, Web of Science, and Embase databases until 5 October 2022. A total of 1022 articles were retrieved using the search strategy. After removing duplicates (n = 566) and examining by title, abstract, and full text, 65 studies reporting monkeypox case reports were included with a detailed description of risk factors, sexually transmitted infections (STIs), site of monkeypox virus-positive specimens, location of skin lesions, and diagnostic test. A total of 4537 confirmed monkeypox cases have been reported, of which 98.72% of the cases were male with a mean age of 36 years, 95.72% had a sexual behavior of being men who have sex with men, and 28.1% had human immunodeficiency virus (HIV). The most frequent locations of lesions in patients diagnosed with monkeypox were: 42.85% on the genitalia and 37.1% in the perianal region. All confirmed monkeypox cases were diagnosed by reverse transcriptase polymerase chain reaction (RT-PCR), and the most frequent locations of samples collected for diagnosis that tested positive for monkeypox virus were: 91.85% from skin lesions, 20.81% from the oropharynx, 3.19% from blood, and 2.43% from seminal fluid. The disease course of the cases with monkeypox was asynchronous, with no severe complications, and most patients did not report specific treatment but simply followed a symptomatic treatment.
1. Introduction
The global resurgence of confirmed cases of monkeypox (MPX) [1] and the rapid spread of cases in different endemic and non-endemic countries of the world have led to a public health emergency of international concern [2,3]. As of 10 December 2022, 82,474 confirmed cases of monkeypox have been reported in 110 countries worldwide [4].
Monkeypox is a reemerging zoonotic viral disease caused by the monkeypox virus (MPXV) [5]. MPXV is a double-stranded DNA virus of the genus Orthopoxvirus of the family Poxviridae known for more than half a century [6] but geographically endemic to Central and West Africa [7,8]. MPXV has two distinct clades: the Central Basin clade and the West African clade [9]. The West African clade exhibits less virulence with a mortality rate of less than 1% [10]. On the other hand, the Central Basin clade (Central African clade) is more lethal, with a mortality rate of up to 10% in unvaccinated children [11]. This is because the two clades are genetically distinct [10]. The Central African clade is reported more frequently than the West African clade and has documented cases of person-to-person transmission, whereas the West African clade does not [12].
Monkeypox is transmitted to humans through close contact with an infected animal or person through lesions, body fluids, respiratory droplets, or virus-contaminated material [13]. The disease has an incubation period of 5 to 21 days [14] with nonspecific clinical manifestations such as fever, lymphadenopathy, headache, malaise, and general lesions [15,16].
Polymerase chain reaction (PCR) using swabs of skin lesions (vesicles, ulcers, and scabs) recommended for confirming infection in symptomatic individuals is used to detect MPXV [17]. In addition, MPXV DNA has been detected by PCR in a wide variety of samples such as throat, nasopharynx, blood, urine, saliva, and semen [18].
It is possible for a person to be infected with MPXV and not show any symptoms [19]. In asymptomatic individuals, MPXV has been found in urethral [20] and anal swabs [20,21,22], according to recent research. In addition, persons with asymptomatic MPX can likely transmit the virus.
Currently, knowledge about the means by which MPXV is spread is rapidly evolving. The rapid increase in MPX cases in non-endemic areas has challenged clinical laboratories, testing has been limited, and results have been delayed [23]. The excretion and transmission of MPXV are poorly understood, and relevant data to support clinical management and public health response are lacking [24].
Therefore, the present study aims to analyze the detection of the monkeypox virus according to the collection site of samples from confirmed monkeypox cases.
2. Materials and Methods
2.1. Protocol and Registration
This protocol follows the recommendations established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, and it has been reported in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42022368207).
2.2. Eligibility Criteria
Published peer-reviewed articles with study designs of case reports, case series, and observational studies (nonrandomized cohort and intervention studies) were included to investigate monkeypox virus detection by the location of specimen collection from confirmed cases. The articles had no language restrictions, and publications up until 5 October 2022, were included. Editorials, letters to the editor, randomized clinical trials, narrative reviews, systematic review publications, and conference proceedings were excluded.
2.3. Information Sources and Search Strategy
The databases of PubMed, Scopus, Web of Science, and Embase were thoroughly searched. The search terms used were: (“Monkeypox” OR “Monkey Pox”) AND “Specimen Handling” OR “Handling, Specimen” OR “Handlings, Specimen” OR “Specimen Handlings” OR “Specimen Collection” OR “Collection, Specimen” OR “Collections, Specimen” OR “Specimen Collections” OR “blood” OR “saliva” OR “skin” OR “semen” OR “genitals” OR “feces”) (Table 1). On October 5, 2022, the searches were finished, and the results were separately assessed by two distinct investigators.

Table 1.
Bibliographic search strategy.
2.4. Study Selection
Based on the electronic searches, three investigators (D.A.L.F., J.J.B. and H.M.S.-C.) assembled a database that was organized with the right management tool (EndNote), and duplicate entries were eliminated. The screening process was then carried out by two researchers (D.K.B.A. and M.J.V.-G.) using Rayyan QCRI. They separately assessed the titles and abstracts provided by the search, selected those that seemed to satisfy the inclusion criteria, and, if necessary, evaluated the complete text. If there is disagreement, the researchers will discuss their differences until a consensus is reached.
To make a choice, the authors (A.J.R.M., E.M.R. and R.S.) examined the full-text reports and considered the inclusion criteria.
2.5. Outcomes
The primary outcome was to analyze the detection of the monkeypox virus according to the collection site of samples from confirmed monkeypox cases.
2.6. Data Collection Process and Data Items
Data from the chosen papers were independently retrieved by three researchers (D.A.L.F., J.J.B., and H.M.S.-C.) and entered into a Microsoft Excel spreadsheet. The following data were extracted from the selected studies: author data, date of publication, study design, country, sex, age, risk factors, sexually transmitted infections (STIs), site of monkeypox virus-positive sample, location of skin lesions, and diagnostic test. A fourth investigator checked for duplicate articles or data in the list of publications and data extractions, and also dealt with disagreements about the inclusion of studies.
3. Results
3.1. Study Selection
A total of 1022 articles were retrieved using the search strategy. The selection strategy is shown in the PRISMA flow chart (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). After the removal of duplicates (n = 566), 456 articles were screened by the authors. After filtering the titles and reading the abstracts, 85 articles were selected for full-text reading, and 65 were considered eligible for inclusion in this systematic review (Figure 1) [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
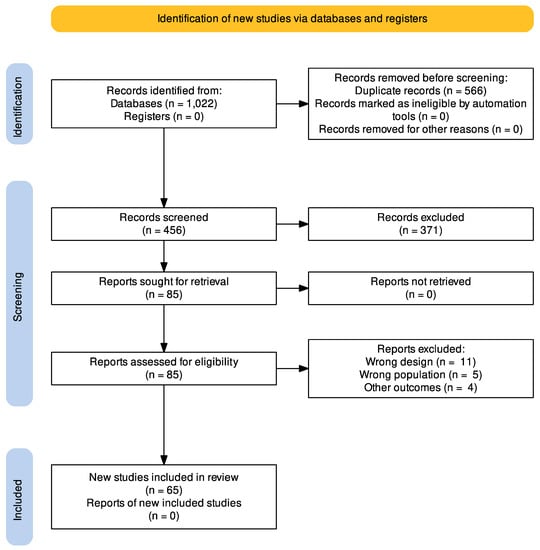
Figure 1.
PRISMA flow chart of the studies selection process.
3.2. Study Characteristics
The main characteristics of the articles included in this review are summarized in Table 2 [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86]. The review included 65 studies published between 1 January and 5 October 2022. The studies (n = 65) reported monkeypox case reports according to the specimen collection site, the number of cases, sexual behavior-oriented risk factor, history of sexually transmitted diseases, site of specimen drawn and positive for monkeypox virus by PCR, location of skin lesions, and method of diagnosis were described in detail (Table 2) [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86]. A total of 4537 confirmed cases of monkeypox were reported distributed in 23 countries: Germany (n = 28) [21,35,47,78], India (n = 2) [84], Korea (n = 1) [70], Iran (n = 1) [39], Canada (n = 2) [55,56], Brazil (n = 4) [36,43,44], Spain (n = 2020) [32,51,72,73,74,76,81], Italy (n = 85) [18,20,26,42,53,54,68,71,80,83,85,86], the United Kingdom (n = 310) [31,33,34,59,69,77,82], Australia (n = 1) [60], Nigeria (n = 19) [30,50,75], the United States (n = 1217) [27,38,40,48,61,67,79], Portugal (n = 29) [25,62,66], France (n = 266) [28,45,63], Israel (n = 1) [29], Cameroon (n = 1) [37], Romania (n = 1) [64], Singapore (n = 15) [41], Central African Republic (n = 2) [57], Greece (n = 1) [49], Sweden (n = 1) [52], the Netherlands (n = 1) [46], the Czech Republic (n = 1) [65], and others (n = 528) [58]. Spain was the country with the highest number of monkeypox cases, followed by the United States and the United Kingdom.

Table 2.
Characteristics of included studies and description of case reports of monkeypox.
3.3. Demographical Characteristics and Diagnostic Method for Monkeypox
Of the total number of cases (n = 4537) reported with monkeypox, 98.72% (n = 4479) of the cases were found to be male [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86]. The mean age of the reported cases of monkeypox was 36 years [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86]. Of the reported cases with monkeypox, 95.72% (n = 4343) [18,20,21,25,26,28,31,32,35,38,40,41,42,43,44,45,47,48,49,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,85,86] had a sexual behavior of being men who have sex with men, and 1.54% (n = 70) [58,61,82] of the cases had a sexual behavior of being gay or bisexual or men who have sex with men. In addition, reported cases of monkeypox with sexually transmitted infections were: 28.1% had HIV (n = 1274) [18,20,21,26,28,31,32,42,44,47,49,53,54,56,58,62,63,64,65,66,68,69,71,72,73,74,75,76,77,79,80,82,85,86], 2% had Syphilis (n = 89) [18,32,38,40,44,50,54,57,58,60,65,67,69,71,72,74,77,78,83,86], 2.1% had Gonorrhea (n = 94) [25,32,35,54,58,69,72,74,77,78,86], and about 1% of cases had Herpes simplex (n = 45) [31,32,40,58,67,69,74,77] and Chlamydia (n = 44) [25,32,35,58,72,74,77]. All confirmed monkeypox cases were diagnosed by reverse transcriptase polymerase chain reaction (RT-PCR) (Table 2) [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
3.4. Location of Lesions, Location of Positive MPX Viral PCR Results, and the Evolution of the Disease
The most frequent locations of lesions in patients diagnosed with monkeypox (n = 4537) were: 42.85% on the genitalia (n = 1944) [18,20,29,32,35,41,42,43,44,45,46,47,48,49,50,51,52,53,54,56,58,59,60,61,62,63,64,66,67,69,70,71,72,73,74,75,76,77,78,79,80,81,83,84,85], 37.1% on the perianal region (n = 1683) [18,20,26,34,41,42,45,47,51,58,61,62,64,65,67,68,69,72,73,74,76,77,79,80,81,82,83], 25.21% on the face (n = 1144) [25,27,29,35,36,37,38,39,43,44,45,46,53,55,56,57,58,59,60,61,68,69,70,73,74,76,77,79,81,85], 19.1% on the upper and lower limbs (n = 867) [18,26,27,28,29,36,37,38,39,40,42,43,45,46,47,50,53,55,56,57,58,60,61,64,66,67,69,71,72,73,74,76,77,78,79,84,85], and 17.63% on the trunk (n = 800) [18,21,28,40,42,43,45,53,54,55,58,60,61,64,68,70,72,73,74,76,77,78,79,86] (Table 2). The diagnosis of monkeypox cases was performed by PCR [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86], and the most frequent locations of samples extracted for diagnosis that tested positive for monkeypox virus (n = 4537) were: 91.85% from skin lesions (n = 4167) [18,20,21,25,26,27,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,81,82,83,84,85,86], 20.81% from oropharynx (n = 944) [18,21,26,28,31,35,36,45,46,47,52,58,60,62,63,64,67,68,70,71,72,73,74,83,84,85,86], 3.19% from blood (n = 145) [18,21,26,30,35,36,38,42,45,46,47,52,58,59,64,68,69,80,84], and 2.43% from seminal fluid (n = 110) [18,21,26,35,42,52,56,58,72,80] (Table 2).
The disease evolution of the cases with monkeypox was asynchronous, with no serious or severe complications [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86], and most patients did not report specific treatment but simply followed symptomatic treatment [18,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
4. Discussion
At a research center in Copenhagen, Denmark, an outbreak of a smallpox-like disease in monkeys led to the discovery and isolation of MPXV in 1958 [87]. The first human case was in 1970, when a smallpox-like disease affected a 9-month-old boy in the Democratic Republic of Congo [88].
Currently, MPXV is slowly emerging as a public health problem of international importance. Uncertainties persist regarding transmission routes; along with epidemiological data, new insights are expected from the virological assessment of the presence of MPXV in different areas of the human body [89]. Therefore, it is crucial to detect them before widespread community transmission [90]. This systematic review aims mainly to analyze the detection of MPXV according to the site of sample collection from confirmed monkeypox cases.
A total of 4537 confirmed cases of monkeypox were reported, and 44.5% and 26.8% of the cases were distributed in Spain and the United States, respectively. It was found that 98.72% of the cases were male with an average age of 36 years, and 95.72% also had a sexual behavior of being men who have sex with men. In the evaluation of sexually transmitted infections, it was reported that 28.1% had HIV, and less than 2% had syphilis and gonorrhea. The location of the most frequent lesions was in the genital and perianal regions at 42.85% and 37.1% respectively. All cases of monkeypox were diagnosed by PCR, and the most frequent samples that tested positive for the monkeypox virus were skin lesions (91.85%) and oropharyngeal lesions (20.81%).
To address the detection of monkeypox virus DNA, according to WHO guidelines, the sample type may be (a) skin lesion material, including lesion exudate swabs, lesion ceilings, and lesion crusts; (b) oropharyngeal swabs; (c) rectal and/or genital swabs; (d) urine; (e) sperm; (f) whole blood; (g) serum; or (h) plasma [91].
The recommended specimen for diagnosis is skin lesion material, because it contains the highest concentration of the virus. In addition, the oropharyngeal swab is recommended for laboratory confirmation of cases [92]. However, because the current outbreak is still under investigation, the collection of additional specimen types for investigative purposes may be considered if permitted by the appropriate ethics review board, and there is sufficient medical and laboratory expertise for their safe collection, handling, and storage [93]. These may include serum, plasma, rectal and vaginal swabs, urine, semen, and blood.
The detection of MPXV in oropharyngeal and perioral lesions [58]. This can be a source of transmission through oral contact (kissing) and saliva exchange [59]. Similarly, the virus has been detected in anal and perianal lesions [58,76,94], generating transmission through insertive anal contact (e.g., penis or finger) or anilingus, since such exposure occurs in intimate physical contact during sexual intercourse. On the other hand, MPXV has been detected in the semen of infected men, which could plausibly transmit the infection [21]. Reda A et al. reported in their systematic review and meta-analysis study that MPXV is highly prevalent in seminal samples of MPX cases, further corroborating the role of sexual transmission of the disease [95].
DNA detection by PCR in samples can last up to 3 weeks. Samples with cycle threshold (Ct) values >35 beyond this duration have been reported for the upper respiratory tract swabs (up to 41 days and possibly 73 days) [96], saliva (up to 76 days) [52], and semen (up to 54 days) [52,58]. In addition, the proportion of replication-competent virus present has been associated with the amounts of viral DNA in clinical samples, suggesting that a higher viral load determined by PCR may indicate a greater potential for infectivity [97].
The recent outbreak of monkeypox in non-endemic regions of the world is of great concern. The current epidemiological statistics reported by WHO shows a predominance of the condition in 97.1% (40,940/42,163) of young men with a median age of 35 years (interquartile range: 29–42 years); furthermore, among cases with declared sexual orientation, 87.9% (18,549/21,099) were identified as men who have sex with men [98].
New routes of transmission of MPXV because most confirmed cases have the risk factor of sexual contact. Thus, the virus could spread rapidly between sexual partners. High viral loads of MPXV from the skin and mucosal sites, including genital and anal sites, imply that transmission is more likely to occur through direct body contact than through the respiratory route or contact with body fluids, which should help improve prevention messages sent to those most exposed to the virus [99].
The situation of this new zoonotic disease, which now appears to be emerging, warrants further study to fully understand the complex effects of this virus, which is currently affecting several continents and may have new transmission pathways, including during the current COVID-19 pandemic.
The results of this systematic review highlight findings of monkeypox virus detection according to the site of specimen collection from confirmed monkeypox cases that can assist healthcare personnel in the early recognition of cases, ensure adequate clinical monitoring and supportive interventions, and prevent further transmission through the implementation of infection control measures [100].
5. Limitations and Strengths
The evidence on monkeypox is constantly changing. This systematic review includes mostly case report studies and case series, which may result in heterogeneity of information. In addition, because of the current monkeypox outbreak, some high-impact studies may not have been included. In terms of strengths, the present study has a rigorous methodology, as it was conducted following the recommendations of the PRISMA guidelines. Likewise, all the processes carried out for the selection of the studies were performed independently by two or more authors.
6. Conclusions
Symphonic smallpox has spread rapidly throughout the world, becoming a public health problem of international importance and generating new routes of transmission with the presence of MPXV in different areas of the human body. Our findings showed that most of the cases in the current outbreak of MPX were male, with a sexual behavior of being men who have sex with men, and the most frequent lesions were in the genital and perianal regions. Finally, the most frequent locations of samples collected for diagnosis that tested positive for monkeypox virus were skin lesions, oropharynx, blood, and seminal fluid.
Author Contributions
Conceptualization, D.A.L.-F., J.J.B., H.M.S.-C., M.J.V.-G. and A.J.R.-M.; methodology, J.J.B., M.J.V.-G., E.M.-R., R.S. and A.J.R.-M.; software, D.A.L.-F., H.M.S.-C., D.K.B.-A. and J.J.B.; validation, D.A.L.-F. and J.J.B.; formal analysis, D.A.L.-F. and A.J.R.-M.; investigation, D.A.L.-F. and A.J.R.-M.; resources, D.A.L.-F.; data curation, D.A.L.-F.; writing—original draft preparation, D.A.L.-F., H.M.S.-C., D.K.B.-A., J.J.B., R.S. and A.J.R.-M.; writing—review and editing, D.A.L.-F., J.J.B., M.J.V.-G. and A.J.R.-M.; visualization, D.A.L.-F., D.K.B.-A., E.M.-R., J.J.B., M.J.V.-G., R.S. and A.J.R.-M.; supervision, D.A.L.-F.; project administration, A.J.R.-M.; and funding acquisition, J.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ilic, I.; Zivanovic Macuzic, I.; Ilic, M. Global Outbreak of Human Monkeypox in 2022: Update of Epidemiology. Trop. Med. Infect. Dis. 2022, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Selvaraj, P.; Halesh, M.B.; Jeyaraman, N.; Nallakumarasamy, A.; Gupta, M.; Maffulli, N.; Gupta, A. Monkeypox: An Emerging Global Public Health Emergency. Life 2022, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- León-Figueroa, D.A.; Barboza, J.J.; Garcia-Vasquez, E.A.; Bonilla-Aldana, D.K.; Diaz-Torres, M.; Saldaña-Cumpa, H.M.; Diaz-Murillo, M.T.; Cruz, O.C.-S.; Rodriguez-Morales, A.J. Epidemiological Situation of Monkeypox Transmission by Possible Sexual Contact: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- CDC. Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 28 October 2022).
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Abdelazeem, B.; Bonilla-Aldana, D.K.; et al. Human Monkeypox Disease (MPX). Infez. Med. 2022, 30, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Aldhaeefi, M.; Rungkitwattanakul, D.; Unonu, J.; Franklin, C.-J.; Lyons, J.; Hager, K.; Daftary, M.N. The 2022 Human Monkeypox Outbreak: Clinical Review and Management Guidance. Am. J. Health Syst. Pharm. 2022, zxac300. [Google Scholar] [CrossRef]
- Mondolfi, A.P.; Guerra, S.; Muñoz, M.; Luna, N.; Hernandez, M.M.; Patino, L.H.; Reidy, J.; Banu, R.; Shrestha, P.; Liggayu, B.; et al. Evaluation and Validation of an RT-PCR Assay for Specific Detection of Monkeypox Virus (MPXV). J. Med. Virol. 2022. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; Satta, E.; Zaniboni, A.; Ranzieri, S.; Baldassarre, A.; Zaffina, S.; Marchesi, F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Infect. Dis. 2022, 7, 135. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic Characterization and Signs of Microevolution in the 2022 Multi-Country Outbreak of Monkeypox Virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Bonilla-Aldana, D.K.; Pachar, M.; Romaní, L.; Saldaña-Cumpa, H.M.; Anchay-Zuloeta, C.; Diaz-Torres, M.; Franco-Paredes, C.; Suárez, J.A.; Ramirez, J.D.; et al. The Never-Ending Global Emergence of Viral Zoonoses after COVID-19? The Rising Concern of Monkeypox in Europe, North America and Beyond. Travel Med. Infect. Dis. 2022, 49, 102362. [Google Scholar] [CrossRef]
- Beer, E.M.; Rao, V.B. A Systematic Review of the Epidemiology of Human Monkeypox Outbreaks and Implications for Outbreak Strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef]
- Nguyen, P.-Y.; Ajisegiri, W.S.; Costantino, V.; Chughtai, A.A.; MacIntyre, C.R. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021, 27, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Lulli, L.G.; Baldassarre, A.; Mucci, N.; Arcangeli, G. Prevention, Risk Exposure, and Knowledge of Monkeypox in Occupational Settings: A Scoping Review. Trop. Med. Infect. Dis. 2022, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Angelo, K.M.; Smith, T.; Camprubí-Ferrer, D.; Balerdi-Sarasola, L.; Díaz Menéndez, M.; Servera-Negre, G.; Barkati, S.; Duvignaud, A.; Huber, K.L.B.; Chakravarti, A.; et al. Epidemiological and Clinical Characteristics of Patients with Monkeypox in the GeoSentinel Network: A Cross-Sectional Study. Lancet Infect. Dis. 2022, in press. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox-A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Chelsky, Z.L.; Dittmann, D.; Blanke, T.; Chang, M.; Vormittag-Nocito, E.; Jennings, L.J. Validation Study of a Direct Real-Time PCR Protocol for Detection of Monkeypox Virus. J. Mol. Diagn. 2022, 24, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Eurosurveillance 2022, 27, 2200421. [Google Scholar] [CrossRef]
- De Baetselier, I.; Van Dijck, C.; Kenyon, C.; Coppens, J.; Michiels, J.; de Block, T.; Smet, H.; Coppens, S.; Vanroye, F.; Bugert, J.J.; et al. Retrospective Detection of Asymptomatic Monkeypox Virus Infections among Male Sexual Health Clinic Attendees in Belgium. Nat. Med. 2022, 28, 2288–2292. [Google Scholar] [CrossRef]
- Moschese, D.; Pozza, G.; Mileto, D.; Giacomelli, A.; Cutrera, M.; Cossu, M.V.; Matone, M.; Beltrami, M.; Salari, F.; Antinori, S.; et al. Isolation of Viable Monkeypox Virus from Anal and Urethral Swabs, Italy, May to July 2022. Eurosurveillance 2022, 27, 2200675. [Google Scholar] [CrossRef]
- Noe, S.; Zange, S.; Seilmaier, M.; Antwerpen, M.H.; Fenzl, T.; Schneider, J.; Spinner, C.D.; Bugert, J.J.; Wendtner, C.-M.; Wölfel, R. Clinical and Virological Features of First Human Monkeypox Cases in Germany. Infection 2022. [Google Scholar] [CrossRef]
- Ferré, V.M.; Bachelard, A.; Zaidi, M.; Armand-Lefevre, L.; Descamps, D.; Charpentier, C.; Ghosn, J. Detection of Monkeypox Virus in Anorectal Swabs from Asymptomatic Men Who Have Sex With Men in a Sexually Transmitted Infection Screening Program in Paris, France. Ann. Intern. Med. 2022, 175, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Porzucek, A.J.; Proctor, A.M.; Klinkhammer, K.E.; Tritsch, S.R.; Robertson, M.A.; Bashor, J.P.; Villani, J.; Sepulveda, J.L.; Mores, C.N. Development of an Accessible and Scalable QPCR Assay for Monkeypox Virus Detection. J. Infect. Dis. 2022, jiac414. [Google Scholar] [CrossRef] [PubMed]
- Hasso, M.; Perusini, S.; Eshaghi, A.; Tang, E.; Olsha, R.; Zhang, H.; Lau, E.; Sullivan, A.; Cronin, K.; Lee, S.; et al. Monkeypox Virus Detection in Different Clinical Specimen Types. Emerg. Infect. Dis. 2022, 28, 2513–2515. [Google Scholar] [CrossRef] [PubMed]
- Brito Caldeira, M.; Fernandes, C. Cutaneous Lesions From Monkeypox Infection. Sex. Transm. Dis. 2022, 49, 595. [Google Scholar] [CrossRef] [PubMed]
- Brundu, M.; Marinello, S.; Scaglione, V.; Ferrari, A.; Franchin, E.; Mazzitelli, M.; Cattelan, A.M. The First Case of Monkeypox Virus and Acute HIV Infection: Should We Consider Monkeypox a New Possible Sexually Transmitted Infection? J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Costello, V.; Sowash, M.; Gaur, A.; Cardis, M.; Pasieka, H.; Wortmann, G.; Ramdeen, S. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1002–1005. [Google Scholar] [CrossRef]
- Davido, B.; D’Anglejan, E.; Baudoin, R.; Dahmane, L.; Chaud, A.; Cortier, M.; Vauloup-Fellous, C.; De Truchis, P.; Ghosn, J. Monkeypox Outbreak 2022: An Unusual Case of Peritonsillar Abscess in a Person Previously Vaccinated against Smallpox. J. Travel Med. 2022, 29, taac082. [Google Scholar] [CrossRef]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980–983. [Google Scholar] [CrossRef]
- Eseigbe, E.E.; Akude, C.; Osagie, I.A.; Eseigbe, P. Human Monkey Pox Virus Infection in Plateau State, North Central Nigeria: A Report of Two Cases. West Afr. J. Med. 2021, 38, 1242–1246. [Google Scholar] [CrossRef]
- Gedela, K.; Da Silva Fontoura, D.; Salam, A.; Gorman, G.; Golden, J.; O’Hara, G.; Elawaidy, A.; Tittle, V.; Girometti, N.; Whitlock, G.; et al. Infectious Proctitis Due to Human Monkeypox. Clin. Infect. Dis. 2022, ciac713. [Google Scholar] [CrossRef]
- Gomez-Garberi, M.; Sarrio-Sanz, P.; Martinez-Cayuelas, L.; Delgado-Sanchez, E.; Bernabeu-Cabezas, S.; Peris-Garcia, J.; Sanchez-Caballero, L.; Nakdali-Kassab, B.; Egea-Sancho, C.; Olarte-Barragan, E.H.; et al. Genitourinary Lesions Due to Monkeypox. Eur. Urol. 2022, 82, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Hobson, G.; Adamson, J.; Adler, H.; Firth, R.; Gould, S.; Houlihan, C.; Johnson, C.; Porter, D.; Rampling, T.; Ratcliffe, L.; et al. Family Cluster of Three Cases of Monkeypox Imported from Nigeria to the United Kingdom, May 2021. Eurosurveillance 2021, 26, 2100745. [Google Scholar] [CrossRef]
- Hofer, U. Case Series of Monkeypox Infections. Nat. Rev. Microbiol. 2022, 20, 445. [Google Scholar] [CrossRef] [PubMed]
- Hornuss, D.; Daehne, T.; Goetz, V.; Mueller, M.; Usadel, S.; Lorz, A.; Mockenhaupt, M.; Huzly, D.; Bierbaum, S.; Fuchs, J.; et al. Transmission Characteristics, Replication Patterns and Clinical Manifestations of Human Monkeypox Virus—An in-Depth Analysis of Four Cases from Germany. Clin. Microbiol. Infect. 2022, in press. [Google Scholar] [CrossRef]
- Carvalho, L.B.; Casadio, L.V.B.; Polly, M.; Nastri, A.C.; Turdo, A.C.; de Araujo Eliodoro, R.H.; Sabino, E.C.; Levin, A.S.; de Proença, A.C.T.; Higashino, H.R. Monkeypox Virus Transmission to Healthcare Worker through Needlestick Injury, Brazil. Emerg. Infect. Dis. 2022, 28. [Google Scholar] [CrossRef] [PubMed]
- Jarman, E.L.; Alain, M.; Conroy, N.; Omam, L.A. A Case Report of Monkeypox as a Result of Conflict in the Context of a Measles Campaign. Public Health Pract. 2022, 4, 100312. [Google Scholar] [CrossRef] [PubMed]
- Karan, A.; Styczynski, A.R.; Huang, C.; Sahoo, M.K.; Srinivasan, K.; Pinsky, B.A.; Salinas, J.L. Human Monkeypox without Viral Prodrome or Sexual Exposure, California, USA, 2022. Emerg. Infect. Dis. 2022, 28, 2121–2123. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Keikha, M. Overlapping Outbreak of COVID-19 and Monkeypox in 2022: Warning for Immediate Preparedness in Iran. Int. J. Surg. 2022, 105, 106892. [Google Scholar] [CrossRef]
- Khan, S.; Razi, S.; Rao, B. It’s Here, Monkeypox: A Case Report. JAAD Case Rep. 2022, 28, 61–63. [Google Scholar] [CrossRef]
- Koh, X.Q.; Chio, M.T.W.; Tan, M.; Leo, Y.S.; Chan, R.K.W. Global Monkeypox Outbreak 2022: First Case Series in Singapore. Ann. Acad. Med. Singap. 2022, 51, 462–472. [Google Scholar] [CrossRef]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox Virus Isolation from a Semen Sample Collected in the Early Phase of Infection in a Patient with Prolonged Seminal Viral Shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.L.d.; Barra, L.A.C.; Borges, L.M.S.; Medeiros, L.A.; Tomishige, M.Y.S.; Santos, L.d.S.L.A.; da Silva, A.J.D.; Rodrigues, C.C.M.; de Azevedo, L.C.F.; Villas-Boas, L.S.; et al. First Case Report of Monkeypox in Brazil: Clinical Manifestations and Differential Diagnosis with Sexually Transmitted Infections. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e54. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.S.; Haddad, G.R.; Miot, H.A. Sexually-Transmitted Monkeypox: Report of Two Cases. An. Bras. Dermatol. 2022, 97, 783–785. [Google Scholar] [CrossRef]
- Mailhe, M.; Beaumont, A.-L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical Characteristics of Ambulatory and Hospitalized Patients with Monkeypox Virus Infection: An Observational Cohort Study. Clin. Microbiol. Infect. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Tutu van Furth, A.M.; van der Kuip, M.; van Els, A.L.; Fievez, L.C.; van Rijckevorsel, G.G.; van den Ouden, A.; Jonges, M.; Welkers, M.R. Paediatric Monkeypox Patient with Unknown Source of Infection, the Netherlands, June 2022. Eurosurveillance 2022, 27, 2200552. [Google Scholar] [CrossRef] [PubMed]
- Nörz, D.; Brehm, T.T.; Tang, H.T.; Grewe, I.; Hermanussen, L.; Matthews, H.; Pestel, J.; Degen, O.; Günther, T.; Grundhoff, A.; et al. Clinical Characteristics and Comparison of Longitudinal QPCR Results from Different Specimen Types in a Cohort of Ambulatory and Hospitalized Patients Infected with Monkeypox Virus. J. Clin. Virol. 2022, 155, 105254. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, Y.; Rodríguez-Morales, A.J.; Franco-Paredes, C.; Chastain, D.B.; Gharamti, A.A.; Vargas Barahona, L.; Henao-Martínez, A.F. Monkeypox-a Description of the Clinical Progression of Skin Lesions: A Case Report from Colorado, USA. Ther. Adv. Infect. Dis. 2022, 9, 20499361221117730. [Google Scholar] [CrossRef] [PubMed]
- Paparizos, V.; Nicolaidou, E.; Tryfinopoulou, K.; Papa, A.; Rigopoulos, D.; Tsiodras, S.; Stratigos, A. Monkeypox Virus Infection: First Reported Case in Greece in a Patient with a Genital Rash. J. Eur. Acad. Dermatol. Venereol. 2022. [Google Scholar] [CrossRef]
- Pembi, E.; Awang, S.; Salaudeen, S.O.; Agaba, I.A.; Omoleke, S. First Confirmed Case of Monkeypox in Adamawa State, Nigeria: A Clinico-Epidemiological Case Report. Pan Afr. Med. J. 2022, 42, 38. [Google Scholar] [CrossRef]
- Pérez-Martín, Ó.G.; Hernández-Aceituno, A.; Dorta-Espiñeira, M.M.; García-Hernández, L.; Larumbe-Zabala, E. Atypical Presentation of Sexually-Transmitted Monkeypox Lesions. Infect. Dis. 2022, 54, 940–943. [Google Scholar] [CrossRef]
- Pettke, A.; Filén, F.; Widgren, K.; Jacks, A.; Glans, H.; Andreasson, S.; Muradrasoli, S.; Helgesson, S.; Hauzenberger, E.; Karlberg, M.L.; et al. Ten-Week Follow-Up of Monkeypox Case-Patient, Sweden, 2022. Emerg. Infect. Dis. 2022, 28, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Pipitò, L.; Cascio, A. Monkeypox Virus Infection and Creatine Phosphokinase Increase: A Case from Italy. Travel Med. Infect. Dis. 2022, 50, 102412. [Google Scholar] [CrossRef] [PubMed]
- Quattri, E.; Avallone, G.; Maronese, C.A.; Cusini, M.; Carrera, C.G.; Marzano, A.V.; Ramoni, S. Unilesional Monkeypox: A Report of Two Cases from Italy. Travel Med. Infect. Dis. 2022, 49, 102424. [Google Scholar] [CrossRef] [PubMed]
- Sukhdeo, S.S.; Aldhaheri, K.; Lam, P.W.; Walmsley, S. A Case of Human Monkeypox in Canada. CMAJ 2022, 194, E1031–E1035. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Jaeranny, S.; Li, M.; Sukhdeo, S.S.; Monge, J.C.; Callejas, M.F.; Hasso, M.; Fattouh, R.; Lalonde, S.D.; Lam, J.; et al. Atypical Clinical Presentation of Monkeypox Complicated by Myopericarditis. Open Forum Infect. Dis. 2022, 9, ofac394. [Google Scholar] [CrossRef]
- Berthet, N.; Nakouné, E.; Whist, E.; Selekon, B.; Burguière, A.-M.; Manuguerra, J.-C.; Gessain, A.; Kazanji, M. Maculopapular Lesions in the Central African Republic. Lancet 2011, 378, 1354. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Heskin, J.; Belfield, A.; Milne, C.; Brown, N.; Walters, Y.; Scott, C.; Bracchi, M.; Moore, L.S.; Mughal, N.; Rampling, T.; et al. Transmission of Monkeypox Virus through Sexual Contact–A Novel Route of Infection. J. Infect. 2022, 85, 334–363. [Google Scholar] [CrossRef]
- Hammerschlag, Y.; MacLeod, G.; Papadakis, G.; Adan Sanchez, A.; Druce, J.; Taiaroa, G.; Savic, I.; Mumford, J.; Roberts, J.; Caly, L.; et al. Monkeypox Infection Presenting as Genital Rash, Australia, May 2022. Eurosurveillance 2022, 27, 2200411. [Google Scholar] [CrossRef]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; et al. Monkeypox Outbreak—Nine States, May 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 764. [Google Scholar] [CrossRef]
- Duque, M.P.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R.; et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance 2022, 27, 2200424. [Google Scholar] [CrossRef]
- Vallée, A.; Farfour, E.; Zucman, D. Monkeypox Virus: A Novel Sexually Transmitted Disease? A Case Report from France. Travel Med. Infect. Dis. 2022, 49, 102394. [Google Scholar] [CrossRef] [PubMed]
- Oprea, C.; Ianache, I.; Piscu, S.; Tardei, G.; Nica, M.; Ceausu, E.; Popescu, C.P.; Florescu, S.A. First Report of Monkeypox in a Patient Living with HIV from Romania. Travel Med. Infect. Dis. 2022, 49, 102395. [Google Scholar] [CrossRef] [PubMed]
- Bížová, B.; Veselý, D.; Trojánek, M.; Rob, F. Coinfection of Syphilis and Monkeypox in HIV Positive Man in Prague, Czech Republic. Travel Med. Infect. Dis. 2022, 49, 102368. [Google Scholar] [CrossRef] [PubMed]
- Patrocinio-Jesus, R.; Peruzzu, F. Monkeypox Genital Lesions. N. Engl. J. Med. 2022, 387, 66. [Google Scholar] [CrossRef]
- Basgoz, N.; Brown, C.M.; Smole, S.C.; Madoff, L.C.; Biddinger, P.D.; Baugh, J.J.; Shenoy, E.S. Case 24-2022: A 31-Year-Old Man with Perianal and Penile Ulcers, Rectal Pain, and Rash. N. Engl. J. Med. 2022, 387, 547–556. [Google Scholar] [CrossRef]
- Mileto, D.; Riva, A.; Cutrera, M.; Moschese, D.; Mancon, A.; Meroni, L.; Giacomelli, A.; Bestetti, G.; Rizzardini, G.; Gismondo, M.R.; et al. New Challenges in Human Monkeypox Outside Africa: A Review and Case Report from Italy. Travel Med. Infect. Dis. 2022, 49, 102386. [Google Scholar] [CrossRef]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and Clinical Characteristics of Confirmed Human Monkeypox Virus Cases in Individuals Attending a Sexual Health Centre in London, UK: An Observational Analysis. Lancet Infect. Dis. 2022, 22, 1321–1328. [Google Scholar] [CrossRef]
- Jang, Y.R.; Lee, M.; Shin, H.; Kim, J.-W.; Choi, M.; Kim, Y.M.; Lee, M.J.; Kim, J.; Na, H.K.; Kim, J.Y. The First Case of Monkeypox in the Republic of Korea. J. Korean Med. Sci. 2022, 37, e224. [Google Scholar] [CrossRef]
- Maronese, C.A.; Beretta, A.; Avallone, G.; Boggio, F.L.; Marletta, D.A.; Murgia, G.; Cusini, M.; Gori, A.; Carrera, C.G.; Di Benedetto, A.; et al. Clinical, Dermoscopic and Histopathological Findings in Localized Human Monkeypox: A Case from Northern Italy. Br. J. Dermatol. 2022, 187, 822–823. [Google Scholar] [CrossRef]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J.; et al. Frequent Detection of Monkeypox Virus DNA in Saliva, Semen, and Other Clinical Samples from 12 Patients, Barcelona, Spain, May to June 2022. Eurosurveillance 2022, 27, 2200503. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.I.; Montalbán, E.G.; Bueno, S.J.; Martínez, F.M.; Juliá, A.N.; Díaz, J.S.; Marín, N.G.; Deorador, E.C.; Forte, A.N.; García, M.A.; et al. Monkeypox Outbreak Predominantly Affecting Men Who Have Sex with Men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance 2022, 27, 2200471. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Yinka-Ogunleye, A. Sexual History of Human Monkeypox Patients Seen at a Tertiary Hospital in Bayelsa, Nigeria. Int. J. STD AIDS 2022, 33, 928–932. [Google Scholar] [CrossRef]
- Orviz, E.; Negredo, A.; Ayerdi, O.; Vázquez, A.; Muñoz-Gomez, A.; Monzón, S.; Clavo, P.; Zaballos, A.; Vera, M.; Sánchez, P.; et al. Monkeypox Outbreak in Madrid (Spain): Clinical and Virological Aspects. J. Infect. 2022, 85, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Fontoura, D.D.S.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef] [PubMed]
- Pfäfflin, F.; Wendisch, D.; Scherer, R.; Jürgens, L.; Godzick-Njomgang, G.; Tranter, E.; Tober-Lau, P.; Stegemann, M.S.; Corman, V.M.; Kurth, F.; et al. Monkeypox In-Patients with Severe Anal Pain. Infection 2022. [Google Scholar] [CrossRef]
- Philpott, D. Epidemiologic and Clinical Characteristics of Monkeypox Cases—United States, May 17–July 22, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1018–1022. [Google Scholar] [CrossRef]
- Raccagni, A.R.; Candela, C.; Mileto, D.; Canetti, D.; Bruzzesi, E.; Rizzo, A.; Castagna, A.; Nozza, S. Monkeypox Infection among Men Who Have Sex with Men: PCR Testing on Seminal Fluids. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Rodríguez, B.S.; Herrador, B.R.G.; Franco, A.D.; Fariñas, M.P.S.-S.; Valero, J.D.A.; Llorente, A.H.A.; de Agreda, J.P.A.P.; Malonda, R.C.; Castrillejo, D.; López, M.D.C.; et al. Epidemiologic Features and Control Measures during Monkeypox Outbreak, Spain, June 2022. Emerg. Infect. Dis. 2022, 28, 1847–1851. [Google Scholar] [CrossRef]
- Vusirikala, A.; Charles, H.; Balasegaram, S.; Macdonald, N.; Kumar, D.; Barker-Burnside, C.; Cumiskey, K.; Dickinson, M.; Watson, M.; Olufon, O.; et al. Epidemiolog y of Early Monkeypox Virus Transmission in Sexual Networks of Gay and Bisexual Men, England, 2022. Emerg. Infect. Dis. 2022, 28, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Ramoni, S.; Maronese, C.A.; Morini, N.; Avallone, G.; Quattri, E.; Carrera, C.G.; Boggio, F.L.; Marzano, A.V. Syphilis and Monkeypox Co-Infection: Coincidence, Synergy or Asymptomatic Carriage? Travel Med. Infect. Dis. 2022, 50, 102447. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. First Two Cases of Monkeypox Virus Infection in Travellers Returned from UAE to India, July 2022. J. Infect. 2022, 85, e145–e148. [Google Scholar] [CrossRef]
- Turco, M.; Mancuso, F.R.; Pisano, L. A Monkeypox Virus Infection Mimicking Primary Syphilis. Br. J. Dermatol. 2022, 187, e194–e195. [Google Scholar] [CrossRef]
- Pisano, L.; Turco, M.; Mancuso, F.R.; Lastrucci, I.; Pimpinelli, N. Atypical Oral Presentation of Monkeypox Virus: A Report of Two Cases from Florence, Italy. Travel Med. Infect. Dis. 2022, 50, 102457. [Google Scholar] [CrossRef]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-Like Disease in Cynomolgus Monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Colavita, F.; Antinori, A.; Nicastri, E.; Focosi, D.; Girardi, E.; Vaia, F.; Maggi, F. Monkeypox Virus in Human Body Sites and Fluids: Evidence for Transmission. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Sitaula, C.; Shahi, T.B. Monkeypox Virus Detection Using Pre-Trained Deep Learning-Based Approaches. J. Med. Syst. 2022, 46, 78. [Google Scholar] [CrossRef]
- Laboratory Testing for the Monkeypox Virus: Interim Guidance. Available online: https://www.who.int/publications-detail-redirect/WHO-MPX-laboratory-2022.1 (accessed on 2 November 2022).
- Gul, I.; Liu, C.; Yuan, X.; Du, Z.; Zhai, S.; Lei, Z.; Chen, Q.; Raheem, M.A.; He, Q.; Hu, Q.; et al. Current and Perspective Sensing Methods for Monkeypox Virus. Bioengineering 2022, 9, 571. [Google Scholar] [CrossRef]
- Laboratory Guidelines for the Detection and Diagnosis of Monkeypox Virus Infection-PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/laboratory-guidelines-detection-and-diagnosis-monkeypox-virus-infection (accessed on 2 November 2022).
- Català, A.; Clavo-Escribano, P.; Riera-Monroig, J.; Martín-Ezquerra, G.; Fernandez-Gonzalez, P.; Revelles-Peñas, L.; Simon-Gozalbo, A.; Rodríguez-Cuadrado, F.J.; Castells, V.G.; de la Torre Gomar, F.J.; et al. Monkeypox Outbreak in Spain: Clinical and Epidemiological Findings in a Prospective Cross-Sectional Study of 185 Cases. Br. J. Dermatol. 2022, 187, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.; Abdelaal, A.; Brakat, A.M.; Lashin, B.I.; Abouelkheir, M.; Abdelazeem, B.; Rodriguez-Morales, A.J.; Sah, R. Monkeypox Viral Detection In Semen Specimens of Confirmed Cases: A Systematic Review and Meta-Analysis. J. Med. Virol. 2022, 95, e28250. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Paran, N.; Yahalom-Ronen, Y.; Shifman, O.; Lazar, S.; Ben-Ami, R.; Yakubovsky, M.; Levy, I.; Wieder-Feinsod, A.; Amit, S.; Katzir, M.; et al. Monkeypox DNA Levels Correlate with Virus Infectivity in Clinical Samples, Israel, 2022. Eurosurveillance 2022, 27, 2200636. [Google Scholar] [CrossRef] [PubMed]
- Multi-Country Outbreak of Monkeypox-External Situation Report 8, Published 19 October 2022-World|ReliefWeb. Available online: https://reliefweb.int/report/world/multi-country-outbreak-monkeypox-external-situation-report-8-published-19-october-2022 (accessed on 2 November 2022).
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. Viral Loads in Clinical Samples of Men with Monkeypox Virus Infection: A French Case Series. Lancet Infect. Dis. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Benites-Zapata, V.A.; Ulloque-Badaracco, J.R.; Alarcon-Braga, E.A.; Hernandez-Bustamante, E.A.; Mosquera-Rojas, M.D.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Clinical Features, Hospitalisation and Deaths Associated with Monkeypox: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).