Improved Detection of Intestinal Helminth Infections with a Formalin Ethyl-Acetate-Based Concentration Technique Compared to a Crude Formalin Concentration Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. FC Method
2.2. FECT
2.3. Microscopic Examination
2.4. Statistical Analysis
2.5. Ethics Statement
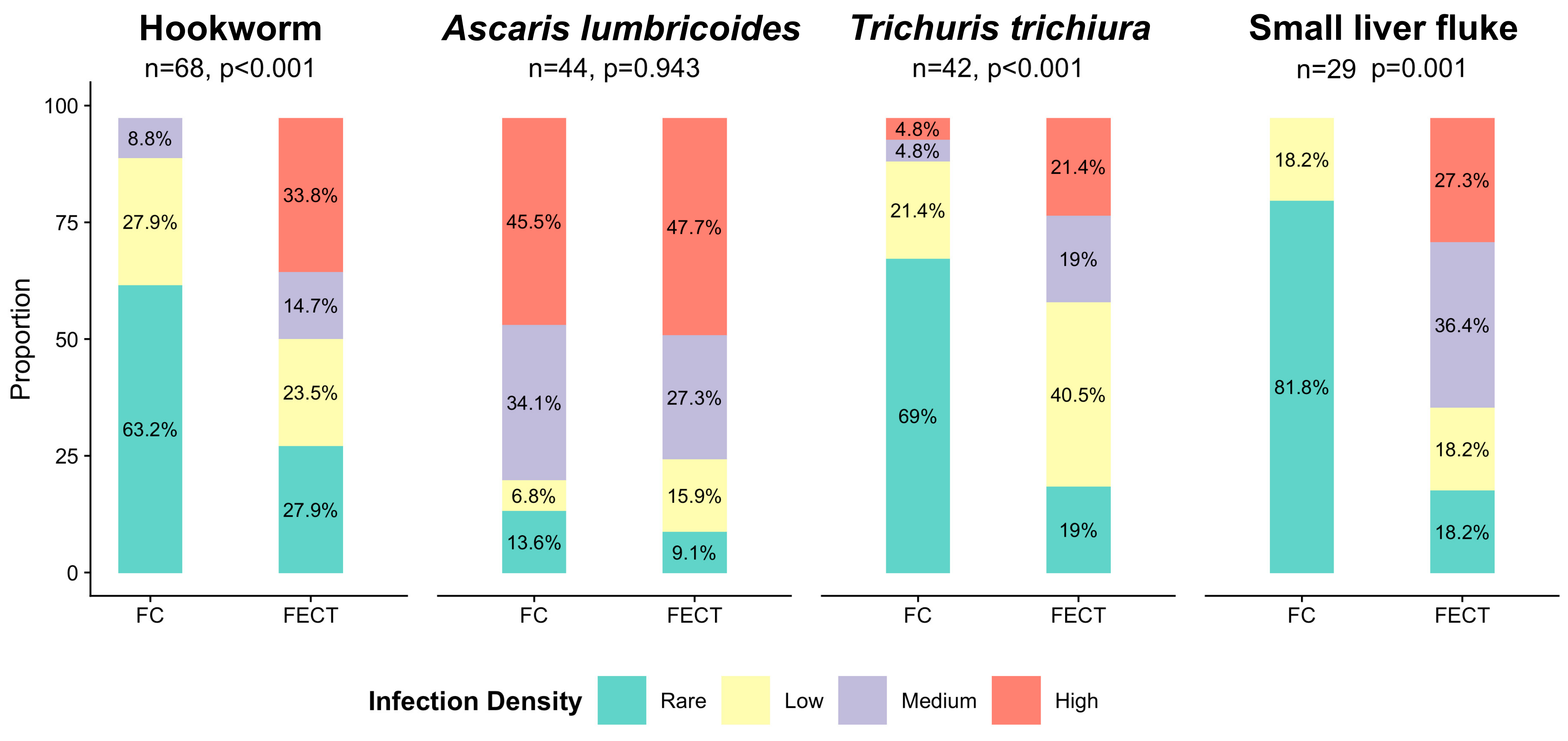
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; Becker, S.; Knopp, S.; Blum, J.; Neumayr, A.; Keiser, J.; Hatz, C. Neglected tropical diseases: Diagnosis, clinical management, treatment and control. Swiss Med. Wkly. 2012, 142, w13727. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases a Roadmap for Implementation. 2012. Available online: https://apps.who.int/iris/handle/10665/70809 (accessed on 2 March 2021).
- WHO World Health Organization. Soil-Transmitted Helminth Infections (Fact Sheet). 2019. Available online: https://Www.Who.Int/News-Room/Fact-Sheets/Detail/Soil-Transmitted-Helminth-Infections (accessed on 4 October 2019).
- DALYs and HALE Collaborators Global. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic snalysis for the global burden of disease study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Brummaier, T.; Tun, N.W.; Min, A.M.; Gilder, M.E.; Archasuksan, L.; Proux, S.; Kiestra, D.; Charunwatthana, P.; Utzinger, J.; Paris, D.H.; et al. Burden of soil-transmitted helminth infection in pregnant refugees and migrants on the Thailand-Myanmar border: Results from a retrospective cohort. PLoS Negl. Trop. Dis. 2021, 15, e0009219. [Google Scholar] [CrossRef] [PubMed]
- Boel, M.; Carrara, V.I.; Rijken, M.; Proux, S.; Nacher, M.; Pimanpanarak, M.; Paw, M.K.; Moo, O.; Gay, H.; Bailey, W.; et al. Complex interactions between soil-transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PLoS Negl. Trop. Dis. 2010, 4, e887. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Bullock, S.L.; Melvin, D.M.; Spruill, C.L. Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J. Clin. Microbiol. 1979, 10, 852–853. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-risk Population Groups. 2017. Available online: https://www.who.int/nutrition/publications/guidelines/deworming/en/ (accessed on 4 January 2021).
- Cox, F.E.G. History of human parasitology. Clin. Microbiol. Rev. 2002, 15, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Telemann, W. Eine Methode zur Erleichterung der Auffindung von Parasiteneiern in den Faeces. DMW—Deutsche Med. Wochenschr. 1908, 34, 1510–1511. [Google Scholar] [CrossRef]
- Xu, B.; Gordon, C.A.; Hu, W.; McManus, D.P.; Chen, H.-G.; Gray, D.J.; Ju, C.; Zeng, X.-J.; Gobert, G.N.; Ge, J.; et al. A novel procedure for precise quantification of Schistosoma japonicum eggs in bovine feces. PLoS Negl. Trop. Dis. 2012, 6, e1885. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Gamer, M.; Lemon, J.; Singh, I.F.P. Irr: Various Coefficients of Interrater Reliability and Agreement. R package Version 0.84.1. 2019. Available online: https://CRAN.R-project.org/package=irr (accessed on 26 March 2021).
- Naaktgeboren, C.A.; Bertens, L.C.M.; van Smeden, M.; de Groot, J.A.H.; Moons, K.G.M.; Reitsma, J.B. Value of composite reference standards in diagnostic research. BMJ 2013, 347, f5605. [Google Scholar] [CrossRef] [PubMed]
- Bogoch, I.I.; Raso, G.; N’Goran, E.K.; Marti, H.P.; Utzinger, J. Differences in microscopic diagnosis of helminths and intestinal protozoa among diagnostic centres. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 344–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stevenson, M.; Sergeant, E.; Nunes, T.; Heuer, C.; Marshall, J.; Sanchez, J.; Thornton, R.; Reiczigel, J.; Robison-Cox, J.; Sebastiani, P.; et al. EpiR: Tools for the Analysis of Epidemiological Data; R package Version 2.0.19. 2018. Available online: https://CRAN.R-project.org/package=epiR (accessed on 26 March 2021).
- Krauth, S.J.; Coulibaly, J.T.; Knopp, S.; Traoré, M.; N’Goran, E.K.; Utzinger, J. An in-depth analysis of a piece of shit: Distribution of Schistosoma mansoni and hookworm eggs in human stool. PLOS Negl. Trop. Dis. 2012, 6, e1969. [Google Scholar] [CrossRef] [PubMed]
- Gisev, N.; Bell, J.S.; Chen, T.F. Interrater agreement and interrater reliability: Key concepts, approaches, and applications. Res. Soc. Adm. Pharm. 2013, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Palmeirim, M.S.; Ali, S.M.; Ame, S.M.; Hattendorf, J.; Keiser, J. Diagnosis of soil-transmitted helminths using the Kato-Katz technique: What is the influence of stirring, storage time and storage temperature on stool sample egg counts? PLoS Negl. Trop. Dis. 2021, 15, e0009032. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, M.R.; Carabin, H.; Joseph, L.; Balolong, E.; Olveda, R.; McGarvey, S.T. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘Gold Standard’. Int. J. Parasitol. 2010, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.; Kurscheid, J.; Williams, G.; Clements, A.; Li, Y.; Zhou, X.-N.; Utzinger, J.; McManus, D.; Gray, D. Asian schistosomiasis: Current status and prospects for control leading to elimination. Trop. Med. Infect. Dis. 2019, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals a Road Map for Neglected Tropical Diseases 2021–2030. 2020. Available online: https://www.who.int/neglected_diseases/resources/who-ucn-ntd-2020.01/en/ (accessed on 2 March 2021).
- Engels, D.; Zhou, X.-N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]

| Hookworm (p < 0.001 ⧧) | Trichuris trichiura (p < 0.001 ⧧) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FECT (sensitivity 87.3%) | FECT (sensitivity 90.8%) | ||||||||
| − | + | Total | − | + | Total | ||||
| FC method (sensitivity 53.6%) | − | 527 | 77 | 604 | FC method (sensitivity 44.1%) | − | 573 | 67 | 640 |
| + | 21 | 68 | 89 | + | 11 | 42 | 53 | ||
| Total | 548 | 145 | 693 | Total | 584 | 109 | 693 | ||
| Kappa = 0.50, PoA = 85.9% | Kappa = 0.46, PoA = 88.7% | ||||||||
| Ascaris lumbricoides(p = 0.546⧧) | Small liver fluke (p < 0.001⧧) | ||||||||
| FECT (sensitivity 79.4%) | FECT (sensitivity 89.5%) | ||||||||
| − | + | Total | − | + | Total | ||||
| FC method (sensitivity 90.5%) | − | 630 | 6 | 636 | FC method (sensitivity 41.1%) | − | 598 | 56 | 654 |
| + | 13 | 44 | 57 | + | 10 | 29 | 39 | ||
| Total | 643 | 50 | 693 | Total | 608 | 85 | 693 | ||
| Kappa = 0.81, PoA = 97.3% | Kappa = 0.30, PoA = 93.5% | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brummaier, T.; Archasuksan, L.; Watthanakulpanich, D.; Paris, D.H.; Utzinger, J.; McGready, R.; Proux, S.; Nosten, F. Improved Detection of Intestinal Helminth Infections with a Formalin Ethyl-Acetate-Based Concentration Technique Compared to a Crude Formalin Concentration Technique. Trop. Med. Infect. Dis. 2021, 6, 51. https://doi.org/10.3390/tropicalmed6020051
Brummaier T, Archasuksan L, Watthanakulpanich D, Paris DH, Utzinger J, McGready R, Proux S, Nosten F. Improved Detection of Intestinal Helminth Infections with a Formalin Ethyl-Acetate-Based Concentration Technique Compared to a Crude Formalin Concentration Technique. Tropical Medicine and Infectious Disease. 2021; 6(2):51. https://doi.org/10.3390/tropicalmed6020051
Chicago/Turabian StyleBrummaier, Tobias, Laypaw Archasuksan, Dorn Watthanakulpanich, Daniel H. Paris, Jürg Utzinger, Rose McGready, Stephane Proux, and François Nosten. 2021. "Improved Detection of Intestinal Helminth Infections with a Formalin Ethyl-Acetate-Based Concentration Technique Compared to a Crude Formalin Concentration Technique" Tropical Medicine and Infectious Disease 6, no. 2: 51. https://doi.org/10.3390/tropicalmed6020051
APA StyleBrummaier, T., Archasuksan, L., Watthanakulpanich, D., Paris, D. H., Utzinger, J., McGready, R., Proux, S., & Nosten, F. (2021). Improved Detection of Intestinal Helminth Infections with a Formalin Ethyl-Acetate-Based Concentration Technique Compared to a Crude Formalin Concentration Technique. Tropical Medicine and Infectious Disease, 6(2), 51. https://doi.org/10.3390/tropicalmed6020051







