Post–Chikungunya Virus Infection Musculoskeletal Disorders: Syndromic Sequelae after an Outbreak
Abstract
1. Introduction
2. Materials and Methods
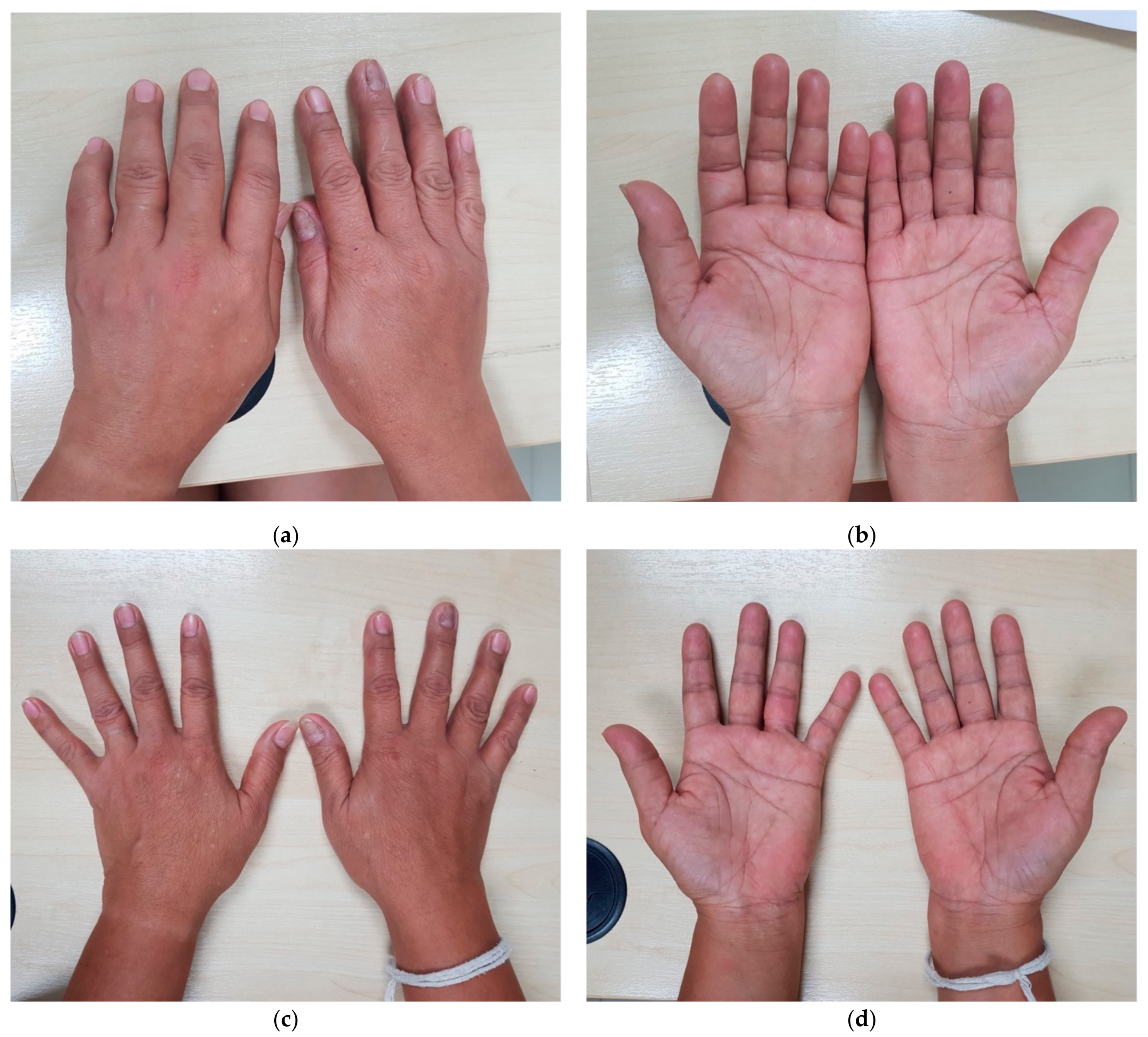
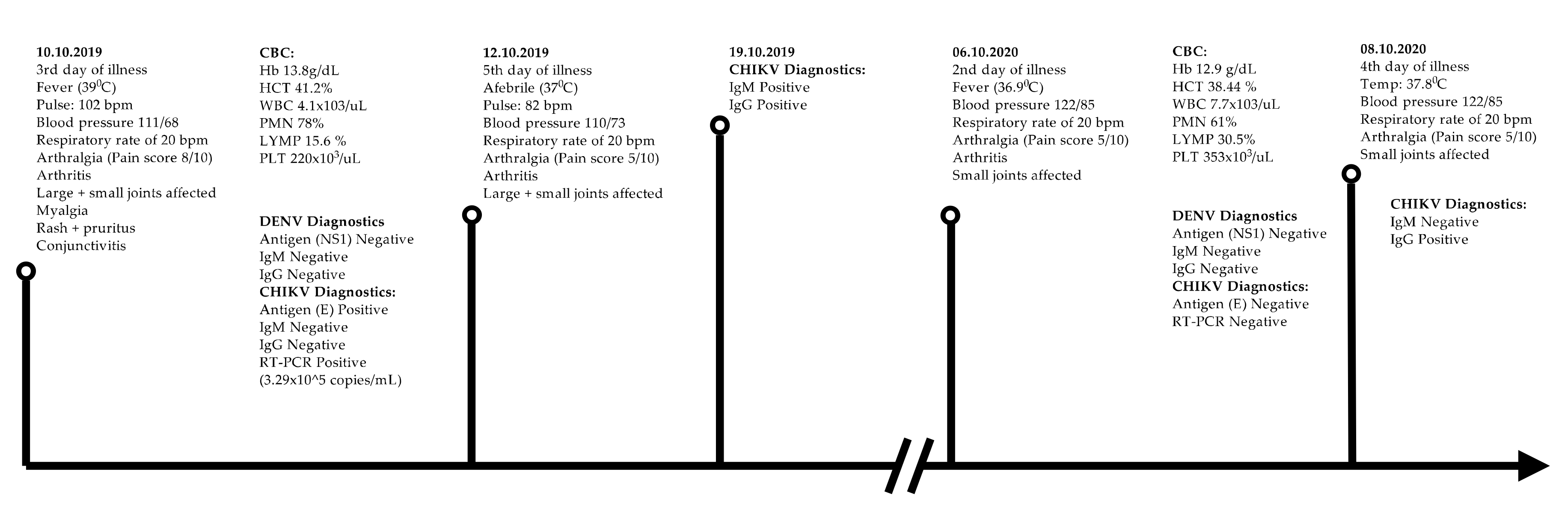
3. Case Report
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Aksoy, S.; Brindley, P.J.; Kamhawi, S. What constitutes a neglected tropical disease? PLoS Negl. Trop. Dis. 2020, 14, e0008001. [Google Scholar] [CrossRef]
- Beltrán-Silva, S.L.; Chacón-Hernández, S.S.; Moreno-Palacios, E.; Pereyra-Molina, J.A. Clinical and differential diagnosis: Dengue, chikungunya and Zika. Rev. Médica Hosp. Gen. México 2018, 81, 146–153. [Google Scholar] [CrossRef]
- Imad, H.; Phadungsombat, J.; Nakayama, E.; Kludkleeb, S.; Matsee, W.; Ponam, T.; Suzuki, K.; Leaungwutiwong, P.; Piyaphanee, W.; Phumratanaprapin, W.; et al. Chikungunya Manifestations and Viremia in Patients WhoPresented to the Fever Clinic at Bangkok Hospital for Tropical Diseases during the 2019 Outbreak in Thailand. Trop. Med. Infect. Dis. 2021, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Imad, H.A.; Phumratanaprapin, W.; Phonrat, B.; Chotivanich, K.; Charunwatthana, P.; Muangnoicharoen, S.; Khusmith, S.; Tantawichien, T.; Phadungsombat, J.; Nakayama, E.; et al. Cytokine Expression in Dengue Fever and Dengue Hemorrhagic Fever Patients with Bleeding and Severe Hepatitis. Am. J. Trop. Med. Hyg. 2020, 102, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Imad, H.A.; Atsawawaranunt, K.; Sharma, C.; Poonam, T.; Piyaphanee, W. Fever, rash, and red eyes in Thailand: A diagnostic challenge. Travel Med. Infect. Dis. 2018, 24, 15. [Google Scholar] [CrossRef]
- Robinson, M.C. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952–1953. Trans. R Soc. Trop. Med. Hyg. 1955, 49, 28–32. [Google Scholar] [CrossRef]
- Jupp, P.G.; Mcintosh, B.M. The Arbovirus: Epidemiology and Ecology; CRC Press: Boca Raton, FL, USA, 1988; Volume II, pp. 137–157. [Google Scholar]
- Phadungsombat, J.; Imad, H.; Rahman, M.; Nakayama, E.E.; Kludkleeb, S.; Ponam, T.; Rahim, R.; Hasan, A.; Poltep, K.; Yamanaka, A.; et al. A Novel Sub-Lineage of Chikungunya Virus East/Central/South African Genotype Indian Ocean Lineage Caused Sequential Outbreaks in Bangladesh and Thailand. Viruses 2020, 12, 1319. [Google Scholar] [CrossRef]
- Appassakij, H.; Khuntikij, P.; Kemapunmanus, M.; Wutthanarungsan, R.; Silpapojakul, K. Viremic profiles in asymptomatic and symptomatic chikungunya fever: A blood transfusion threat? Transfusion 2013, 53, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Javelle, E.; Cabie, A.; Bouquillard, E.; Troisgros, O.; Gentile, G.; Leparc-Goffart, I.; Hoen, B.; Gandjbakhch, F.; Rene-Corail, P.; et al. French guidelines for the management of chikungunya (acute and persistent presentations). Médecine Mal. Infect. 2015, 45, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Couturier, E.; Guillemin, F.; Mura, M.; Léon, L.; Virion, J.-M.; Letort, M.-J.; De Valk, H.; Simon, F.; Vaillant, V. Impaired quality of life after chikungunya virus infection: A 2-year follow-up study. Rheumatology 2012, 51, 1315–1322. [Google Scholar] [CrossRef]
- Schilte, C.; Staikovsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Albert, M.L.; Lecuit, M.; Michault, A. Chikungunya virus-associated long-term arthralgia: A 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- Van Aalst, M.; Nelen, C.M.; Goorhuis, A.; Stijnis, C.; Grobusch, M.P. Long-term sequelae of chikungunya virus disease: A systematic review. Travel Med. Infect. Dis. 2017, 15, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Paixao, E.S.; Rodrigues, L.C.; Costa, M.D.C.N.; Itaparica, M.; Barreto, F.; Gérardin, P.; Teixeira, M.G. Chikungunya chronic disease: A systematic review and meta-analysis. Trans. R Soc. Trop. Med. Hyg. 2018, 112, 301–316. [Google Scholar] [CrossRef]
- Clarris, B.J. Viral Arthritis and the Possible Role of Viruses in Rheumatoid Arthritis. Aust. N. Z. J. Med. 1978, 8 (Suppl. S1), 40–43. [Google Scholar] [CrossRef] [PubMed]
- Fourie, E.D.; Morrison, J.G. Rheumatoid arthritic syndrome after chikungunya fever. South Afr. Med. J. 1979, 56, 130–132. [Google Scholar]
- Chaaitanya, I.K.; Muruganandam, N.; Sundaram, S.G.; Kawalekar, O.; Sugunan, A.P.; Manimunda, S.P.; Ghosal, S.R.; Muthumani, K.; Vijayachari, P. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011, 24, 265–271. [Google Scholar] [CrossRef]
- Miner, J.J.; Yeang, H.X.A.; Fox, J.M.; Taffner, S.; Malkova, O.N.; Oh, S.T.; Kim, A.H.J.; Diamond, M.S.; Lenschow, D.J.; Yokoyama, W.M. Brief Report: Chikungunya Viral Arthritis in the United States: A Mimic of Seronegative Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Bouquillard, É.; Combe, B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Jt. Bone Spine 2009, 76, 654–657. [Google Scholar] [CrossRef]
- Suzuki, K.; Huits, R.; Phadungsombat, J.; Tuekprakhon, A.; Nakayama, E.E.; Berg, R.V.D.; Barbé, B.; Cnops, L.; Rahim, R.; Hasan, A.; et al. Promising application of monoclonal antibody against chikungunya virus E1-antigen across genotypes in immunochromatographic rapid diagnostic tests. Virol. J. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kishishita, N.; Sasayama, M.; Takeda, N.; Sa-Ngasang, A.; Anuegoonpipat, A.; Anantapreecha, S. Neutralization Activity of Patient Sera Collected during the 2008-2009 Chikungunya Outbreak in Thailand. J. Clin. Microbiol. 2015, 53, 184–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aiempanakit, K. Cutaneous Manifestations in Chikungunya Disease. J. Health Sci. Med. Res. 2019, 37, 1–3. [Google Scholar] [CrossRef]
- Suhrbier, A. Rheumatic manifestations of chikungunya: Emerging concepts and interventions. Nat. Rev. Rheumatol. 2019, 15, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Foissac, M.; Javelle, E.; Ray, S.; Guérin, B.; Simon, F. Post-Chikungunya Rheumatoid Arthritis, Saint Martin. Emerg. Infect. Dis. 2015, 21, 530–532. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Calvache-Benavides, C.E.; Giraldo-Gómez, J.; Hurtado-Hurtado, N.; Yepes-Echeverri, M.C.; García-Loaiza, C.J.; Patiño-Barbosa, A.M.; Sabogal-Roman, J.A.; Patiño-Valencia, S.; Hidalgo-Zambrano, D.M.; et al. Post-chikungunya chronic arthralgia: Results from a retrospective follow-up study of 131 cases in Tolima, Colombia. Travel Med. Infect. Dis. 2016, 14, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Edington, F.; Varjão, D.; Melo, P. Incidence of articular pain and arthritis after chikungunya fever in the Americas: A systematic review of the literature and meta-analysis. Jt. Bone Spine 2018, 85, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.L.; Grilli, E.; Corvetta, A.; Silvi, G.; Angelini, R.; Mascella, F.; Miserocchi, F.; Sambo, P.; Finarelli, A.; Sambri, V.; et al. Long-term chikungunya infection clinical manifestations after an outbreak in Italy: A prognostic cohort study. J. Infect. 2012, 65, 165–172. [Google Scholar] [CrossRef]
- Marimoutou, C.; Ferraro, J.; Javelle, E.; Deparis, X.; Simon, F. Chikungunya infection: Self-reported rheumatic morbidity and impaired quality of life persist 6 years later. Clin. Microbiol. Infect. 2015, 21, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Duvignaud, A.; Fianu, A.; Bertolotti, A.; Jaubert, J.; Michault, A.; Poubeau, P.; Fred, A.; Méchain, M.; Gaüzère, B.-A.; Favier, F.; et al. Rheumatism and chronic fatigue, the two facets of post-chikungunya disease: The TELECHIK cohort study on Reunion island. Epidemiol. Infect. 2018, 146, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Imad, H.A.; Phadungsombat, J.; Nakayama, E.E.; Kludkleeb, S.; Matsee, W.; Ponam, T.; Suzuki, K.; Leaungwutiwong, P.; Piyaphanee, W.; Phumratanaprapin, W.; et al. Clinical features of acute chikungunya virus infection in children and adults during an outbreak in the Maldives. Am. J. Trop. Med. Hyg. 2021, 6, 12, (under review). [Google Scholar]
- Mehta, R.; Gerardin, P.; de Brito, C.A.A.; Soares, C.N.; Ferreira MLBSolomon, T. The neurological complications of chikungunya virus: A systematic review. Rev. Med. Virol. 2018, 28, e1978. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Stoermer, K.A.; Montgomery, S.A.; Pal, P.; Oko, L.; Diamond, M.S.; Morrison, T.E. Chronic Joint Disease Caused by Persistent Chikungunya Virus Infection Is Controlled by the Adaptive Immune Response. J. Virol. 2013, 87, 13878–13888. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, J.J.; Bandjee, M.C.J.; Trotot, P.K.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Martins, K.A.; Encinales, L.; Reid, S.P.; Acuña, M.; Encinales, C.; Matranga, C.B.; Pacheco, N.; Cure, C.; Shukla, B.; et al. Chikungunya Arthritis Mechanisms in the Americas: A Cross-Sectional Analysis of Chikungunya Arthritis Patients Twenty-Two Months After Infection Demonstrating No Detectable Viral Persistence in Synovial Fluid. Arthritis Rheumatol. 2018, 70, 585–593. [Google Scholar] [CrossRef]
- Prow, N.A.; Tang, B.; Gardner, J.; Le, T.T.; Taylor, A.; Poo, Y.S.; Nakayama, E.; Hirata, T.D.; Nakaya, H.I.; Slonchak, A.; et al. Lower temperatures reduce type I interferon activity and promote alphaviral arthritis. PLoS Pathog. 2017, 13, e1006788. [Google Scholar] [CrossRef] [PubMed]
- Malvy, D.; Ezzedine, K.; Mamani-Matsuda, M.; Autran, B.; Tolou, H.; Receveur, M.-C.; Pistone, T.; Rambert, J.; Moynet, D.; Mossalayi, D. Destructive arthritis in a patient with chikungunya virus infection with persistent specific IgM antibodies. BMC Infect. Dis. 2009, 9, 200. [Google Scholar] [CrossRef]
- Grivard, P.; Le Roux, K.; Laurent, P.; Fianu, A.; Perrau, J.; Gigan, J.; Hoarau, G.; Grondin, N.; Staikowsky, F.; Favier, F.; et al. Molecular and serological diagnosis of Chikungunya virus infection. Pathol. Biol. 2007, 55, 490–494. [Google Scholar] [CrossRef]
- Javelle, E.; Ribera, A.; Degasne, I.; Gaüzere, B.-A.; Marimoutou, C.; Simon, F. Specific Management of Post-Chikungunya Rheumatic Disorders: A Retrospective Study of 159 Cases in Reunion Island from 2006-2012. PLoS Negl. Trop. Dis. 2015, 9, e0003603. [Google Scholar] [CrossRef]
- Suhrbier, A.; Jaffar-Bandjee, M.C.; Gasque, P. Arthritogenic alphaviruses—An overview. Nat. Rev. Rheumatol. 2012, 8, 420–429. [Google Scholar] [CrossRef]
- Rezza, G.; Chen, R.; Weaver, S.C. O’nyong-nyong fever: A neglected mosquito-borne viral disease. Pathog. Glob. Health 2017, 111, 271–275. [Google Scholar] [CrossRef] [PubMed]
| Year of Presentation | 2019 | 2020 | |
|---|---|---|---|
| Day of illness | 3rd day | 5th day | 2nd day |
| History of fever | Yes | No | Yes |
| Temperature °C | 39 | 37 | 36.9 |
| Pulse, beats per minute | 102 | 110 | 122 |
| Systolic blood pressure, mmHg | 111 | 110 | 122 |
| Diastolic blood pressure, mmHg | 68 | 73 | 85 |
| Arthralgia | Yes | Yes | Yes |
| Arthralgia pain score, (0/10) | 8/10 | 5/10 | 5/10 |
| Arthritis | Yes | No | Yes |
| Myalgia | Yes | No | No |
| Fatigue | Yes | No | No |
| Rash | Yes | No | No |
| Pruritus | Yes | No | No |
| Headache | Yes | No | Yes |
| Diarrhea | No | No | No |
| Vomiting | No | No | No |
| Nausea | No | No | No |
| Conjunctivitis | Yes | No | No |
| Dengue NS1 antigen | Negative | Negative | |
| Dengue IgM antibody | Negative | Negative | |
| Dengue IgG antibody | Negative | Negative | |
| Chikungunya E1 antigen | Positive | Negative | |
| Chikungunya IgM | Negative | Positive | Negative |
| Chikungunya IgG | Negative | Positive | Positive |
| CHIKV real time RT-PCR (Ct value) | 19.43 | Negative | |
| CHIKV viral load, copies/mL | 3.29 × 105 | Not detected | |
| Hemoglobin g/dL | 13.8 | 12.9 | |
| Hematocrit % | 41.2 | 38.3 | |
| Leukocytes/µL | 4100 | 7700 | |
| Neutrophils/µL | 3218 | 4750 | |
| Lymphocytes/µL | 639 | 2348 | |
| Monocytes/µL | 231 | 250 | |
| Eosinophils/µL | 54 | 53 | |
| Basophils/µL | 54 | 16 | |
| Platelets/µL | 220,000 | 353,000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imad, H.A.; Matsee, W.; Kludkleeb, S.; Asawapaithulsert, P.; Phadungsombat, J.; Nakayama, E.E.; Suzuki, K.; Leaungwutiwong, P.; Piyaphanee, W.; Phumratanaprapin, W.; et al. Post–Chikungunya Virus Infection Musculoskeletal Disorders: Syndromic Sequelae after an Outbreak. Trop. Med. Infect. Dis. 2021, 6, 52. https://doi.org/10.3390/tropicalmed6020052
Imad HA, Matsee W, Kludkleeb S, Asawapaithulsert P, Phadungsombat J, Nakayama EE, Suzuki K, Leaungwutiwong P, Piyaphanee W, Phumratanaprapin W, et al. Post–Chikungunya Virus Infection Musculoskeletal Disorders: Syndromic Sequelae after an Outbreak. Tropical Medicine and Infectious Disease. 2021; 6(2):52. https://doi.org/10.3390/tropicalmed6020052
Chicago/Turabian StyleImad, Hisham A., Wasin Matsee, Sajikapon Kludkleeb, Punyisa Asawapaithulsert, Juthamas Phadungsombat, Emi E. Nakayama, Keita Suzuki, Pornsawan Leaungwutiwong, Watcharapong Piyaphanee, Weerapong Phumratanaprapin, and et al. 2021. "Post–Chikungunya Virus Infection Musculoskeletal Disorders: Syndromic Sequelae after an Outbreak" Tropical Medicine and Infectious Disease 6, no. 2: 52. https://doi.org/10.3390/tropicalmed6020052
APA StyleImad, H. A., Matsee, W., Kludkleeb, S., Asawapaithulsert, P., Phadungsombat, J., Nakayama, E. E., Suzuki, K., Leaungwutiwong, P., Piyaphanee, W., Phumratanaprapin, W., & Shioda, T. (2021). Post–Chikungunya Virus Infection Musculoskeletal Disorders: Syndromic Sequelae after an Outbreak. Tropical Medicine and Infectious Disease, 6(2), 52. https://doi.org/10.3390/tropicalmed6020052







