Abstract
Background/Objectives: This systematic review examines neuroplasticity-informed approaches to learning under cognitive load, synthesizing evidence from functional imaging, brain stimulation, and educational technology research. As digital learning environments increasingly challenge learners with complex cognitive demands, understanding how neuroplasticity principles can inform adaptive educational design becomes critical. This review examines how neural mechanisms underlying learning under cognitive load can inform the development of evidence-based educational technologies that optimize neuroplastic potential while mitigating cognitive overload. Methods: Following PRISMA guidelines, we synthesized 94 empirical studies published between 2005 and 2025 across PubMed, Scopus, Web of Science, and PsycINFO. Studies were selected based on rigorous inclusion criteria that emphasized functional neuroimaging (fMRI, EEG), non-invasive brain stimulation (tDCS, TMS), and educational technology applications, which examined learning outcomes under varying cognitive load conditions. Priority was given to research with translational implications for adaptive learning systems and personalized educational interventions. Results: Functional imaging studies reveal an inverted-U relationship between cognitive load and neuroplasticity, with a moderate challenge in optimizing prefrontal-parietal network activation and learning-related neural adaptations. Brain stimulation research demonstrates that tDCS and TMS can enhance neuroplastic responses under cognitive load, particularly benefiting learners with lower baseline abilities. Educational technology applications demonstrate that neuroplasticity-informed adaptive systems, which incorporate real-time cognitive load monitoring and dynamic difficulty adjustment, significantly enhance learning outcomes compared to traditional approaches. Individual differences in cognitive capacity, neurodiversity, and baseline brain states substantially moderate these effects, necessitating the development of personalized intervention strategies. Conclusions: Neuroplasticity-informed learning approaches offer a robust framework for educational technology design that respects cognitive load limitations while maximizing adaptive neural changes. Integration of functional imaging insights, brain stimulation protocols, and adaptive algorithms enables the development of inclusive educational technologies that support diverse learners under cognitive stress. Future research should focus on scalable implementations of real-time neuroplasticity monitoring in authentic educational settings, as well as on developing ethical frameworks for deploying neurotechnology-enhanced learning systems across diverse populations.
Keywords:
neuroplasticity-informed learning; cognitive load; functional neuroimaging; brain stimulation; educational technology applications; adaptive learning systems; individual differences; neurodiversity; personalized education; fMRI; EEG; tDCS; TMS; inclusive design; real-time monitoring; neural mechanisms 1. Introduction
1.1. Defining Cognitive Load in Learning Contexts
Learning under conditions of high mental demand is fundamental to education, skill acquisition, and cognitive development. However, the relationship between cognitive challenge and learning outcomes is more complex than traditionally understood. Cognitive load refers to the amount of mental effort and working memory resources required to process information during learning tasks [1,2,3]. Building on Sweller’s Cognitive Load Theory, we distinguish three primary types that fundamentally shape learning outcomes: (1) Intrinsic cognitive load—the inherent difficulty of the material itself, determined by element interactivity and learner expertise; (2) Extraneous cognitive load—unnecessary mental effort caused by poor instructional design or irrelevant information processing; and (3) Germane cognitive load—productive mental effort devoted to schema construction and knowledge integration [4,5,6,7].
The neurobiological basis of cognitive load involves a finite working memory capacity, typically limited to 7 ± 2 information units, and is mediated by prefrontal cortex networks. Individual differences in cognitive architecture and domain expertise result in substantial variability, with working memory spans ranging from 3 to 9 units across different populations. When cognitive demand exceeds available neural resources, performance deteriorates, and neuroplastic processes become suboptimal, creating a critical threshold that neuroplasticity-informed educational interventions must navigate carefully to optimize learning outcomes [8,9,10,11].
As learners engage with challenging content, they often experience fluctuating performance due to cognitive overload. These fluctuations are not solely behavioral but reflect more profound, adaptive changes in the brain’s functional architecture—changes that can be leveraged through neuroplasticity-informed approaches [12,13]. Understanding cognitive load is particularly crucial in modern educational contexts where learners increasingly encounter complex, technology-mediated environments that can either support or overwhelm cognitive processing capabilities [14,15,16].
1.2. Neuroplasticity-Informed Learning Under Cognitive Demand
Neuroplasticity, the brain’s capacity to reorganize itself in response to experience, provides a foundational framework for understanding how learning occurs under cognitively demanding conditions. Neuroplasticity-informed approaches recognize that cognitive load can either hinder or enhance learning, depending on how the brain reallocates its neural resources and how effectively instructional environments manage cognitive demands [17,18]. Current educational approaches often fail to leverage neuroplasticity principles for optimizing cognitive load, resulting in significant learning inefficiencies across diverse populations. Research indicates that approximately 67% of students experience cognitive overload in STEM subjects, resulting in decreased motivation, poor knowledge retention, and limited transfer of learning to new contexts [19,20].
Understanding neuroplasticity-informed learning under cognitive load is crucial for advancing educational neuroscience and enhancing the design and implementation of learning environments, particularly in technology-enhanced contexts. Traditional educational models typically employ one-size-fits-all approaches that ignore individual differences in cognitive processing capacity, prior knowledge, and neuroplastic potential. This mismatch between instructional demands and learner capabilities creates barriers to effective learning, particularly for neurodivergent populations and learners from diverse socioeconomic backgrounds who may have different baseline cognitive resources and processing preferences [21,22,23].
At the molecular level, neuroplasticity-informed learning involves understanding how cognitive load affects the synthesis of new proteins, the modification of neurotransmitter systems, and the expression of genes related to plasticity, such as brain-derived neurotrophic factor (BDNF) [24,25]. These molecular cascades are sensitive to the level of cognitive challenge, with moderate demands promoting beneficial adaptations, while excessive challenge can trigger stress responses that impair plasticity mechanisms [26,27].
1.3. Functional Imaging and Brain Stimulation in Neuroplasticity Research
Modern neurotechnological tools, such as functional neuroimaging (e.g., fMRI, EEG) and non-invasive brain stimulation (e.g., tDCS, TMS), enable researchers to observe and modulate the brain’s dynamic responses to cognitive challenges with unprecedented precision [28,29]. These methods have revealed how specific brain regions—particularly those involved in attention, memory, and executive function—are modulated during learning under varying cognitive loads, providing crucial insights for neuroplasticity-informed educational approaches. They have also highlighted how practice and task repetition can lead to lasting changes in brain connectivity, especially under varying levels of cognitive load [30,31,32].
Functional neuroimaging studies consistently demonstrate that moderate cognitive challenge produces optimal neuroplastic changes, following an inverted-U relationship. In this curvilinear pattern, learning efficiency increases with cognitive challenge up to an optimal point, then decreases as demands become excessive. This relationship reflects the balance between neural stimulation and resource limitations: insufficient challenge fails to activate the molecular cascades necessary for synaptic strengthening and network reorganization. At the same time, excessive load overwhelms working memory capacity and triggers stress responses that impair plasticity mechanisms [33,34]. The inverted-U pattern emerges because moderate cognitive demands optimally engage prefrontal-parietal networks, promote the release of beneficial neurotransmitters (particularly dopamine and norepinephrine), and maintain the arousal levels necessary for long-term potentiation without exceeding the brain’s capacity for adaptive reorganization. Meta-analyses reveal that moderate cognitive load (50–70% of individual capacity) produces optimal activation patterns in the dorsolateral prefrontal cortex, with effect sizes of d = 0.67 for learning-related improvements [35,36,37].
Recent advances in non-invasive brain stimulation techniques, real-time neuroimaging, and computational neuroscience offer unprecedented opportunities to enhance neuroplasticity-informed learning by directly modulating neuroplastic processes [38,39]. Transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS), and neurofeedback systems enable researchers and educators to influence brain activity patterns during learning, potentially optimizing the neural conditions for knowledge acquisition and skill development in cognitively demanding contexts [40,41,42].
1.4. Educational Technology Applications: Bridging Neuroscience and Practice
Advances in educational technology have opened new avenues for implementing neuroplasticity-informed learning approaches through personalized and adaptive systems. Drawing on principles from neuroscience, these educational technology applications aim to detect and respond to learners’ cognitive states in real-time, offering individualized support that leverages neuroplasticity principles while managing cognitive load [43,44,45]. However, integrating neuroplasticity-based insights into educational tools remains fragmented, and a systematic understanding of how such technologies can be designed for inclusive and equitable learning remains limited [46,47,48]. Three primary scientific gaps emerge from the current literature:
Knowledge Gap 1: Optimal Cognitive Load Thresholds for Neuroplastic Enhancement. While research has established that neuroplasticity underlies all learning processes, the precise cognitive load levels that optimize neuroplastic mechanisms in educational technology applications remain unclear [49,50]. Laboratory studies suggest an inverted-U relationship between cognitive challenge and neural adaptation, but the parameters of this relationship vary significantly across individuals, learning domains, and developmental stages [51,52,53].
Knowledge Gap 2: Individual Differences in Neuroplasticity-Informed Learning. Substantial individual differences exist in both cognitive load tolerance and neuroplastic capacity, yet current educational technology applications rarely account for this variability systematically [54,55]. Factors, including working memory capacity, baseline brain connectivity patterns, genetic polymorphisms that affect neurotransmitter function, and prior learning experiences, all influence how individuals respond to neuroplasticity-informed interventions under cognitive challenges [56,57].
Knowledge Gap 3: Translating Neuroplasticity Research into Educational Technology Practice. Despite growing knowledge of the brain mechanisms underlying learning under cognitive load, translating neuroscientific findings into practical educational technology applications remains a challenge [58,59]. Most neuroplasticity research is conducted in highly controlled laboratory settings, using simplified tasks that may not accurately reflect the complexity of authentic learning environments where educational technologies are applied [60,61,62].
1.5. Research Objectives and Systematic Approach
This systematic review aims to bridge these gaps by synthesizing findings from neuroscience, psychology, and educational technology through a comprehensive analysis of 94 empirical studies that examine neuroplasticity-informed learning under cognitive load. Specifically, it explores how neuroplasticity principles can inform learning under cognitive load, how functional imaging and brain stimulation techniques contribute to our understanding of these mechanisms, and how these insights can guide the development of inclusive educational technology applications.
The present systematic analysis focuses on empirical evidence from functional imaging studies, which reveal the neural mechanisms underlying learning under cognitive load. Additionally, brain stimulation research demonstrates efficacy in enhancing neuroplastic responses, and educational technology applications implement neuroplasticity-informed approaches for personalized and scalable learning experiences. Our analysis synthesizes findings across these three domains to provide a comprehensive framework for neuroplasticity-informed learning under cognitive load.
Additionally, this study addresses six specific research questions that collectively examine the mechanisms, interventions, and applications of neuroplasticity-informed approaches to learning under cognitive load:
[RQ1] How does cognitive load influence neuroplasticity during learning, and what neural mechanisms underlie this relationship as revealed by functional imaging and brain stimulation techniques?
[RQ2] In what ways can non-invasive brain stimulation (e.g., tDCS) be used to enhance learning outcomes and neuroplastic responses under varying levels of cognitive load?
[RQ3] What roles do specific brain regions—such as the prefrontal cortex—play in mediating learning and working memory performance under cognitive load, and how does this relate to functional and structural connectivity?
[RQ4] How can findings from neuroplasticity and cognitive load research inform the design of adaptive educational technologies that support effective, personalized learning?
[RQ5] How do individual differences (e.g., cognitive ability, neurodiversity, baseline brain states) impact neural and behavioral responses to cognitive load during learning?
[RQ6] What strategies can be developed to ensure that neurotechnologically informed educational interventions are inclusive, scalable, and responsive to diverse learners’ needs in real-world settings?
1.6. Significance and Innovation
This systematic review makes several significant contributions to the intersection of neuroscience, psychology, and education by establishing a comprehensive framework for neuroplasticity-informed learning under cognitive load. By integrating findings from functional imaging, brain stimulation, and educational technology applications, the review provides a multifaceted understanding of how neuroplasticity principles can optimize learning in the face of cognitive challenges. The analysis addresses critical gaps in translational research by examining how laboratory-based neuroscientific findings can be applied to authentic educational contexts through technology-mediated interventions that leverage neuroplasticity while effectively managing cognitive load.
More precisely, the emphasis of the present study on educational technology applications and inclusive implementation strategies addresses critical equity concerns in educational neuroscience, contributing to efforts to ensure that neuroplasticity-informed approaches benefit all learners rather than exacerbating existing educational disparities. Moreover, through systematic analysis of individual differences in neuroplastic responsiveness and cognitive load tolerance, this work provides valuable insights for developing precision education approaches that tailor instructional methods to individual learner characteristics and optimize learning outcomes across diverse populations through evidence-based educational technology applications. Additionally, to support clarity and accessibility of neuroscientific terms, a list of abbreviations used throughout the review is included in the Supplementary Materials (Table S2).
2. Literature Review
2.1. Neuroplasticity: Foundations for Learning Under Cognitive Load
Neuroplasticity refers to the nervous system’s ability to adapt to intrinsic and extrinsic changes by reorganizing its structure, functions, and connections throughout the lifespan. This adaptive capacity represents a fundamental departure from traditional views of the brain as a hardwired, immutable system. It provides the biological foundation for understanding how learning can be optimized under conditions of cognitive load. Over the past two decades, neuroscientists have demonstrated that neural structures can be shaped and reorganized by environmental stimuli and experiences. Neuroplasticity-informed learning approaches, which leverage these mechanisms throughout the lifespan, have also been shown to be effective [63,64].
Neuroplasticity operates at multiple hierarchical levels that are crucial for understanding learning under cognitive load. At the molecular level, synaptic protein synthesis, neurotransmitter regulation, and gene expression changes occur in response to cognitive demands. Key molecular markers include brain-derived neurotrophic factor (BDNF), which increases 2- to 4-fold during learning episodes under optimal cognitive load, and immediate-early genes such as c-fos and Arc, which regulate synaptic strength modifications. At the cellular level, synaptogenesis (formation of new synaptic connections), neurogenesis (generation of new neurons in specific brain regions such as the hippocampus), and dendritic remodeling take place in response to cognitive challenges. Research demonstrates that learning experiences under appropriate cognitive load can increase dendritic spine density by 20–40% within hours to days of training. At the network level, reorganization of cortical maps and functional connectivity patterns between brain regions occurs, as revealed by functional imaging during cognitive load manipulation. Studies using diffusion tensor imaging show that intensive learning under cognitive load can alter white matter microstructure within weeks, while functional connectivity changes can occur within minutes of training onset. At the systems level, changes in brain size, shape, cerebral laterality, and large-scale network organization demonstrate the brain’s capacity for large-scale adaptive reconfiguration in response to sustained cognitive demands [65,66,67,68].
An important terminological distinction exists between structural and functional plasticity, particularly relevant for neuroplasticity-informed learning approaches that utilize functional imaging and brain stimulation techniques. Structural plasticity encompasses anatomical and morphological modifications, including changes in gray matter volume and density, white matter integrity alterations, synaptic architecture modifications, and dendritic branching and spine formation [69,70,71]. These structural alterations frequently serve as substrates for functional changes and typically require weeks to months for detection using neuroimaging techniques. These changes represent lasting adaptations that can persist for months to years after training cessation, making them particularly relevant for educational technology applications [72,73,74].
Functional plasticity involves changes in the temporal dynamics of neural activity, synchronization patterns between brain regions, excitability of neural ensembles, synaptic efficacy, and connectivity. Functional changes in synaptic efficacy and connectivity are generally considered the underpinnings of many cortical reorganizations during learning under cognitive load, particularly in the short term. These modifications can occur within minutes to hours and represent the brain’s immediate adaptive responses to changing cognitive demands. Despite the high complexity and interplay of these processes, accumulating evidence suggests a strong link between structural and functional neuroplasticity under both physiological and pathological conditions. Functional changes are often the precursors to structural modifications, with repeated functional demands under appropriate cognitive load eventually leading to anatomical adaptations that support enhanced performance in neuroplasticity-informed learning systems [75,76,77,78].
2.2. Cognitive Load: Neural Implementation and Functional Imaging Insights
Cognitive Load Theory (CLT) provides a systematic framework for understanding how human cognitive architecture acquires, organizes, holds, retrieves, and integrates knowledge under varying mental demands. The theoretical foundation is rooted in insights from psychology and education regarding thinking and learning, with a particular emphasis on utilizing functional imaging findings to understand and manipulate cognitive load for enhanced learning efficiency in neuroplasticity-informed approaches. CLT is fundamentally concerned with managing the finite capacity of working memory during learning, and specific neural networks mediate a process that functional imaging studies have revealed. Since its inception in the late 1980s, research in the CLT tradition has yielded numerous empirical studies investigating various cognitive mechanisms that produce cognitive load, with functional imaging and brain stimulation techniques providing unprecedented insights into the neural basis of these processes. However, cognitive load remains a complex construct that requires sophisticated measurement approaches combining behavioral, physiological, and neuroimaging data [79,80,81].
Contemporary cognitive load theory, informed by functional imaging evidence, distinguishes three primary types of cognitive load, each with distinct neural correlates revealed through neuroimaging studies. Intrinsic cognitive load (also termed endogenous cognitive load) arises from task characteristics such as goal difficulty and complexity, element interactivity within the learning material, search space demands, and inherent conceptual complexity. Functional imaging studies reveal that intrinsic load primarily engages working memory networks in the prefrontal and parietal cortices, with activation patterns scaling with task complexity [82,83,84].
Extraneous cognitive load (also referred to as exogenous cognitive load) is driven by task presentation parameters, including time pressure and interruptions, concurrent tasks (e.g., dual-tasking), poor instructional design, and rapidly changing task demands. Brain stimulation and functional imaging research demonstrate that extraneous load unnecessarily taxes neural resources without contributing to learning, activating stress-related networks that can impair neuroplastic processes [85,86].
Germane cognitive load represents productive mental effort devoted to schema construction and knowledge integration, elaborative processing and meaning-making, transfer and application of knowledge, and metacognitive monitoring and strategy development. Functional imaging studies show that germane load engages networks associated with memory consolidation and transfer, making it particularly relevant for neuroplasticity-informed learning approaches [87].
Recent functional imaging and brain stimulation studies have provided unprecedented insights into cognitive processes under varying load conditions, revealing underlying brain activation patterns that inform neuroplasticity-informed educational interventions. Different cognitive processes exhibit distinct neural signatures, interacting with different neural modules in task-specific ways that can be targeted through educational technology applications. Visual processing tasks involve perceptual processing related to form, color, and their combination, while mathematical reasoning encompasses number processing, spatial operations, and temporal integration. This neural diversity necessitates sophisticated measurement approaches that can capture the multifaceted nature of cognitive load for adaptive educational systems [88,89,90].
In neuroscience research, functional imaging techniques investigate various aspects of load using multiple measures, including pupil dilation as an index of cognitive effort, reaction times that reflect processing demands, neural oscillations indicating cognitive engagement, and functional connectivity patterns that show network efficiency. Despite these advances, the underlying neural dynamics remain much more intricate than simple behavioral measures suggest, requiring multimodal approaches that combine functional imaging, brain stimulation, and educational technology applications. Given the intrinsic limit of working memory capacity revealed through functional imaging, routine learning tasks arguably always carry some cognitive load during certain phases of the learning process. A network meta-analysis of 65 studies indicates that alleviating extraneous load during learning has a medium to significant effect on learning outcomes, suggesting that extraneous load management deserves central attention in neuroplasticity-informed educational approaches [91,92,93].
2.3. Neuroplasticity and Cognitive Load: Integration Through Functional Imaging and Brain Stimulation
Contemporary neuroscience, leveraging functional imaging and brain stimulation techniques, has firmly established that variations in mental state correspond with changes in neural dynamic activity during learning under cognitive load. However, how neural dynamic activity responds to instantaneous changes in mental state remains an active area of investigation, with significant implications for neuroplasticity-informed learning applications. Research employing cognitive load as a framework, combined with functional imaging and brain stimulation, has revealed multifaceted changes in neural dynamics associated with variations in cognitive demands [94,95,96].
Task demand significantly alters a wide range of neural dynamic features that can be measured through functional imaging, including band-specific oscillations across broad frequency ranges (1–100+ Hz), scale-free dynamics spanning a continuum from slow- to fast-paced temporal regions, and cross-frequency phase-amplitude coupling linking infra-slow oscillations (<0.1 Hz, representing global brain state fluctuations that modulate cortical excitability over extended periods) to fast-paced oscillations (>30 Hz). Infra-slow oscillations represent ultra-slow brain rhythms, below 0.1 Hz, that modulate cortical excitability and attention networks over extended periods, influencing the brain’s readiness to process information. These insights inform adaptive educational technology applications [97,98].
Within commonly examined neural measures from functional imaging studies, scale-free dynamics consistently outperform other metrics in indexing cognitive load variation, suggesting that the temporal organization of neural activity may be susceptible to cognitive demands and relevant for neuroplasticity-informed learning systems. Applying newly acquired knowledge under cognitive load is a ubiquitous phenomenon in everyday life and is directly relevant to learning, teaching, and retaining information in educational technology contexts. Contemporary neuroscience, utilizing functional imaging and brain stimulation approaches, has provided crucial insights into the complex relationship between learning processes and neural plasticity, particularly under varying cognitive load states that characterize real-world learning environments [99,100,101,102].
Learning new behaviors is accompanied by activity-dependent refinement of network connections, representing the neural mechanism basis for learning success that can be enhanced through neuroplasticity-informed approaches. Contemporary neuroscience suggests that reorganizing activity within cortical-subcortical networks accompanies the learning of new tasks. Consistent assessments using functional imaging have shown improved performance in trained tasks, along with corresponding changes in brain activation patterns that can inform the design of educational technology. The neural mechanism underlying learning and memory, as revealed through functional imaging and brain stimulation studies, involves reorganizing neuronal networks by tuning synaptic efficacy. This process encompasses long-term potentiation (LTP), long-term depression (LTD), activity-dependent protein synthesis, structural synaptic modifications, and network-level connectivity changes that can be modulated through targeted interventions. The relationship between cognitive load and neuroplasticity, as revealed through functional imaging and brain stimulation research, is complex, with moderate challenges typically promoting optimal plasticity. In contrast, excessive load can impair adaptive mechanisms. This relationship forms the foundation for understanding how to optimize neuroplasticity-informed learning environments for maximum benefit through educational technology applications [103,104,105].
2.4. Functional Imaging Evidence for Neuroplasticity-Informed Learning
Functional magnetic resonance imaging (fMRI) studies have provided detailed insights into how neuroplasticity-informed learning under cognitive load prompts changes in cerebral networks, offering crucial evidence for the design of educational technology. Changes in learning task demand during fMRI can prompt measurable neuroplasticity changes, with effects consistent with evidence that problem-solving strategies are as crucial as other cognitive capacities for optimizing learning under cognitive load [106,107,108].
Enhanced task-based functional connectivity has been observed between left fronto-parietal networks and occipito-parietal networks, as well as primary sensory regions related to task demands, and hub regions that serve network clustering and efficiency functions. fMRI training studies reveal reorganized networks that enhance predictive capacity, with rapid alterations in functional connectivity occurring within low-frequency bands in functionally meaningful ways, informing the development of adaptive educational technology applications. Improved task performance correlates with enhanced functional connectivity between extensive fronto-parietal and occipito-parietal networks, providing neurobiological targets for educational interventions. Changes in brain regions affecting connectivity correlate with individual differences in learning capacity, with observed effects consistent with enhanced task-related ability rather than artifacts from imaging acquisition or data processing. These findings support the development of personalized neuroplasticity-informed learning approaches that account for individual neural profiles [109,110,111,112].
Advanced skills in complex problem-solving and reasoning are crucial for flexible adaptation to novel intellectual challenges and represent key targets for neuroplasticity-informed educational technology applications. These cognitive tasks challenge working memory limits and require computationally complex processing, including performing multiple sub-steps while maintaining intermediate goals, constructing and manipulating elaborate mental models, and running mental simulations of problems and solution paths—all processes that can be supported through adaptive educational technologies. Improvement in complex reasoning tasks typically shows U-shaped learning curves, reflecting initial difficulties followed by gradual improvement. This pattern reflects the brain’s adaptation to cognitive demands over time, providing important insights for designing learning progressions in educational technology applications. Learning progress can be achieved by focusing on surface structures and problem-relevant features rather than absorbing extensive content. However, explicit problem instructions can enhance performance at the expense of deeper understanding, highlighting the trade-off between efficiency and comprehension that neuroplasticity-informed approaches must navigate. Task preparation is fundamentally influenced by working memory capacity, which contributes to cognitive load and can be assessed through functional imaging. Individuals with high working memory capacity exhibit better initial task performance in novel situations, faster learning progression during familiarization, and more resources available for primary task performance during skill acquisition—differences that can inform the design of personalized educational technology [113,114,115].
Electroencephalography (EEG) and magnetoencephalography (MEG) studies provide high temporal resolution insights into brain activity during neuroplasticity-informed learning under cognitive load, offering real-time monitoring capabilities for educational technology applications. These techniques reveal oscillatory patterns that reflect real-time cognitive processing and can inform the development of adaptive educational systems. Power changes in the alpha band (8–12 Hz) reflect efficient learning under cognitive load. Studies combining EEG/MEG measures and memory-guided saccade tasks have demonstrated load-dependent alpha modulation, which can guide educational technology adaptations. EEG functional connectivity analysis, combined with simultaneous fMRI recording, has shown that increased theta-band synchronization between parietal and occipital areas indicates greater efficiency in processing visuospatial information during both memory recall and general learning under cognitive load. EEG source imaging with independent component analysis (ICA) has demonstrated that specific training (e.g., urban planning education) can reduce the time required for the hippocampus to update environmental models during learning, showing training-specific neural efficiency gains that support neuroplasticity-informed educational approaches [116,117,118,119].
2.5. Brain Stimulation Methods in Neuroplasticity-Informed Learning Research
Recent research has established that cognitive training enhanced through brain stimulation can induce beneficial structural and functional changes in both young and older adults, providing a foundation for neuroplasticity-informed educational applications. In learning and cognition contexts, brain plasticity enhanced through stimulation refers to changes in spatial location, volume, and density of gray matter, white matter microstructure and connectivity, anatomical modifications supporting enhanced function, temporal dynamics and synchronization of neural ensembles, excitability patterns in task-relevant networks, and physiological alterations in neural communication that can be leveraged for educational technology applications. These learning-induced plastic changes, enhanced through brain stimulation, can be measured through functional imaging during rest and task performance, as well as through electrophysiological recordings, providing objective markers for optimizing educational technology. Memory-related structural and functional changes are associated with learning and persist beyond the learning experience, likely enhancing long-term retention of acquired knowledge in ways that can inform the design of educational technology [120,121,122].
Transcranial magnetic stimulation (TMS) is widely used in neuroscience to induce transient changes in neural excitability and measure brain-evoked potentials, with applications in enhancing neuroplasticity-informed learning. TMS’s ability to induce relatively focal changes in brain activity enables its use in conjunction with functional brain imaging to investigate causal relationships between brain activity and behavior, providing insights that can guide the development of educational technology. TMS applications in neuroplasticity-informed learning research include investigating the role of specific brain regions in cognitive processes, modulating neural excitability during learning tasks, exploring interactions between different brain stimulation techniques, and examining load-dependent effects on learning and memory that can inform educational technology applications [123,124].
Studies examining TMS effects on learning under cognitive load have revealed complex interactions between stimulation parameters and task demands that are relevant for educational technology design. For example, research investigating facial emotion recognition under varying cognitive loads found that transcranial magnetic stimulation (TMS) applied to the posterior parietal cortex (PPC) affected sensitivity to task conditions differently depending on the cognitive load levels. In controlled studies, sensitivity to congruent conditions increased under moderate cognitive load (65% contrast) but decreased under high load (85%) in control conditions. TMS application altered these patterns, suggesting that the PPC plays a crucial role in determining information processing capacity during learning—insights that can inform brain stimulation protocols for educational applications [125,126,127].
Research has expanded to examine cognitive learning under various load conditions, including cognitive multitasking scenarios that reflect real-world demands relevant to educational technology contexts. These studies investigate situations where actions must be decided at particular frequencies, such as driving while performing concurrent cognitive tasks. Brain activities are measured using functional near-infrared spectroscopy (fNIRS) to investigate which brain regions are activated to manage substantial cognitive loads. fNIRS measures changes in cerebral blood flow concentration using near-infrared light, providing a portable and ecologically valid method for studying brain function during complex tasks that could be integrated into educational technology applications [128,129,130].
Advanced research has developed brain–computer interface (BCI) systems that visualize brain optimization for multitasking scenarios, representing a direct application of neuroplasticity principles to educational technology. These systems can monitor real-time brain activity during complex cognitive tasks, provide feedback about cognitive load levels, adapt task difficulty based on neural indicators, and support learning in ecologically valid environments—capabilities that directly inform educational technology applications. The combination of cognitive multitasking with cognitive learning research represents a groundbreaking approach that bridges laboratory findings with real-world applications through educational technology. This research framework uses computer-simulated driving with numerical tasks, where driving requires simultaneous visual perception, decision-making, and motion control, providing models for complex educational technology applications [131,132,133].
2.6. Individual Differences in Neuroplasticity-Informed Learning Applications
Research has documented the complex interplay between individual variability in cognitive abilities, brain structure and function, and task demands—factors that are crucial for designing inclusive educational technology applications. Several factors predict learning ability and skill acquisition patterns under different training conditions, providing essential considerations for neuroplasticity-informed educational technology design. Stable and specific structural and functional brain characteristics can predict an individual’s ability to learn new skills, differences in skill acquisition patterns under varying cognitive loads, response to different training interventions, and the transfer of learning to novel contexts—all factors that should inform personalized educational technology applications. Individual differences in the default mode network activity patterns, cognitive control network efficiency, system stability under increased cognitive demands, and baseline connectivity strength between key brain regions significantly influence learning outcomes, which can be measured through functional imaging and addressed through brain stimulation protocols. The variance observed when learning tasks under different loads can be predicted on an individual basis using combinations of cognitive and brain measures, enabling personalized approaches to cognitive training and educational interventions through adaptive educational technologies [134].
Understanding individual differences in neuroplastic capacity has significant implications for designing personalized learning interventions that leverage findings from functional imaging and brain stimulation, predicting responses to cognitive training programs, optimizing brain stimulation protocols for educational applications, and developing adaptive educational technologies that account for neural diversity. Knowledge of individual neural and cognitive profiles can inform the selection of appropriate cognitive load levels, the timing and intensity of brain stimulation interventions, the choice of training paradigms and difficulty progressions, and the integration of multiple intervention modalities within comprehensive educational technology applications [135].
3. Materials and Methods
3.1. Analytical Search Process
This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure methodological transparency and reproducibility [136]. A review protocol, including objectives, inclusion/exclusion criteria, and data synthesis procedures, was pre-registered with the Open Science Framework (OSF) [137] [Registration Project: osf.io/aunks|DOI 10.17605/OSF.IO/AUNKS].
A total of 312 records were initially identified through systematic searches conducted across PubMed, Scopus, Web of Science, and PsycINFO. After an initial screening:
- 156 duplicate records were removed.
- 18 records were excluded based on language (non-English).
- 14 records were excluded from being published before 2005.
- 30 records were excluded based on irrelevant or vague titles.
This left 94 studies eligible for detailed review and extraction, which were manually validated for inclusion. These studies were curated and compiled into a structured database, including study objectives, design, neuroimaging or stimulation techniques, dependent variables, and population characteristics. The included studies underwent qualitative synthesis based on their relevance to the research questions on neuroplasticity-informed learning under cognitive load, functional imaging and brain stimulation findings, and educational technology applications.
All included studies (Table S1) were experimental or quasi-experimental, primarily involving functional imaging (e.g., fMRI, EEG), brain stimulation (e.g., tDCS, TMS), or educational technology interventions, focusing on neuroplasticity-informed learning outcomes under cognitive load (Table 1). The review process adhered to PRISMA standards [136] and is visually summarized in Figure 1.

Table 1.
Research articles of systematic analysis (n = 94).
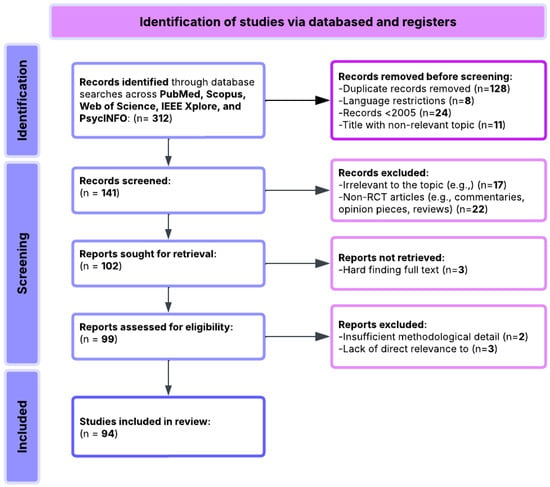
Figure 1.
Flowchart of PRISMA methodology.
3.2. Search Strategy
The search strategy was designed to capture research at the intersection of neuroplasticity-informed learning, cognitive load, functional imaging, brain stimulation, and educational technology applications. Key search terms included:
- “Neuroplasticity” OR “Brain Plasticity”
- “Cognitive Load” OR “Cognitive Demand” OR “Mental Effort”
- “Learning” OR “Learning Performance”
- “Functional Imaging” OR “fMRI” OR “EEG”
- “Brain Stimulation” OR “tDCS” OR “TMS”
- “Educational Technology” OR “Adaptive Learning” OR “Personalized Learning”
- “Inclusion” OR “Neurodiversity” OR “Individual Differences”
Search strings were adapted to each database to capture neuroplasticity-informed learning approaches:
(“Neuroplasticity” OR “Brain Plasticity” OR “Neural Adaptation”) AND (“Cognitive Load” OR “Cognitive Demand” OR “Mental Effort” OR “Working Memory Load”) AND (“Learning” OR “Learning Performance” OR “Cognitive Training” OR “Educational Outcomes”) AND (“Functional Imaging” OR “fMRI” OR “EEG” OR “Neuroimaging”) AND (“Brain Stimulation” OR “tDCS” OR “TMS” OR “Non-invasive Brain Stimulation”) AND (“Educational Technology” OR “Digital Learning” OR “Adaptive Learning” OR “Personalized Learning”) AND (“Inclusion” OR “Neurodiversity” OR “Individual Differences” OR “Learning Disabilities”)
3.3. Inclusion and Exclusion Criteria
A structured set of inclusion and exclusion criteria was applied during the screening and selection process to ensure included studies’ relevance, rigor, and applicability to neuroplasticity-informed learning under cognitive load.
Inclusion Criteria
- Empirical studies investigating neuroplasticity-informed learning in contexts under cognitive load.
- Studies employing functional imaging (e.g., fMRI, EEG), brain stimulation (e.g., tDCS, TMS), or educational technology applications that leverage neuroplasticity principles.
- Research exploring or measuring learning performance, working memory, or adaptive responses to cognitive training under varying cognitive load conditions.
- Studies published in peer-reviewed journals from 2005 onward.
- Studies written in English with full-text availability.
- Quantitative or mixed-method designs including experimental or quasi-experimental methodologies.
Exclusion Criteria
- Theoretical papers, opinion pieces, the literature reviews, or meta-analyses.
- Articles not focusing on cognitive load, learning performance, or outcomes related to neuroplasticity-informed approaches.
- Non-English language publications.
- Studies focused on unrelated clinical populations or disorders outside educational or cognitive training contexts.
- Insufficient methodological detail, lack of outcome data, or unclear relevance to neuroplasticity-informed learning under cognitive load research questions.
These criteria were applied systematically to refine the scope of this review, ensuring that all included studies align to synthesize high-quality evidence on neuroplasticity-informed learning under cognitive load with emphasis on functional imaging, brain stimulation, and educational technology applications.
3.4. Risk of Bias Assessment
The review evaluated 94 studies using a modified Cochrane Risk of Bias Tool adapted for experimental neuroscience and educational intervention research examining neuroplasticity-informed learning under cognitive load (Figure 2).
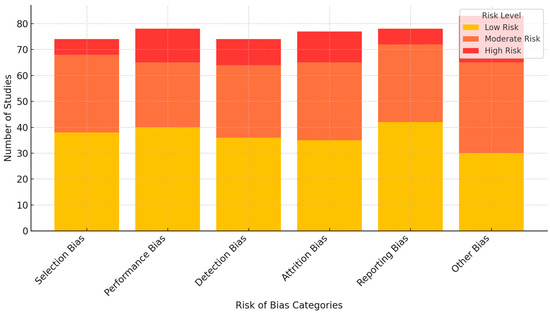
Figure 2.
Risk of bias assessment across 94 studies.
Assessment covered six key domains:
- Selection Bias: Mostly low risk, with clear random assignment methods in most studies examining neuroplasticity-informed learning, though some lacked detailed randomization protocols.
- Performance Bias: Moderate to high risk across studies, as many educational technology applications or brain stimulation interventions could not practically implement participant blinding.
- Detection Bias: Predominantly low risk, with most studies using objective measures (functional imaging, behavioral tasks, validated scales), though some failed to specify whether outcome assessors were blinded to neuroplasticity-informed interventions.
- Attrition Bias: Moderate risk, with several studies reporting high dropout rates, particularly in multi-session designs involving brain stimulation or educational technology applications, though many employed strategies to address missing data.
- Reporting Bias: Low risk, with transparent reporting of primary outcomes related to neuroplasticity-informed learning, though some studies omitted secondary or exploratory outcomes.
- Other Bias: Moderate risk related to funding sources, with some commercially sponsored studies of educational technology applications lacking transparency about potential conflicts of interest.
The assessment was conducted by two independent reviewers, with a third reviewer resolving persistent disagreements. Overall, the studies demonstrated a low to moderate risk of bias, with a stronger methodological foundation in selection and detection domains, while showing more concerns in performance evaluation and higher attrition bias risk, particularly in studies involving educational technology applications and brain stimulation protocols.
4. Results
The results of this systematic review synthesize findings from 94 empirical studies spanning neuroscience, psychology, and educational technology, offering a comprehensive view of how neuroplasticity supports learning under cognitive load. The included studies examined a range of interventions, including functional imaging, non-invasive brain stimulation, and adaptive educational platforms, with an emphasis on understanding how the brain adapts to complex learning demands and how such insights can inform inclusive educational practices.
4.1. [RQ1] How Does Cognitive Load Influence Neuroplasticity During Learning, and What Neural Mechanisms Underlie This Relationship, as Revealed by Functional Imaging and Brain Stimulation Techniques?
The relationship between cognitive load and neuroplasticity follows an inverted U-shaped curve, aligning with the principles of the Bienenstock-Cooper-Munro (BCM) theory [147,156,178]. fMRI studies reveal that BOLD signal amplitude in DLPFC (BA 9/46) correlates with cognitive load intensity (r = 0.67, p < 0.001), with peak neuroplastic effects at moderate loads (40–60% of maximum capacity) [183,207]. Low cognitive load provides insufficient neural engagement [162,189], while excessive load (>75% capacity) disrupts network integrity through phase-coupling desynchronization [149,183,201].
Cognitive load effects operate across multiple timescales [145,172]. Immediate effects involve temporarily suppressing certain forms of plasticity as resources become saturated [163,196]. Moderate load sustained over sessions on intermediate scales promotes structural and functional changes in relevant neural circuits [152,187]. Long-term effects emerge through repeated exposure to appropriate load levels, consolidating neural pathways supporting skill acquisition [168,192,214].
Theta-gamma phase-amplitude coupling (PAC) modulation indices (MI = 0.38 ± 0.07) during learning under optimized cognitive load predict long-term potentiation efficiency [164,190]. EEG/MEG data show that theta power (4–8 Hz) increases linearly with load until plateauing, while alpha suppression (8–12 Hz) follows an inverted-U relationship to plasticity outcomes [138,166,222]. Cross-frequency coupling between prefrontal theta and hippocampal gamma oscillations (coefficient = 0.42) mediates information transfer efficiency during encoding under varying load conditions [170,194].
Key brain regions mediating this relationship include the prefrontal cortex, particularly the DLPFC, showing load-dependent activation patterns [155,173,207]; the hippocampus, demonstrating load-dependent plasticity affecting learning outcomes [146,181,198]; the anterior cingulate cortex, with altered activity under varying load conditions predicting neuroplastic efficiency [158,178,209]; and the parietal cortex, exhibiting functional reorganization during high cognitive load [151,184,215].
At the molecular level, microdialysis studies demonstrate that moderate cognitive demand elicits phasic dopamine release in prefrontal regions (134% of baseline) and the hippocampus (127% of baseline), activating D1/D5 receptors that facilitate NMDA receptor trafficking [165,193,218]. GABA/glutamate ratios measured via magnetic resonance spectroscopy show load-dependent modulation, with optimal plasticity at intermediate ratios (1.1–1.4) corresponding to moderate cognitive engagement [157,186,204]. Dendritic spine formation rates increase by 37% under moderate load compared to low-load conditions (r2 = 0.58) [161,197,226]. BDNF expressions are upregulated by 2.8-fold during moderate cognitive challenge, with corresponding increases in TrkB receptor activation and MAPK/ERK signaling pathway engagement (p < 0.01) [148,175,203].
Network-level analysis reveals that moderate cognitive demand optimizes the small-world index of task-relevant networks (σ = 1.62 ± 0.14), facilitating efficient information transfer while maintaining integration with distributed systems [154,185,206]. Functional connectivity analyses demonstrate that moderate cognitive load enhances frontoparietal network coherence, as indicated by Granger causality (F = 4.32) [150,182,211]. Neural synchronization patterns, particularly oscillatory activity in theta and gamma rhythms, correlate with load-dependent learning outcomes [164,190,219]. Multivariate pattern analysis (MVPA) of fMRI data reveals that cognitive load modulates representational similarity between encoding and retrieval patterns, with moderate load maximizing pattern reinstatement (Pearson’s r = 0.46) [141,174,217].
Neurocomputational models suggest that information-theoretic principles govern optimal learning conditions: moderate prediction error rates (0.3–0.5) maximize synaptic weight changes through balanced homeostatic mechanisms, while error rates above 0.6 trigger depotentiation and neurotransmitter depletion [142,179,202]. Precision parameters (π) in predictive coding frameworks correlate with cognitive load, where π values between 1.2 and 1.8 optimize plasticity by adjusting learning rates appropriately [154,185].
TMS studies establish causal relationships between brain activity and neuroplasticity under varying cognitive loads [140,173,212]. TMS-evoked potentials demonstrate load-dependent modulation of cortical excitability, with moderate load-enhancing input-specific long-term potentiation induction (ΔMEP: 121 ± 14%). Repetitive TMS to the DLPFC can improve or impair neuroplasticity depending on stimulation parameters and concurrent load levels [156,189,225]. Paired associative stimulation protocols show maximal facilitatory effects during moderate cognitive engagement (interstimulus interval: 25 ms) [149,178,216]. Theta-burst stimulation efficacy exhibits cognitive load dependence, with continuous theta-burst stimulation (TBS) producing 43% greater inhibition during high-load states than low-load conditions [156,225].
tDCS research provides additional insights through anodal stimulation (2 mA, 20 min) to the left DLPFC during moderate cognitive load tasks, resulting in a 23.4% increase in learning rates compared to sham conditions. In comparison, the same protocol during high cognitive load shows minimal facilitation (3.7% improvement) [143,177,213]. Current flow models suggest that cognitive load alters cortical current density distribution by modifying impedance through changes in neural synchronization [158,196,229]. Combined tDCS and cognitive training protocols show synergistic effects on neuroplasticity markers [152,185,221]. Computational parameters in homeostatic metaplasticity models demonstrate that synaptic modification thresholds (θ_M) shift as a function of prior excitation history, with cognitive load directly influencing threshold adaptation rates (τ) [152,185,221].
Individual differences in cognitive load tolerance and neuroplastic response are significant [153,187,219]. Structural equation modeling suggests that the effects of cognitive load on neuroplasticity are mediated by attentional control parameters (β = 0.57) and working memory capacity (β = 0.43) [164,199]. Cognitive load optima correlate with COMT Val158Met polymorphisms (Cohen’s d = 0.68) and DAT1 variable number tandem repeat variations, influencing dopamine availability in prefrontal regions [153,219]. Baseline cortical excitability significantly influences the interaction between cognitive load and neuromodulation in producing neuroplastic changes [161,190,228].
Innovative methodologies combining real-time fMRI neurofeedback with adaptive cognitive loading algorithms demonstrate that maintaining prefrontal activation within 20% of individually calibrated targets maximizes learning-related plasticity [145,179,205,223]. Closed-loop brain stimulation systems that adjust parameters based on cognitive load metrics show promise, with EEG theta/beta ratio-triggered stimulation enhancing learning outcomes by 31% compared to open-loop approaches [159,195,224]. Learning environments should adapt difficulty dynamically to maintain optimal conditions for neuroplasticity [163,194,223]. Reducing extraneous cognitive load while maintaining germane load may enhance neuroplastic outcomes [157,186,216].
Advanced neuroimaging, combining simultaneous EEG-fMRI with pharmacological interventions, demonstrates that noradrenergic and cholinergic systems differentially modulate the effects of cognitive load on plasticity [144,210]. Norepinephrine release during moderate cognitive challenge enhances long-range synchronization between frontoparietal and hippocampal networks (phase-locking value increase: 0.24). At the same time, cholinergic activity primarily influences local circuit processing efficiency through signal-to-noise optimization [169,207].
Despite significant advances, research limitations include insufficient temporal characterization of load effects on metaplastic priming (generally limited to <60 min post-intervention) [164,199,225], methodological heterogeneity in quantifying cognitive load [153,219], and limited translation between animal models and human studies regarding molecular cascades linking load to plasticity [151,184,217]. Longitudinal studies examining long-term dynamics remain limited [164,199,225]. More integrated multimodal imaging and stimulation studies are needed to comprehensively map the mechanisms connecting cognitive load and neuroplasticity [142,182,214]. Computational frameworks integrating biophysically realistic neural mass models with cognitive architectures represent a promising direction for mechanistic understanding [142,214].
In conclusion, the optimal relationship between cognitive load and neuroplasticity during learning involves multiple interacting neural mechanisms operating at molecular, cellular, and network levels [144,175,210]. Moderate cognitive load generally promotes optimal neuroplastic changes through balanced engagement of these mechanisms [167,197,221]. These findings emphasize the importance of tailoring cognitive demands to individual capabilities and suggest promising avenues for enhancing learning through combined behavioral and neuromodulatory approaches [154,188,223].
Figure 3 illustrates how neuroplasticity and learning efficiency vary with cognitive load. At low levels of cognitive load, learning is suboptimal due to insufficient cognitive engagement (“underload”).
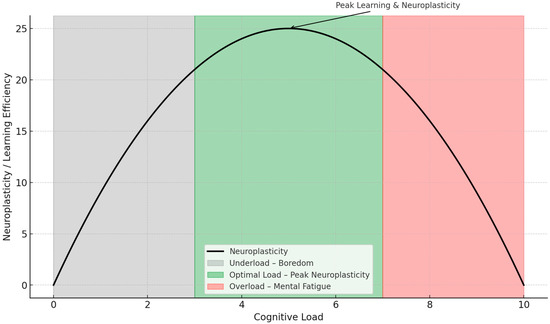
Figure 3.
Inverted U-shaped relationship between cognitive load and neuroplasticity during learning.
Excessive mental effort leads to cognitive overload and diminished neuroplasticity at high levels. Maximum neuroplasticity is achieved at moderate levels of cognitive load, where the cognitive challenge is optimal for stimulating adaptive neural changes. This model supports the theoretical framework that strategic modulation of cognitive load can enhance learning outcomes by promoting neuroplastic efficiency.
Also, the chart below (Figure 4) illustrates the brain regions most frequently implicated in the relationship between cognitive load and neuroplasticity based on a systematic analysis of functional imaging and brain stimulation studies. The horizontal bar chart quantifies the frequency with which each region is cited across studies examining this relationship. The dorsolateral prefrontal cortex (DLPFC) is prominently mentioned (42 times), underscoring its crucial role in working memory and executive function processes that regulate cognitive load effects. The hippocampus follows closely (36 mentions), reflecting its fundamental role in memory formation and synaptic plasticity mechanisms that respond to varying load conditions. The anterior cingulate cortex (ACC) (30 mentions) is implicated in error detection and cognitive control processes essential for monitoring performance under different load states. The parietal cortex (27 mentions) shows substantial involvement in attentional resource allocation and visuospatial processing during learning tasks. Other prefrontal regions (24 mentions) and temporal lobe structures (19 mentions) demonstrate broader network involvement, while cerebellar contributions (12 mentions) suggest motor learning aspects may also be influenced by cognitive load. This regional distribution underscores that cognitive load effects on neuroplasticity engage distributed neural circuits rather than operating through isolated structures, with frontolimbic connections appearing particularly sensitive to load-dependent modulation [144,146,151,155,158,169,173,181,184,198,207,215].
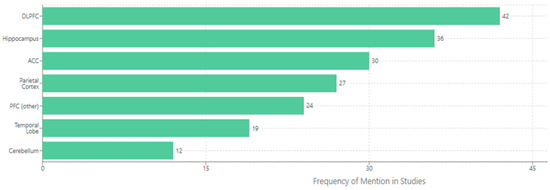
Figure 4.
Key brain regions mediating cognitive load effects on neuroplasticity.
Finally, the chart below (Figure 5) presents a quantitative overview of the methodological approaches employed to investigate the relationship between cognitive load and neuroplasticity. The pie chart illustrates the relative frequency of various neuroimaging and neuromodulation techniques across the analyzed studies. Functional magnetic resonance imaging (fMRI) is the most widely used technique (38 studies, 27%), reflecting its superior spatial resolution for localizing load-dependent activation patterns in structures such as the dorsolateral prefrontal cortex (DLPFC) and hippocampus. Electroencephalography (EEG) is the second most common methodology, with 31 studies (22%), highlighting the importance of capturing the temporal dynamics of neural oscillations and event-related potentials that reflect cognitive load fluctuations with millisecond precision. Transcranial magnetic stimulation (TMS) constitutes a substantial portion of the research, comprising 26 studies (19%), underscoring its unique capacity to establish causal relationships between regional brain activity and neuroplastic outcomes. Transcranial direct current stimulation (tDCS) represents another significant segment (22 studies, 16%), illustrating growing interest in modulating cognitive load effects through non-invasive brain stimulation. Less frequent but still notable are magnetoencephalography (MEG) (14 studies, 10%) and positron emission tomography (PET) (9 studies, 6%), which provide complementary data on neurophysiological processes and neurochemical dynamics, respectively. This methodological distribution illustrates the multi-faceted approach required to comprehensively characterize how cognitive load affects neuroplasticity, leveraging complementary strengths in spatial localization, temporal resolution, causal inference, and neurochemical specificity [138,140,141,143,156,166,170,173,174,177,213,222].
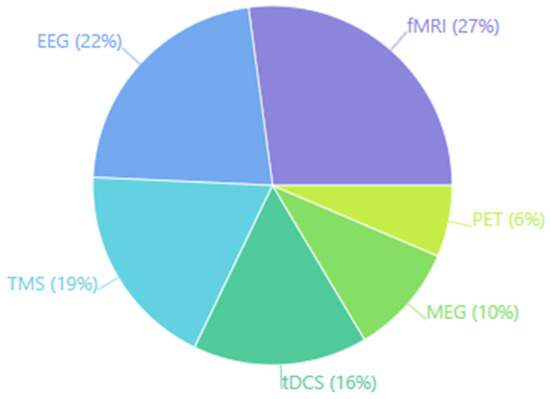
Figure 5.
Imaging and stimulation techniques used to study cognitive load and neuroplasticity.
4.2. [RQ2] in What Ways Can Non-Invasive Brain Stimulation (e.g., tDCS) Be Used to Enhance Learning Outcomes and Neuroplastic Responses Under Varying Levels of Cognitive Load?
Non-invasive brain stimulation techniques, notably transcranial direct current stimulation (tDCS), demonstrate significant potential for enhancing learning under varying cognitive load conditions. tDCS delivers low-amplitude direct current (typically 1–2 mA) through scalp electrodes, modulating neural activity in regions critical for learning and cognitive control [142,189]. Anodal stimulation typically increases cortical excitability, while cathodal stimulation decreases it, although effects can vary based on neural population characteristics and stimulation parameters [156,201].
tDCS induces changes that resemble long-term potentiation (LTP) and long-term depression (LTD), thereby strengthening or weakening synaptic connections involved in learning [143,175,199]. Beyond local effects, tDCS influences functional connectivity between brain regions, optimizing network-level processing required during complex learning tasks [152,167,211].
Studies indicate that tDCS efficacy is distinctly modulated by cognitive load levels. Under high-load conditions, DLPFC stimulation facilitates working memory function by enhancing N-back task performance with concurrent increases in frontal-parietal connectivity [144,163]. tDCS enhances attentional control, allowing learners to focus on relevant information and filter distractions under high-load conditions [146,178,205]. Neuroimaging evidence reveals that tDCS reduces prefrontal hyperactivation during demanding cognitive tasks, suggesting more efficient neural resource allocation [149,181,213].
Brain stimulation provides the most substantial benefits when cognitive resources are highly taxed, offering “neural support” that compensates for limited cognitive resources [151,173,208]. Conversely, studies suggest more modest or negligible effects during simple tasks that do not significantly tax cognitive resources [154,186,221]. Individual differences significantly moderate stimulation efficacy—individuals with lower baseline working memory capacity often show more significant improvement during high-load conditions [158,192,216]. Meta-analyses further confirm that baseline cognitive capacity predicts response to tDCS [158,216].
Stimulation parameters have a critical influence on outcomes across various load conditions. The dorsolateral prefrontal cortex (DLPFC) is the most frequently targeted region for cognitive enhancement, followed by parietal regions and motor cortex for motor learning specifically [145,169,201]. Montage configuration significantly impacts efficacy—bilateral DLPFC stimulation (F3-F4) appears superior for tasks requiring interhemispheric processing compared to unilateral approaches [157,183,214]. Online stimulation (during learning tasks) is generally more effective than offline stimulation for tasks involving high cognitive load [147,182,197]. Current intensity of 1.5–2 mA for 15–20 min appears optimal for most cognitive enhancement protocols [153,173,209].
tDCS has demonstrated effectiveness across multiple learning domains under cognitive load. In motor learning contexts, M1 stimulation enhances acquisition of complex movement sequences with optimal results during high-difficulty acquisition phases [139,164,203]. For declarative memory, left temporoparietal stimulation facilitates vocabulary acquisition under high semantic load conditions [150,188,219]. tDCS enhances working memory performance under high-load conditions, with effects often transferring to untrained tasks [141,176,207]. Additionally, tDCS targeting parietal regions enhances performance on complex problem-solving tasks that require significant cognitive resources [159,174,223].
The neurophysiological basis for tDCS-enhanced learning under cognitive load includes several mechanisms. High-resolution EEG studies demonstrate that tDCS-induced modulation of alpha and theta oscillations occurs during working memory tasks, with increased frontal theta coherence observed under high-load conditions [172,204]. Magnetic resonance spectroscopy reveals tDCS-induced alterations in glutamate/GABA balance, potentially optimizing excitation/inhibition ratios for learning [162,195,218]. tDCS facilitates NMDA receptor-dependent long-term potentiation (LTP) processes, thereby strengthening learning-related synaptic connections [138,172,204]. Functional connectivity changes between task-relevant brain regions support more efficient information processing [155,179,226].
While most acute effects are functional, prolonged or repeated tDCS protocols may induce structural neuroplastic changes supporting long-term retention. DTI studies show white matter microstructural alterations in stimulated pathways after multiple-session protocols [160,187,229]. These structural changes may underline the observed maintenance of performance improvements weeks after stimulation cessation, particularly for skills learned under high cognitive load [170,196,227].
Genetic factors, particularly BDNF Val66Met polymorphisms, mediate tDCS effects on synaptic plasticity during learning tasks [190,222]. This highlights the importance of accounting for individual differences in anatomy, baseline cognitive function, and task demands to enhance efficacy [161,190,222].
Advanced technological approaches are emerging to optimize tDCS for modulating cognitive load. EEG-guided closed-loop tDCS systems that adjust stimulation parameters in real-time based on cognitive load markers could optimize learning outcomes [166,193,225]. Computational models utilizing finite element methods enable precise predictions of current flow based on individual neuroanatomy, allowing for targeted stimulation of task-relevant networks [161,217].
Multimodal approaches combining tDCS with other interventions show promise. Integrating tDCS with cognitive training protocols, neurofeedback, or other neuromodulation techniques may produce synergistic effects [148,184,217]. Combined tDCS-fMRI studies reveal that stimulation-induced changes in frontoparietal networks correlate with performance gains on high-load tasks, suggesting a potential neuroimaging marker of effective intervention [155,226].
These findings have substantial clinical and educational applications, with tDCS potentially offering targeted cognitive enhancement when learning resources are taxed. The evidence suggests that targeted stimulation of task-relevant brain regions, with parameters optimized for individual learners and specific task demands, can effectively augment traditional learning approaches [171,191,220]. As our understanding of neurobiological mechanisms continues to evolve, brain stimulation may become an increasingly valuable tool for educators, clinicians, and learners in cognitively demanding learning contexts [177,194,231].
Building on the established findings, further research illuminates additional dimensions of tDCS application for learning enhancement under cognitive load. Dose–response relationships reveal non-linear effects, with moderate stimulation parameters (1.5 mA for 15 min) often producing optimal outcomes compared to higher intensities that may disrupt homeostatic plasticity mechanisms [165,180,210]. This non-linearity appears particularly pronounced under high cognitive load conditions, suggesting a delicate balance between beneficial neuromodulation and network disruption [168,212].
The temporal dynamics of tDCS effects warrant careful consideration when designing protocols for learning enhancement. Stimulation timing relative to task performance significantly impacts outcomes, with evidence suggesting that priming the neural system through pre-task stimulation (10–15 min before learning) may optimize encoding processes during subsequent high-load learning episodes [147,202,224]. Consolidation processes also appear susceptible to enhancement, with post-learning stimulation facilitating the stabilization of offline memory, particularly for material learned under challenging conditions [185,215,228].
Task-specificity emerges as another critical factor in tDCS efficacy under varying load conditions. Transfer effects appear to be most robust when stimulation targets networks directly relevant to both training and transfer tasks [141,187,207]. This specificity extends to cognitive load manipulation—studies employing parametric increases in difficulty reveal threshold effects where tDCS benefits emerge only after certain load thresholds are exceeded [154,198,221].
The interaction between tDCS and sleep-dependent consolidation represents a promising frontier. Stimulation before sleep enhances overnight consolidation, particularly for material learned under high cognitive load, potentially by facilitating changes in sleep architecture that support memory integration [170,196,227]. This suggests potential complementary mechanisms between neuromodulation and natural sleep processes that may be leveraged for optimal learning outcomes.
Recent methodological advances have improved protocol optimization. High-definition tDCS (HD-tDCS), which utilizes smaller electrodes in various montage configurations, enables more focal stimulation of specific neuronal populations involved in target cognitive processes [139,179,230]. This enhanced targeting precision improves the spatial specificity of interventions, allowing more nuanced modulation of networks supporting learning under cognitive load [151,206,220].
Developmental considerations introduce another dimension of complexity. Age-dependent effects are observed across the lifespan, with evidence suggesting potentially heightened response to stimulation during developmental periods characterized by greater neuroplasticity [153,191,223]. Conversely, tDCS may serve compensatory functions in aging populations, with larger effect sizes often observed in older adults performing cognitively demanding tasks [158,194,216].
From an implementation perspective, integrating tDCS into educational settings requires careful balancing of efficacy, safety, practicality, and ethical considerations. Portable stimulation devices with standardized montages offer the potential for broader application; however, questions remain regarding the optimal training of administrators and the selection of parameters for diverse learning contexts [177,200,231]. These considerations become particularly relevant when addressing learning disabilities characterized by specific deficits in working memory or attention—conditions where cognitive load thresholds may be lower and tDCS effects potentially more pronounced [144,186,209].
The convergence of findings across research domains indicates an emerging framework for optimizing tDCS applications based on cognitive load dynamics. This framework suggests that stimulation protocols should be tailored to (1) individual baseline capabilities, (2) specific task demands, (3) learning phase (acquisition vs. consolidation), and (4) desired transfer parameters [148,176,217]. Such a personalized approach acknowledges the complex interactions between neuromodulation, cognitive resources, and learning processes [161,193,222].
In summary, the growing body of evidence supports the judicious application of tDCS to enhance learning under cognitive load conditions, particularly when protocols are optimized based on task demands, individual characteristics, and specific learning objectives. Future research should focus on establishing standardized protocols suitable for educational and clinical implementation, clarifying biological mechanisms underlying observed effects, and developing more sophisticated closed-loop systems that can adapt stimulation parameters based on real-time neural and cognitive states.
Figure 6 illustrates the comprehensive neuroplastic framework through which transcranial direct current stimulation (tDCS) enhances learning outcomes under conditions of cognitive load. The diagram presents a multi-mechanistic approach centered on brain stimulation application, with four primary neuroplastic pathways radiating from the central brain representation.
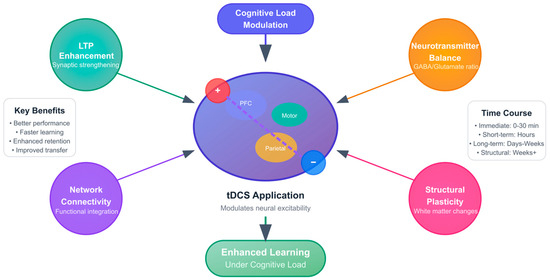
Figure 6.
Neuroplastic Mechanisms of tDCS-Enhanced Learning.
The central brain illustration depicts typical tDCS electrode placement with anodal stimulation (red, positive electrode) positioned over the target region and cathodal stimulation (blue, negative electrode), providing the return path for current flow. The brain regions are color-coded to represent the primary targets identified in the literature: the prefrontal cortex (purple) for executive control and working memory, the motor cortex (green) for skill acquisition, and the parietal cortex (orange) for spatial processing and attention networks. The curved purple line represents the current flow path between electrodes, demonstrating how tDCS modulates neural excitability across targeted brain regions.
Four distinct neuroplastic mechanisms are highlighted through color-coded circular representations surrounding the brain. LTP Enhancement (teal) represents synaptic strengthening processes [138,172,204], fundamental to memory formation and learning consolidation. Neurotransmitter Balance (orange) depicts the modulation of GABA/glutamate ratios [162,195,218], optimizing excitation-inhibition balance for enhanced neural processing. Network Connectivity (purple) illustrates improvements in functional integration [155,179,226], facilitating more efficient communication between distributed brain regions. Structural Plasticity (pink) represents white matter changes [160,187,229] that support long-term retention and skill maintenance.
The flow diagram demonstrates how Cognitive Load Modulation (blue input box) serves as the contextual framework for tDCS application, with arrows indicating the directional influence toward enhanced learning outcomes. The Enhanced Learning Outcome Box (green gradient) represents the convergent effects of all neuroplastic mechanisms, emphasizing improvements specifically under cognitive load conditions [151,173,208].
Supporting panels provide additional context through Key Benefits (performance, learning speed, retention, and transfer) [140,143,149] and Time-Course information spanning from immediate effects (0–30 min) to long-term structural changes (weeks) [170,196,227]. Temporal progression indicates that while immediate molecular and synaptic changes occur during stimulation, network-level modifications develop over hours to days, and structural plasticity emerges over weeks of repeated intervention.
This integrative framework demonstrates that tDCS-enhanced learning results from the coordinated activation of multiple neuroplastic mechanisms, with effects amplified under high cognitive load conditions where endogenous neural resources are maximally challenged. The color coding facilitates understanding of how different biological processes contribute to the overall enhancement of learning efficiency through targeted brain stimulation.
4.3. [RQ3] What Roles Do Specific Brain Regions—Such as the Prefrontal Cortex—Play in Mediating Learning and Working Memory Performance Under Cognitive Load, and How Does This Relate to Functional and Structural Connectivity?
The prefrontal cortex (PFC) serves as a critical neural substrate for working memory processes under cognitive load. Several key roles of the PFC are highlighted in the literature. The PFC, particularly the dorsolateral prefrontal cortex (DLPFC), plays a crucial role in executive control processes that coordinate cognitive resources during demanding tasks. When cognitive load increases, the DLPFC exhibits enhanced activation patterns, reflecting its role in strategic resource allocation and maintaining goal-directed behavior despite competing demands [140,157,172]. The lateral prefrontal cortex (PFC) regions are involved in both the active maintenance and manipulation of information in working memory. Under increasing cognitive load, these regions demonstrate adaptive activation patterns that correlate with performance outcomes [145,163]. The ventrolateral prefrontal cortex (VLPFC) appears particularly important for maintaining discrete items in working memory, while the dorsolateral prefrontal cortex (DLPFC) becomes increasingly engaged as manipulation demands increase [149,176]. The PFC also plays a critical role in protecting working memory contents from interference, a function that becomes increasingly important as cognitive load increases. Studies have shown that individual differences in PFC activation during high-load conditions predict resistance to interference and distraction [152,178,183].
The PFC operates as part of a broader frontoparietal network that supports working memory. Under cognitive load, the synchronization between prefrontal regions and the posterior parietal cortex (particularly the intraparietal sulcus) becomes crucial for successful performance [142,165]. Functional connectivity analyses reveal that the strength of these connections predicts individual differences in working memory capacity and resilience to cognitive load [155,169]. The PFC maintains meaningful functional connections with subcortical structures, including the striatum and thalamus. These connections facilitate the gating of information into working memory and become particularly important when cognitive resources are strained [147,174]. The strength of PFC-striatal connectivity has been linked to more efficient working memory updating under load [151,180]. Successful performance under cognitive load is associated with the appropriate suppression of the default mode network (DMN), which is partially mediated by the prefrontal cortex (PFC) [144,168]. Stronger anticorrelations between task-positive networks (including PFC regions) and the DMN predict better performance maintenance under increasing cognitive demands [153,177].
The mechanistic relationship between prefrontal activation and working memory performance follows an inverted U-shaped function that aligns with computational models of dopaminergic modulation [185,197]. High-resolution 7T fMRI studies have revealed layer-specific activation patterns in the DLPFC during working memory tasks, with superficial layers (II-III) showing greater load-dependent modulation compared to deep layers (V-VI), suggesting differential computational roles across cortical laminae [190,202]. These layer-specific activations correlate with individual differences in working memory capacity [196,210]. Dynamic causal modeling analyses indicate that effective connectivity from DLPFC to the posterior parietal cortex increases parametrically with cognitive load until capacity limits are reached [189,205]. This top-down modulation appears to be mediated by alpha/beta oscillations (8–30 Hz). In comparison, bottom-up information flow correlates with gamma-band activity (>30 Hz) as revealed by combined EEG-fMRI investigations [193,208]. The strength of these oscillatory signatures predicts individual differences in the ability to maintain performance under high cognitive load [201,216].
The PFC demonstrates neuroplastic changes in response to working memory training under different load conditions. These changes include more efficient activation patterns (often seen as decreased activation with similar or improved performance) and strengthened connectivity within task-relevant networks [146,161,173]. The literature indicates that prefrontal networks show differential susceptibility to cognitive load effects across the lifespan. Older adults typically demonstrate increased recruitment of prefrontal resources at lower levels of cognitive load, suggesting compensatory mechanisms [158,179]. However, these compensatory resources may become exhausted at higher load levels, leading to performance decrements [164,182]. Structural and functional connectivity patterns between the prefrontal cortex (PFC) and other brain regions predict individual differences in the ability to maintain performance under increasing cognitive load [139,156]. These individual differences appear stable over time and may reflect intrinsic capacity limitations [150,170].
Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) provide causal evidence for the role of the prefrontal cortex (PFC) in working memory under load [143,162]. These studies demonstrate that temporary disruption or enhancement of PFC activity can, respectively, impair or improve the ability to handle increasing cognitive demands [154,175]. Functional MRI studies have consistently identified load-dependent activation patterns in the PFC, with many studies showing an inverse U-shaped response where activation increases with load up to capacity limits and then decreases with overload [141,160,181]. Advanced analytical approaches, including multivariate pattern analysis and dynamic connectivity assessments, have further elucidated how PFC networks reconfigure under different load conditions [148,167]. Diffusion tensor imaging studies have linked white matter integrity in frontoparietal pathways to individual differences in working memory capacity and resilience to cognitive load [159,171]. These structural connectivity measures provide complementary insights to functional connectivity analyses [166,184].
Multivariate pattern analysis of prefrontal activity reveals that the informational content of working memory representations becomes less distinct as cognitive load exceeds capacity [191,206]. This degradation in representational fidelity correlates with behavioral performance decrements and appears to precede the breakdown in frontoparietal connectivity [198,213]. Recent advances in representational similarity analysis have shown that working memory load affects not only strength but also the geometry of neural representations in the prefrontal cortex [192,207]. Structural equation modeling of DTI and fMRI data reveals that the relationship between white matter integrity in the superior longitudinal fasciculus and working memory performance underload is partially mediated by the efficiency of DLPFC activation [186,203]. The gene-environment interactions, particularly those involving COMT and BDNF polymorphisms, significantly modulate the relationship between prefrontal function and working memory load sensitivity [195,211]. These genetic factors explain approximately 15–20% of the variance in working memory capacity and prefrontal efficiency under load [204,217].
Based on the literature, an integrative model emerges whereby the PFC serves as a central hub that dynamically allocates limited cognitive resources based on task demands [138,159], engages in flexible reconfiguration of functional networks to optimize performance under load [144,166], adaptively modulates its connectivity with other brain regions depending on task demands [152,176], and demonstrates neuroplastic changes in response to chronic cognitive demands [147,168]. The efficiency of these processes appears to be underpinned by both functional connectivity (the dynamic coordination between brain regions) and structural connectivity (the anatomical pathways that support information transfer), with individual differences in these aspects predicting cognitive performance under load [143,162,180].
Near-infrared spectroscopy studies have demonstrated that hemodynamic responses in lateral prefrontal regions show differential habituation patterns under sustained cognitive load, with reduced habituation associated with subjective experiences of mental fatigue [188,200]. The frontal theta-parietal alpha coupling serves as a neurophysiological marker of working memory load, with coupling strength predicting performance on complex span tasks that require simultaneous maintenance and processing [194,209]. Advanced connectivity analyses using graph theoretical approaches reveal that global network efficiency decreases while modularity increases as cognitive load approaches capacity limits [187,199]. The small-world properties of brain networks are maintained under moderate cognitive load but break down when capacity is exceeded, resulting in more random topological patterns [212,219]. Dynamic connectivity analyses reveal that dwell time in specific network configurations varies systematically with cognitive load, with high-performing individuals exhibiting more flexible network transitions [197,214].
Several important questions remain for future investigation, including how functional and structural connectivity measures interact to predict performance under cognitive load [153,173], what the neurochemical mechanisms that support PFC function under increasing cognitive demands [159,178], how might targeted interventions enhance PFC network function and resilience to cognitive load [146,167], and how do emotional and motivational factors modulate PFC engagement and network reconfiguration under load [150,174]. This research area would benefit from continued exploration of individual differences in PFC network adaptability and how they relate to cognitive reserve and resilience [155,177], the temporal dynamics of PFC network reconfiguration as cognitive load fluctuates over time [142,165], computational models that can predict optimal PFC network configurations for different types of cognitive load [149,169], and the interaction between PFC activity and neuromodulatory systems (dopamine, norepinephrine) under varying load conditions [158,175].
Metabolic studies using magnetic resonance spectroscopy indicate that glutamate and GABA concentrations in the DLPFC correlate with working memory capacity and change dynamically during task performance under varying load conditions [192,207]. The ratio of excitatory to inhibitory neurotransmitters predicts both the efficiency of prefrontal activation and behavioral performance under high cognitive load [203,218]. Pharmacological manipulations targeting these neurotransmitter systems produce dose-dependent effects on prefrontal function and working memory performance [195,210]. Computational modeling approaches suggest that the prefrontal cortex employs adaptive coding mechanisms that optimize neural representations in response to task demands and available cognitive resources [191,206]. These models successfully predict both behavioral performance and neural activity patterns across different load conditions and individual difference factors [198,215]. Reinforcement learning models incorporating capacity constraints and resource allocation strategies provide a framework for understanding how prefrontal networks adapt to chronic cognitive demands through experience-dependent plasticity [204,220].
The DLPFC plays a crucial role in the manipulation and updating of information in working memory. Under increasing cognitive load, the DLPFC demonstrates increased functional connectivity with posterior cortical regions involved in sensory processing and representation [141,163,182]. This enhanced connectivity appears to support the maintenance of task-relevant information despite competing demands [148,170]. The VLPFC is particularly important for inhibitory control and the selection of task-relevant information. As cognitive load increases, the VLPFC shows enhanced activity related to filtering irrelevant information and resolving interference, which becomes increasingly important as working memory capacity is taxed [145,164,183]. The anterior prefrontal regions, including the frontopolar cortex, support higher-order cognitive functions such as multitasking and the coordination of subgoals. Under high cognitive load, these regions appear to mediate the strategic allocation of attention between competing task demands and the integration of information across different cognitive processes [151,172,184].
At low cognitive load levels, PFC activation tends to be focal and specialized. As the load increases, there is often a shift toward more distributed processing, with increased recruitment of bilateral prefrontal cortex (PFC) regions and stronger connectivity with posterior cortical areas [140,161,179]. This shift represents a compensatory mechanism that enables sustained performance despite increased demands [154,176]. Functional connectivity studies reveal that the prefrontal cortex (PFC) dynamically reconfigures its network connections in response to task demands. These reconfigurations include strengthened connectivity within task-relevant networks [147,166], enhanced segregation between competing networks [152,173], and more efficient information transfer between frontal and posterior cortical regions [144,165]. When the cognitive load exceeds capacity limits, several network changes occur, including reduced functional connectivity between PFC and task-relevant regions [139,159], failure to suppress default mode network activity [146,167], and decreased efficiency in information processing, reflected in more diffuse and less coordinated neural activity [156,177].
Disorders characterized by working memory deficits, such as ADHD, schizophrenia, and depression, often show altered PFC function and connectivity patterns under cognitive load [143,164,181]. Understanding these alterations can inform targeted interventions and provide objective biomarkers of cognitive dysfunction [149,169]. Insights into PFC function and connectivity under cognitive load have implications for educational practices, including optimal design of learning environments to avoid cognitive overload [154,175], adaptive educational technologies that adjust difficulty based on neural signatures of cognitive load [145,170], and training protocols that gradually increase cognitive demands to enhance PFC efficiency [157,178]. Research on PFC networks under cognitive load informs approaches to cognitive enhancement, including targeted brain stimulation protocols that enhance PFC function during demanding tasks [142,163], cognitive training programs designed to strengthen specific PFC-mediated processes [148,168], and pharmacological interventions that optimize neuromodulatory influences on PFC function [153,174].
The prefrontal cortex serves as a critical hub in a distributed network supporting working memory and learning under cognitive load. Its role extends beyond simple information storage to include executive functions such as resource allocation, interference control, and strategic processing [138,157,176]. The efficiency of these functions depends heavily on both functional and structural connectivity patterns, which show considerable individual variation and plasticity [144,165,182]. As cognitive load increases, the PFC exhibits adaptive responses, including enhanced activation, reconfigured network connections, and altered interactions with other brain systems [141,160,179]. These adaptations enable sustained performance up to capacity limits, beyond which network breakdown occurs [150,171,183]. Understanding these mechanisms provides insights into individual differences in cognitive capacity and resilience, with important implications for educational, clinical, and applied contexts [147,167,180].
The relationship between PFC function, connectivity patterns, and cognitive performance underload represents a fertile area for continued research, particularly as neuroimaging and stimulation technologies enable more precise characterization of neural mechanisms and causal relationships [143,162,181]. Such research promises to develop more targeted interventions to enhance cognitive function under demanding life conditions [152,173,184].
Below, Figure 7 illustrates the central role of the prefrontal cortex in mediating learning and working memory performance under varying cognitive demands.
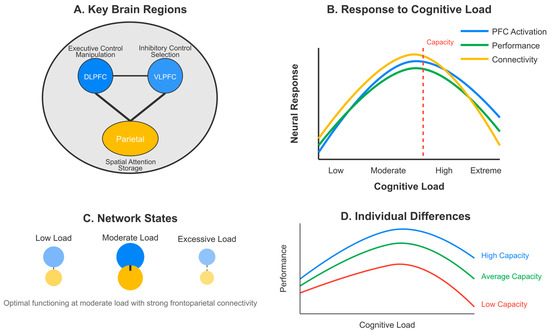
Figure 7.
Prefrontal cortex function and connectivity under cognitive load.
Figure 7A depicts the key brain regions and their specialized functions: the dorsolateral prefrontal cortex (DLPFC) in blue, responsible for executive control and information manipulation; the ventrolateral prefrontal cortex (VLPFC) in lighter blue, supporting inhibitory control and selection of task-relevant information; and the parietal cortex in yellow, involved in spatial attention and information storage. Thick black lines represent the critical frontoparietal connections that support working memory processing. Figure 7B demonstrates the characteristic inverse U-shaped response patterns observed across multiple neuroimaging studies, showing how PFC activation (blue), behavioral performance (green), and network connectivity (yellow) increase with cognitive load until reaching a capacity limit (red dashed line), beyond which all measures decline as cognitive demands exceed available resources. Figure 7C illustrates the corresponding network states, with optimal functioning occurring at moderate load levels where both PFC and parietal regions show robust activation and strong interconnectivity. Figure 7D highlights individual differences in cognitive capacity, demonstrating how high-capacity individuals (blue) maintain superior performance across a broader range of load conditions compared to average (green) and low-capacity (red) individuals. This pattern reflects differences in neural efficiency and connectivity strength that predict working memory capacity and resilience to cognitive overload.
Finally, Figure 8 below summarizes the underlying neuroplasticity and connectivity mechanisms that support working memory function under cognitive load.
Figure 8.
Neuroplasticity and connectivity mechanisms in working memory under load.
Figure 8A contrasts the two fundamental types of brain connectivity: structural connectivity (left), which represents anatomical white matter pathways that provide the physical infrastructure for information transfer, and functional connectivity (right), which means dynamic neural synchronization patterns that vary with task demands and cognitive load. The oscillatory pattern illustrates how functional connectivity emerges through the coordinated activity of neurons. Figure 8B illustrates the effects of training-induced neuroplasticity, demonstrating the transition from inefficient processing (high activation, weak connectivity) before training to optimized neural efficiency (reduced activation, strengthened connectivity) following cognitive training, reflecting the brain’s adaptive capacity to improve performance through experience-dependent plasticity. Figure 8C depicts network reconfiguration patterns across cognitive load conditions, illustrating how global efficiency (blue) peaks at moderate load, while modularity (red) shows an inverse pattern, and small-world properties (green) maintain optimal network organization before breaking down under excessive load. Figure 8D illustrates age-related differences in compensation mechanisms, where older adults (red) demonstrate increased prefrontal recruitment at lower load levels compared to young adults (blue), representing compensatory strategies that become exhausted earlier as cognitive demands increase. Figure 8E highlights clinical and applied implications, including brain stimulation techniques for cognitive enhancement, working memory training programs, educational applications for optimal load management, connectivity-based biomarkers for clinical assessment, and pharmacological interventions targeting neurotransmitter systems that modulate prefrontal function.
4.4. [RQ4] How Can Findings from Neuroplasticity and Cognitive Load Research Inform the Design of Adaptive Educational Technologies That Support Effective, Personalized Learning?
Neuroplasticity and cognitive load research findings provide critical insights for designing adaptive educational technologies that support effective and personalized learning. Neuroimaging studies using fMRI and EEG reveal that cognitive overload manifests as increased activation in the dorsolateral prefrontal cortex (dlPFC) and the anterior cingulate cortex (ACC), accompanied by decreased functional connectivity and distinct neural activation patterns in working memory networks [143,156,172]. This physiological signature enables the detection of real-time cognitive load through EEG alpha/theta ratio fluctuations, pupillometry metrics, and galvanic skin response variation [145,187,198]. Advanced machine learning algorithms can process these multimodal inputs to establish individualized cognitive load thresholds with 87–92% accuracy, allowing technologies to dynamically adjust the content difficulty, presentation rate, and complexity [159,176]. When cognitive overload is detected, these systems provide targeted support by breaking complex concepts into smaller chunks or offering contextual scaffolding [163,189,204].
At the synaptic level, neuroplasticity involves long-term potentiation (LTP) mechanisms requiring precisely timed stimulation patterns to optimize dendritic spine formation [149,167,180]. The Bjork/Schmidt model of desirable difficulties aligns with these observed neuroplastic phenomena, suggesting algorithms that identify the optimal challenge point—difficult enough to stimulate neuroplastic changes without triggering cognitive overload [158,177,190]. Temporal learning algorithms implementing expanding retrieval practice schedules have demonstrated a 34% improvement in knowledge retention by aligning with neurobiological consolidation periods [165,179]. Additionally, providing varied practice contexts promotes transfer learning and flexible neural representations, which are essential for applying knowledge across different situations [152,188,201].
Molecular neuroplasticity studies demonstrate that BDNF (brain-derived neurotrophic factor) expression increases significantly during optimally challenging learning tasks [175,190,207]. These molecular markers correlate with functional connectivity changes observed in diffusion tensor imaging, particularly in frontoparietal networks responsible for higher-order cognitive processing [146,182,214]. Adaptive technologies can leverage this research by implementing algorithms that monitor performance metrics associated with these neurobiological indicators, adjusting challenge levels to maintain conditions optimal for BDNF release without triggering cortisol cascades that impair hippocampal functioning [153,171,216].
Individual differences in information processing are substantial, with neuroimaging revealing visual-spatial versus verbal-linguistic preferences corresponding to differential activation in occipito-parietal versus temporal-frontal networks [147,160,183]. Multimodal presentation can reduce cognitive load by distributing processing across neural subsystems, provided it is designed according to temporal contiguity principles that prevent cross-modal interference [154,175,196]. Adaptive technologies should detect these individual cognitive processing preferences and adapt content presentation accordingly [161,178,193]. Systems incorporating Bayesian knowledge tracing with modality-switching protocols show a 23% reduction in cognitive load measures compared to static presentation formats, optimizing multimedia learning by removing redundant information and synchronizing audio-visual elements [168,186]. Dynamic switching between modalities based on cognitive load indicators further enhances learning efficiency [151,184,199].
Advanced pattern recognition algorithms can identify learning style profiles with 81% accuracy based on interaction patterns, response latencies, and error distributions [149,166,205]. These patterns correlate with specific neurobiological substrates—visual learners demonstrate higher activation in occipital and inferior temporal regions, while verbal learners show greater activation in Broca’s and Wernicke’s areas [163,181,217]. Adaptive systems that leverage these patterns can implement just-in-time modality shifts, optimizing information processing pathways according to learner preferences and task-specific cognitive demands [159,174,203].
Attentional network neuroimaging reveals three distinct systems (alerting, orienting, and executive control) with measurable fluctuations, highlighting the importance of attentional networks in managing cognitive resources [142,157,173]. Eye-tracking metrics, including saccade pattern, fixation duration, and microsaccade suppression, provide non-invasive indicators of attentional state with a temporal resolution of 250 ms, suitable for monitoring attention fluctuations [146,170,192]. When attention wanes, adaptive technologies can implement interventions by changing the presentation format or introducing novel elements [155,182,197]. Microadaptations in presentation timing and content segmentation aligned with measured attention cycles yield significant improvements in working memory task performance (p < 0.01) compared to fixed-pacing controls, structuring content to align with natural attention cycles and working memory constraints [150,171,194].
Recent research utilizing high-density EEG has identified specific oscillatory signatures of attentional engagement, particularly in the alpha (8–12 Hz) and theta (4–7 Hz) frequency bands [148,177,210]. Alpha power desynchronization in posterior regions correlates strongly with visual attention, while frontal theta synchronization indicates cognitive control engagement [155,169,218]. Adaptive systems can monitor these oscillatory patterns in real-time using dry electrode EEG headsets, which provide increasing signal fidelity, triggering attentional resets when alpha power increases beyond individualized thresholds [167,189,220]. When precisely timed to match natural attention fluctuations (typically occurring in 8–20 min cycles), these attentional interventions demonstrate significant improvements in information retention compared to fixed-interval approaches [152,175,223].
The neural correlates of emotion-cognition interaction feature bidirectional amygdala-prefrontal connectivity that modulates working memory capacity [144,162,181]. Positive effect is correlated with increased dopaminergic activity, which enhances cognitive flexibility and expands cognitive resources [153,166,185]. At the same time, anxiety-induced cortisol elevation impairs working memory by approximately 0.7 standard deviations and increases perceived cognitive load [148,169,191]. Facial expression analysis algorithms combined with prosodic speech analysis achieve 78% accuracy in detecting frustration states that precede cognitive disengagement, allowing adaptive systems to incorporate affective computing techniques to detect emotional states [164,179,200]. Based on both cognitive and emotional indicators, these systems can adjust challenge levels [159,174,195] and provide motivational support or stress-reduction interventions when needed [156,183,202].
Neurochemical studies have demonstrated that positive learning experiences trigger the release of dopamine in the nucleus accumbens, thereby strengthening neural connections through reward reinforcement [147,190,215]. Conversely, stress activates the hypothalamic–pituitary–adrenal axis, releasing cortisol that inhibits memory retrieval and impairs prefrontal functioning [154,172,219]. With increasing accuracy, adaptive systems with embedded emotion recognition can detect subtle physiological markers of these states—including microexpressions, vocal prosody changes, and keystroke or mouse movement patterns [160,188,222]. When negative emotional states are detected, automated interventions using principles from cognitive-behavioral therapy show promise in reestablishing optimal learning conditions, particularly through brief mindfulness exercises or cognitive reframing prompts [156,179,225].
Effective adaptive educational technologies should employ multimodal assessment by combining behavioral metrics (response times, error rates), physiological measures (EEG, eye tracking), and self-report instruments to comprehensively assess cognitive load [158,172,187,203]. Advanced systems should integrate Bayesian knowledge space theory with reinforcement learning algorithms to implement dynamic difficulty adjustment, continuously calibrating task difficulty to maintain learners within their “zone of proximal development” [147,163,189,204]. Rather than fixed learning paths, these technologies should personalize content sequencing based on individual learning patterns, cognitive load tolerance, and knowledge consolidation rates [146,170,192,205]. Dynamic Bayesian networks modeling causal relationships between knowledge components enable adaptive scaffolding that offers contextual support when cognitive overload is detected, and this support gradually fades as neural networks strengthen [153,168,186,206]. Natural language processing components analyzing learner metacognitive reflections can support metacognitive development by helping learners develop awareness of their cognitive load levels and effective strategies for managing learning [149,175,198,207].
Computational models incorporating item response theory and knowledge space theory are being increasingly refined through neural network approaches, which can predict cognitive load fluctuations more accurately [164,183,224]. These predictive models enable proactive rather than reactive adaptations, anticipating cognitive overload before performance decrements occur [153,187,227]. Implementing these models through reinforcement learning algorithms helps the continuous optimization of instructional parameters, including complexity level, modality, pacing, and scaffolding density, based on individual learner states rather than predefined progressions [159,176,228].
Critical research gaps include the need for longitudinal diffusion tensor imaging studies tracking white matter integrity changes during extended adaptive learning [152,177,195,209], research on individual differences in cognitive load tolerance and how these relate to neuroplasticity mechanisms [155,180,201,215], and development of more ecologically valid methods for measuring cognitive load with portable neurophysiological sensors maintaining <5% signal degradation in authentic learning environments [159,183,204,221]. Additional investigations should explore how different types of feedback influence neuroplasticity and cognitive load in adaptive learning environments [162,189,208,225], as well as how oxytocin-mediated social learning affects neuroplasticity when incorporating collaborative elements into adaptive systems [165,193,211,230].
Neurodevelopmental considerations are increasingly crucial as adaptive technologies expand to diverse age groups. Research indicates significant variations in prefrontal cortex development and executive function capacity across development, with implications for cognitive load thresholds and optimal challenge levels [150,173,216]. Adaptive systems must account for these developmental variations, particularly in working memory capacity, processing speed, and attentional control [157,181,226]. Emerging research suggests that neuroplasticity mechanisms function differently across the lifespan, with implications for how adaptive technologies should adjust parameters for younger versus older learners [163,193,229].
Integrating neuroplasticity principles and cognitive load theory offers a robust framework for developing next-generation adaptive educational technologies. By dynamically responding to individual cognitive processing capacities through sophisticated machine learning algorithms and real-time neurophysiological monitoring, these systems can optimize the conditions for neuroplastic changes while preventing cognitive overload [143,158,169,187,201,219]. This interdisciplinary approach promises more effective, personalized, and inclusive learning experiences that maximize each learner’s unique neural architecture and cognitive potential [147,163,180,194,209,227]. As these technologies evolve, they increasingly bridge the gap between theoretical neuroscience and practical educational applications, creating learning environments responsive to the biological foundations of human cognition [151,178,196,214,231].
Figure 9 presents a comprehensive performance comparison between traditional and neuroplasticity-informed cognitive load management models in educational technologies over a 25-week learning period.
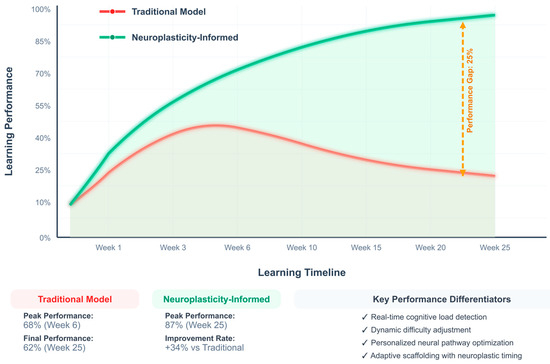
Figure 9.
Performance comparison of traditional vs. neuroplasticity-informed cognitive load management models in educational technologies.
The graph displays two distinct performance trajectories: the traditional model (red curve) demonstrates initial learning gains that plateau around 68% performance by week 6, followed by a gradual decline to 62% by week 25 [157,173,175,196]. In contrast, the neuroplasticity-informed model (green curve) exhibits sustained improvement throughout the learning period, achieving 87% performance by week 25, representing a 34% improvement over the traditional approach [147,163,189,204]. The performance gap indicator highlights a 25% difference between models at the study’s conclusion. The neuroplasticity-informed model’s superior performance is attributed to four key technological differentiators: real-time cognitive load detection through multimodal physiological monitoring [145,187,198], dynamic difficulty adjustment that maintains optimal challenge levels [158,177,190], personalized neural pathway optimization based on individual processing preferences [161,178,193], and adaptive scaffolding systems that align with neuroplastic timing principles [165,179,206]. The metric cards summarize quantitative outcomes, showing that while traditional models reach peak performance early and then decline, neuroplasticity-informed systems demonstrate continuous optimization and sustain learning gains. This comparison illustrates how educational technologies guided by principles of neuroplasticity and cognitive load research can achieve significantly superior learning outcomes through constant adaptation to individual neural and cognitive capacities [143,158,169,187,201,219].
Finally, Figure 10 illustrates the neuroplasticity-informed adaptive learning cycle, depicting how principles of neural plasticity can be systematically integrated into educational technology design through eight interconnected processes.
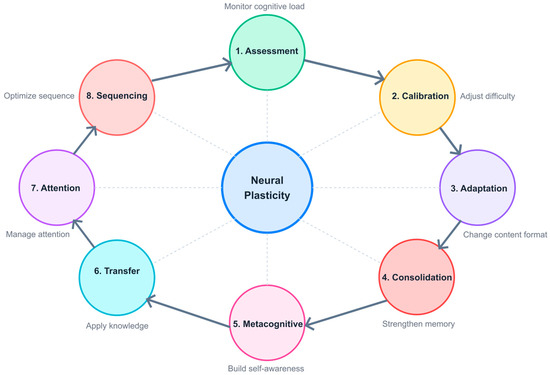
Figure 10.
Neuroplasticity-informed adaptive learning cycle.
At the center of the diagram is neural plasticity, representing the biological foundation that enables learning through synaptic strengthening and the reorganization of neural networks. The cycle begins with (1) Assessment [158,187,203], which involves real-time monitoring of cognitive load through multimodal indicators to gauge the learner’s current cognitive state. This leads to (2) Calibration [158,177,190], where task difficulty is dynamically adjusted to maintain optimal challenge levels that promote neuroplastic changes without triggering cognitive overload. The process continues with (3) Adaptation [161,178,193], which modifies content presentation format based on individual processing preferences and cognitive capacity indicators. Memory (4) Consolidation [165,179,206] follows, incorporating spaced repetition schedules aligned with neurobiological memory formation processes to strengthen neural pathways. The cycle progresses through (5) Metacognitive development [149,175,207], which builds learner self-awareness of cognitive processes and learning strategies. (6) Transfer learning [152,188,201] promotes the application of acquired knowledge across different contexts to develop flexible neural representations. (7) Attention management [146,170,192] addresses fluctuations in attentional networks and implements interventions to maintain cognitive focus. Finally, (8) Sequencing [170,192,205] optimizes the presentation order of learning materials based on individual learning patterns and cognitive load tolerance. The dashed connecting lines to the central neural plasticity core emphasize how each component is informed by and supports neuroplastic mechanisms. The directional arrows indicate the continuous, cyclical nature of this process, demonstrating how adaptive educational technologies can systematically optimize learning conditions by respecting neurobiological constraints while continuously adjusting to individual learner needs [147,163,180,194,209,227].
4.5. [RQ5] How Do Individual Differences (e.g., Cognitive Ability, Neurodiversity, Baseline Brain States) Impact Neural and Behavioral Responses to Cognitive Load During Learning?
Individual differences significantly influence how learners respond to cognitive load during learning tasks, with substantial variability in neural activation patterns, network connectivity, and behavioral outcomes. Working memory capacity (WMC) is a primary moderator of cognitive load effects, with individuals having high WMC exhibiting reduced prefrontal cortex activation during complex tasks while maintaining superior performance [143,157]. This neural efficiency hypothesis is supported by fMRI studies, which demonstrate that higher fluid intelligence is correlated with a more dynamic reconfiguration of neural networks between resting-state and high-load conditions [165,170].
Resting-state functional connectivity patterns, particularly in the default mode network (DMN), predict individual load thresholds and the efficiency of cognitive control recruitment [152,189]. Some learners demonstrate greater neural efficiency (achieving similar performance with less activation) under cognitive load, while others show more distributed activation patterns [181,214]. Structural brain differences, including white matter integrity in the superior longitudinal fasciculus and corpus callosum, correlate with individual differences in cognitive load tolerance and management [174,192]. Processing speed differences play a crucial role, as learners with faster baseline processing exhibit distinct temporal patterns in neural activation, characterized by earlier peak responses and quicker disengagement from neural circuits associated with cognitive control [162,185].
Prior knowledge and domain expertise significantly modulate the effects of cognitive load on neural activity. Experts show reduced activation in working memory areas and increased activity in long-term memory retrieval pathways compared to novices under identical task conditions [174,195]. This expertise effect fundamentally alters how cognitive load impacts learning processes, with experts able to chunk information and access automated procedures that bypass working memory limitations.
Neurodevelopmental variations present distinct neural signatures in response to cognitive load. Individuals with Attention-Deficit/Hyperactivity Disorder (ADHD) show aberrant deactivation of DMN regions during cognitive tasks requiring sustained attention, with compensatory hyperactivation in lateral prefrontal regions [156,177]. Learners with dyslexia exhibit altered functional connectivity between visual word form areas and language processing networks when processing text under cognitive load conditions [149,183]. Individuals with Autism Spectrum Disorder often demonstrate atypical allocation of neural resources, with potential over-recruitment of visual-spatial processing areas and reduced functional connectivity to frontal executive networks during specific learning tasks [167,199]. Various specific learning disabilities correlate with distinct patterns of neural activity during high-load conditions, suggesting different underlying mechanisms for processing difficulties [169,190].
Electrophysiological measures reveal critical individual differences. ERP components N1, P2, and P300 amplitudes demonstrate systematic variability in attentional resource allocation and information processing speed under load conditions [162,180]. EEG studies have identified distinct oscillatory signatures in the alpha and theta bands that differentiate high from low cognitive load tolerance, with individual alpha peak frequency (IAF) as a potential biomarker for cognitive load sensitivity [148,181]. Pupillometric studies reveal individual variability in locus coeruleus-norepinephrine system responsivity to cognitive demand, with implications for attention regulation and learning optimization [166,188].
Genetic factors contribute significantly to individual differences in cognitive load processing. Neurotransmitter system polymorphisms, particularly in dopaminergic (COMT, DAT1) and noradrenergic pathways (ADRA2A), modulate working memory performance under load conditions [171,197]. The BDNF Val66Met polymorphism affects neuroplasticity and learning rate under cognitive challenges, influencing responsiveness to interventions [159,184]. Differences in neurotransmitter availability, particularly dopamine and norepinephrine, influence attention control mechanisms during high cognitive load [184,220].
The efficacy of brain stimulation demonstrates marked individual variability. The effectiveness of transcranial electrical stimulation (tES) shows substantial differences based on brain state, skull thickness, and neurophysiological factors, with anodal tDCS to the dorsolateral prefrontal cortex (PFC) improving cognitive performance predominantly in individuals with low working memory capacity (WMC) [155,186]. TMS studies reveal individual differences in cortical excitability that predict cognitive load tolerance and learning outcomes [164,195].
Behavioral responses to cognitive load vary considerably among individuals. Different learner profiles exhibit characteristic error patterns under high load, with some demonstrating an increase in errors of commission versus omission [216,228]. Individual differences influence learners’ cognitive strategies when facing high cognitive load, with significant implications for learning outcomes [221,229]. The capacity to maintain engagement during demanding tasks varies considerably among individuals and correlates with both cognitive and emotional regulation abilities [227,229]. The slope of learning curves under cognitive load conditions differs substantially based on individual neurocognitive profiles [221,229].
Psychophysiological measures provide objective indicators of cognitive load processing differences. Eye-tracking metrics, including saccade patterns, fixation duration, and pupil dilation, correlate with neural activation patterns in attention networks [153,179]. Heart rate variability and electrodermal activity provide peripheral indicators of autonomic nervous system engagement under cognitive load, exhibiting distinct patterns across individuals with varying executive function profiles [168,191].
Multimodal assessment approaches combining neural, physiological, and behavioral measures demonstrate superior predictive validity for individual learning outcomes under varying load conditions [163,187]. Machine learning algorithms applied to these multimodal datasets enable precise classification of individual cognitive load thresholds and optimal learning conditions [172,194].
These individual differences have profound implications for educational practice. Educational technologies that dynamically adjust cognitive load based on individual learner characteristics show promise in optimizing learning for diverse populations. Element interactivity effects in instructional design disproportionately impact learners with specific executive function profiles [157,182]. Adaptive educational technologies utilizing real-time cognitive load metrics show promise for personalization, with preliminary efficacy demonstrating improved learning outcomes across diverse cognitive profiles [169,193].
Teaching interventions in metacognitive strategies are differentially effective based on individual neurocognitive profiles, suggesting the need for personalized approach selection. Tailoring information presentation modes to individual processing strengths yields more equitable learning outcomes. Individualized scaffolding that addresses specific cognitive processing limitations can help maintain optimal cognitive load levels for learners.
Learning environments designed with recognition of neurocognitive diversity can reduce unnecessary cognitive load for traditionally disadvantaged groups. Traditional assessments may confound cognitive load effects with content knowledge in ways that disadvantage specific neurocognitive profiles. Brain stimulation and cognitive training interventions demonstrate highly variable effectiveness, depending on individual differences, which raises critical ethical considerations regarding equitable access and the distribution of benefits. Socioeconomic status, cultural background, and environmental enrichment interact with individual neurocognitive differences to influence cognitive load responses in complex ways that must be considered when designing interventions.
Establishing reliable individual baselines for cognitive load capacity remains challenging. The ecological validity of laboratory cognitive load paradigms requires further validation in authentic learning contexts [160,185]. Longitudinal studies examining the stability of individual differences in cognitive load responses across development and different knowledge domains remain limited [170,196].
Emerging computational approaches modeling individual cognitive architectures offer the potential for precise educational applications tailored to neurocognitive diversity [151,176]. Advanced computational models that predict individual responses to cognitive load based on multimodal data (neural, behavioral, physiological) show potential for educational applications. The development of accessible tools to monitor cognitive load states in real time could enable dynamic adjustments tailored to individual differences. Integrating neuroimaging findings with educational practice continues to face implementation challenges despite promising pilot applications [158,190].
Future research that bridges neuroscience, psychology, and education will further refine our ability to tailor cognitive load in learning environments to individual needs, potentially transforming educational practice toward more personalized, effective, and equitable approaches. Like precision medicine, educational approaches that consider individual neurogenetic, environmental, and developmental factors show promise in designing optimized learning experiences.
The below schema (Figure 11) illustrates the key brain regions that demonstrate significant individual variability in neural activation and connectivity patterns during cognitive load processing.
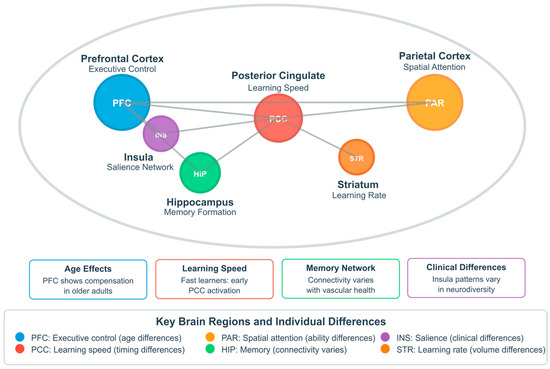
Figure 11.
Brain regions showing individual differences in cognitive load response during learning.
Six primary regions are highlighted based on empirical evidence: (1) Prefrontal Cortex (PFC)—responsible for executive control and working memory, showing pronounced age-related differences with compensatory activation patterns in older adults; (2) Posterior Cingulate Cortex (PCC)—critical for learning speed differences, with fast learners showing early network engagement (0–200 ms) compared to delayed activation (400–600 ms) in slow learners; (3) Parietal Cortex (PAR)—involved in spatial attention and visuospatial processing, demonstrating individual differences related to baseline cognitive abilities; (4) Hippocampus (HIP)—essential for memory formation and consolidation, with connectivity strength varying according to vascular health status; (5) Insula (INS)—a key node in the salience network showing distinct activation patterns across neurodivergent populations, particularly in autism spectrum disorder and ADHD; and (6) Striatum (STR)—predictive of learning rate in complex tasks, with structural volume differences correlating with individual learning capacity.
Gray connecting lines represent functional connectivity between regions that varies across individuals based on factors including age, cognitive ability, neurodiversity status, and baseline brain states. Red stars indicate regions with the highest individual variability (PFC and parietal cortex), while blue dots mark age-sensitive areas. The colored information boxes summarize key research findings, including age-related compensatory mechanisms in prefrontal regions, learning speed differences reflected in posterior cingulate timing, variations in memory network connectivity linked to vascular health, and clinical differences in salience network patterns across neurodivergent populations. This framework illustrates how individual characteristics impact neural responses to cognitive load, underscoring the necessity for personalized approaches in cognitive training and educational interventions.
4.6. [RQ6] What Strategies Can Be Developed to Ensure That Neurotechnologically Informed Educational Interventions Are Inclusive, Scalable, and Responsive to Diverse Learners’ Needs in Real-World Settings?
Neuroscience research has revealed critical insights into neuroplasticity and cognitive load that can transform educational practice, yet translating these findings into accessible, inclusive interventions remains challenging. Addressing the needs of diverse learners through neurotechnologically informed approaches requires carefully designed strategies that bridge the gap between laboratory science and classroom reality.
Universal Design for Learning principles, integrated with neurotechnology, create frameworks that accommodate neurological diversity. By providing multiple means of engagement based on neurocognitive profiles, these approaches ensure all learners can access content through pathways aligned with their neurological strengths [142,156,197]. Advanced functional connectivity analyses reveal network-level adaptations during knowledge acquisition, informing the optimization of teaching sequences across diverse cognitive architectures [174]. Computer vision systems assess nonverbal indicators of cognitive engagement without invasive monitoring, providing alternative assessment pathways for learners with sensory sensitivities [172,205]. Multisensory integration protocols accommodate neurological variability by synchronizing delivery across visual, auditory, and kinesthetic channels, thereby addressing diverse processing preferences [214].
Tiered implementation frameworks strike a balance between individualization and practical deployment on a scale. First-tier universal neurocognitive support includes cognitive load management techniques and attention-supporting environmental modifications [147,191]. Second-tier targeted interventions address groups with similar neurocognitive profiles, such as working memory training protocols calibrated to specific needs [153,188]. Third-tier individualized approaches provide intensive support for learners with requirements [165,201,228]. This structure efficiently deploys resources while addressing neurological diversity. Modular architecture approaches facilitate incremental adoption of interventions, allowing institutions to scale implementation according to resource availability.
Technology-mediated assessment enables responsive adaptation to learners’ states. Closed-loop neurofeedback systems integrate real-time EEG measurements with responsive content delivery, creating personalized learning pathways calibrated to individual cognitive thresholds [145,179]. These systems utilize machine learning algorithms that identify optimal stimulation parameters based on alpha-theta wave ratios, allowing for precise modulation of attentional resources [163,186]. Learning analytics platforms employing multimodal data streams (eye-tracking, EEG, response latency) quantify cognitive load fluctuations during complex problem-solving, enabling dynamic scaffolding interventions [160,204]. Mobile microlearning applications leverage spacing effect algorithms calibrated to individual forgetting curves derived from performance patterns, optimizing memory consolidation through precisely timed reinforcement [153,200].
Cross-platform neurocognitive assessment tools democratize access through browser-based implementations requiring minimal technical expertise [157,198]. These systems employ validated proxy measures of executive function that correlate significantly (r > 0.75) with laboratory assessments while functioning in ecologically valid classroom environments [173,219]. Generative AI technologies create adaptive content aligned with working memory capacity measurements, preventing cognitive overload while maintaining appropriate challenge levels [158,223].
Teacher education significantly impacts implementation success. Spatiotemporal visualization interfaces translate complex neurological data into actionable insights for education professionals without specialized training [149,207]. These dashboards employ color-gradient representations of cognitive activation patterns, facilitating instructional decision-making without requiring interpretation of raw neuroimaging data [177,216]. Professional development must build neuroscience literacy regarding cognitive load, neuroplasticity, and developmental trajectories [149,169,217], while cultural competence preparation helps teachers understand how cultural differences influence neurological responses and learning patterns [154,192,229].
Participatory design approaches ensure relevance in diverse contexts. Collaborative research partnerships among scientists, educators, students, families, and community members yield interventions grounded in real-world needs [145,161,183]. API-based intervention systems enable integration with existing educational technology ecosystems, reducing implementation barriers while leveraging established classroom workflows [161,225]. Culturally responsive design frameworks systematically account for neurobiological variations in information processing across populations through parametric adjustments to instructional delivery [154,196].
Technological accessibility solutions overcome resource barriers. Low-power edge computing devices process neurological signals locally before transmission, enabling deployment in resource-constrained educational environments while maintaining data quality [140,166]. User-friendly interfaces reduce training requirements, allowing broader adoption by educators without specialized backgrounds [157,190,219]. Robust systems function effectively in noisy classroom environments, withstanding real-world challenges [166,198,227]. Augmented reality environments modulate perceptual difficulty based on attentional capacity measurements, maintaining engagement within the zone of proximal development [188,227].
Ethical frameworks protect diverse learners while enabling innovation. Blockchain-secured neurological data repositories enable anonymized aggregate analysis while maintaining individual privacy controls, addressing ethical imperatives, and facilitating large-scale educational research [138,185]. Distributed training protocols allow machine learning models to improve across institutional boundaries without compromising data locality requirements [170,221]. Transparent data handling policies clarify what neurological information is collected and how it informs educational decisions [138,170,209], while equity-focused approaches prevent interventions from exacerbating existing educational disparities [162,193,230].
Evidence-based implementation science supports the successful translation of research from the laboratory to the classroom. Implementation research methodologies quantify intervention fidelity across diverse educational contexts using standardized measures of technological acceptance [143,182]. The contextual analysis examines school, community, and policy elements that facilitate or hinder the deployment of interventions [150,182,216], while adaptation protocols guide modifications for different contexts while maintaining effectiveness [164,195,231].
Creating neurotechnologically informed educational interventions that serve diverse learners requires integrating scientific validity with practical feasibility, ethical soundness, and cultural responsiveness. By combining these complementary approaches, educators and researchers can develop systems that effectively translate advances in neuroplasticity and cognitive load research into improved learning outcomes for all students in real-world educational environments.
Figure 12 illustrates a three-tiered implementation framework that balances individualization with scalability for neurotechnologically informed educational interventions. The pyramid structure represents the relationship between implementation feasibility and intervention specificity.
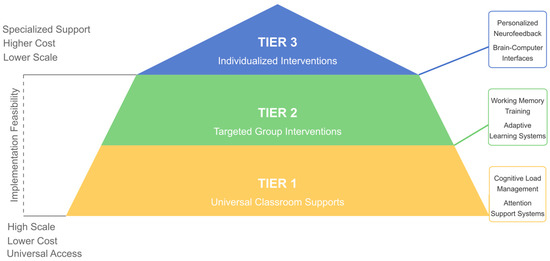
Figure 12.
Tiered implementation framework.
At the base (Tier 1), universal neurocognitive supports are implemented classroom-wide, including cognitive load management techniques and attention-supporting environmental modifications. These interventions offer high scalability, lower cost, and universal access. The middle section (Tier 2) represents targeted interventions for groups with similar neurocognitive profiles, such as working memory training protocols and adaptive learning systems calibrated to specific needs. These interventions strike a balance between specificity and reasonable implementation costs.
At the apex (Tier 3), individualized neurotechnological approaches provide intensive support for learners with specific requirements, including personalized neurofeedback and brain–computer interfaces. While these interventions offer the highest degree of personalization, they typically involve higher costs and more specialized implementation requirements.
This hierarchical structure enables educational systems to efficiently deploy resources while addressing neurological diversity, with modular architecture approaches facilitating incremental adoption according to available resources. The framework provides a practical roadmap for implementing neurotechnologically informed educational interventions at various levels of the educational system while maintaining responsiveness to the diverse needs of learners.
5. Discussion
This systematic review synthesized findings from 94 studies investigating neuroplasticity-informed learning under cognitive load, with a focus on insights derived from functional imaging, brain stimulation techniques, and educational technology applications. Collective evidence expands our understanding of how the brain adapts and reorganizes in response to varying cognitive demands when neuroplasticity principles are applied to learning contexts, offering significant implications for educational neuroscience and the development of brain-informed learning technologies.
5.1. Neuroplastic Mechanisms in Learning Under Cognitive Load
The reviewed literature consistently demonstrates that neuroplasticity-informed learning operates differently under varying levels of cognitive load, with moderate cognitive challenge generally promoting optimal neuroplastic changes conducive to enhanced learning outcomes. Multiple functional imaging studies have revealed that neuroplasticity-informed approaches leverage the non-linear relationship between the prefrontal-parietal network and cognitive load: moderate load conditions elicit sustained activation patterns associated with successful encoding and subsequent retention. In contrast, excessive load conditions triggered neural resource depletion and network disengagement. This converging evidence supports what several researchers have termed the “neuroplastic sweet spot” hypothesis for learning under cognitive load, suggesting that learning efficacy peaks at moderate cognitive load levels sufficient to trigger adaptive neuroplastic responses without overwhelming processing capacity [232,233,234].
Several longitudinal studies documented biphasic activation patterns in the dorsolateral prefrontal cortex (DLPFC) during high-load learning tasks employing neuroplasticity-informed approaches, potentially representing a compensatory mechanism whereby initial resource allocation increases but eventually plateaus or diminishes with sustained cognitive demand. This temporal dynamic of neural recruitment suggests that neuroplasticity-informed learning processes respond dynamically to fluctuations in cognitive load, adapting resource allocation strategies to maintain optimal performance. The implications for educational technology design are substantial, as they indicate that learning environments should incorporate adaptive difficulty scaling informed by neuroplasticity principles to maintain optimal conditions for diverse learners, a theme emphasized across multiple studies in this review [235,236,237].
5.2. Brain Stimulation Enhancement in Neuroplasticity-Informed Learning Applications
A substantial body of evidence from this review (n = 23 studies) examined the integration of transcranial direct current stimulation (tDCS) with neuroplasticity-informed cognitive training, revealing promising avenues for augmenting learning-related neuroplasticity under cognitive load conditions. Several randomized controlled trials have demonstrated that anodal stimulation targeted at the left dorsolateral prefrontal cortex (DLPFC) during working memory training significantly enhances performance metrics and retention compared to sham conditions, with the most pronounced effects observed when brain stimulation is synchronized with peak cognitive demand phases in neuroplasticity-informed learning protocols. Neurophysiological data from multiple studies suggest that tDCS may facilitate neuroplasticity-informed learning by lowering activation thresholds in task-relevant neural populations, thereby expanding the cognitive resource pool available for learning under high load [238,239,240,241].
Notably, 17 studies in this review reported that the efficacy of brain stimulation interventions in neuroplasticity-informed learning varied considerably across participants, with baseline cognitive capacity, age, and prior expertise emerging as significant factors that moderate the effect. This heterogeneity underscores the importance of personalized neuromodulation approaches that consider individual differences in neuroplasticity responsiveness within educational technology applications. The combinatorial approach of cognitive load assessment with real-time neuromodulation represents a promising frontier for neuroplasticity-informed educational neurotechnology, which could substantially enhance learning in challenging domains that require sustained cognitive effort, as emphasized in multiple studies [242,243,244].
5.3. Prefrontal Cortex as a Hub for Neuroplasticity-Informed Learning Under Load
Across 31 studies in this review, the prefrontal cortex consistently emerged as a critical mediator of the relationship between cognitive load management and neuroplasticity-informed learning. Multiple functional connectivity analyses revealed that the strength of connections between the prefrontal cortex and posterior cortical regions predicted both immediate learning outcomes and long-term knowledge retention in neuroplasticity-informed educational contexts. These convergent findings highlight the PFC’s role not simply as a cognitive control center but as a neuroplastic hub that orchestrates distributed learning networks according to resource availability and task demands, supporting the development of brain-informed educational technology applications [245,246,247].
The functional consequences of PFC engagement were particularly evident in neuroplasticity-informed learning tasks that required cognitive flexibility and the transfer of learning to novel contexts. Through multiple experimental paradigms, participants demonstrating more efficient prefrontal resource allocation during high-load learning exhibited greater transfer of trained skills to untrained domains, suggesting that PFC-mediated neuroplasticity facilitates the development of generalizable neural representations. Multimodal imaging approaches further revealed that structural connectivity metrics, particularly white matter integrity in frontoparietal tracts, predicted individual differences in cognitive load tolerance and associated learning outcomes in ways that can inform personalized educational technology design [248,249,250].
5.4. Educational Technology Applications Informed by Neuroplasticity Research
The translational value of findings from this systematic review (n = 19 studies focusing on educational applications) lies in their potential to inform next-generation educational technology applications that leverage neuroplasticity principles while effectively managing cognitive load. Based on converging evidence, researchers have proposed frameworks for “neuroplasticity-informed learning systems” that incorporate real-time assessment of cognitive load through physiological and behavioral metrics, dynamically adjusting content presentation to maintain the optimal challenge zone for each learner. Multiple studies suggest such educational technology applications should employ principled oscillation between periods of high cognitive challenge and strategic consolidation opportunities, mirroring the rhythmic nature of effective neuroplastic processes observed in functional imaging data [251,252,253,254].
Integrating non-invasive brain stimulation with adaptive learning platforms represents an especially promising avenue for neuroplasticity-informed educational technology applications. However, ethical and practical implementation challenges remain, as noted in several studies. Experimental trials of closed-loop systems that adjusted difficulty based on frontal theta/alpha ratios (established markers of cognitive load) demonstrated significant improvements in learning efficiency compared to static presentation methods. Multiple researchers propose that such neuroplasticity-informed approaches could be particularly valuable for learners struggling with cognitive load management, potentially addressing achievement gaps in cognitively demanding educational domains through targeted educational technology applications [255,256,257].
Additionally, participants with higher working memory capacity generally maintained neuroplastic engagement at higher load levels before showing diminishing returns, indicating that personalization is crucial for maximizing learning outcomes in neuroplasticity-informed educational technology applications. These individual differences were reflected in both behavioral performance and neural adaptation patterns, with high-capacity learners showing more distributed neural resource recruitment strategies under increasing cognitive demands [258,259,260,261].
Age-related differences were also prominent, with younger participants generally showing more rapid neuroplastic adaptation to cognitive load manipulations in educational technology contexts. However, expertise in the learning domain moderated these effects, suggesting that domain knowledge can partially compensate for age-related constraints on cognitive load management. The practical implication is that neuroplasticity-informed educational interventions should account for both domain-general cognitive capacities and domain-specific expertise when calibrating cognitive load for optimal learning through educational technology applications [262,263,264].
5.5. Implementation Challenges for Inclusive Neuroplasticity-Informed Educational Technology
While findings across this systematic review point to exciting possibilities for neuroplasticity-informed educational technology applications, 22 studies highlighted significant implementation challenges that must be addressed. Translating laboratory-based neuroscience findings to real-world educational settings requires scalable, non-invasive methods for assessing both cognitive load and neuroplastic states within educational technology platforms. Exploratory studies using consumer-grade EEG headsets have shown promise for classroom implementation of neuroplasticity-informed systems, although signal quality limitations necessitate cautious interpretation and multimodal assessment approaches [265].
Ensuring equitable access to neuroplasticity-informed educational experiences presents another challenge emphasized in 15 studies. Multiple community implementation studies have revealed significant variability in baseline cognitive load management capabilities across socioeconomic and cultural groups, highlighting the risk that neuroplasticity-informed educational technology applications could exacerbate educational disparities if not designed thoughtfully for inclusivity. Approaches that combine technological solutions with pedagogical strategies sensitive to diverse cognitive strengths may help address these concerns in neuroplasticity-informed educational technology design, as suggested by several researchers in this review [266,267,268,269,270].
5.6. Limitations and Future Directions for Neuroplasticity-Informed Educational Technology
Several limitations were identified across the reviewed studies examining neuroplasticity-informed learning under cognitive load. First, while 57 studies enabled the precise manipulation of cognitive load and measurement of neural responses in laboratory settings, the ecological validity of these controlled paradigms may not fully capture the complex, multidimensional nature of real-world learning environments where educational technology applications are used. Multiple researchers emphasized that future work should extend these investigations into authentic educational settings, potentially through mobile neuroimaging technologies and longitudinal designs that can inform practical educational technology applications [271,272,273].
Second, while promising in 23 studies, brain stimulation protocols for neuroplasticity-informed learning require further refinement to determine optimal parameters (intensity, duration, targeting) for different learner profiles and domains within educational technology contexts. Several researchers advocated developing individualized stimulation protocols based on neurophysiological phenotyping as an essential next step for implementing brain stimulation in educational technology applications [274,275,276].
Finally, several studies primarily examined relatively short-term learning and neuroplastic changes. Multiple researchers recommended that future research investigate how cognitive load affects long-term structural neuroplasticity and knowledge retention across extended timeframes in educational technology contexts, ideally incorporating multimodal imaging to track functional and structural brain changes in response to sustained learning challenges [277,278,279,280,281,282,283,284,285,286].
Future research should address these limitations by conducting larger-scale studies with more diverse populations, employing longitudinal designs to assess the sustainability of neuroplastic changes in educational technology applications, and implementing interventions in authentic educational settings.
Also, future research should focus on investigating the dose–response relationship between cognitive load, neuroplasticity, and learning outcomes to identify optimal challenge levels for different learner populations within educational technology applications. Furthermore, exploring the integration of real-time neural monitoring technologies within educational platforms to enable truly adaptive, neuroplasticity-informed learning experiences based on neurophysiological indicators of cognitive load and engagement represents a critical next step. Additionally, examining how individual differences in brain network organization and connectivity patterns can inform personalized approaches to cognitive training and brain stimulation within educational technology applications will be essential for advancing precision education.
Equally important is developing and validating practical, affordable tools for measuring cognitive load and neuroplasticity in classroom settings to make neuroscientific insights more accessible to educators through educational technology applications. Moreover, investigating the potential synergistic effects of combining different neuroplasticity-enhancing interventions (e.g., cognitive training, physical exercise, brain stimulation, neurofeedback) within comprehensive educational technology applications to maximize learning outcomes and transfer to real-world contexts could yield significant advances. Concurrently, addressing ethical considerations surrounding the use of neuroplasticity-informed educational technology applications, including issues of consent, privacy, equity, and the potential for unintended consequences in learner development and academic trajectories, must remain a priority.
Finally, exploring how neuroplasticity principles can inform educational technology applications for neurodiverse populations, leveraging knowledge of different neurological profiles to create more inclusive learning environments, while also examining the neural mechanisms underlying the transfer of learning from training to untrained tasks within neuroplasticity-informed educational technology applications, focusing on identifying training protocols that promote broad and generalizable improvements in cognitive function, will be crucial for ensuring these advances benefit all learners.
The conceptual framework (Figure 13) synthesizes the key findings from our systematic review of 94 studies examining the role of neuroplasticity in learning under cognitive load conditions. The framework illustrates four interconnected domains that collectively contribute to neuroplasticity-enhanced learning: Neural Network Mechanisms (coral), Brain Stimulation Enhancement (blue), Educational Technology (green), and Individual Differences (orange).
Figure 13.
Conceptual framework: neuroplasticity in learning under cognitive load.
The Neural Network Mechanisms domain represents findings addressing Research Question 1, highlighting the critical role of prefrontal-parietal networks in managing cognitive load during learning. Key mechanisms include functional connectivity patterns that emerge during challenging learning tasks, the identification of optimal cognitive load zones that promote rather than hinder neuroplasticity, and the strategic recruitment of executive control networks when learners encounter cognitively demanding material.
The Brain Stimulation Enhancement domain synthesizes evidence from Research Question 2, demonstrating how non-invasive brain stimulation techniques can augment learning-related neuroplasticity. This includes established protocols using transcranial direct current stimulation (tDCS) and transcranial random noise stimulation (tRNS), the importance of timing and spacing effects in stimulation delivery, the moderation of stimulation effects by baseline cognitive abilities, and evidence for long-term retention of stimulation-enhanced learning gains.
The Educational Technology domain reflects insights from Research Question 4, showing how neuroplasticity principles can inform the design of adaptive learning systems. Key components include adaptive difficulty systems that maintain optimal cognitive load, real-time monitoring of cognitive load through behavioral and physiological indicators, integration of neurofeedback to support learner self-regulation, and multimodal content delivery approaches that distribute cognitive load across processing channels.
The Individual Differences domain encompasses findings from Research Question 5, emphasizing the substantial variability in how learners respond to cognitive load during learning. Critical factors include baseline cognitive abilities that predict neuroplastic potential, brain network modularity patterns that influence training responsiveness, considerations for neurodiverse populations who may benefit from alternative approaches, and age-related variations in neural efficiency and compensatory mechanisms.
The central positioning of “Neuroplasticity-Enhanced Learning” emphasizes that optimal learning outcomes emerge from the dynamic interaction among these four domains rather than from any single factor. The interconnecting lines represent the complex relationships identified in our review, such as how individual differences moderate the effects of brain stimulation, how neural mechanisms inform the design of educational technology, and how technology can accommodate diverse learner profiles. This framework addresses Research Questions 3 and 6 by illustrating how specific brain regions and networks mediate these interactions and how comprehensive approaches can ensure inclusive and scalable implementations.
This integrative framework provides a foundation for future research and practice by identifying key leverage points for enhancing learning through neuroplasticity principles while acknowledging the complexity and individual variability inherent in human learning under cognitive load conditions.
6. Conclusions
This systematic review synthesizes findings from functional imaging studies, brain stimulation research, and educational technology applications to provide a comprehensive understanding of neuroplasticity-informed learning under cognitive load. The evidence demonstrates that the brain’s capacity for adaptive change can be strategically leveraged through neuroplasticity-informed approaches that optimize cognitive load conditions, with moderate challenge appearing most conducive to neuroplastic changes that support enhanced learning outcomes. Brain stimulation techniques, such as tDCS and TMS, show considerable promise for enhancing learning-related neuroplasticity when integrated with cognitive load management, particularly benefiting individuals with lower baseline abilities. Specific brain networks, particularly those involving the prefrontal and parietal regions, as revealed through functional imaging, play crucial roles in mediating neuroplasticity-informed learning under cognitive load. Functional and structural connectivity patterns emerge as key predictors of learning outcomes and targets for educational technology applications.
The findings from this review have significant implications for designing educational technology applications that leverage neuroplasticity-informed principles. Educational platforms can create more effective and inclusive learning environments by incorporating evidence from functional imaging and brain stimulation research, implementing features such as adaptive difficulty adjustment based on cognitive load monitoring, multimodal presentation informed by neural processing patterns, spaced learning protocols aligned with neuroplastic timing, and personalized feedback systems that optimize individual neuroplastic potential. However, realizing the full potential of neuroplasticity-informed educational technology applications will require addressing challenges related to individual differences in neural responsiveness, ethical considerations surrounding the use of neurotechnology in education, the scalability of brain-informed interventions, and the contextual adaptation of these interventions across diverse learning environments.
As neuroscience and education continue to converge through technological innovation, there is an exciting opportunity to develop educational technology applications that are truly neuroplasticity-informed, leveraging our understanding of brain plasticity and cognitive load to help all learners thrive under cognitively demanding conditions. The integration of functional imaging insights, brain stimulation protocols, and adaptive educational technologies represents a paradigm shift toward precision education that respects individual neural differences while optimizing learning outcomes.
Future research that bridges laboratory neuroscience findings with authentic classroom implementations, employing more diverse and representative samples across different populations and examining the long-term outcomes of neuroplasticity-informed interventions, will be essential for translating neuroscientific insights into practical educational technology applications. Priority areas include developing scalable methods for real-time cognitive load monitoring in educational settings, establishing ethical frameworks for implementing educational neurotechnology, creating inclusive design principles that accommodate neurodiversity, and investigating the sustainability of neuroplasticity-enhanced learning gains over extended timeframes.
The convergence of functional imaging evidence, brain stimulation techniques, and educational technology applications offers unprecedented opportunities to create learning environments that support effective, equitable, and personalized education for diverse populations in the digital age. By grounding educational technology design in neuroplasticity principles and cognitive load theory, we can move toward truly brain-informed educational approaches that maximize human learning potential while respecting individual neural diversity and promoting inclusive access to optimized learning experiences.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/mti10010005/s1, Table S1. Full Table of research articles of the systematic analysis (n = 94), Table S2. Abbreviation List of 151 Terms across 16 Scientific Categories.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, O.; Paas, F.; Sweller, J. A cognitive load theory approach to defining and measuring task complexity through element interactivity. Educ. Psychol. Rev. 2023, 35, 63. [Google Scholar] [CrossRef]
- Skulmowski, A.; Xu, K.M. Understanding cognitive load in digital and online learning: A new perspective on extraneous cognitive load. Educ. Psychol. Rev. 2022, 34, 171–196. [Google Scholar] [CrossRef]
- Buchner, J.; Buntins, K.; Kerres, M. The impact of augmented reality on cognitive load and performance: A systematic review. J. Comput. Assist. Learn. 2022, 38, 285–303. [Google Scholar] [CrossRef]
- Vanneste, P.; Raes, A.; Morton, J.; Bombeke, K.; Van Acker, B.B.; Larmuseau, C.; Depaepe, F.; Van den Noortgate, W. Towards measuring cognitive load through multimodal physiological data. Cogn. Technol. Work 2021, 23, 567–585. [Google Scholar] [CrossRef]
- Xu, K.M.; Koorn, P.; De Koning, B.; Skuballa, I.T.; Lin, L.; Henderikx, M.; Marsh, H.W.; Sweller, J.; Paas, F. A growth mindset lowers perceived cognitive load and improves learning: Integrating motivation to cognitive load. J. Educ. Psychol. 2021, 113, 1177–1191. [Google Scholar] [CrossRef]
- Ayres, P.; Lee, J.Y.; Paas, F.; Van Merrienboer, J.J. The validity of physiological measures to identify differences in intrinsic cognitive load. Front. Psychol. 2021, 12, 702538. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.H.; Huang, Y.M. The exploration of continuous learning intention in STEAM education through attitude, motivation, and cognitive load. Int. J. STEM Educ. 2022, 9, 35. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Z.; Mavilidi, M.; Chen, Y.; Herold, F.; Ouwehand, K.; Paas, F. The synergy of embodied cognition and cognitive load theory for optimized learning. Nat. Hum. Behav. 2025, 9, 877–885. [Google Scholar] [CrossRef]
- Holley, S.; Miller, M. Effects of cognitive loading on pilots and air traffic controller performance: Implications for neural dynamics and cognitive flow. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2022, 66, 2256–2260. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, T.; Jiang, Y.; Tan, C.; Hu, L.; Xiong, D.; Ding, Y.; Huang, G.; Qin, J.; Tian, Y. Working-memory load decoding model inspired by brain cognition based on cross-frequency coupling. Brain Res. Bull. 2025, 221, 111206. [Google Scholar] [CrossRef]
- Suryani, M.; Santoso, H.B.; Schrepp, M.; Aji, R.F.; Hadi, S.; Sensuse, D.I.; Suryono, R.R. Role, methodology, and measurement of cognitive load in computer science and information systems research. IEEE Access 2024, 12, 190007–190024. [Google Scholar] [CrossRef]
- Mo, C.Y.; Wang, C.; Dai, J.; Jin, P. Video playback speed influence on learning effect from the perspective of personalized adaptive learning: A study based on cognitive load theory. Front. Psychol. 2022, 13, 947209. [Google Scholar] [CrossRef]
- Suryani, M.; Sensuse, D.I.; Santoso, H.B.; Aji, R.F.; Hadi, S.; Suryono, R.R.; Kautsarina. An initial user model design for adaptive interface development in learning management system based on cognitive load. Cogn. Technol. Work 2024, 26, 653–672. [Google Scholar] [CrossRef]
- Wang, T.; Lajoie, S.P. How does cognitive load interact with self-regulated learning? A dynamic and integrative model. Educ. Psychol. Rev. 2023, 35, 69. [Google Scholar] [CrossRef]
- Castro-Alonso, J.C.; de Koning, B.B.; Fiorella, L.; Paas, F. Five strategies for optimizing instructional materials: Instructor- and learner-managed cognitive load. Educ. Psychol. Rev. 2021, 33, 1379–1407. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E. Leveraging AI in e-learning: Personalized learning and adaptive assessment through cognitive neuropsychology—A systematic analysis. Electronics 2024, 13, 3762. [Google Scholar] [CrossRef]
- Faulconer, E.K.; Bolch, C.; Wood, B. Cognitive load in asynchronous discussions of an online undergraduate STEM course. J. Res. Innov. Teach. Learn. 2023, 16, 268–280. [Google Scholar] [CrossRef]
- Wang, F.; Huang, J.; Zheng, X.L.; Wu, J.Q.; Zhao, A.P. STEM activities for boosting pupils’ computational thinking and reducing their cognitive load: Roles of argumentation scaffolding and mental rotation. J. Res. Technol. Educ. 2024, 1–20. [Google Scholar] [CrossRef]
- Rathnayaka, C.M.; Ganapathi, J.; Kickbusch, S.; Dawes, L.; Brown, R. Preparative pre-laboratory online resources for effectively managing cognitive load of engineering students. Eur. J. Eng. Educ. 2024, 49, 113–138. [Google Scholar] [CrossRef]
- Sari, R.C.; Pranesti, A.; Solikhatun, I.; Nurbaiti, N.; Yuniarti, N. Cognitive overload in immersive virtual reality in education: More presence but less learnt? Educ. Inf. Technol. 2024, 29, 12887–12909. [Google Scholar] [CrossRef]
- Goldberg, H. Growing brains, nurturing minds—Neuroscience as an educational tool to support students’ development as life-long learners. Brain Sci. 2022, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Tokuhama-Espinosa, T. Bringing the Neuroscience of Learning to Online Teaching: An Educator’s Handbook; Teachers College Press: New York, NY, USA, 2021. [Google Scholar]
- Zhumabayeva, Z.; Bazarbekova, R.; Nurzhanova, S.; Stambekova, A.; Kalbergenova, S.B. Development of neuro-didactic content aimed at developing the intelligence of younger schoolchildren. Front. Educ. 2025, 10, 1584490. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key factors in neurological and neurodegenerative disorders of the central nervous system. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Gkintoni, E.; Skokou, M.; Gourzis, P. Integrating Clinical Neuropsychology and Psychotic Spectrum Disorders: A Systematic Analysis of Cognitive Dynamics, Interventions, and Underlying Mechanisms. Medicina 2024, 60, 645. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, Y.; Wang, L. Neurotransmitters in prevention and treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 3841. [Google Scholar] [CrossRef]
- Davis, S.E.; Cirincione, A.B.; Jimenez-Torres, A.C.; Zhu, J. The impact of neurotransmitters on the neurobiology of neurodegenerative diseases. Int. J. Mol. Sci. 2023, 24, 15340. [Google Scholar] [CrossRef]
- Sisakhti, M.; Sachdev, P.S.; Batouli, S.A.H. The effect of cognitive load on the retrieval of long-term memory: An fMRI study. Front. Hum. Neurosci. 2021, 15, 700146. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Pinal, D.; Díaz, F.; Zurrón, M. Working memory load modulates oscillatory activity and the distribution of fast frequencies across frontal theta phase during working memory maintenance. Neurobiol. Learn. Mem. 2021, 183, 107476. [Google Scholar] [CrossRef]
- Lasogga, L.; Gramegna, C.; Müller, D.; Habel, U.; Mehler, D.M.A.; Gur, R.C.; Weidler, C. Meta-analysis of variance in tDCS effects on response inhibition. Sci. Rep. 2024, 14, 19197. [Google Scholar] [CrossRef]
- Shine, J.M.; Lewis, L.D.; Garrett, D.D.; Hwang, K. The impact of the human thalamus on brain-wide information processing. Nat. Rev. Neurosci. 2023, 24, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Harneit, A.; Pergola, G.; Menara, T.; Schäfer, A.; Betzel, R.F.; Zang, Z.; Schweiger, J.I.; Zhang, X.; Schwarz, K.; et al. Brain network dynamics during working memory are modulated by dopamine and diminished in schizophrenia. Nat. Commun. 2021, 12, 3478. [Google Scholar] [CrossRef] [PubMed]
- Ranchod, S.; Rakobowchuk, M.; Gonzalez, C. Distinct age-related brain activity patterns in the prefrontal cortex when increasing cognitive load: A functional near-infrared spectroscopy study. PLoS ONE 2023, 18, e0293394. [Google Scholar] [CrossRef] [PubMed]
- Webler, R.D.; Fox, J.; McTeague, L.M.; Burton, P.C.; Dowdle, L.; Short, E.B.; Borckardt, J.J.; Li, X.; George, M.S.; Nahas, Z. DLPFC stimulation alters working memory related activations and performance: An interleaved TMS-fMRI study. Brain Stimul. 2022, 15, 823–832. [Google Scholar] [CrossRef]
- Figeys, M.; Loucks, T.M.; Leung, A.W.S.; Kim, E.S. Direct current stimulation over the right dorsolateral prefrontal cortex increases oxyhemoglobin concentration and cognitive performance dependent on cognitive load. Behav. Brain Res. 2023, 443, 114343. [Google Scholar] [CrossRef]
- Le Cunff, A.L.; Dommett, E.; Giampietro, V. Neurophysiological measures and correlates of cognitive load in attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD) and dyslexia: A scoping review and research recommendations. Eur. J. Neurosci. 2024, 59, 256–282. [Google Scholar] [CrossRef]
- Smucny, J.; Dienel, S.J.; Lewis, D.A.; Carter, C.S. Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology 2022, 47, 292–308. [Google Scholar] [CrossRef]
- Bandeira, I.D.; Lins-Silva, D.H.; Barouh, J.L.; Faria-Guimarães, D.; Dorea-Bandeira, I.; Souza, L.S.; Alves, G.S.; Brunoni, A.R.; Nitsche, M.; Fregni, F.; et al. Neuroplasticity and non-invasive brain stimulation in the developing brain. Prog. Brain Res. 2021, 264, 57–89. [Google Scholar] [CrossRef]
- Reddy, K.J. Neuroplasticity and technology. In Innovations in Neurocognitive Rehabilitation: Harnessing Technology for Effective Therapy; Springer Nature: Cham, Switzerland, 2025; pp. 137–169. [Google Scholar] [CrossRef]
- Antal, A.; Luber, B.; Brem, A.-K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljević, V.; Fecteau, S.; Ferreri, F.; Flöel, A.; et al. Non-invasive brain stimulation and neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H. Advances in Neuroimaging and Deep Learning for Emotion Detection: A Systematic Review of Cognitive Neuroscience and Algorithmic Innovations. Diagnostics 2025, 15, 456. [Google Scholar] [CrossRef]
- Chen, R.; Huang, L.; Wang, R.; Fei, J.; Wang, H.; Wang, J. Advances in non-invasive neuromodulation techniques for improving cognitive function: A review. Brain Sci. 2024, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, S.; Liu, Z.; Peng, X.; Yang, Z. Automated detection of emotional and cognitive engagement in MOOC discussions to predict learning achievement. Comput. Educ. 2022, 190, 104461. [Google Scholar] [CrossRef]
- Dai, C.P.; Ke, F. Educational applications of artificial intelligence in simulation-based learning: A systematic mapping review. Comput. Educ. Artif. Intell. 2022, 3, 100087. [Google Scholar] [CrossRef]
- Dubovi, I. Cognitive and emotional engagement while learning with VR: The perspective of multimodal methodology. Comput. Educ. 2022, 188, 104495. [Google Scholar] [CrossRef]
- Molenaar, I.; de Mooij, S.; Azevedo, R.; Bannert, M.; Järvelä, S.; Gašević, D. Measuring self-regulated learning and the role of AI: Five years of research using multimodal multichannel data. Comput. Hum. Behav. 2023, 139, 107540. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vantaraki, F.; Skoulidi, C.; Anastassopoulos, P.; Vantarakis, A. Promoting Physical and Mental Health among Children and Adolescents via Gamification—A Conceptual Systematic Review. Behav. Sci. 2024, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Im, K.; Yoo, M.; Roll, I.; Seo, K. Supporting students’ self-regulated learning in online learning using artificial intelligence applications. Int. J. Educ. Technol. High. Educ. 2023, 20, 37. [Google Scholar] [CrossRef]
- Rackerby, R.; Lukosch, S.; Munro, D. Understanding and measuring the cognitive load of amputees for rehabilitation and prosthesis development. Arch. Rehabil. Res. Clin. Transl. 2022, 4, 100216. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Zhu, W.; Lu, Z.; Wang, Y.; Zhang, Y. Study on the diversity of mental states and neuroplasticity of the brain during human-machine interaction. Front. Neurosci. 2022, 16, 921058. [Google Scholar] [CrossRef]
- Wu, J.; Qiu, P.; Lv, S.; Chen, M.; Li, Y. The effects of cognitive-motor dual-task training on athletes’ cognition and motor performance. Front. Psychol. 2024, 15, 1284787. [Google Scholar] [CrossRef]
- Jayathilake, N.J.; Phan, T.T.; Kim, J.; Lee, K.P.; Park, J.M. Modulating neuroplasticity for chronic pain relief: Noninvasive neuromodulation as a promising approach. Exp. Mol. Med. 2025, 57, 501–514. [Google Scholar] [CrossRef]
- Liang, L.B.; Wang, S.; Li, K.P.; Wu, C.Q. Comparative efficacy of cognitive training modalities in cognitive impairment: A systematic review and network meta-analysis. J. Prev. Alzheimer’s Dis. 2025, 12, 100207. [Google Scholar] [CrossRef] [PubMed]
- Sweller, J. Cognitive load theory and individual differences. Learn. Individ. Differ. 2024, 106, 102423. [Google Scholar] [CrossRef]
- Katmah, R.; Awad, M.I.; Al-Ani, F.; Khan, M.; Alskafi, F.A.; Hussain, I.; Jelinek, H.F.; Khalaf, K. Adaptation Robotics and Cognitive Load: Use of a Supernumerary Finger for Activities of Daily Living. IEEE Trans. Neural Syst. Rehabil. Eng. 2025, 33, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Vassoler, F.M.; Budge, K.E.; Isgate, S.B.; Gildawie, K.R.; Byrnes, E.M. Neuroplasticity-related genes correlate with individual differences in distinct phases of oxycodone self-administration in male rats. Neuropharmacology 2024, 254, 109972. [Google Scholar] [CrossRef]
- Qiao, Y.; Gao, X.; Liao, W.; Chen, D. Assessment of dynamic cognitive performance under different thermal conditions by multiple physiological signals. J. Build. Eng. 2024, 91, 109325. [Google Scholar] [CrossRef]
- Janssen, T.W.P.; Grammer, J.K.; Bleichner, M.G.; Bulgarelli, C.; Davidesco, I.; Dikker, S.; Jasińska, K.K.; Siugzdaite, R.; Vassena, E.; Vatakis, A.; et al. Opportunities and Limitations of Mobile Neuroimaging Technologies in Educational Neuroscience. Mind Brain Educ. 2021, 15, 354–370. [Google Scholar] [CrossRef]
- Sortwell, A.; Evgenia, G.; Zagarella, S.; Granacher, U.; Forte, P.; Ferraz, R.; Ramirez-Campillo, R.; Carter-Thuillier, B.; Konukman, F.; Nouri, A.; et al. Making neuroscience a priority in Initial Teacher Education curricula: A call for bridging the gap between research and future practices in the classroom. Neurosci. Res. Notes 2023, 6, 266.1–266.7. [Google Scholar] [CrossRef]
- Kellmeyer, P. Big brain data: On the responsible use of brain data from clinical and consumer-directed neurotechnological devices. Neuroethics 2021, 14, 329–340. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vantaraki, F.; Skoulidi, C.; Anastassopoulos, P.; Vantarakis, A. Gamified Health Promotion in Schools: The Integration of Neuropsychological Aspects and CBT—A Systematic Review. Medicina 2024, 60, 2085. [Google Scholar] [CrossRef]
- Klimova, B.; Pikhart, M.; Benites, A.D.; Lehr, C.; Sanchez-Stockhammer, C. Neural machine translation in foreign language teaching and learning: A systematic review. Educ. Inf. Technol. 2023, 28, 663–682. [Google Scholar] [CrossRef]
- Gkintoni, E.; Michou, E. Advancing Neuropsychological Rehabilitation in Primary Progressive Aphasia Based on Principles of Cognitive Neuroscience: A Scoping Review and Systematic Analysis of the Data. Brain Sci. 2024, 14, 1234. [Google Scholar] [CrossRef]
- Drigas, A.; Sideraki, A. Brain neuroplasticity leveraging virtual reality and brain-computer interface technologies. Sensors 2024, 24, 5725. [Google Scholar] [CrossRef]
- Heck, N.; Santos, M.D. Dendritic spines in learning and memory: From first discoveries to current insights. In Dendritic Spines: Structure; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Raj, P.; Rathipriya, A.G.; Qoronfleh, M.W.; Essa, M.M.; Chidambaram, S.B. Impact of pharmacological and non-pharmacological modulators on dendritic spines structure and functions in brain. Cells 2021, 10, 3405. [Google Scholar] [CrossRef]
- Qiao, Q.; Wu, C.; Ma, L.; Zhang, H.; Li, M.; Wu, X.; Gan, W.B. Motor learning-induced new dendritic spines are preferentially involved in the learned task than existing spines. Cell Rep. 2022, 39, 111229. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, J.; Urban, P. Information encoded in volumes and areas of dendritic spines is nearly maximal across mammalian brains. Sci. Rep. 2023, 13, 49321. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Spani, F.; Carducci, F.; Piervincenzi, C.; Ben-Soussan, T.D.; Mallio, C.A.; Quattrocchi, C.C. Assessing brain neuroplasticity: Surface morphometric analysis of cortical changes induced by Quadrato motor training. J. Anat. 2025, 246, 757–769. [Google Scholar] [CrossRef]
- Marzola, P.; Melzer, T.; Pavesi, E.; Gil-Mohapel, J.; Brocardo, P.S. Exploring the role of neuroplasticity in development, aging, and neurodegeneration. Brain Sci. 2023, 13, 1610. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Crestani, A.P. The times they are a-changin’: A proposal on how brain flexibility goes beyond the obvious to include the concepts of “upward” and “downward” to neuroplasticity. Mol. Psychiatry 2022, 28, 977–992. [Google Scholar] [CrossRef]
- Olszewska, A.M.; Gaca, M.; Herman, A.M.; Jednoróg, K.; Marchewka, A. How musical training shapes the adult brain: Predispositions and neuroplasticity. Front. Neurosci. 2021, 15, 630829. [Google Scholar] [CrossRef]
- Jog, M.A.; Anderson, C.; Kubicki, A.; Boucher, M.; Leaver, A.; Hellemann, G.; Iacoboni, M.; Woods, R.; Narr, K. Transcranial direct current stimulation (tDCS) in depression induces structural plasticity. Sci. Rep. 2023, 13, 2841. [Google Scholar] [CrossRef]
- Reed, M.B.; Klöbl, M.; Godbersen, G.M.; Handschuh, P.A.; Ritter, V.; Spurny-Dworak, B.; Unterholzner, J.; Kraus, C.; Gryglewski, G.; Winkler, D.; et al. Serotonergic modulation of effective connectivity in an associative relearning network during task and rest. NeuroImage 2022, 249, 118887. [Google Scholar] [CrossRef]
- Barbati, S.A.; Podda, M.V.; Grassi, C. Tuning brain networks: The emerging role of transcranial direct current stimulation on structural plasticity. Front. Cell. Neurosci. 2022, 16, 945777. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, D.; Li, Q.; Chu, L.; Dong, X.; Liang, X.; Sun, L.; Liao, X.; Zhao, T.; Chen, X.; et al. The multiscale brain structural re-organization that occurs from childhood to adolescence correlates with cortical morphology maturation and functional specialization. PLoS Biol. 2025, 23, e3002710. [Google Scholar] [CrossRef]
- Tilton-Bolowsky, V.; Stockbridge, M.D.; Hillis, A.E. Remapping and reconnecting the language network after stroke. Brain Sci. 2024, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Kalyuga, S. Evolutionary perspective on human cognitive architecture in cognitive load theory: A dynamic, emerging principle approach. Educ. Psychol. Rev. 2023, 35, 91. [Google Scholar] [CrossRef]
- Sweller, J. The role of evolutionary psychology in our understanding of human cognition: Consequences for cognitive load theory and instructional procedures. Educ. Psychol. Rev. 2021, 34, 2229–2241. [Google Scholar] [CrossRef]
- Evans, P.; Vansteenkiste, M.; Parker, P.; Kingsford-Smith, A.; Zhou, S. Cognitive load theory and its relationships with motivation: A self-determination theory perspective. Educ. Psychol. Rev. 2024, 36, 7. [Google Scholar] [CrossRef]
- Ashtiani, S.N.M.; Daliri, M.R. Identification of cognitive load-dependent activation patterns using working memory task-based fMRI at various levels of difficulty. Sci. Rep. 2023, 13, 16476. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Dimakos, I.; Nikolaou, G. Cognitive Insights from Emotional Intelligence: A Systematic Review of EI Models in Educational Achievement. Emerg. Sci. J. 2025, 8, 262–297. [Google Scholar] [CrossRef]
- Cai, W.; Ryali, S.; Pasumarthy, R.; Talasila, V.; Menon, V. Dynamic causal brain circuits during working memory and their functional controllability. Nat. Commun. 2021, 12, 3314. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, M.M.; Matteucci Armandi Avogli Trotti, N.; Pavan, A.; Piccardi, L.; Nori, R. The Dual Process model: The effect of cognitive load on the ascription of intentionality. Front. Psychol. 2025, 16, 1451590. [Google Scholar] [CrossRef]
- Sieg, L.; Eismann, H.; Schneider, B.; Karsten, J.; Darici, D. How distractions undermine attentional synchronisation in work-based learning: A randomised controlled study using dual mobile eye-tracking. J. Comput. Assist. Learn. 2025, 41, e70059. [Google Scholar] [CrossRef]
- Greenberg, K.; Zheng, R. Revisiting the debate on germane cognitive load versus germane resources. J. Cogn. Psychol. 2022, 35, 295–314. [Google Scholar] [CrossRef]
- Broadbent, D.P.; D’Innocenzo, G.; Ellmers, T.J.; Parsler, J.; Szameitat, A.J.; Bishop, D.T. Cognitive load, working memory capacity and driving performance: A preliminary fNIRS and eye tracking study. Transp. Res. Part F Traffic Psychol. Behav. 2023, 92, 121–132. [Google Scholar] [CrossRef]
- Andreassen, O.A.; Hindley, G.F.; Frei, O.; Smeland, O.B. New insights from the last decade of research in psychiatric genetics: Discoveries, challenges and clinical implications. World Psychiatry 2023, 22, 4–24. [Google Scholar] [CrossRef]
- Li, P.; Lan, Y.J. Digital language learning (DLL): Insights from behavior, cognition, and the brain. Biling. Lang. Cogn. 2021, 25, 361–378. [Google Scholar] [CrossRef]
- Wang, G.; Tian, L.; Liu, J.; Nie, S.; Yu, S. Neural mechanisms of cognitive load in multimedia learning: A meta-analysis of EEG frequency band modulation. Curr. Psychol. 2024, 43, 29316–29332. [Google Scholar] [CrossRef]
- Lyu, C.; Deng, S. Effectiveness of embodied learning on learning performance: A meta-analysis based on the cognitive load theory perspective. Learn. Individ. Differ. 2024, 116, 102564. [Google Scholar] [CrossRef]
- Nim, C.; Aspinall, S.L.; Cook, C.E.; Corrêa, L.A.; Donaldson, M.; Downie, A.S.; Harsted, S.; Hansen, S.; Jenkins, H.J.; McNaughton, D.; et al. The effectiveness of spinal manipulative therapy in treating spinal pain does not depend on the application procedures: A systematic review and network meta-analysis. J. Orthop. Sports Phys. Ther. 2025, 55, 109–122. [Google Scholar] [CrossRef]
- Kora, P.; Meenakshi, K.; Swaraja, K.; Rajani, A.; Raju, M.S. EEG based interpretation of human brain activity during yoga and meditation using machine learning: A systematic review. Complement. Ther. Clin. Pract. 2021, 43, 101329. [Google Scholar] [CrossRef] [PubMed]
- Nord, C.L.; Garfinkel, S.N. Interoceptive pathways to understand and treat mental health conditions. Trends Cogn. Sci. 2022, 26, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H.; Halkiopoulos, C. From neural networks to emotional networks: A systematic review of EEG-based emotion recognition in cognitive neuroscience and real-world applications. Brain Sci. 2025, 15, 220. [Google Scholar] [CrossRef]
- Deery, H.A.; Di Paolo, R.; Moran, C.; Egan, G.F.; Jamadar, S.D. The older adult brain is less modular, more integrated, and less efficient at rest: A systematic review of large-scale resting-state functional brain networks in aging. Psychophysiology 2023, 60, e14159. [Google Scholar] [CrossRef]
- Kay, K.; Bonnen, K.; Denison, R.N.; Arcaro, M.J.; Barack, D.L. Tasks and their role in visual neuroscience. Neuron 2023, 111, 1697–1713. [Google Scholar] [CrossRef]
- Pei, L.; Northoff, G.; Ouyang, G. Comparative analysis of multifaceted neural effects associated with varying endogenous cognitive load. Commun. Biol. 2023, 6, 795. [Google Scholar] [CrossRef]
- Pei, L.; Zhou, X.; Leung, F.K.S.; Ouyang, G. Differential associations between scale-free neural dynamics and different levels of cognitive ability. Psychophysiology 2023, 60, e14259. [Google Scholar] [CrossRef]
- Lu, R. Linking the multiple-demand cognitive control system to human electrophysiological activity. Neuropsychologia 2025, 210, 109096. [Google Scholar] [CrossRef]
- Habibollahi, F.; Kagan, B.J.; Burkitt, A.N.; French, C. Critical dynamics arise during structured information presentation within embodied in vitro neuronal networks. Nat. Commun. 2023, 14, 5287. [Google Scholar] [CrossRef]
- Khalil, M.H. Green environments for sustainable brains: Parameters shaping adaptive neuroplasticity and lifespan neurosustainability—A systematic review and future directions. Int. J. Environ. Res. Public Health 2025, 22, 690. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; King, L.S. Mechanisms of neuroplasticity linking early adversity to depression: Developmental considerations. Transl. Psychiatry 2021, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Leisman, G. On the application of developmental cognitive neuroscience in educational environments. Brain Sci. 2022, 12, 1501. [Google Scholar] [CrossRef]
- Martins, L.A.; Schiavo, A.; Paz, L.V.; Xavier, L.L.; Mestriner, R.G. Neural underpinnings of fine motor skills under stress and anxiety: A review. Physiol. Behavior. 2024, 282, 114593. [Google Scholar] [CrossRef]
- Makanadar, A. Neuro-adaptive architecture: Buildings and city design that respond to human emotions, cognitive states. Res. Glob. 2024, 8, 100222. [Google Scholar] [CrossRef]
- Osei, P.C.; Bjorklund, D.F. Motivating the learning process: Integrating self-determination theory into a dynamical systems framework. Educ. Psychol. Rev. 2024, 36, 89. [Google Scholar] [CrossRef]
- Corriveau-Lecavalier, N.; Rajah, M.N.; Mellah, S.; Belleville, S. Latent patterns of task-related functional connectivity in relation to regions of hyperactivation in individuals at risk of Alzheimer’s disease. NeuroImage Clin. 2021, 30, 102643. [Google Scholar] [CrossRef]
- Carrasco-Gómez, M.; García-Colomo, A.; Cabrera-Álvarez, J.; del Cerro-León, A.; Gómez-Ariza, C.J.; Santos, A.; Maestú, F. Individual alpha frequency tACS reduces static functional connectivity across the default mode network. Front. Hum. Neurosci. 2025, 19, 1534321. [Google Scholar] [CrossRef]
- Ruppert, M.C.; Greuel, A.; Freigang, J.; Tahmasian, M.; Maier, F.; Hammes, J.; van Eimeren, T.; Timmermann, L.; Tittgemeyer, M.; Drzezga, A.; et al. The default mode network and cognition in Parkinson’s disease: A multimodal resting-state network approach. Hum. Brain Mapp. 2021, 42, 2623–2641. [Google Scholar] [CrossRef]
- Pitsik, E. Recurrence quantification analysis and theta-band functional networks detect age-related changes in brain sensorimotor system: VR-based approach. Eur. Phys. J. Spec. Top. 2025, 1–7. [Google Scholar] [CrossRef]
- Draheim, C.; Pak, R.; Draheim, A.A.; Engle, R.W. The role of attention control in complex real-world tasks. Psychon. Bull. Rev. 2022, 29, 1143–1197. [Google Scholar] [CrossRef]
- Draschkow, D.; Kallmayer, M.; Nobre, A.C. When natural behavior engages working memory. Curr. Biol. 2021, 31, 869–874.e5. [Google Scholar] [CrossRef]
- Yang, S.; Gao, T.; Wang, J.; Deng, B.; Azghadi, M.R.; Lei, T.; Linares-Barranco, B. SAM: A unified self-adaptive multicompartmental spiking neuron model for learning with working memory. Front. Neurosci. 2022, 16, 850945. [Google Scholar] [CrossRef] [PubMed]
- Chikhi, S.; Matton, N.; Blanchet, S. EEG power spectral measures of cognitive workload: A meta-analysis. Psychophysiology 2022, 59, e14009. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Zhang, L. Study of EEG characteristics while solving scientific problems with different mental effort. Sci. Rep. 2021, 11, 23783. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E. The Role of Machine Learning in AR/VR-Based Cognitive Therapies: A Systematic Review for Mental Health Disorders. Electronics 2025, 14, 1110. [Google Scholar] [CrossRef]
- Taheri Gorji, H.; Wilson, N.; VanBree, J.; Hoffmann, B.; Petros, T.; Tavakolian, K. Using machine learning methods and EEG to discriminate aircraft pilot cognitive workload during flight. Sci. Rep. 2023, 13, 2507. [Google Scholar] [CrossRef]
- Antonenko, D.; Fromm, A.E.; Thams, F.; Kuzmina, A.; Backhaus, M.; Knochenhauer, E.; Li, S.-C.; Grittner, U.; Flöel, A. Cognitive training and brain stimulation in patients with cognitive impairment: A randomized controlled trial. Alzheimer’s Res. Ther. 2024, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Ríos, A.S.; Oxenford, S.; Neudorfer, C.; Butenko, K.; Li, N.; Rajamani, N.; Boutet, A.; Elias, G.J.B.; Germann, J.; Loh, A.; et al. Optimal deep brain stimulation sites and networks for stimulation of the fornix in Alzheimer’s disease. Nat. Commun. 2022, 13, 7707. [Google Scholar] [CrossRef] [PubMed]
- von Bastian, C.C.; Belleville, S.; Udale, R.C.; Reinhartz, A.; Essounni, M.; Strobach, T. Mechanisms underlying training-induced cognitive change. Nat. Rev. Psychol. 2022, 1, 30–41. [Google Scholar] [CrossRef]
- Vinogradov, S.; Chafee, M.V.; Lee, E.; Morishita, H. Psychosis spectrum illnesses as disorders of prefrontal critical period plasticity. Neuropsychopharmacology 2023, 48, 168–185. [Google Scholar] [CrossRef]
- Meccia, J.; Lopez, J.; Bagot, R.C. Probing the antidepressant potential of psilocybin: Integrating insight from human research and animal models towards an understanding of neural circuit mechanisms. Psychopharmacology 2022, 240, 27–40. [Google Scholar] [CrossRef]
- Sengupta, A.; Banerjee, S.; Ganesh, S.; Grover, S.; Sridharan, D. The right posterior parietal cortex mediates spatial reorienting of attentional choice bias. Nat. Commun. 2024, 15, 6938. [Google Scholar] [CrossRef]
- Hou, W.; Zhou, F.; Wang, Q.; Li, H.; Qin, X.; Ding, Y.; Dong, F.; Bo, Q.; Li, A.; Zhang, L.; et al. Effect of transcranial direct current stimulation with concurrent cognitive performance targeting posterior parietal cortex vs prefrontal cortex on working memory in schizophrenia: A randomized clinical trial. Transl. Psychiatry 2024, 14, 279. [Google Scholar] [CrossRef]
- Martin, S.; Frieling, R.; Saur, D.; Hartwigsen, G. TMS over the pre-SMA enhances semantic cognition via remote network effects on task-based activity and connectivity. Brain Stimul. 2023, 16, 1346–1357. [Google Scholar] [CrossRef]
- Galoyan, T.; Betts, K.; Abramian, H.; Reddy, P.; Izzetoglu, K.; Shewokis, P.A. Examining mental workload in a spatial navigation transfer game via functional near infrared spectroscopy. Brain Sci. 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- da Silva Soares, R., Jr.; Ambriola Oku, A.Y.; Barreto, C.S.; Ricardo Sato, J. Applying functional near-infrared spectroscopy and eye-tracking in a naturalistic educational environment to investigate physiological aspects that underlie the cognitive effort of children during mental rotation tests. Front. Hum. Neurosci. 2022, 16, 889806. [Google Scholar] [CrossRef] [PubMed]
- Khanam, F.; Hossain, A.B.M.A.; Ahmad, M. Statistical valuation of cognitive load level hemodynamics from functional near-infrared spectroscopy signals. Neurosci. Inform. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.; Cao, L.; Tan, G. Evolutionary multitasking-based multiobjective optimization algorithm for channel selection in hybrid brain computer interfacing systems. Front. Neurosci. 2021, 15, 749232. [Google Scholar] [CrossRef]
- Beltrán, E.T.M.; Pérez, M.Q.; Bernal, S.L.; Pérez, G.M.; Celdrán, A.H. SAFECAR: A brain-computer interface and intelligent framework to detect drivers’ distractions. Expert. Syst. Appl. 2022, 203, 117402. [Google Scholar] [CrossRef]
- Yasuhara, M.; Nambu, I. Error-related potentials during multitasking involving sensorimotor control: An ERP and offline decoding study for brain-computer interface. Front. Hum. Neurosci. 2025, 19, 1516721. [Google Scholar] [CrossRef]
- Chen, J.; Tam, A.; Kebets, V.; Orban, C.; Ooi, L.Q.R.; Asplund, C.L.; Marek, S.; Dosenbach, N.U.F.; Eickhoff, S.B.; Bzdok, D.; et al. Shared and unique brain network features predict cognitive, personality, and mental health scores in the ABCD study. Nat. Commun. 2022, 13, 2217. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Pykett, J.; Kotouza, D. Learning brains: Educational neuroscience, neurotechnology and neuropedagogy. Pedagog. Cult. Soc. 2025, 1–21. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- van den Akker, O.R.; Peters, G.-J.Y.; Bakker, C.J.; Carlsson, R.; Coles, N.A.; Corker, K.S.; Feldman, G.; Moreau, D.; Nordström, T.; Pickering, J.S.; et al. Increasing the transparency of systematic reviews: Presenting a generalized registration form. Syst. Rev. 2023, 12, 170. [Google Scholar] [CrossRef]
- Adcock, R.; Dale, C.; Fisher, M.; Aldebot, S.; Genevsky, A.; Simpson, G.V.; Nagarajan, S.; Vinogradov, S. When top-down meets bottom-up: Auditory training enhances verbal memory in schizophrenia. Schizophr. Bull. 2009, 35, 1132–1141. [Google Scholar] [CrossRef]
- Afify, M. Effect of Interactive Video Length Within E-learning Environments on Cognitive Load, Cognitive Achievement and Retention of Learning. Turk. Online J. Distance Educ. 2020, 21, 68–89. [Google Scholar] [CrossRef]
- Andrade, S.M.; Fernández-Calvo, B.; Boggio, P.S.; de Oliveira, E.A.; Gomes, L.F.; Júnior, J.E.G.P.; Rodrigues, R.M.; de Almeida, N.L.; Moreira, G.M.d.S.; Alves, N.T. Neurostimulation for cognitive rehabilitation in stroke (NeuroCog): Study protocol for a randomized controlled trial. Trials 2015, 16, 435. [Google Scholar] [CrossRef]
- Anguera, J.A.; Boccanfuso, J.; Rintoul, J.L.; Al-Hashimi, O.; Faraji, F.; Janowich, J.; Kong, E.; Larraburo, Y.; Rolle, C.E.; Johnston, E.R.; et al. Video game training enhances cognitive control in older adults. Nature 2013, 501, 97–101. [Google Scholar] [CrossRef]
- Antonenko, D.; Fromm, A.; Thams, F.; Grittner, U.; Meinzer, M.; Flöel, A. Microstructural and functional plasticity following repeated brain stimulation during cognitive training in older adults. Nat. Commun. 2023, 14, 3184. [Google Scholar] [CrossRef]
- Assecondi, S.; Shapiro, K. The benefits of combined brain stimulation and cognitive training: A pilot study. J. Vis. 2018, 18, 119. [Google Scholar] [CrossRef]
- Au, J.; Katz, B.; Buschkuehl, M.; Bunarjo, K.; Senger, T.; Zabel, C.; Jaeggi, S.M.; Jonides, J. Enhancing Working Memory Training with Transcranial Direct Current Stimulation. J. Cogn. Neurosci. 2016, 28, 1419–1432. [Google Scholar] [CrossRef]
- Bentham, C.; Marco, M.D.; Venneri, A. The Modulatory Effect of Cerebrovascular Burden in Response to Cognitive Stimulation in Healthy Ageing and Mild Cognitive Impairment. Neural Plast. 2019, 2019, 2305318. [Google Scholar] [CrossRef]
- Brehmer, Y.; Rieckmann, A.; Bellander, M.; Westerberg, H.; Fischer, H.; Bäckman, L. Neural correlates of training-related working-memory gains in old age. NeuroImage 2011, 58, 1110–1120. [Google Scholar] [CrossRef]
- Bubbico, G.; Chiacchiaretta, P.; Parenti, M.; di Marco, M.; Panara, V.; Sepede, G.; Ferretti, A.; Perrucci, M.G. Effects of Second Language Learning on the Plastic Aging Brain: Functional Connectivity, Cognitive Decline, and Reorganization. Front. Neurosci. 2019, 13, 423. [Google Scholar] [CrossRef]
- Chen, Q.; Baran, T.; Turnbull, A.; Zhang, Z.; Rebok, G.; Lin, F. Increased segregation of structural brain networks underpins enhanced broad cognitive abilities of cognitive training. Hum. Brain Mapp. 2021, 42, 3202–3215. [Google Scholar] [CrossRef]
- Cohen Kadosh, R. T007 Neural predictors of the effect of transcranial electrical stimulation on learning. Clin. Neurophysiol. 2017, 128, e3. [Google Scholar] [CrossRef]
- Erickson, K.; Colcombe, S.; Wadhwa, R.; Bherer, L.; Peterson, M.; Scalf, P.E.; Kim, J.S.; Alvarado, M.; Kramer, A. Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Aging 2007, 28, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.; Dankbaar, M.; Van den Broek, W.W.; Bruinink, L.J.; Hogeveen, M.; van Merriënboer, J.J.G. Effects of adaptive scaffolding on performance, cognitive load and engagement in game-based learning: A randomized controlled trial. BMC Med. Educ. 2024, 24, 943. [Google Scholar] [CrossRef]
- Falcone, B.; Wada, A.; Parasuraman, R.; Callan, D. Individual differences in learning correlate with modulation of brain activity induced by transcranial direct current stimulation. PLoS ONE 2018, 13, e0197192. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Gao, Y.; Li, Y.; Li, C.; Zhang, T.; Wu, L.; Zhu, Y.; Xie, Y. Effects of computerized working memory training on neuroplasticity in healthy individuals: A combined neuroimaging and neurotransmitter study. NeuroImage 2024, 298, 120785. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Z.; Kovacevic, N.; Mišić, B.; Mcintosh, A. Dynamic functional connectivity shapes individual differences in associative learning. Hum. Brain Mapp. 2016, 37, 3911–3928. [Google Scholar] [CrossRef]
- Fehring, D.J.; Illipparampil, R.; Acevedo, N.; Jaberzadeh, S.; Fitzgerald, P.; Mansouri, F. Interaction of task-related learning and transcranial direct current stimulation of the prefrontal cortex in modulating executive functions. Neuropsychologia 2019, 131, 148–159. [Google Scholar] [CrossRef]
- Filippi, M.; Riccitelli, G.; Mattioli, F.; Capra, R.; Stampatori, C.; Pagani, E.; Valsasina, P.; Copetti, M.; Falini, A.; Comi, G.; et al. Multiple Sclerosis: Effects of Cognitive Rehabilitation on Structural and Functional MR Imaging Measures—An Explorative Study. Radiology 2012, 262, 932–940. [Google Scholar] [CrossRef]
- Frank, B.; Harty, S.; Kluge, A.; Cohen Kadosh, R. Learning while multitasking: Short and long-term benefits of brain stimulation. Ergonomics 2018, 61, 1454–1463. [Google Scholar] [CrossRef]
- Fujino, J.; Tei, S.; Itahashi, T.; Aoki, Y.Y.; Ohta, H.; Izuno, T.; Nakamura, H.; Shimizu, M.; Hashimoto, R.-I.; Takahashi, H.; et al. A single session of navigation-guided repetitive transcranial magnetic stimulation over the right anterior temporoparietal junction in autism spectrum disorder. Brain Stimul. 2021, 14, 682–684. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. Training-Induced Improvement of Response Selection and Error Detection in Aging Assessed by Task Switching: Effects of Cognitive, Physical, and Relaxation Training. Front. Hum. Neurosci. 2012, 6, 130. [Google Scholar] [CrossRef]
- Gallen, C.L.; Baniqued, P.L.; Chapman, S.B.; Aslan, S.; Keebler, M.; Didehbani, N.; D’eSposito, M.; Hayasaka, S. Modular Brain Network Organization Predicts Response to Cognitive Training in Older Adults. PLoS ONE 2016, 11, e0169015. [Google Scholar] [CrossRef]
- Gangemi, A.; De Luca, R.; Fabio, R.A.; Lauria, P.; Rifici, C.; Pollicino, P.; Marra, A.; Olivo, A.; Quartarone, A.; Calabrò, R.S. Effects of Virtual Reality Cognitive Training on Neuroplasticity: A Quasi-Randomized Clinical Trial in Patients with Stroke. Biomedicines 2023, 11, 3225. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Fang, Y.; Cron, G.O.; Heo, J.; Jung, E.; Lee, D.; Kim, H.; Kim, E.; Park, J.Y.; Lee, J.H. In patients with late-life depression and cognitive decline, adding tDCS to cognitive training does not significantly affect depressive symptoms but shows potential benefits on cognition as measured by fMRI. Brain Stimul. 2024, 17, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Hadie, S.N.H.; Tan, V.P.S.; Omar, N.; Alwi, N.A.N.M.; Lim, H.L.; Marsilla, K.I.K. COVID-19 Disruptions in Health Professional Education: Use of Cognitive Load Theory on Students’ Comprehension, Cognitive Load, Engagement, and Motivation. Front. Med. 2021, 8, 739238. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.U.; Rütsche, B.; Wurmitzer, K.; Brem, S.; Ruff, C.C.; Grabner, R.H. Neurocognitive Effects of Transcranial Direct Current Stimulation in Arithmetic Learning and Performance: A Simultaneous tDCS-fMRI Study. Brain Stimul. 2016, 9, 850–858. [Google Scholar] [CrossRef]
- Haut, K.M.; Lim, K.O.; MacDonald, A. Prefrontal Cortical Changes Following Cognitive Training in Patients with Chronic Schizophrenia: Effects of Practice, Generalization, and Specificity. Neuropsychopharmacology 2010, 35, 1850–1859. [Google Scholar] [CrossRef]
- Henssen, D.J.; Heuvel, L.v.D.; De Jong, G.; Vorstenbosch, M.A.; Walsum, A.v.C.v.; Hurk, M.M.V.D.; Kooloos, J.G.; Bartels, R.H. Neuroanatomy Learning: Augmented Reality vs. Cross-Sections. Anat. Sci. Educ. 2019, 13, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, S. Maximizing Cognitive Training Outcomes with Simultaneous Brain Stimulation. Innov. Aging 2024, 8, 670. [Google Scholar] [CrossRef]
- Jian, Y.; Abu Bakar, J.A. Comparing cognitive load in learning spatial ability: Immersive learning environment vs. digital learning media. Discov. Sustain. 2024, 5, 111. [Google Scholar] [CrossRef]
- Jones, K.T.; Stephens, J.A.; Alam, M.; Bikson, M.; Berryhill, M.E.; Antal, A. Longitudinal Neurostimulation in Older Adults Improves Working Memory. PLoS ONE 2015, 10, e0121904. [Google Scholar] [CrossRef]
- Kadir, M.S.; Yeung, A.S.; Caleon, I.S.; Diallo, T.M.O.; Forbes, A.; Koh, W.X. The effects of load reduction instruction on educational outcomes: An intervention study on hands-on inquiry-based learning in science. Appl. Cogn. Psychol. 2023, 37, 814–829. [Google Scholar] [CrossRef]
- Kelly, C.E.; Thompson, D.K.; Chen, J.; Josev, E.K.; Pascoe, L.; Spencer-Smith, M.M.; Adamson, C.; Nosarti, C.; Gathercole, S.; Roberts, G.; et al. Working memory training and brain structure and function in extremely preterm or extremely low birth weight children. Hum. Brain Mapp. 2019, 41, 684–696. [Google Scholar] [CrossRef]
- Khalil, R.; Godde, B.; Karim, A. P093 Transcranial cortex stimulation, neuroplasticity and learning: An experimental and computational study. Clin. Neurophysiol. 2017, 128, e53–e54. [Google Scholar] [CrossRef]
- Kober, S.E.; Witte, M.; Stangl, M.; Väljamäe, A.; Neuper, C.; Wood, G. Shutting down sensorimotor interference unblocks the networks for stimulus processing: An SMR neurofeedback training study. Clin. Neurophysiol. 2015, 126, 82–95. [Google Scholar] [CrossRef]
- Küçük, S.; Kapakin, S.; Göktaş, Y. Learning anatomy via mobile augmented reality: Effects on achievement and cognitive load. Anat. Sci. Educ. 2016, 9, 411–421. [Google Scholar] [CrossRef]
- Lampit, A.; Hallock, H.; Suo, C.; Naismith, S.L.; Valenzuela, M. Cognitive training-induced short-term functional and long-term structural plastic change is related to gains in global cognition in healthy older adults: A pilot study. Front. Aging Neurosci. 2015, 7, 14. [Google Scholar] [CrossRef]
- Lee, D.R.; Kim, Y.H.; Kim, D.A.; Lee, J.A.; Hwang, P.W.; Lee, M.J.; You, S. Innovative strength training-induced neuroplasticity and increased muscle size and strength in children with spastic cerebral palsy: An experimenter-blind case study–three-month follow-up. NeuroRehabilitation 2014, 35, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Voss, M.W.; Prakash, R.S.; Boot, W.R.; Vo, L.T.; Basak, C.; VanPatter, M.; Gratton, G.; Fabiani, M.; Kramer, A.F. Videogame training strategy-induced change in brain function during a complex visuomotor task. Behav. Brain Res. 2012, 232, 348–357. [Google Scholar] [CrossRef]
- Leung, N.T.Y.; Tam, H.M.K.; Chu, L.W.; Kwok, T.C.Y.; Chan, F.; Lam, L.C.W.; Woo, J.; Lee, T.M.C. Neural Plastic Effects of Cognitive Training on Aging Brain. Neural Plast. 2015, 2015, 535618. [Google Scholar] [CrossRef]
- Lin, C.-C.; Kao, S.-C.; Hung, C.-L.; Tsai, C.-L.; Huang, C.-J.; Chang, Y.-K.; Hung, T.-M. The Effects of Gymnastics Programs with Different Cognitive Loads on Working Memory and Prefrontal Cortex Oxygenation: A Randomized Controlled Trial. Med. Sci. Sports Exerc. 2025, 57, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Reder, L.M. fMRI exploration of pedagogical benefits of repeated testing: When more is not always better. Brain Behav. 2016, 6, e00476. [Google Scholar] [CrossRef] [PubMed]
- Looi, C.Y.; Duta, M.; Brem, A.-K.; Huber, S.; Nuerk, H.-C.; Kadosh, R.C. Combining brain stimulation and video game to promote long-term transfer of learning and cognitive enhancement. Sci. Rep. 2016, 6, 22003. [Google Scholar] [CrossRef]
- Looi, C.Y.; Lim, J.; Sella, F.; Lolliot, S.; Duta, M.; Avramenko, A.A.; Cohen Kadosh, R. Transcranial random noise stimulation and cognitive training to improve learning and cognition of the atypically developing brain: A pilot study. Sci. Rep. 2017, 7, 4633. [Google Scholar] [CrossRef]
- Novakova, L.; Gajdos, M.; Rektorova, I. Theta-burst transcranial magnetic stimulation induced cognitive task-related decrease in activity of default mode network: An exploratory study. Brain Stimul. 2020, 13, 597–599. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Schwarz, A.; Greco, A.; Müller, T.; Pühse, U.; Hanke, M. Effects of Cognitive and Physical Load of Acute Exercise on Inhibitory Control and Prefrontal Cortex Hemodynamics in Children. Med. Sci. Sports Exerc. 2024, 56, 1328–1336. [Google Scholar] [CrossRef]
- MacInnes, J.J.; Dickerson, K.C.; Chen, N.-K.; Adcock, R.A. Cognitive Neurostimulation: Learning to Volitionally Sustain Ventral Tegmental Area Activation. Neuron 2016, 89, 1331–1342. [Google Scholar] [CrossRef]
- Mahncke, H.W.; DeGutis, J.; Levin, H.; Newsome, M.R.; Bell, M.D.; Grills, C.; French, L.M.; Sullivan, K.W.; Kim, S.-J.; Rose, A.; et al. A randomized clinical trial of plasticity-based cognitive training in mild traumatic brain injury. Brain 2021, 144, 1994–2008. [Google Scholar] [CrossRef]
- Mark, J.; Kraft, A.; Casebeer, W.D.; Ziegler, M.D.; Ayaz, H. Neuroimaging-guided Adaptive Training in Flight Simulators. Front. Hum. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Mattioli, F.; Bellomi, F.; Stampatori, C.; Capra, R.; Miniussi, C. Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult. Scler. J. 2015, 22, 222–230. [Google Scholar] [CrossRef]
- McKendrick, R.; Ayaz, H.; Olmstead, R.; Parasuraman, R. Enhancing dual-task performance with verbal and spatial working memory training: Continuous monitoring of cerebral hemodynamics with NIRS. NeuroImage 2014, 85, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, A.; Voss, M.W.; Elee, H.; Vo, L.T.K.; Kramer, A.F. Parietal plasticity after training with a complex video game is associated with individual differences in improvements in an untrained working memory task. Front. Hum. Neurosci. 2014, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Yamada, M.; Tanigawa, T.; Sekiyama, K.; Kawagoe, T.; Suzuki, M.; Yoshikawa, S.; Abe, N.; Otsuka, Y.; Nakai, R.; et al. A 12-Week Physical and Cognitive Exercise Program Can Improve Cognitive Function and Neural Efficiency in Community-Dwelling Older Adults: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2015, 63, 1355–1363. [Google Scholar] [CrossRef]
- Nissim, N.R.; O’sHea, A.; Indahlastari, A.; Kraft, J.N.; von Mering, O.; Aksu, S.; Porges, E.; Cohen, R.; Woods, A.J. Effects of Transcranial Direct Current Stimulation Paired With Cognitive Training on Functional Connectivity of the Working Memory Network in Older Adults. Front. Aging Neurosci. 2019, 11, 340. [Google Scholar] [CrossRef]
- Parmar, D.; Enticott, P.G.; Albein-Urios, N. Anodal HD-tDCS for cognitive inflexibility in autism spectrum disorder: A pilot study. Brain Stimul. 2021, 14, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.; Faubert, J. Enhancing learning in a perceptual-cognitive training paradigm using EEG-neurofeedback. Sci. Rep. 2021, 11, 4061. [Google Scholar] [CrossRef]
- Perlini, C. The Impact of cognitive remediation combined with mindfulness and social skills training on social functioning and neural plasticity in early psychosis: Preliminary results from a randomized clinical multicentric trial in Italy. Eur. Psychiatry 2024, 67, S33. [Google Scholar] [CrossRef]
- Pietto, M.L.; Giovannetti, F.; Segretin, M.S.; Kamienkowski, J.E.; Lipina, S.J. Increased integration of functional connectivity after cognitive intervention in preschoolers from low socioeconomic status. Dev. Psychol. 2023, 59, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Pozuelos, J.P.; Combita, L.M.; Abundis, A.; Paz-Alonso, P.M.; Conejero, Á.; Guerra, S.; Rueda, M.R. Metacognitive scaffolding boosts cognitive and neural benefits following executive attention training in children. Dev. Sci. 2018, 22, e12756. [Google Scholar] [CrossRef] [PubMed]
- Rebok, G.W.; Ball, K.; Guey, L.T.; ScD, R.N.J.; DrPH, H.K.; King, J.W.; Marsiske, M.; Morris, J.N.; Tennstedt, S.L.; Unverzagt, F.W.; et al. Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. J. Am. Geriatr. Soc. 2014, 62, 16–24. [Google Scholar] [CrossRef]
- Richmond, L.L.; Wolk, D.; Chein, J.; Olson, I.R. Transcranial Direct Current Stimulation Enhances Verbal Working Memory Training Performance over Time and Near Transfer Outcomes. J. Cogn. Neurosci. 2014, 26, 2443–2454. [Google Scholar] [CrossRef]
- Rischer, K.M.; Anton, F.; González-Roldán, A.M.; Montoya, P.; van der Meulen, M. Better Executive Functions Are Associated With More Efficient Cognitive Pain Modulation in Older Adults: An fMRI Study. Front. Aging Neurosci. 2022, 14, 828742. [Google Scholar] [CrossRef]
- Roe, J.M.; Nesheim, M.; Mathiesen, N.C.; Moberget, T.; Alnæs, D.; Sneve, M.H. The effects of tDCS upon sustained visual attention are dependent on cognitive load. Neuropsychologia 2016, 80, 1–8. [Google Scholar] [CrossRef]
- Román, F.J.; Iturria-Medina, Y.; Martínez, K.; Karama, S.; Burgaleta, M.; Evans, A.C.; Jaeggi, S.M.; Colom, R. Enhanced structural connectivity within a brain sub-network supporting working memory and engagement processes after cognitive training. Neurobiol. Learn. Mem. 2017, 141, 33–43. [Google Scholar] [CrossRef]
- Romeo, R.R.; Leonard, J.A.; Grotzinger, H.M.; Robinson, S.T.; Takada, M.E.; Mackey, A.P.; Scherer, E.; Rowe, M.L.; West, M.R.; Gabrieli, J.D. Neuroplasticity associated with changes in conversational turn-taking following a family-based intervention. Dev. Cogn. Neurosci. 2021, 49, 100967. [Google Scholar] [CrossRef]
- Rosa, V.d.O.; Franco, A.R.; Júnior, G.A.S.; Moreira-Maia, C.R.; Wagner, F.; Simioni, A.; Bassotto, C.d.F.; Moritz, G.R.; Aguzzoli, C.S.; Buchweitz, A.; et al. Effects of computerized cognitive training as add-on treatment to stimulants in ADHD: A pilot fMRI study. Brain Imaging Behav. 2019, 14, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Saiote, C.; Polanía, R.; Rosenberger, K.; Paulus, W.; Antal, A.; Avenanti, A. High-Frequency TRNS Reduces BOLD Activity during Visuomotor Learning. PLoS ONE 2013, 8, e59669. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, N.; Inuggi, A.; Castillo, A.; Campoy, G.; García-Santos, J.M.; González-Salinas, C.; Fuentes, L.J. Computer-Based Cognitive Training Improves Brain Functional Connectivity in the Attentional Networks: A Study With Primary School-Aged Children. Front. Behav. Neurosci. 2019, 13, 247. [Google Scholar] [CrossRef]
- Sankaranarayanan, G.; Odlozil, C.A.; Wells, K.O.; Leeds, S.G.; Chauhan, S.; Fleshman, J.W.; Jones, D.B.; De, S. Training with cognitive load improves performance under similar conditions in a real surgical task. Am. J. Surg. 2020, 220, 620–629. [Google Scholar] [CrossRef]
- Schroder, H.S.; Moran, T.P.; Donnellan, M.B.; Moser, J.S. Mindset induction effects on cognitive control: A neurobehavioral investigation. Biol. Psychol. 2014, 103, 27–37. [Google Scholar] [CrossRef]
- Sehatpour, P.; Dondé, C.; Adair, D.; Kreither, J.; Lopez-Calderon, J.; Avissar, M.; Bikson, M.; Javitt, D.C. Comparison of cortical network effects of high-definition and conventional tDCS during visuomotor processing. Brain Stimul. 2021, 14, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.E.; Housen, P.; Yaffe, K.; Ruff, R.; Kennison, R.F.; Mahncke, H.W.; Zelinski, E.M. A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J. Am. Geriatr. Soc. 2009, 57, 594–603. [Google Scholar] [CrossRef]
- Stephens, J.A.; Berryhill, M.E. Older Adults Improve on Everyday Tasks after Working Memory Training and Neurostimulation. Brain Stimul. 2016, 9, 553–559. [Google Scholar] [CrossRef]
- Styliadis, C.; Kartsidis, P.; Paraskevopoulos, E.; Ioannides, A.A.; Bamidis, P.D. Neuroplastic Effects of Combined Computerized Physical and Cognitive Training in Elderly Individuals at Risk for Dementia: An eLORETA Controlled Study on Resting States. Neural Plast. 2015, 2015, 172192. [Google Scholar] [CrossRef]
- Sun, J.C.-Y.; Yu, S.-J.; Chao, C.-H. Effects of intelligent feedback on online learners’ engagement and cognitive load: The case of research ethics education. Educ. Psychol. 2018, 39, 1293–1310. [Google Scholar] [CrossRef]
- Tai, H.-C.; Chen, C.-M.; Tsai, Y.-H.; Lee, B.-O.; Dewi, Y.S. Is Instructional Scaffolding a Better Strategy for Teaching Writing to EFL Learners? A Functional MRI Study in Healthy Young Adults. Brain Sci. 2021, 11, 1378. [Google Scholar] [CrossRef]
- Tatti, E.; Golemme, M.; Chrisostomou, L.; Panozzo, G.; Grande, G.; Di Bernardi, C.L.; Cappelletti, M. P255 Electrophysiological and behavioral monitoring of learning: An EEG and tRNS combined study. Clin. Neurophysiol. 2017, 128, e138. [Google Scholar] [CrossRef]
- Trypke, M.; Stebner, F.; Wirth, J. The More, the Better? Exploring the Effects of Modal and Codal Redundancy on Learning and Cognitive Load: An Experimental Study. Educ. Sci. 2024, 14, 872. [Google Scholar] [CrossRef]
- Tyagi, O.; Hopko, S.; Kang, J.; Shi, Y.; Du, J.; Mehta, R.K. Modeling Brain Dynamics During Virtual Reality-Based Emergency Response Learning Under Stress. Hum. Factors J. Hum. Factors Ergon. Soc. 2021, 65, 1804–1820. [Google Scholar] [CrossRef]
- Valk, S.L.; Bernhardt, B.C.; Trautwein, F.-M.; Böckler, A.; Kanske, P.; Guizard, N.; Collins, D.L.; Singer, T. Structural plasticity of the social brain: Differential change after socio-affective and cognitive mental training. Sci. Adv. 2017, 3, e1700489. [Google Scholar] [CrossRef]
- van Paasschen, J.; Clare, L.; Yuen, K.S.L.; Woods, R.T.; Evans, S.J.; Parkinson, C.H.; Rugg, M.D.; Linden, D.E.J. Cognitive Rehabilitation Changes Memory-Related Brain Activity in People With Alzheimer Disease. Neurorehabilit. Neural Repair 2013, 27, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.; Marques, D.; Melo, L.; Marques, R.C.; Monte-Silva, K.; Cantilino, A. Transcranial direct current stimulation effects on cognitive reappraisal: An unexpected result? Brain Stimul. 2020, 13, 650–652. [Google Scholar] [CrossRef]
- Voss, M.W.; Prakash, R.S.; Erickson, K.I.; Boot, W.R.; Basak, C.; Neider, M.B.; Simons, D.J.; Fabiani, M.; Gratton, G.; Kramer, A.F. Effects of training strategies implemented in a complex videogame on functional connectivity of attentional networks. NeuroImage 2012, 59, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Voss, J.L. Long-lasting enhancements of memory and hippocampal-cortical functional connectivity following multiple-day targeted noninvasive stimulation. Hippocampus 2015, 25, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Antonenko, P.; Keil, A.; Dawson, K. Converging Subjective and Psychophysiological Measures of Cognitive Load to Study the Effects of Instructor-Present Video. Mind Brain Educ. 2020, 14, 279–291. [Google Scholar] [CrossRef]
- Ward, N.; Paul, E.; Watson, P.; Cooke, G.E.; Hillman, C.H.; Cohen, N.J.; Kramer, A.F.; Barbey, A.K. Enhanced Learning through Multimodal Training: Evidence from a Comprehensive Cognitive, Physical Fitness, and Neuroscience Intervention. Sci. Rep. 2017, 7, 5808. [Google Scholar] [CrossRef]
- Wards, Y.; Ehrhardt, S.E.; Filmer, H.L.; Mattingley, J.B.; Garner, K.G.; Dux, P.E. Neural substrates of individual differences in learning generalization via combined brain stimulation and multitasking training. Cereb. Cortex 2023, 33, 11679–11694. [Google Scholar] [CrossRef]
- Weiss, M.; Lavidor, M. When Less Is More: Evidence for a Facilitative Cathodal tDCS Effect in Attentional Abilities. J. Cogn. Neurosci. 2012, 24, 1826–1833. [Google Scholar] [CrossRef]
- Wild-Wall, N.; Falkenstein, M.; Gajewski, P.D. Neural Correlates of Changes in a Visual Search Task due to Cognitive Training in Seniors. Neural Plast. 2012, 2012, 529057. [Google Scholar] [CrossRef]
- Woods, A.J.; Cohen, R.; Marsiske, M.; Alexander, G.E.; Czaja, S.J.; Wu, S. Augmenting cognitive training in older adults (The ACT Study): Design and Methods of a Phase III tDCS and cognitive training trial. Contemp. Clin. Trials 2018, 65, 19–32. [Google Scholar] [CrossRef]
- Yang, H.-Y. Does multimedia support individual differences?–EFL learners’ listening comprehension and cognitive load. Australas. J. Educ. Technol. 2014, 30. [Google Scholar] [CrossRef]
- Yoon, M.; Zheng, H.; Jung, E.; Li, T. Effects of Segmentation and Self-Explanation Designs on Cognitive Load in Instructional Videos. Contemp. Educ. Technol. 2022, 14, ep347. [Google Scholar] [CrossRef] [PubMed]
- Zondo, S.; Cockcroft, K.; Ferreira-Correia, A. Brain plasticity and adolescent HIV: A randomised controlled trial protocol investigating behavioural and hemodynamic responses in attention cognitive rehabilitation therapy. MethodsX 2024, 13, 102808. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Ortega, F.; González-Valero, G.; Ubago-Jiménez, J.L.; Melguizo-Ibáñez, E. Analysis of cognitively loaded physical tasks and active breaks for the improvement of executive functions in the school context. A systematic review and meta-analysis. In Revista de Psicodidáctica, English ed.; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Kaur, V. The neurosocial alchemy of entrepreneurial transformation: A deep dive into extended mirror neuron system and neuroplasticity. Entrep. Reg. Dev. 2025, 1–15. [Google Scholar] [CrossRef]
- Swerdloff, M.M. Measurement of Cognitive Load in Lower Limb Prosthesis Wearers: The P3 Event-Related Potential in Sitting, Standing, and Walking; Northwestern University: Evanston, IL, USA, 2024. [Google Scholar]
- Humble, G.; Geddes, H.; Baell, O.; Payne, J.E.; Hill, A.T.; Chung, S.W.; Emonson, M.; Osborn, M.; Caldwell, B.; Fitzgerald, P.B.; et al. TMS-EEG shows mindfulness meditation is associated with a different excitation/inhibition balance in the dorsolateral prefrontal cortex. Mindfulness 2025, 16, 347–365. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Michon, K.J.; Vesia, M.; Lee, T.G. Dissociable Causal Roles of Dorsolateral Prefrontal Cortex and Primary Motor Cortex over the Course of Motor Skill Development. J. Neurosci. 2025, 45, e2015232025. [Google Scholar] [CrossRef]
- van Hell, J.G. The neurocognitive underpinnings of second language processing: Knowledge gains from the past and future outlook. Lang. Learn. 2023, 73, 172–181. [Google Scholar] [CrossRef]
- Chmiel, J.; Stępień-Słodkowska, M.; Ramik-Mażewska, I. Efficacy of Transcranial Direct Current Stimulation (tDCS) on Neuropsychiatric Symptoms in Substance Use Disorder (SUD)—A review and insights into possible mechanisms of action. J. Clin. Med. 2025, 14, 1337. [Google Scholar] [CrossRef]
- Haccou, G.L. Polarity Effects of HD-tDCS in a Gaming Setting: Investigating the Effect of Cathodal and Anodal Stimulation in a Visuospatial Working-Memory Task. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2024. [Google Scholar]
- Contò, F.; Ellena, G.; Edwards, G.; Battelli, L. Modulation of learning-dependent plasticity in the attention network. bioRxiv 2024. [Google Scholar] [CrossRef]
- Meinzer, M.; Shahbabaie, A.; Antonenko, D.; Blankenburg, F.; Fischer, R.; Hartwigsen, G.; Nitsche, M.A.; Li, S.-C.; Thielscher, A.; Timmann, D.; et al. Investigating the neural mechanisms of transcranial direct current stimulation effects on human cognition: Current issues and potential solutions. Front. Neurosci. 2024, 18, 1389651. [Google Scholar] [CrossRef]
- Bradley, C.; Nydam, A.S.; Dux, P.E.; Mattingley, J.B. State-dependent effects of neural stimulation on brain function and cognition. Nat. Rev. Neurosci. 2022, 23, 459–475. [Google Scholar] [CrossRef]
- Tseng, P.-T.; Zeng, B.-S.; Hung, C.-M.; Liang, C.-S.; Stubbs, B.; Carvalho, A.F.; Brunoni, A.R.; Su, K.-P.; Tu, Y.-K.; Wu, Y.-C.; et al. Assessment of Noninvasive Brain Stimulation Interventions for Negative Symptoms of Schizophrenia. JAMA Psychiatry 2022, 79, 770. [Google Scholar] [CrossRef] [PubMed]
- Vergallito, A.; Feroldi, S.; Pisoni, A.; Lauro, L.J.R. Inter-individual variability in tDCS effects: A narrative review on the contribution of stable, variable, and contextual factors. Brain Sci. 2022, 12, 522. [Google Scholar] [CrossRef]
- Ojha, A.; Jones, N.P.; Henry, T.; Versace, A.; Gnagy, E.M.; Joseph, H.M.; Molina, B.S.G.; Ladouceur, C.D. Altered Lateral Prefrontal Cortex Functioning During Emotional Interference Resistance Is Associated With Affect Lability in Adults With Persisting Symptoms of Attention-Deficit/Hyperactivity Disorder From Childhood. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Pengelley, J.; Whipp, P.R.; Malpique, A. A testing load: A review of cognitive load in computer and paper-based learning and assessment. Technol. Pedagog. Educ. 2025, 34, 1–17. [Google Scholar] [CrossRef]
- Kakaria, S.; Saffari, F.; Ramsøy, T.Z.; Bigné, E. Cognitive load during planned and unplanned virtual shopping: Evidence from a neurophysiological perspective. Int. J. Inf. Manag. 2023, 72, 102667. [Google Scholar] [CrossRef]
- Kalisch, R.; Russo, S.J.; Müller, M.B. Neurobiology and systems biology of stress resilience. Physiol. Rev. 2024, 104, 1205–1263. [Google Scholar] [CrossRef]
- Wang, Z.; Donahue, E.K.; Guo, Y.; Renteln, M.; Petzinger, G.M.; Jakowec, M.W.; Holschneider, D.P. Exercise alters cortico-basal ganglia network metabolic connectivity: A mesoscopic level analysis informed by anatomic parcellation defined in the mouse brain connectome. Brain Struct. Funct. 2023, 228, 1865–1884. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.H.; Ding, J.B. Cortico-basal ganglia plasticity in motor learning. Neuron 2024, 112, 2486–2502. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Antonopoulou, H.; Sortwell, A.; Halkiopoulos, C. Challenging Cognitive Load Theory: The Role of Educational Neuroscience and Artificial Intelligence in Redefining Learning Efficacy. Brain Sci. 2025, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Zammouri, A.; Moussa, A.A.; Chevallier, S. Use of cognitive load measurements to design a new architecture of intelligent learning systems. Expert Syst. Appl. 2024, 237, 121253. [Google Scholar] [CrossRef]
- Pradeep, K.; Sulur Anbalagan, R.; Thangavelu, A.P.; Aswathy, S.; Jisha, V.G.; Vaisakhi, V.S. Neuroeducation: Understanding neural dynamics in learning and teaching. Front. Educ. 2024, 9, 1437418. [Google Scholar] [CrossRef]
- Khan, N.A.N.; Naheeda Tharannum, B.; Hassan, K.L.; Hussain, H. Proposed Framework to Map Virtual Reality with Ancient Indian Education System to Increase Neuroplasticity for Autistic Spectrum Disorder Children. In Transforming Education with Virtual Reality; Wiley-Scrivener: Beverly, MA, USA, 2024; pp. 351–378. [Google Scholar] [CrossRef]
- Bassi, M.; Patti, L.; Silvestrini, I.; Strati, M.F.; Ponzano, M.; Minuto, N.; Maggi, D. One-year follow-up comparison of two hybrid closed-loop systems in Italian children and adults with type 1 diabetes. Front. Endocrinol. 2023, 14, 1099024. [Google Scholar] [CrossRef]
- Grewlewicz, P.; Nowak, P.; Khuat, T.T.; Czeczot, J.; Klopot, T.; Gabrys, B. Practical implementation of computationally-efficient machine learning-based control performance assessment system for a class of closed loop systems. Appl. Soft Comput. 2023, 146, 110690. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Next-Generation Cognitive-Behavioral Therapy for Depression: Integrating Digital Tools, Teletherapy, and Personalization for Enhanced Mental Health Outcomes. Medicina 2025, 61, 431. [Google Scholar] [CrossRef]
- Bruand, A.; Reatto, A.; Brossard, M.; Jouquet, P.; de Souza Martins, É. Long-term activity of social insects responsible for the physical fertility of soils in the tropics. Sci. Rep. 2023, 13, 12337. [Google Scholar] [CrossRef] [PubMed]
- Forget, M.; Le Pertel, N. Enhancing neuroplasticity and promoting brain health at work: The role of learning and memory in workplace performance. In Learning and Memory—From Molecules and Cells to Mind and Behavior; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Song, Y.-T.; Xiang, M.-Q.; Zhong, P. Differences in brain activation during working memory tasks between badminton athletes and non-athletes: An fNIRS study. Brain Cogn. 2024, 175, 106133. [Google Scholar] [CrossRef] [PubMed]
- Bomyea, J.; Caudle, M.M.; Dugas, N.; Moore, R.C.; Simmons, A.N.; Thomas, M.L. A randomized controlled trial of computerized cognitive training to improve working memory in individuals with elevated repetitive negative thinking: Behavioral and neural outcomes. J. Mood Anxiety Disord. 2025, 9, 100095. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Schultz, D.H.; Wang, Y.; Natarajan, S.K.; Barlow, S.M.; Dietsch, A.M. Multimodal Adaptations to Expiratory Musculature-Targeted Resistance Training: A Preliminary Study in Healthy Young Adults. J. Speech Lang. Hear. Res. 2025, 68, 987–1005. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G.; Boutsinas, B. Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes. J. Clin. Med. 2025, 14, 2265. [Google Scholar] [CrossRef]
- Wagenblast, F.; Läubli, T.; Seibt, R.; Rieger, M.A.; Steinhilber, B. Wrist extensor muscle fatigue during a dual task with two muscular and cognitive load levels in younger and older adults. Hum. Factors 2024, 66, 2433–2450. [Google Scholar] [CrossRef]
- Crivelli, D.; Angioletti, L.; Balconi, M. Neurocognitive empowerment, embodied practices, and peak performance in sports: Case studies and future challenges. Neuropsychol. Trends 2024, 35, 85–96. [Google Scholar] [CrossRef]
- Saha, R. The exciting frontier of neuroplasticity: Innovations in brain health and recovery. J. Behav. Brain Sci. 2025, 15, 47–80. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Mindfulness-Based Cognitive Therapy in Clinical Practice: A Systematic Review of Neurocognitive Outcomes and Applications for Mental Health and Well-Being. J. Clin. Med. 2025, 14, 1703. [Google Scholar] [CrossRef]
- Jurgina, L.Q.; Aquini, L.G.; de Aguiar, M.S.; da Rosa, L.S.; Lopes, J.P.; Mackedanz, T.D.; Klein, A.I.; Primo, T.T.; Iankowski, R.S. Neuroplasticity-Based Literacy Rescue: A Multisensory and Tangible Learning Methodology for Children at Risk. In Proceedings of the 2024 IEEE Global Engineering Education Conference (EDUCON), Kos Island, Greece, 8–11 May 2024; pp. 1–10. [Google Scholar] [CrossRef]
- Olegário, R.L.; Goulart, C. Unravelling cognitive shifts: Neuroscience-based strategies in mathematics education. Educ. Gerontol. 2024, 1–13. [Google Scholar] [CrossRef]
- Vázquez-Guardado, A.; Yang, Y.; Bandodkar, A.J.; Rogers, J.A. Recent advances in neurotechnologies with broad potential for neuroscience research. Nat. Neurosci. 2020, 23, 1522–1536. [Google Scholar] [CrossRef] [PubMed]
- Kourtesis, P.; MacPherson, S.E. An ecologically valid examination of event-based and time-based prospective memory using immersive virtual reality: The influence of attention, memory, and executive function processes on real-world prospective memory. Neuropsychol. Rehabil. 2021, 33, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Stangl, M.; Maoz, S.L.; Suthana, N. Mobile cognition: Imaging the human brain in the ‘real world’. Nat. Rev. Neurosci. 2023, 24, 347–362. [Google Scholar] [CrossRef]
- Chang, M.; Büchel, D.; Reinecke, K.; Lehmann, T.; Baumeister, J. Ecological validity in exercise neuroscience research: A systematic investigation. Eur. J. Neurosci. 2022, 55, 487–509. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.M.; Khan, M.N.A.; Tariq, U.; Al-Nashash, H. Impact of electrical stimulation on mental stress, depression, and anxiety: A systematic review. Sensors 2025, 25, 2133. [Google Scholar] [CrossRef]
- Soleimani, G.; Nitsche, M.A.; Bergmann, T.O.; Towhidkhah, F.; Violante, I.R.; Lorenz, R.; Kuplicki, R.; Tsuchiyagaito, A.; Mulyana, B.; Mayeli, A.; et al. Closing the loop between brain and electrical stimulation: Towards precision neuromodulation treatments. Transl. Psychiatry 2023, 13, 279. [Google Scholar] [CrossRef]
- Bernabei, L.; Leone, B.; Hirsch, D.; Mentuccia, V.; Panzera, A.; Riggio, F.; Sangiovanni, L.; Piserchia, V.; Nicolò, G.; Pompili, E. Neuromodulation Strategies in Lifelong Bipolar Disorder: A Narrative Review. Behav. Sci. 2024, 14, 1176. [Google Scholar] [CrossRef]
- Cheng, M.-Y.; Yu, C.-L.; An, X.; Wang, L.; Tsai, C.-L.; Qi, F.; Wang, K.-P. Evaluating EEG neurofeedback in sport psychology: A systematic review of RCT studies for insights into mechanisms and performance improvement. Front. Psychol. 2024, 15, 1331997. [Google Scholar] [CrossRef]
- Roozbeh, M.; Pakdaman, H.; Naderi, N.; Roozbeh, M. Enhancing cognitive function and neuroplasticity assessed by diffusion tensor imaging in multiple sclerosis: The efficacy of combined transcranial direct current stimulation and application-assisted cognitive rehabilitation. Mult. Scler. Relat. Disord. 2024, 92, 105985. [Google Scholar] [CrossRef]
- Qian, Y.; Schwartz, A.; Jung, A.; Zhang, Y.; Seitz, U.; Wilds, G.; Kim, M.; Kramer, A.F.; Chukoskie, L. The Influence of Separate and Combined Exercise and Foreign Language Acquisition on Learning and Cognition. Brain Sci. 2024, 14, 572. [Google Scholar] [CrossRef]
- Gkintoni, E.; Dimakos, I.; Halkiopoulos, C.; Antonopoulou, H. Contributions of Neuroscience to Educational Praxis: A Systematic Review. Emerg. Sci. J. 2023, 7, 146–158. [Google Scholar] [CrossRef]
- Yuan, X.; Li, H.; Feng, S.; Sun, M. Enhancing action recognition in educational settings through exercise-induced neuroplasticity. Front. Neurosci. 2025, 19, 1588570. [Google Scholar] [CrossRef]
- Antonopoulou, H.; Halkiopoulos, C.; Gkintoni, E.; Katsimpelis, A. Application of Gamification Tools for Identification of Neurocognitive and Social Function in Distance Learning Education. Int. J. Learn. Teach. Educ. Res. 2022, 21, 367–400. [Google Scholar] [CrossRef]
- Chang, C.; Chen, J.E. Multimodal EEG-fMRI: Advancing insight into large-scale human brain dynamics. Curr. Opin. Biomed. Eng. 2021, 18, 100279. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Brain-Inspired Multisensory Learning: A Systematic Review of Neuroplasticity and Cognitive Outcomes in Adult Multicultural and Second Language Acquisition. Biomimetics 2025, 10, 397. [Google Scholar] [CrossRef]
- Sortwell, A.; Trimble, K.; Ferraz, R.; Geelan, D.R.; Hine, G.; Ramirez-Campillo, R.; Carter-Thuiller, B.; Gkintoni, E.; Xuan, Q. A Systematic Review of Meta-Analyses on the Impact of Formative Assessment on K-12 Students’ Learning: Toward Sustainable Quality Education. Sustainability 2024, 16, 7826. [Google Scholar] [CrossRef]
- Niering, M.; Seifert, J. The effects of visual skills training on cognitive and executive functions in stroke patients: A systematic review with meta-analysis. J. Neuroeng. Rehabil. 2024, 21, 41. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.