Abstract
COVID-19 has proved to be a serious, and consequential disease that has affected millions of people globally. Previously, the adverse effects of proton pump inhibitors (PPI) have been observed with increasing the risk of pneumonia and COVID-19. This meta-analysis aims to address the relationship between the use of PPI and the severity of COVID-19 infection. We conducted a systemic literature search from PUBMED, Science Direct, and Cinahl from December 2019 to January 2022. Published and unpublished randomized control trials and cohort studies were included. Review Manager was used for all statistical analyses. In total, 14 studies were included in this systemic review and meta-analysis. Outcomes of interest include: (1) susceptibility of COVID-19 infection and (2) severity of COVID-19 (defined as the composite of poor outcomes: ICU admission, need for oxygen therapy, need for a ventilator, or death), and (3) mortality due to COVID-19. PPI use was marginally associated with a nominal but statistically significant increase in the risk of COVID-19 infection (OR 1.05 [1.01, 1.09]; I2 97%, p = 0.007). PPI use also increased the risk of the composite poor outcome (OR 1.84 [1.71, 1.99]; I2 98%, p < 0.00001) and mortality (OR 1.12 [1.00, 1.25]; I2 84%, p = 0.05) in patients with COVID-19.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness that has been responsible for the loss of 5.3 million lives globally (as of December 2021) [1]. To control this pandemic, it is crucial to seek preventative methods by identifying any risk factors for increased morbidity or mortality in COVID-19 infection. It has been shown that routinely used medications can variably influence the susceptibility to COVID-19 infection, as well as the severity of the disease. Treatment of COVID-19 infection with antihistamines and azithromycin, in symptomatic as well as asymptomatic and susceptible patients, is associated with reduced severity and mortality—irrespective of patients’ age and risk factors [2]. ACE inhibitors and ARBs appear to have no effect on the incidence and prognosis of COVID-19 [3]. Systemic corticosteroids and biologics used in treating asthmatic patients are not associated with increased susceptibility to COVID-19 infection [4]. On the other hand, the adverse effects of opioids can potentially increase the severity of COVID-19 infection, especially by lowering the immune response [5]. Obesity is also another risk factor for worsening the COVID-19 disease across various ethnicities [6]. In addition, hypertensive patients had been observed to have a higher proportion of pulmonary infection and an increased risk for severe adverse COVID-19 outcomes [7].
Proton pump inhibitors (PPI) are chiefly prescribed over the counter for upper gastrointestinal acid-related disorders, such as gastroesophageal reflux disease (GERD), non-erosive reflux disease, and peptic ulcer disease. Due to the high prevalence of upper GI acid-related disorders, high efficacy, favorable safety profile, and affordability, PPI are prescribed frequently and at higher doses for longer durations [8]. In fact, PPI are one of the top ten most used drugs in the world and are prescribed without any clear indication in up to 70% of cases [9]. PPI can negatively affect several organ systems in the body. For instance, associations have been found between PPI and an increased risk of developing chronic kidney disease, which can progress to end-stage renal disease and pneumonia due to PPI-induced hypochloremia [10,11]. Due to the inhibition of acid secretion, hepatotoxicity, neuroendocrine neoplasms, and cancers in the gastrointestinal tract have also been reported with PPI administration [12,13,14].
Given that several medications have been shown to influence COVID-19 incidence and outcomes, and that PPI have systemic effects—it is essential to investigate the relationship between PPI use and COVID-19 susceptibility and outcomes. Previous studies have shown inconsistent associations [15,16]. For instance, Almario et al. [16] reported an increase in the likelihood of developing COVID-19 infection after PPI administration. In contrast, Xiang Y et al. [17] concluded that PPI decrease the susceptibility to COVID-19. Furthermore, Ramchandran et al. [18] observed increased mortality with PPI usage whereas Fan X [19] did not find an association between PPI and mortality due to COVID-19 infection. This meta-analysis was conducted in order to resolve these inconsistencies and provide a holistic, well-powered assessment of the effect of PPI use on the incidence and prognosis of COVID-19.
2. Materials and Methods
2.1. Data Sources and Search Strategy
We undertook a systematic review and meta-analysis investigating the effects of PPI in patients diagnosed with COVID-19. Articles eligible for inclusion were observational cohort, case control, or randomized controlled trials (RCTs) characterizing severity and mortality of COVID-19 infection in patients on PPI therapy. This meta-analysis was carried out as per the recommended guidelines for systematic review and meta-analyses in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]. We designed a high sensitivity search strategy, Supplementary Table S1 [21], combining free text and keyword (COVID-19, Coronavirus, Proton pump inhibitor, severity, morbidity, mortality) search term synonym clusters for COVID-19, combined with clusters for PPI.
PUBMED, Cinahl, and Science Direct were screened from December 2019 to January 2022, using the various names of all PPI and keywords related to COVID-19. The retrieved articles were manually screened to identify relevant studies.
2.2. Study Selection
The following inclusion criteria were used to select studies: (a) published and unpublished cohort and case control studies linking PPI usage to outcomes of COVID-19 infection; (b) published or unpublished cohort or case control studies linking PPI use with COVID-19 severity or mortality. For inclusion, the studies had to report at least one of the following outcomes of interest: (1) susceptibility of COVID-19 infection, and (2) severity of COVID-19 (defined as the composite of poor outcomes: ICU admission, need for oxygen therapy, need for a ventilator, or death), and (3) mortality due to COVID-19.
The following exclusion criteria were used: (a) commentaries, perspectives, and editorials that give a subjective impact of PPI on the topic without clinical patient data were excluded; (b) articles that were not in the English language.
In total, 14 studies fulfilled the eligibility criteria and were included in this meta-analysis. The characteristics of the included studies are mentioned in Table 1, and the baseline characteristics of the patients and their PPI usage are mentioned in Table 2 and Table 3, respectively.

Table 1.
Characteristics of included studies.

Table 2.
Baseline patient characteristics.

Table 3.
Patient proton pump inhibitor (PPI) use.
2.3. Data Extraction and Assessment of Study Quality
EndNote Reference Library was used to remove duplicates. Next, the articles were thoroughly screened and evaluated by two reviewers (AS and SL), and only those studies that fulfilled the aforementioned criteria were included. Firstly, studies were shortlisted based on their titles and abstracts, followed by a detailed review of the full article to ensure relevance. Then, a third investigator (AJ) was consulted to resolve any discrepancies. The studies yielded by our search strategy did not include any randomized control trials (RCTs). The Newcastle-Ottawa Scale was used to assess the quality and risk of bias for cohorts and case control studies, Supplementary Table S2 [21]. Studies were rated on a scale of 0–9. Studies with a cumulative score of 5 or less were considered to be low quality, those with a score of 6–7 were considered to be of moderate quality, and a study with a score of 8 or more was considered to be of high quality. Only moderate and high quality studies were included in our analysis.
2.4. Statistical Analysis
A statistical analysis was performed using Review Manager (version 5.3). The outcomes from the studies were stated as odds ratios (ORs) with 95% confidence intervals (CIs) and were pooled using a random-effects model. Forest plots were generated to visually represent the results. The chi-square test was performed to assess for differences between the subgroups. Heterogeneity across studies was evaluated using Higgins I2 [31]. For I2, values from 25–50% indicated low heterogeneity, 50–75% indicated moderate heterogeneity, and more than 75% indicated severe heterogeneity. A p-value of ≤0.05 was regarded as significant in all cases.
3. Results
3.1. Search Result
Our search string produced 304 studies from our primary databases (33 from Cinahl, 115 PUBMED, 156 Science Direct), additional records of 216 studies were also identified through other sources. After an exclusion of duplicates, 278 studies were screened which yielded a total of 39 studies for full assessment. Of these, eight studies were excluded for being previous meta-analyses and another 17 were removed for not having sufficient data to permit calculations. The remaining studies were carefully evaluated in detail. The final number of studies included in the qualitative synthesis and quantitative synthesis were (n = 14). The PRISMA flow diagram of the selection process is provided in Figure 1.
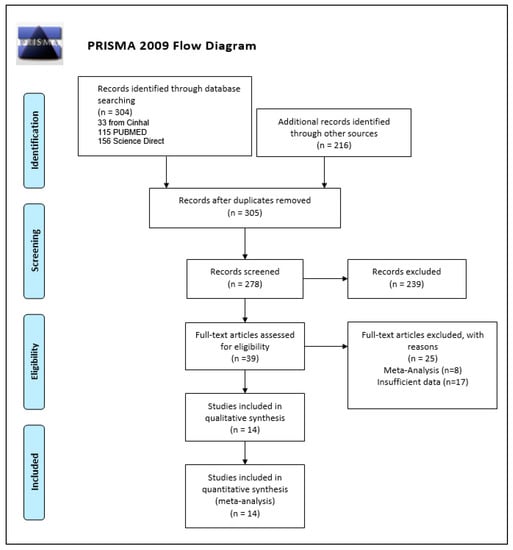
Figure 1.
Prisma flow diagram.
3.2. Study Characteristics and Quality Assessment
Out of the 14 studies we selected for this meta-analysis and systematic review, 10 were cohort studies with 461,003 patients in total, and the other four of them were case control, which included one retrospective case control and one matched case control, with 16,154 patients in total. From the 14 articles, a total of 477,157 patients with laboratory-confirmed COVID-19 infection were identified. Individual study characteristics and patient demographics are presented in Table 1 and Table 2, respectively. The Newcastle-Ottawa Scale (NOS) (range from 0 to 9 points) was used to evaluate quality and risk of bias for all the 14, non-randomized included studies, Supplementary Table S2 [21]. The risk of bias appeared higher for some included studies in comparison to others; however, no important difference was noted in sensitivity analyses by excluding studies at higher risk of bias. Quality assessment was completed independently by two reviewers, and any disagreements were settled by discussion.
3.3. Outcomes
3.3.1. PPI Usage and Susceptibility to COVID-19
Seven out of the 14 included studies provided evidence for this outcome. Overall, our pooled analysis shows that PPI use results in a nominal but statistically significant increased risk of developing COVID-19 (OR 1.05 [1.01, 1.09]; I2 97%, p = 0.007), Figure 2. In the forest plots, the individual point estimates for each study are illustrated by blocks and lines and the diamond represents the meta-analysis of each outcome using the chosen effect measure [32].
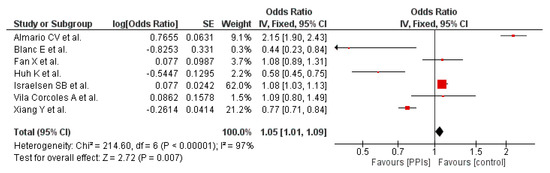
Figure 2.
Probability of developing COVID-19 in patients on PPI versus not on PPI.
3.3.2. PPI Usage and Composite Poor Outcome
The composite poor outcome (ICU admission, need for oxygen therapy, need for a ventilator, or death) was reported in three studies, [22,23,24]. Our pooled analysis shows that PPI use is associated with a statistically significant increase in the risk of the composite poor outcome (OR 1.84 [1.71, 1.99]; I2 98%, p < 0.00001), Figure 3.
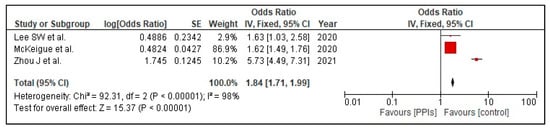
Figure 3.
Risk of poor composite outcome in patients with COVID-19 on PPI versus not on PPI.
3.3.3. PPI Usage and Mortality
Six out of the 14 included studies reported the association between PPI use and mortality. Before carrying out a leave-one-out sensitivity analysis, the association between use of PPI and mortality was found to be borderline significant (OR 1.12 [1.00, 1.25]; I2 85%, p = 0.05), Figure 4.
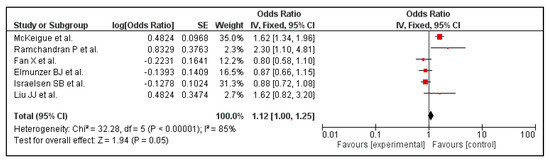
Figure 4.
Risk of mortality in patients with COVID-19 on PPI versus not on PPI.
3.4. Publication Bias
As all the outcomes included studies less than 10, a funnel plot was not retrieved, and publication bias was not assessed [33].
3.5. Sensitivity Analysis
A sensitivity analysis was performed in order to tackle heterogeneity. This was performed for the outcome of mortality in COVID-19 patients with PPI use. One of the studies was excluded, namely McKeigue et al. [26]. Then, the analysis was repeated to obtain a forest plot (Figure 4). This sensitivity analysis brought a change in the final odds ratio from (OR 1.12 [1.00, 1.25] p = 0.05) to (OR 0.92 [0.80, 1.05] p = 0.22), Figure 5, for the outcome of mortality. The heterogeneity reduced from I2 = 85% to a moderate value of I2 = 58% when the study was excluded.
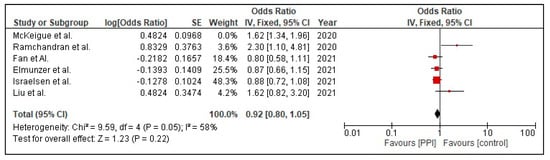
Figure 5.
Risk mortality in patients with COVID-19 on PPI versus not on PPI after sensitivity analysis.
4. Discussion
This is the largest meta-analysis conducted on this topic comprising a total of 477,157 COVID-19 positive patients from 14 studies. According to our results, PPI use is associated with a nominal but statistically significant increase in the risk of developing COVID-19. Moreover, PPI use increased the severity of COVID-19 infection (i.e., the combined risk of ICU admission, need for oxygen therapy, mechanical ventilators, or death). Additionally, we found a borderline significant association between PPI use and mortality in patients with COVID-19.
A 2020 meta-analysis conducted by Israelson et al. [15] showed that PPI increased the risk of COVID-19 infection. An update of this analysis in 2021 by Pranta et al. [34] demonstrated no significant relationship between PPI use and susceptibility to COVID-19. The current meta-analysis consolidates the idea that PPI use is associated with an increased risk of developing COVID-19; however, the risk is only marginal. A recent meta-analysis by Hariyanto et al. [35] correlated with an increased risk of secondary outcomes such as ARDS, gastric tumors, cardiovascular diseases, and kidney diseases. Decreased enzymatic activity of dimethylarginine dimethylaminohydrolase (DDAH) inhibits nitric oxide synthase leading to thrombosis which is a potential cause of cardiovascular diseases. PPI have also been reported to cause nephritis and a humoral and cell-mediated hypersensitivity reaction. The aforementioned events contribute to the severe outcomes and mortality as a result of COVID-19 infection. Inhibition of neutrophil function causes a decrease in the eradication of the virus thus increasing the severity of infection. Irreversible proton pump inhibition is the primary effect of PPI, which diminishes gastric output in the body. Gastric acid is a partial barrier that limits the entry of SARS-CoV-2 viruses into the remaining gastrointestinal tract. When this restrictive acid barrier is withdrawn, by a PPI dosage, it raises the gastric pH to 6.0 from its normal levels of 1.5–3 [36]. The hypochlorhydria subsequent to PPI use is linked with a greater risk of susceptibility for viral infections like COVID-19. Prolonged survival of the COVID-19 virus in the stomach of individuals consuming PPI creates better provision for invasion of epithelial cells in the gastrointestinal tract. Excessive suppression of gastric acid due to PPI also means ingested pathogens are not readily removed from the stomach, with alteration of various immune-modulatory and anti-inflammatory effects [37]. It has been established that the COVID-19 virus, using the spike-like proteins on its surface, binds to ACE-2 of alveolar type 2, intestinal, and other types of cells [38]. This means that a higher expression of ACE-2 will lead to greater binding with the virus, and thus, a higher level of viral entry into the cell causing much more severity of the infection [39]. The GI tract demonstrates greater levels of ACE-2, hence individuals using PPI may be more vulnerable to infection with lower viral loads [38]. This idea is supported by the link between PPI use and increased risk of other respiratory infections—in a meta-analysis of clinical trials conducted by Nabil Sultan et al. the absolute incidence of respiratory infections was estimated to be 1.3% higher in patients receiving PPI than in those receiving a placebo [40].
This study also demonstrates an association between PPI use and adverse clinical outcomes in patients with COVID-19. PPI may increase the severity of COVID-19 by promoting suppression of the immune system [41]. Previously present experimental data propose that gastric acid suppression consequent to the use of PPI affects the immune system in a way that promotes infections [42]. One way the immune system is suppressed is by hampering cytotoxic T lymphocytes, natural killer cells, and polymorphonuclear neutrophil cell activities in the body [43]. PPI can also interfere with the signaling of tumor necrosis factor alpha (TNFα), IL-6, and nuclear factor kappa B (NFκB), and interleukin-1 beta (IL-1β) [44]. PPI also diminish bactericidal activity due to the neutrophil and monocyte inhibition they cause, coupled with intra and extra cellular reduction of nitric oxide and neutrophil reactive oxygen [45]. A cohort study conducted by Sheng Hong Lin et al. in 2021 has provided evidence that PPI have an influence on the gut microbiota [46]. The gut microbiota specifically can play a vital role in other enteric infections by boosting or restricting pathogen colonization [47]. Alteration of the microbiome, or host–microbiota interaction, has a major influence in how the host’s immune system develops and responds. Therefore, manipulation of the intestinal microbiota (which stems from the use of PPI) is considered an approach to inhibit the immune response of the body [48].
Certain limitations must be kept in mind when interpreting the findings of this study. A high heterogeneity was seen in the outcomes—this was expected due to methodological differences between studies, and a random-effects model was used in order to account for it. The methodological differences responsible for heterogeneity may include multiple features of the population such as age and gender, intervention factors such as PPI dosage, timing, or duration of usage before testing for COVID-19, the severity of COVID-19 infection, and wide variations in comorbidities (i.e., hypertension, diabetes, cardiovascular disease, renal disease, and respiratory disease). In addition, the COVID-19 protocols differed between countries which may have contributed to differences between studies that could not be accounted for in our results.
Moreover, reduced reliability of the included studies’ outcomes due to the variability in definitions of the severity of illness from COVID-19 also accounts for a higher heterogeneity. Due to the large sample size included by Israelsen et al. [15] and vast age group of participants catered by Seung Won Lee et al. [23], a higher heterogeneity in our secondary outcomes, that is, the incidence rate of COVID-19 infection and severity (ICU admission and death comparison) of COVID-19 infection in association with PPI usage, was encountered. Furthermore, as no clinical trials have been published, our meta-analysis only included observational studies and that constituted two problems that have led to the increase in heterogeneity: (1) studies did not have comparable follow-up times, (2) there were not enough studies to perform meta-regression. However, to avoid systematic errors and find the source of heterogeneity, we conducted a sensitivity analysis (i.e., removal of individual studies and observing any change in overall heterogeneity). It was observed that the removal of McKeigue et al. [27] led to a decrease in heterogeneity from 85% to 58%. This study was found to have a high bias due to various confounding variables such as age and the presence of comorbidities in the fatality group. Removal of this study changed the OR from 1.12 (95% CI 1.00, 1.25) to 0.92 (95% CI 0.80, 1.05). Thus, the finding of this study wherein PPI use was associated with an increased risk of mortality should be viewed as exploratory, and further studies are required to test this association.
5. Conclusions
PPI use was marginally associated with a nominal but statistically significant in-crease in the risk of COVID-19 infection. PPI use also increased the risk of the composite poor outcomes in patients with COVID-19.
In the ongoing COVID-19 pandemic, with newer variants emerging, it is crucial to know the factors influencing the risk of COVID-19. The increased risk of COVID-19 infection in PPI users appears to be only marginal and thus does not merit prophylactic discontinuation of PPI in patients for whom this medication is indicated. This study suggests that PPI increase the risk of poor clinical outcomes in patients with COVID-19; thus, PPI should be initiated with caution in this population. Moreover, patients with COVID-19 who are PPI users, should be monitored very closely for severe diseases. The current evidence is not sufficient to recommend discontinuation of PPI in patients with COVID-19. Further studies are required to consolidate out findings. Moreover, future studies should investigate whether the associations between PPI use and COVID-19 susceptibility and prognosis are influenced by the variant of COVID-19. The role of PPI and the duration of use should also be considered as important measures to take into account in these studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/tropicalmed7030037/s1, Table S1: Search Strategy used in each database searched; Table S2: Quality assessment of included observational studies using Newcastle-Ottawa Scale.
Author Contributions
Conceptualization, K.F.; methodology, A.A.; software, S.L.; validation, A.S., A.J. and A.M.; formal analysis, S.L.; investigation, A.S.; resources, Z.A. and A.S.; data curation, S.A.Q. and S.G.; writing—original draft preparation, A.R. and A.A.; writing—review and editing, A.S. and A.A.; visualization, A.S.; supervision, A.M. and K.F.; project administration, A.J and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable as our systematic review and meta-analysis did not use original patient data.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- COVID Live-Coronavirus Statistics-Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 20 February 2022).
- Morán Blanco, J.I.; Alvarenga Bonilla, J.A.; Homma, S.; Suzuki, K.; Fremont-Smith, P.; Villar Gómez de Las Heras, K. Antihistamines and azithromycin as a treatment for COVID-19 on primary health care-A retrospective observational study in elderly patients. Pulm. Pharmacol. Ther. 2021, 67, 101989. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; Siddiqi, T.J.; Khan, M.S.; Ahmed, A.; Ali, S.S.; Michos, E.D.; Hall, M.E.; Krasuski, R.A.; Greene, S.J.; Butler, J.; et al. A Meta-analysis of the Relationship Between Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19. Am. J. Cardiol. 2020, 130, 159–161. [Google Scholar] [CrossRef]
- Adir, Y.; Humbert, M.; Saliba, W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: Nationwide real-world evidence. J. Allergy Clin. Immunol. 2021, 148, 361–367.e13. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.E.; Adab, P.; Cheng, K.K. COVID-19: Risk factors for severe disease and death. BMJ 2020, 368, m1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattar, N.; Valabhji, J. Obesity as a Risk Factor for Severe COVID-19: Summary of the Best Evidence and Implications for Health Care. Curr. Obes. Rep. 2021, 10, 282–289. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Du, H.; Ma, R.; Nan, Y.; Zhang, T. Risk Factors for COVID-19 in Patients with Hypertension. Can. J. Infect. Dis. Med. Microbiol. J. Can. Mal. Infect. Microbiol. Med. 2021, 2021, 5515941. [Google Scholar] [CrossRef]
- Ksiądzyna, D.; Szeląg, A.; Paradowski, L. Overuse of proton pump inhibitors. Pol. Arch. Med. Wewn. 2015, 125, 289–298. [Google Scholar] [CrossRef]
- Charpiat, B.; Bleyzac, N.; Tod, M. Proton Pump Inhibitors are Risk Factors for Viral Infections: Even for COVID-19? Clin. Drug Investig. 2020, 40, 897–899. [Google Scholar] [CrossRef]
- Li, T.; Xie, Y.; Al-Aly, Z. The association of proton pump inhibitors and chronic kidney disease: Cause or confounding? Curr. Opin. Nephrol. Hypertens. 2018, 27, 182–187. [Google Scholar] [CrossRef]
- Haastrup, P.F.; Thompson, W.; Søndergaard, J.; Jarbøl, D.E. Side Effects of Long-Term Proton Pump Inhibitor Use: A Review. Basic Clin. Pharmacol. Toxicol. 2018, 123, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Caplin, M.; Khan, K.; Savage, K.; Rode, J.; Varro, A.; Michaeli, D.; Grimes, S.; Brett, B.; Pounder, R.; Dhillon, A. Expression and processing of gastrin in hepatocellular carcinoma, fibrolamellar carcinoma and cholangiocarcinoma. J. Hepatol. 1999, 30, 519–526. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Ciafardini, C.; Massironi, S. Gastric neuroendocrine neoplasms and proton pump inhibitors: Fact or coincidence? Scand. J. Gastroenterol. 2015, 50, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Wahlin, K.; Engstrand, L.; Lagergren, J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: A nationwide population-based cohort study in Sweden. BMJ Open 2017, 7, e017739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israelsen, S.B.; Ernst, M.T.; Lundh, A.; Lundbo, L.F.; Sandholdt, H.; Hallas, J.; Benfield, T. Proton Pump Inhibitor Use Is Not Strongly Associated With SARS-CoV-2 Related Outcomes: A Nationwide Study and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 1845–1854.e6. [Google Scholar] [CrossRef] [PubMed]
- Almario, C.V.; Chey, W.D.; Spiegel, B.M.R. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am. J. Gastroenterol. 2020, 115, 1707–1715. [Google Scholar] [CrossRef]
- Xiang, Y.; Wong, K.C.-Y.; So, H.-C. Exploring Drugs and Vaccines Associated with Altered Risks and Severity of COVID-19: A UK Biobank Cohort Study of All ATC Level-4 Drug Categories Reveals Repositioning Opportunities. Pharmaceutics 2021, 13, 1514. [Google Scholar] [CrossRef]
- Ramachandran, P.; Perisetti, A.; Gajendran, M.; Jean-Louis, F.; Bansal, P.; Dwivedi, A.K.; Goyal, H. Pre-hospitalization proton pump inhibitor use and clinical outcomes in COVID-19. Eur. J. Gastroenterol. Hepatol. 2020, 34, 137–141. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Miyata, T.; Dasarathy, S.; Rotroff, D.M.; Wu, X.; Poulsen, K.L.; Nagy, L.E. Effect of Acid Suppressants on the Risk of COVID-19: A Propensity Score-Matched Study Using UK Biobank. Gastroenterology 2021, 160, 455–458.e5. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 62, e1–e34. [Google Scholar]
- Strategy, S. Supplementary Table 1. Search Strategy Used in Each Database Searched. 2019. Available online: https://www.ijhpm.com/jufile?ar_sfile=44803 (accessed on 4 November 2021).
- Elmunzer, B.J.; Wolf, B.J.; Scheiman, J.M.; Tierney, W.M.; Taylor, J.R. Association Between Preadmission Acid Suppressive Medication Exposure and Severity of Illness in Patients Hospitalized With COVID-19. Gastroenterology 2021, 160, 1417–1422.e14. [Google Scholar] [CrossRef]
- Lee, S.W.; Ha, E.K.; Yeniova, A.Ö.; Moon, S.Y.; Kim, S.Y.; Koh, H.Y.; Yang, J.M.; Jeong, S.J.; Moon, S.J.; Cho, J.Y.; et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matching. Gut 2021, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Sloan, M.E.; Owings, A.H.; Figgins, E.; Gauthier, J.; Gharaibeh, R.; Robinson, T.; Williams, H.; Sindel, C.B.; Backus, F.; et al. Increased ACE2 Levels and Mortality Risk of Patients With COVID-19 on Proton Pump Inhibitor Therapy. Am. J. Gastroenterol. 2021, 116, 1638–1645. [Google Scholar] [CrossRef]
- Lee, S.W.; Yang, J.M.; Yoo, I.K.; Moon, S.Y.; Ha, E.K.; Yeniova, A.Ö.; Cho, J.Y.; Kim, M.S.; Shin, J.I.; Yon, D.K. Proton pump inhibitors and the risk of severe COVID-19: A post-hoc analysis from the Korean nationwide cohort. Gut 2021, 70, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Lee, S.; Wu, W.K.K.; Cheung, B.M.Y.; Zhang, Q.; Tse, G. Proton pump inhibitor or famotidine use and severe COVID-19 disease: A propensity score-matched territory-wide study. Gut 2021, 70, 2012–2013. [Google Scholar] [CrossRef] [PubMed]
- McKeigue, P.M.; Kennedy, S.; Weir, A.; Bishop, J.; McGurnaghan, S.J.; McAllister, D.; Robertson, C.; Wood, R.; Lone, N.; Murray, J.; et al. Relation of severe COVID-19 to polypharmacy and prescribing of psychotropic drugs: The REACT-SCOT case-control study. BMC Med. 2021, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Blanc, F.; Waechter, C.; Vogel, T.; Schorr, B.; Demuynck, C.; Martin-Hunyadi, C.; Meyer, M.; Mutelica, D.; Bougaa, N.; Fafi-Kremer, S.; et al. Interest of Proton Pump Inhibitors in Reducing the Occurrence of COVID-19: A Case-Control Study. Preprints 2020, 2020050016. [Google Scholar] [CrossRef]
- Huh, K.; Ji, W.; Kang, M.; Hong, J.; Bae, G.H.; Lee, R.; Na, Y.; Choi, H.; Gong, S.Y.; Jung, J. Association of previous medications with the risk of COVID-19: A nationwide claims-based study from South Korea. MedRxiv 2020, 1–24. [Google Scholar] [CrossRef]
- Vila-Corcoles, A.; Satue-Gracia, E.; Ochoa-Gondar, O.; Torrente-Fraga, C.; Gomez-Bertomeu, F.; Vila-Rovira, A.; Hospital-Guardiola, I.; de Diego-Cabanes, C.; Bejarano-Romero, F.; Rovira-Veciana, D.; et al. Use of distinct anti-hypertensive drugs and risk for COVID-19 among hypertensive people: A population-based cohort study in Southern Catalonia, Spain. J. Clin. Hypertens. 2020, 22, 1379–1388. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February 2021); Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 7 November 2021).
- Thornton, A.; Lee, P. Publication bias in meta-analysis: Its causes and consequences. J. Clin. Epidemiol. 2000, 53, 207–216. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Lawrensia, S.; Henrina, J.; Lim, M.A.; Lukito, A.A.; Kuswardhani, R.A.T.; Wibawa, I.D.N. Proton pump inhibitor on susceptibility to COVID-19 and its severity: A systematic review and meta-analysis. Pharmacol. Rep. 2021, 73, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Prasetya, I.B.; Kurniawan, A. Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver. 2020, 52, 1410–1412. [Google Scholar] [CrossRef]
- Price, E.; Treacher, D.F. Reduced gastric acidity, proton pump inhibitors and increased severity of COVID-19 infections. Crit. Care. 2021, 25, 73. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Stanciu, C.; Girleanu, I.; Stoica, O.C.; Singeap, A.M.; Maxim, R.; Chiriac, S.A.; Ciobica, A.; Boiculese, L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 6500–6515. [Google Scholar] [CrossRef]
- Arendse, L.B.; Danser, A.H.J.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C.J.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Sultan, N.; Nazareno, J.; Gregor, J. Association between proton pump inhibitors and respiratory infections: A systematic review and meta-analysis of clinical trials. Can. J. Gastroenterol. 2008, 22, 761–766. [Google Scholar] [CrossRef]
- Biswas, S.; Benedict, S.H.; Lynch, S.G.; LeVine, S.M. Potential immunological consequences of pharmacological suppression of gastric acid production in patients with multiple sclerosis. BMC Med. 2012, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palencia-Herrejón, E.; Sánchez, B.; Escobar, I.; Gómez-Lus, M.L. Proton pump inhibitors and infection risk. Rev. Esp. Quimioter. Publ. Of. Soc. Esp. Quimioter. 2011, 24, 4–12. [Google Scholar]
- Laheij, R.J.F.; Sturkenboom, M.C.J.M.; Hassing, R.-J.; Dieleman, J.; Stricker, B.H.C.; Jansen, J.B.M.J. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004, 292, 1955–1960. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; Cooke, J.P.; Gerhart, W.; Griego, C.; Brower, J.B.; Doyle-Eisele, M.; Moeller, B.C.; Zhou, Q.; Ho, L.; de Andrade, J.; et al. Pleiotropic effect of the proton pump inhibitor esomeprazole leading to suppression of lung inflammation and fibrosis. J. Transl. Med. 2015, 13, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zedtwitz-Liebenstein, K.; Wenisch, C.; Patruta, S.; Parschalk, B.; Daxböck, F.; Graninger, W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit. Care Med. 2002, 30, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Chang, Y.-S.; Lin, T.-M.; Hu, L.-F.; Hou, T.-Y.; Hsu, H.-C.; Shen, Y.-C.; Kuo, P.-I.; Chen, W.-S.; Lin, Y.-C.; et al. Proton Pump Inhibitors Increase the Risk of Autoimmune Diseases: A Nationwide Cohort Study. Front. Immunol. 2021, 12, 736036. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgibbon, G.; Mills, K.H.G. The microbiota and immune-mediated diseases: Opportunities for therapeutic intervention. Eur. J. Immunol. 2020, 50, 326–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).