Antioxidant Activity and Oxidative Stability of Flaxseed and Its Processed Products: A Review
Abstract
1. Introduction
2. Research Methodology
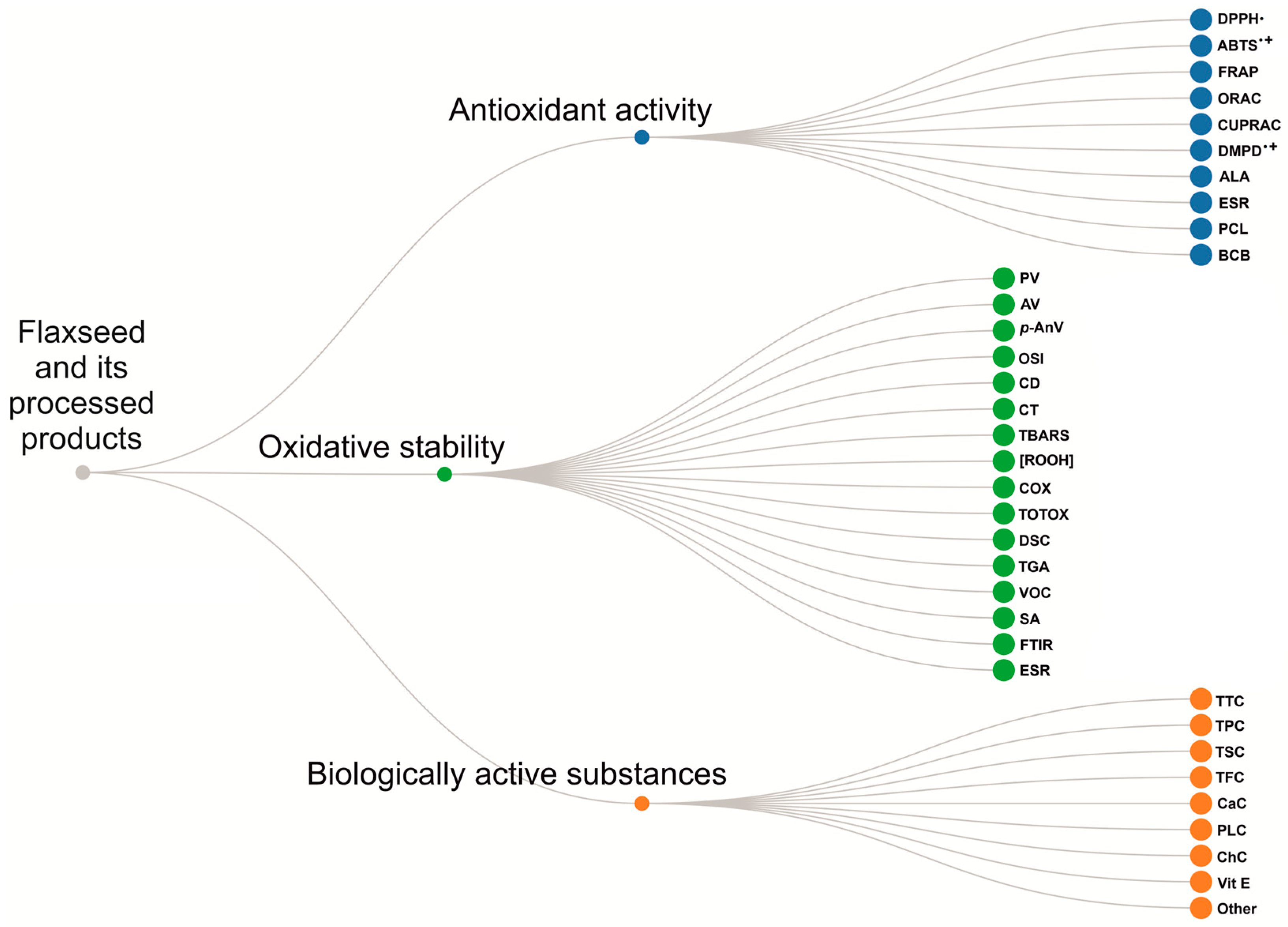
3. Study of Antioxidant Activity and Oxidative Stability of Flaxseeds
4. Flaxseed Oil: Methods of Production, Antioxidant Activity, and Oxidative Stability
4.1. Methods of Production of Flaxseed Oil
4.2. Investigation of Antioxidant Activity and Oxidative Stability of Flaxseed Oil Under In Vitro Conditions
4.3. The Addition of Biologically Active Substances as a Factor in Increasing the Oxidative Stability of Flaxseed Oil
5. Study of the Antioxidant Activity and Oxidative Stability of Flaxseed Processing Products: Flaxseed Meal, Cake, and Hull
6. Main Methodological Approaches to Studying the Antioxidant Activity and Oxidative Stability of Flaxseed and Its Processed Products
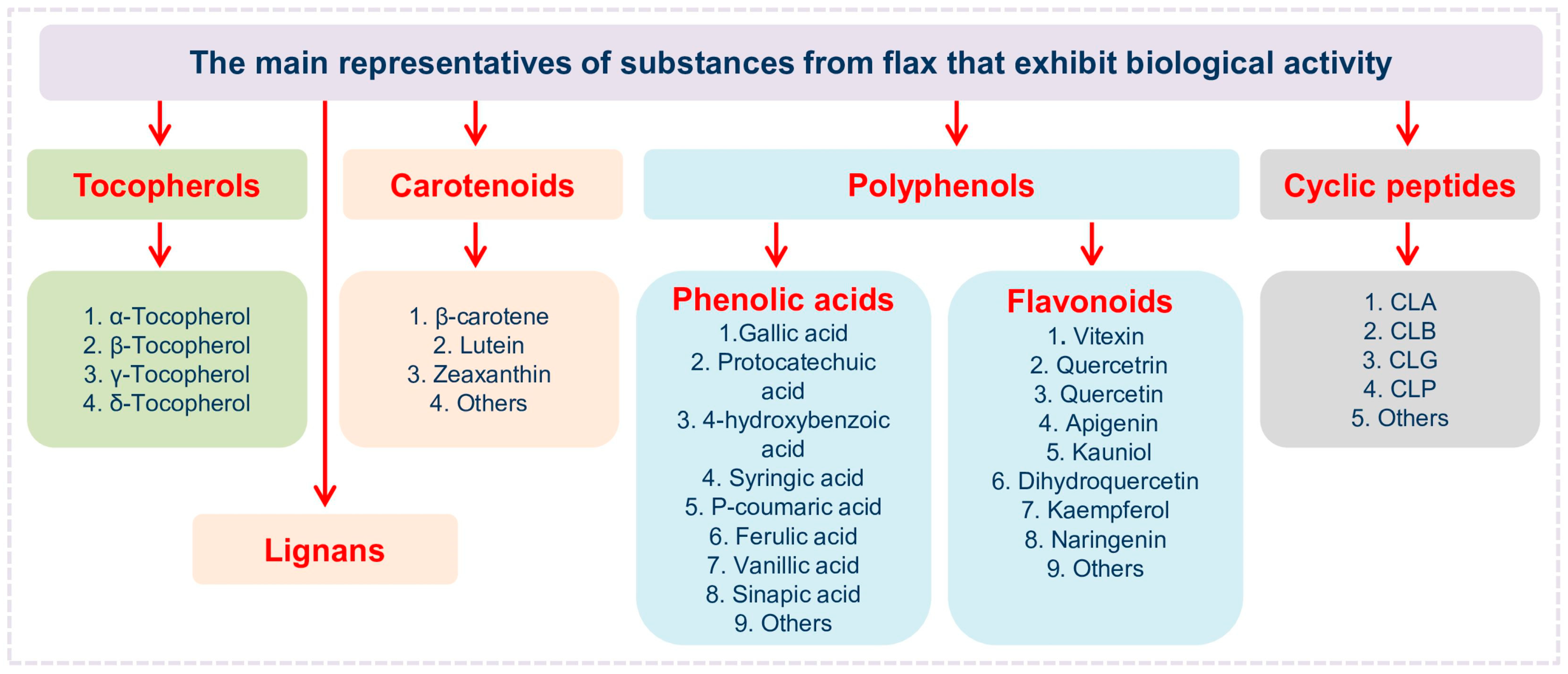
7. Prospects for Research on Flax-Derived Compounds with Antioxidant Activity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS•+ | radical 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) |
| ALA | antioxidant activity in linoleic acid |
| AOA | antioxidant activity |
| AV | acid value |
| BAS | biologically active substance |
| BCB | β-carotene bleaching activity |
| BHA | butylated hydroxy anisole |
| BHT | butylhydroxytoluene |
| CA | chelating activity |
| CaC | carotenoid content |
| CD | conjugated diene |
| CE | catechin |
| ChC | chlorophyll content |
| CLs | cyclolinopeptides |
| CoQ | coenzyme Q content |
| CouAG | coumaric acid glucoside |
| COX | calculated oxidizability value |
| CT | conjugated triene |
| CUPRAC | cupric ion (Cu2+) reducing power assay |
| DFC | defatted flaxseed cake |
| DFM | defatted flaxseed meal |
| DMPD•+ | N,N-dimethyl-p-phenylenediamine |
| DPPH• | radical 1,1-diphenyl-2-picrylhydrazyl |
| DSC | differential scanning calorimetry |
| DTBHQ | 2,5-di-tert-butyl hydroquinone |
| DW | dry weight |
| ESR | electron paramagnetic resonance spectrometry |
| FC | flaxseed cake |
| FeAG | ferulic acid glucoside |
| FH | flaxseed hull |
| FM | flaxseed meal |
| FO | flaxseed oil |
| FOC | cold-pressed oil |
| FOCD | cold-pressed flaxseed oil subjected to desorption |
| FOE | oil extracted using solvents |
| FOCG | cold-pressed oil from germinated seeds |
| FOCU | unrefined flaxseed oil |
| FOD | flaxseed oil subjected to desorption |
| FOEG | oil extracted from germinated seeds |
| FOH | oil from flaxseed hull |
| FOSC | oil extracted by supercritical CO2 |
| FOSE | Soxhlet extraction of oil |
| FR | fluorometric analysis |
| FRAP | ferric reducing antioxidant power |
| FSO | cold-pressed oil with preliminary heat treatment |
| FTIR | Fourier transform infrared spectroscopy method |
| GA | gallic acid |
| LARI | lariciresinol |
| MATA | matairesinol |
| MDA | malondialdehyde |
| NMR | nuclear magnetic resonance |
| ORAC | oxygen radical absorbance capacity |
| OS | oxidative stability |
| OSI | oxidative stability index |
| p-AnV | para-anisidine value |
| PBN | α-Phenyl-tert-butyl nitrone |
| PCL | photochemiluminescence assay |
| PLC | phospholipid content |
| PSC | phytosterol content |
| PV | peroxide value |
| QE | quercetin |
| [ROOH] | peroxide concentration |
| RP | reducing antioxidant power |
| SA | sensory analysis |
| SDG | secoisolariciresinol diglucoside |
| SECO | secoisolariciresinol |
| SqC | squalene content |
| TAC | total antioxidant capacity |
| TAS | total antioxidant status |
| TBARS | thiobarbituric acid reactive substance |
| TBHQ | tert-butyl hydroquinone |
| TE | trolox |
| TFC | total flavonoid content |
| TGA | thermogravimetric analyzer |
| TOTOX | total oxidation |
| TTC | total tocopherol content |
| TPC | total phenolic content |
| TSC | total sterol content |
| Vit E | vitamin E |
| VOC | volatile organic compound |
References
- Mueed, A.; Ali, A.; Madjirebaye, P.; Li, J.; Deng, Z. Metabolomic and chemometrics depict to understand the nutraceutical potential of different flaxseed varieties. Food Biosci. 2024, 59, 103876. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed bioactive compounds: Chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Zou, X.G.; Chen, X.L.; Hu, J.N.; Wang, Y.F.; Gong, D.M.; Zhu, X.M.; Deng, Z.Y. Comparisons of proximate compositions, fatty acids profile and micronutrients between fiber and oil flaxseeds (Linum usitatissimum L.). J. Food Compost. Anal. 2017, 62, 168–176. [Google Scholar] [CrossRef]
- Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 September 2025).
- Waghmode, M.; Gunjal, A.; Patil, N. Probiotic sugar confectionery fortified with flax seeds (Linum usitatissimum L.). J. Food Sci. Technol. 2020, 57, 1964–1970. [Google Scholar] [CrossRef]
- Roozegar, M.H.; Shahedi, M.; Keramet, J.; Hamdami, N.; Roshanak, S. Effect of coated and uncoated ground flaxseed addition on rheological, physical and sensory properties of Taftoon bread. J. Food Sci. Technol. 2015, 52, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed cake as a tool for the improvement of nutraceutical and sensorial features of sourdough bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Marand, M.A.; Amjadi, S.; Marand, M.A.; Roufegarinejad, L.; Jafari, S.M. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technol. 2020, 359, 76–84. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, P.; Li, Z. Antibacterial properties of cyclolinopeptides from flaxseed oil and their application on beef. Food Chem. 2022, 385, 132715. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; McClements, D.J.; Hou, T.; Geng, F.; Chen, P.; Chen, H.; Xie, B.; Sun, Z.; Tang, H.; et al. Composition, processing, and quality control of whole flaxseed products used to fortify foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 587–614. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wen, C.; Duan, Y.; Deng, Q.; Peng, D.; Zhang, H.; Ma, H. The composition, extraction, analysis, bioactivities, bioavailability and applications in food system of flaxseed (Linum usitatissimum L.) oil: A review. Trends Food Sci. Technol. 2021, 118, 252–260. [Google Scholar] [CrossRef]
- Puligundla, P.; Lim, S. A review of extraction techniques and food applications of flaxseed mucilage. Foods 2022, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Kouamé, K.J.E.P.; Bora, A.F.M.; Li, X.; Sun, Y.; Liu, L. Novel trends and opportunities for microencapsulation of flaxseed oil in foods: A review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Mercier, S.; Villeneuve, S.; Moresoli, C.; Mondor, M.; Marcos, B.; Power, K.A. Flaxseed-enriched cereal-based products: A review of the impact of processing conditions. Compr. Rev. Food Sci. Food Saf. 2014, 13, 400–412. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Gui, B.; Wang, Y.; Reaney, M.J. Flaxseed (Linum usitatissimum L.) oil processing and selected products. Trends Food Sci. Technol. 2015, 43, 162–177. [Google Scholar] [CrossRef]
- Herchi, W.; Arráez-Román, D.; Trabelsi, H.; Bouali, I.; Boukhchina, S.; Kallel, H.; Segura-Carretero, A.; Fernández-Gutierrez, A. Phenolic compounds in flaxseed: A review of their properties and analytical methods. An overview of the last decade. J. Oleo Sci. 2014, 63, 7–14. [Google Scholar] [CrossRef]
- Lorenc, F.; Jarošová, M.; Bedrníček, J.; Smetana, P.; Bárta, J. Structural characterization and functional properties of flaxseed hydrocolloids and their application. Foods 2022, 11, 2304. [Google Scholar] [CrossRef]
- Sharav, O.; Shim, Y.Y.; Okinyo-Owiti, D.P.; Sammynaiken, R.; Reaney, M.J. Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J. Agric. Food Chem. 2014, 62, 88–96. [Google Scholar] [CrossRef]
- Stepień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Oxidant and Anti-Cancer Properties of Flaxseed. Int. J. Mol. Sci. 2025, 26, 1226. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Tufail, T.; Bader Ul Ain, H.; Ali, A.; Aadil, R.M.; Nemat, A.; Manzoor, M.F. Antioxidant activity and phytochemical analysis of fennel seeds and flaxseed. Food Sci. Nutr. 2023, 11, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Shah, M.A.; Sanches Silva, A. Flaxseed in Diet: A Comprehensive Look at Pros and Cons. Molecules 2025, 30, 1335. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, H.; Guo, Y.; Long, S.; Wang, Y.; Abbasi, A.M.; Guo, X.; Jarvis, D.I. Comparison of fatty acid composition, phytochemical profile and antioxidant activity in four flax (Linum usitatissimum L.) varieties. Oil Crop Sci. 2020, 5, 136–141. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2016, 242, 777–786. [Google Scholar] [CrossRef]
- Chera, E.I.; Pop, R.M.; Pârvu, M.; Sorițău, O.; Uifălean, A.; Cătoi, F.A.; Cecan, A.; Negoescu, A.G.; Achimaș-Cadariu, P.; Pârvu, A.E. Flaxseed ethanol extracts’ antitumor, antioxidant, and anti-inflammatory potential. Antioxidants 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Alawlaqi, M.M.; Al-Rajhi, A.M.; Abdelghany, T.M.; Ganash, M.; Moawad, H. Evaluation of biomedical applications for linseed extract: Antimicrobial, antioxidant, anti-diabetic, and anti-inflammatory activities in vitro. J. Funct. Biomater. 2023, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Senila, L.; Neag, E.; Cadar, O.; Kovacs, M.H.; Becze, A.; Senila, M. Chemical, nutritional and antioxidant characteristics of different food seeds. Appl. Sci. 2020, 10, 1589. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Karamać, M.; Kancheva, V.; Amarowicz, R. Antioxidant activity of flaxseed extracts in lipid systems. Molecules 2015, 21, 17. [Google Scholar] [CrossRef]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). ACTA Sci. Pol. Technol. Aliment. 2012, 11, 293–302. [Google Scholar]
- Hanaa, M.H.; Ismail, H.A.; Mahmoud, M.E.; Ibrahim, H.M. Antioxidant activity and phytochemical analysis of flaxseeds (Linum usitatisimum L.). Minia J. Agric. Res. Develop. 2017, 37, 129–140. [Google Scholar]
- Gebremeskal, Y.H.; Nadtochii, L.A.; Eremeeva, N.B.; Mensah, E.O.; Kazydub, N.G.; Soliman, T.N.; Baranenko, D.A.; El-Messery, T.M.; Tantawy, A.A. Comparative analysis of the nutritional composition, phytochemicals, and antioxidant activity of chia seeds, flax seeds, and psyllium husk. Food Biosci. 2024, 61, 104889. [Google Scholar] [CrossRef]
- Deng, Q.; Yu, X.; Ma, F.; Xu, J.; Huang, F.; Huang, Q.; Sheng, F. Comparative analysis of the in-vitro antioxidant activity and bioactive compounds of flaxseed in China according to variety and geographical origin. Int. J. Food Prop. 2017, 20 (Suppl. 3), S2708–S2722. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uslu, N. Investigation of changes in some chemical properties, bioactive compounds, antioxidant activity, phenolic and fatty acid profiles of flaxseed and oils. J. Food Process. Preserv. 2022, 46, e17091. [Google Scholar] [CrossRef]
- Quezada, N.; Cherian, G. Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur. J. Lipid Sci. Technol. 2012, 114, 974–982. [Google Scholar] [CrossRef]
- Brodowska, K.; Catthoor, R.I.K.; Brodowska, A.J.; Symonowicz, M.; Lodyga-Chruscinska, E. A comparison of antioxidant properties of extracts from defatted and non-defatted flax (Linum usitatissimum) seeds. Albanian J. Agric. Sci. 2014, 13, 16. [Google Scholar]
- Huang, X.; Wang, N.; Ma, Y.; Liu, X.; Guo, H.; Song, L.; Zhao, Q.; Hai, D.; Cheng, Y.; Bai, G.; et al. Flaxseed polyphenols: Effects of varieties on its composition and antioxidant capacity. Food Chem. X 2024, 23, 101597. [Google Scholar] [CrossRef]
- Al-Temimi, W.K.; Al-Garory, N.H.; Khalaf, A.A. Diagnose the bioactive compounds in flaxseed extract and its oil and use their mixture as an antioxidant. Basrah J. Agric. Sci. 2020, 33, 172–188. [Google Scholar] [CrossRef]
- Sargi, S.C.; Silva, B.C.; Santos, H.M.C.; Montanher, P.F.; Boeing, J.S.; Santos Júnior, O.O.; Souza, N.E.; Visentainer, J.V. Antioxidant capacity and chemical composition in seeds rich in omega-3: Chia, flax, and perilla. Food Sci. Technol. 2013, 33, 541–548. [Google Scholar] [CrossRef]
- Hussain, S. Antioxidant potential and phenolic contents of various flaxseed cultivars from different agro-industrial regions. Pol. J. Environ. Stud. 2021, 30, 4325–4330. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of extraction method on the phenolic and cyanogenic glucoside profile of flaxseed extracts and their antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Koçak, M.Z. Phenolic compounds, fatty acid composition, and antioxidant activities of some flaxseed (Linum usitatissimum L.) varieties: A comprehensive analysis. Processes 2024, 12, 689. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Machado, S.; Espírito Santo, L.; Nunes, M.A.; Costa, A.S.G.; Álvarez-Ortí, M.; Pardo, J.E.; Alves, R.C.; Oliveira, M.B.P.P. Defatted flaxseed flour as a new ingredient for foodstuffs: Comparative analysis with whole flaxseeds and updated composition of cold-pressed oil. Nutrients 2024, 16, 3482. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Dong, S.; Li, Y.; Wei, L.; Zhao, C.; Li, J.; Liu, X.; Wang, Y. Effects of germination on tocopherol, secoisolarlciresinol diglucoside, cyanogenic glycosides and antioxidant activities in flaxseed (Linum usitatissimum L.). Int. J. Food Sci. Technol. 2019, 54, 2346–2354. [Google Scholar] [CrossRef]
- Khare, B.; Sangwan, V.; Rani, V. Influence of sprouting on proximate composition, dietary fiber, nutrient availability, antinutrient, and antioxidant activity of flaxseed varieties. J. Food Process. Preserv. 2021, 45, e15344. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- Malcolmson, L.J.; Przybylski, R.; Daun, J.K. Storage stability of milled flaxseed. J. Am. Oil Chem. Soc. 2000, 77, 235–238. [Google Scholar] [CrossRef]
- Findlay, C.R.; Singh, J.; Nadimi, M.; Paliwal, J. Advanced oxidative decontamination of flax and its impacts on storage. Food Bioproc. Technol. 2023, 16, 2935–2946. [Google Scholar] [CrossRef]
- Gopalakrishnan, N.; Cherian, G.; Sim, J.S. Chemical changes in the lipids of canola and flax seeds during storage. Lipid/Fett 1996, 98, 168–171. [Google Scholar] [CrossRef]
- Mundhada, S.; Chaudhry, M.M.A.; Erkinbaev, C.; Paliwal, J. Development of safe storage guidelines for prairie-grown flaxseed. J. Stored Prod. Res. 2022, 97, 101965. [Google Scholar] [CrossRef]
- Simbalista, R.L.; Frota, K.D.M.G.; Soares, R.A.M.; Arêas, J.A.G. Effect of storage and processing of Brazilian flaxseed on lipid and lignan contents. Food Sci. Technol. 2012, 32, 374–380. [Google Scholar] [CrossRef]
- Schorno, A.L.; Manthey, F.A.; Hall III, C.A. Effect of particle size and sample size on lipid stability of milled flaxseed (Linum usitatissimum L.). J. Food Process. Preserv. 2010, 34, 167–179. [Google Scholar] [CrossRef]
- Bechlin, T.R.; Granella, S.J.; Christ, D.; Coelho, S.R.M.; Viecelli, C.A. Evaluation of grain and oil quality of packaged and ozonized flaxseed. J. Stored Prod. Res. 2019, 83, 311–316. [Google Scholar] [CrossRef]
- Tomaszewska-Gras, J.; Islam, M.; Grzeca, L.; Kaczmarek, A.; Fornal, E. Comprehensive thermal characteristics of different cultivars of flaxseed oil (Linum usittatissimum L.). Molecules 2021, 26, 1958. [Google Scholar] [CrossRef]
- Pointner, T.; Rauh, K.; Auñon-Lopez, A.; Veličkovska, S.K.; Mitrev, S.; Arsov, E.; Pignitter, M. Comprehensive analysis of oxidative stability and nutritional values of germinated linseed and sunflower seed oil. Food Chem. 2024, 454, 139790. [Google Scholar] [CrossRef]
- Zamani Ghaleshahi, A.; Ezzatpanah, H.; Rajabzadeh, G.; Ghavami, M. Comparison and analysis characteristics of flax, perilla and basil seed oils cultivated in Iran. J. Food Sci. Technol. 2020, 57, 1258–1268. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharyya, D.K.; Ghosh, M. Comparative study of chemical characteristics, phytochemical contents and antioxidant activity of polar and non-polar solvent extracted flaxseed oil. ACTA Pharm. Sci. 2019, 57, 65–74. [Google Scholar] [CrossRef]
- Oomah, B.D.; Sitter, L. Characteristics of flaxseed hull oil. Food Chem. 2009, 114, 623–628. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Dong, G.; Chen, X.; Wang, S.; Lei, F.; Su, X.; Bai, Q. Effect of different extraction methods on quality characteristics of rapeseed and flaxseed oils. J. Food Qual. 2022, 2022, 8296212. [Google Scholar] [CrossRef]
- Kasote, D.M.; Badhe, Y.S.; Hegde, M.V. Effect of mechanical press oil extraction processing on quality of linseed oil. Ind. Crops Prod. 2013, 42, 10–13. [Google Scholar] [CrossRef]
- Herchi, W.; Ammar, K.B.; Bouali, I.; Abdallah, I.B.; Guetet, A.; Boukhchina, S. Heating effects on physicochemical characteristics and antioxidant activity of flaxseed hull oil (Linum usitatissimum L.). Food Sci. Technol. 2016, 36, 97–102. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Liu, L.; Li, M.; Zhang, R. A rapid method for evaluating the edible oil oxidative stability during ambient storage by FTIR spectroscopy using a mesh cell. Anal. Methods 2016, 8, 5117–5122. [Google Scholar] [CrossRef]
- Farag, M.A.; Elimam, D.M.; Afifi, S.M. Outgoing and potential trends of the omega-3 rich linseed oil quality characteristics and rancidity management: A comprehensive review for maximizing its food and nutraceutical applications. Trends Food Sci. Technol. 2021, 114, 292–309. [Google Scholar] [CrossRef]
- Azad, M.; Nadeem, M.; Gulzar, N.; Imran, M. Impact of fractionation on fatty acids composition, phenolic compounds, antioxidant characteristics of olein and super olein fractions of flaxseed oil. J. Food Process. Preserv. 2021, 45, e15369. [Google Scholar] [CrossRef]
- Teh, S.S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Composit. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity and oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, X.; Qu, Z.; Gao, Y.; Li, Q.; Yu, X. Mechanism, indexes, methods, challenges, and perspectives of edible oil oxidation analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 4901–4915. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Momot-Ruppert, M.; Stawicka, B.; Szydłowska-Czerniak, A. Health benefits, antioxidant activity, and sensory attributes of selected cold-pressed oils. Molecules 2023, 28, 5484. [Google Scholar] [CrossRef]
- Gundev, P.; Chauhan, K.; Sachdev, D. To study the Antioxidative potential of Flaxseed oil at varied temperatures. Int. J. Adv. Sci. Res. Manag. 2019, 4, 409–413. [Google Scholar]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Oxidative stability and the chemical composition of market cold-pressed linseed oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1700055. [Google Scholar] [CrossRef]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and antioxidant potential of cold-pressed oils—Possible strategies to improve oil stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef]
- Herchi, W.; Bahashwan, S.; Sebei, K.; Saleh, H.B.; Kallel, H.; Boukhchina, S. Effects of germination on chemical composition and antioxidant activity of flaxseed (Linum usitatissimum L.) oil. Grasas y Aceites 2015, 66, e057. [Google Scholar] [CrossRef]
- Huimin, L.; Yongfu, L.; Ju, Q. Characterization of the evolution of free radicals and TALAs in linseed oil during heat treatment. Heliyon 2024, 10, e27168. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, E.; Szczucinska, A.; Gwardiak, H.; Szulc, A.; Winiarska, A. Comparative studies of oxidative stability of linseed oil. Thermochim. Acta 2001, 370, 135–140. [Google Scholar] [CrossRef]
- Jaswir, I.; Kitts, D.D.; Man, Y.B.C.; Hassan, T.H. Physico-chemical stability of flaxseed oil with natural antioxidant mixtures during heating. J. Oleo Sci. 2005, 54, 71–79. [Google Scholar] [CrossRef][Green Version]
- Cantele, C.; Bertolino, M.; Bakro, F.; Giordano, M.; Jędryczka, M.; Cardenia, V. Antioxidant effects of hemp (Cannabis sativa L.) inflorescence extract in stripped linseed oil. Antioxidants 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, G.; Özdemir, K.S. Effects of black elderberry and spirulina extracts on the chemical stability of cold pressed flaxseed oil during accelerated storage. J. Food Meas. Charact. 2021, 15, 4838–4847. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Keramat, M.; Zare Darniyani, L. A kinetic approach to the oxidation of linseed oil as influenced by fruit peel and seeds of pomegranate. Eur. J. Lipid Sci. Technol. 2020, 122, 1900084. [Google Scholar] [CrossRef]
- Kūka, M.; Čakste, I.; Kūka, P. Inhibition of formation of conjugated dienes in linseed oil. Proc. Latv. Acad. Sci. 2018, 72, 80. [Google Scholar] [CrossRef]
- Drini, Z.; Mudri, J.; Zduni, G.; Bigovi, D.; Šavikin, K. Investigation of stability of cold pressed linseed (Linum usitatissimum L.) oil. Lek. Sirovine 2019, 39, 40. [Google Scholar] [CrossRef]
- Shadyro, O.; Sosnovskaya, A.; Edimecheva, I. Effect of biologically active substances on oxidative stability of flaxseed oil. J. Food Sci. Technol. 2020, 57, 243–252. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Wang, J.H.; Xie, H.K.; Wang, Y.F.; Zhao, Q.; Zhou, D.Y.; Shahidi, F. Improving oxidative stability of flaxseed oil with a mixture of antioxidants. J. Food Process. Preserv. 2020, 44, e14355. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Pilarski, W.; Gęsiński, K.; Tańska, M. Effect of Ferulic acid and its derivatives on cold-pressed flaxseed oil oxidative stability and bioactive compounds retention during oxidation. Foods 2023, 12, 1088. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Arslan, D.; Tontul, İ.; Polak, T.; Ulrih, N.P. Use of Sinapic Acid Alkyl Esters as Antioxidants in Microencapsulated Flaxseed Oil. Food Bioproc. Tech. 2025, 18, 449–459. [Google Scholar] [CrossRef]
- Symoniuk, E.; Marczak, Z.; Brzezińska, R.; Janowicz, M.; Ksibi, N. Effect of the freeze-dried mullein flower extract (Verbascum nigrum L.) addition on oxidative stability and antioxidant activity of selected cold-pressed oils. Foods 2023, 12, 2391. [Google Scholar] [CrossRef] [PubMed]
- Arslan, D.; Polak, T.; Poklar Ulrih, N. Antioxidative effects of alkyl esters of sinapic acid on flaxseed oil and its fatty acid methyl esters. J. Am. Oil Chem. Soc. 2024, 101, 675–687. [Google Scholar] [CrossRef]
- Odeh, D.; Kraljić, K.; Benussi Skukan, A.; Škevin, D. Oxidative stability, microbial safety, and sensory properties of flaxseed (Linum usitatissimum L.) oil infused with spices and herbs. Antioxidants 2021, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, H.D.; Almasi, H.; Zeynali, F.; Hamishehkar, H.; Amjadi, S. Improving oxidative stability of flaxseed oil by Zein-Basil seed gum electrospun nanofibers activated by thyme essential oil loaded nanostructured lipid carriers. Appl. Food Res. 2024, 4, 100616. [Google Scholar] [CrossRef]
- Barrett, A.H.; Porter, W.L.; Marando, G.; Chinachoti, P. Effect of various antioxidants, antioxidant levels, and encapsulation on the stability of fish and flaxseed oils: Assessment by fluorometric analysis. J. Food Process. Preserv. 2011, 35, 349–358. [Google Scholar] [CrossRef]
- Shadyro, O.I.; Sosnovskaya, A.A.; Edimecheva, I.P. Flaxseed oil stabilization using natural and synthetic antioxidants. Eur. J. Lipid Sci. Technol. 2017, 119, 1700079. [Google Scholar] [CrossRef]
- Ma, G.; Wang, J.; Wang, X.; Gan, S.; Yang, F.; Dong, G. Assessing the influence of Secoisolariciresinol diglucoside multi-antioxidants on flaxseed oil’s oxidative stability. Eur. J. Lipid Sci. Technol. 2024, 126, 2300159. [Google Scholar] [CrossRef]
- Edimecheva, I.P.; Sosnovskaya, A.A.; Shadyro, O.I. The application of natural and synthetic antioxidants to increase the oxidation resistance of linseed oil. Food Ind. Sci. Technol. 2020, 13, 41–51. [Google Scholar] [CrossRef]
- Misharina, T.A.; Alinkina, E.S.; Terenina, M.B.; Krikunova, N.I.; Kiseleva, V.I.; Medvedeva, I.B.; Semenova, M.G. Inhibition of autoxidation of flaxseed oil by essential oils and extracts of aromatic plants. Appl. Biochem. Microbiol. 2015, 51, 417. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Shadyro, O.I.; Sosnovskaya, A.A.; Edimecheva, I.P. Application of plant raw materials to protect flaxseed oil from oxidation. Proc. Natl. Acad. Sci. Belarus. Agric. Sci. Ser. 2016, 1, 114–118. [Google Scholar]
- Srivastava, Y.; Singh, B.; Kaur, B.; Ubaid, M.; Semwal, A.D. Kinetic study of thermal degradation of flaxseed oil and moringa oil blends with physico-chemical, oxidative stability index (OSI) and shelf-life prediction. J. Food Sci. Technol. 2024, 61, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P. Physicochemical characteristics, oxidative stability, pigments, fatty acid profile and antioxidant properties of co-pressed oil from blends of peanuts, flaxseed and black cumin seeds. Food Chem. Adv. 2023, 2, 100231. [Google Scholar] [CrossRef]
- Belhoussaine, O.; El Kourchi, C.; Amakhmakh, M.; Ullah, R.; Iqbal, Z.; Goh, K.W.; Gallo, M.; Harhar, H.; Bouyahya, A.; Tabyaoui, M. Oxidative stability and nutritional quality of stored Linum usitatissmium L. and Argania spinosa L., oil blends: Chemical compositions, properties and nutritional value. Food Chem. X 2024, 23, 101680. [Google Scholar] [CrossRef]
- Talwar, B.; Chopra, R.; Taneja, N.K.; Chand, M.; Homroy, S.; Dhiman, A.; Singh, P.K.; Chaudhary, S. Use of flaxseed cake as a source of nutrients in the food industry and possible health benefits—A review. Food Prod. Process. Nutr. 2025, 7, 22. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B. The effect of roasting on the protein profile and antiradical capacity of flaxseed meal. Foods 2020, 9, 1383. [Google Scholar] [CrossRef]
- Barthet, V.J.; Klensporf-Pawlik, D.; Przybylski, R. Antioxidant activity of flaxseed meal components. Can. J. Plant Sci. 2014, 94, 593–602. [Google Scholar] [CrossRef]
- Akl, E.M.; Mohamed, S.S.; Hashem, A.I.; Taha, F.S. Biological activities of phenolic compounds extracted from flaxseed meal. Bull. Natl. Res. Cent. 2020, 44, 27. [Google Scholar] [CrossRef]
- Kasote, D.M.; Hegde, M.V.; Deshmukh, K.K. Antioxidant activity of phenolic components from n-butanol fraction (PC-BF) of defatted flaxseed meal. Am. J. Food Technol. 2011, 6, 604–612. [Google Scholar] [CrossRef]
- Tavarini, S.; De Leo, M.; Matteo, R.; Lazzeri, L.; Braca, A.; Angelini, L.G. Flaxseed and camelina meals as potential sources of health-beneficial compounds. Plants 2021, 10, 156. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, X.; Ma, Y.; Huang, X.; Zhang, X.; Liu, J.; Song, L.; Qiao, M.; Li, T.; Wang, T. Effects of different processing methods on phenolic compounds in flaxseed meal. Food Chem. X 2024, 24, 101934. [Google Scholar] [CrossRef] [PubMed]
- Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (flour, meals, and groats) from the vegetable oil industry as a potential source of antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef]
- Krimer Malešević, V.; Vaštag, Ž.; Popović, L.; Popović, S.; Peričin-Starčevič, I. Characterisation of black cumin, pomegranate and flaxseed meals as sources of phenolic acids. Int. J. Food Sci. Technol. 2014, 49, 210–216. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B.; Kaur, A. Dry-air roasting impact on physicochemical, functional, antioxidant properties, phenolic profile and Maillard reaction products of flaxseed flour and cake flour. Food Chem. 2024, 442, 138571. [Google Scholar] [CrossRef] [PubMed]
- Herchi, W.; Al Hujaili, A.D.; Sakouhi, F.; Sebei, K.; Trabelsi, H.; Kallel, H.; Boukhchina, S. Flaxseed hull: Chemical composition and antioxidant activity during development. J. Oleo Sci. 2014, 63, 681–689. [Google Scholar] [CrossRef]
- Hao, M.; Beta, T. Qualitative and quantitative analysis of the major phenolic compounds as antioxidants in barley and flaxseed hulls using HPLC/MS/MS. J. Sci. Food Agric. 2012, 92, 2062–2068. [Google Scholar] [CrossRef]
- Han, H.; Yilmaz, H.; Gulcin, I. Antioxidant activity of flaxseed (Linum usitatissimum L.) shell and analysis of its polyphenol contents by LC-MS/MS. Rec. Nat. Prod. 2018, 12, 397–402. [Google Scholar] [CrossRef]
- Mannucci, A.; Castagna, A.; Santin, M.; Serra, A.; Mele, M.; Ranieri, A. Quality of flaxseed oil cake under different storage conditions. LWT 2019, 104, 84–90. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Wang, X. Composition and antioxidant ability of extract from different flaxseed cakes and its application in flaxseed oil. J. Oleo Sci. 2023, 72, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.S.; Bekhit, A.E.D.; Birch, J. Antioxidative polyphenols from defatted oilseed cakes: Effect of solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef]
- Majed, M.; Galala, A.A.; Amer, M.M.; Selmar, D.; Abouzeid, S. Oilseed Cakes: A Promising Source of Antioxidant, and Anti-Inflammatory Agents—Insights from Lactuca sativa. Int. J. Mol. Sci. 2024, 25, 11077. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B.; Kaur, A.; Singh, N. Proximate, mineral, amino acid composition, phenolic profile, antioxidant and functional properties of oilseed cakes. Int. J. Food Sci. Technol. 2021, 56, 6732–6741. [Google Scholar] [CrossRef]
- Teh, S.S.; Niven, B.E.; Bekhit, A.E.D.A.; Carne, A.; Birch, J. Optimization of polyphenol extraction and antioxidant activities of extracts from defatted flax seed cake (Linum usitatissimum L.) using microwave-assisted and pulsed electric field (PEF) technologies with response surface methodology. Food Sci. Biotechnol. 2015, 24, 1649–1659. [Google Scholar] [CrossRef]
- Singh, S.; Shahi, N.C.; Lohani, U.C.; Bhat, M.I.; Sirohi, R.; Singh, S. Process optimization for the extraction of bioactive compounds from defatted flaxseed cake (Linum usitatissimu) using ultrasound-assisted extraction method and its characterization. J. Food Process Eng. 2023, 46, e14217. [Google Scholar] [CrossRef]
- Ribas, J.C.R.; Ferreira, G.F.; Clemente, S.G.B.; Marinho, M.T.; Pedrosa, V.B.; Martins, A.D.S. Oxidative Stability of Flaxseed (Linum usitatissimum L.) Cake and its Inclusion in the Diet of Lactating Cows. Braz. Arch. Biol. Technol. 2024, 67, e24240603. [Google Scholar] [CrossRef]
- Pag, A.I.; Radu, D.G.; Draganescu, D.; Popa, M.I.; Sirghie, C. Flaxseed cake—A sustainable source of antioxidant and antibacterial extracts. Cellul. Chem. Technol. 2014, 48, 265–273. [Google Scholar]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Influence of microwave roasting on chemical composition, oxidative stability and fatty acid composition of flaxseed (Linum usitatissimum L.) oil. Food Chem. 2020, 326, 126974. [Google Scholar] [CrossRef]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The influence of edible oils’ composition on the properties of beeswax-based oleogels. Gels 2020, 8, 48. [Google Scholar] [CrossRef]
- Punia, J.; Deen, M.K. Comparative evaluation of phenols, flavonoids and antioxidant activity of flax seed from two locations. Asian J. Chem. 2016, 28, 2038. [Google Scholar] [CrossRef]
- Sharav, O. Antioxidant Activity of Cyclolinopeptides. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2013. [Google Scholar]
- Makarenko, M.A.; Malinkin, A.D.; Bessonov, V.V.; Sarkisyan, V.A.; Kochetkova, A.A. Secondary lipid oxidation products. Human health risks evaluation (Article 1). Vopr. Pitan. 2018, 87, 125–138. [Google Scholar] [CrossRef]
- Rajesha, J.; Murthy, K.N.C.; Kumar, M.K.; Madhusudhan, B.; Ravishankar, G.A. Antioxidant potentials of flaxseed by in vivo model. J. Agric. Food Chem. 2006, 54, 3794–3799. [Google Scholar] [CrossRef]
- Króliczewska, B.; Miśta, D.; Ziarnik, A.; Żuk, M.; Szopa, J.; Pecka-Kiełb, E.; Zawadzki, W.; Króliczewski, J. The effects of seed from Linum usitatissimum cultivar with increased phenylpropanoid compounds and hydrolysable tannin in a high cholesterol-fed rabbit. Lipids Health Dis. 2018, 17, 76. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, Y.Y.; Wang, J.P.; Yan, B.X.; Huang, Y.Q.; Wang, Z.X. Effect of flaxseed on the fatty acid profile of egg yolk and antioxidant status of their neonatal offspring in Huoyan geese. Animal 2015, 9, 1749–1755. [Google Scholar] [CrossRef]
- Sobeková, A.; Piešová, E.; Maková, Z.; Szabóová, R.; Sopková, D.; Andrejčáková, Z.; Vlčková, R.; Faixová, D.; Faixová, Z. Duration of the flaxseed supplementation affects antioxidant defence mechanisms and the oxidative stress of fattening pigs. Vet. Sci. 2023, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Pilar, B.; Güllich, A.; Oliveira, P.; Ströher, D.; Piccoli, J.; Manfredini, V. Protective role of flaxseed oil and flaxseed lignan secoisolariciresinol diglucoside against oxidative stress in rats with metabolic syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef]
- Singh, M.; Mollier, R.T.; Sharma, P.R.; Kadirvel, G.; Doley, S.; Sanjukta, R.K.; Rajkhowa, D.J.; Kandpal, B.K.; Kumar, D.; Khan, M.H.; et al. Dietary flaxseed oil improve boar semen quality, antioxidant status and in-vivo fertility in humid sub-tropical region of North East India. Theriogenology 2021, 159, 123–131. [Google Scholar] [CrossRef]
- Kaithwas, G.; Majumdar, D.K. In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur. J. Lipid Sci. Technol. 2012, 114, 1237–1245. [Google Scholar] [CrossRef]
- Perumal, P.; Sunder, J.; De, A.K.; Alyethodi, R.R.; Vikram, R.; Upadhyay, V.R.; Mayuri, S.C.; Bhattacharya, D. Flaxseed oil modulates testicular biometrics, hormone, libido, antioxidant and semen profiles in endangered Teressa goat of Andaman and Nicobar Islands. Reprod. Biol. 2023, 23, 100730. [Google Scholar] [CrossRef]
- Palla, A.H.; Iqbal, N.T.; Minhas, K.; Gilani, A.H. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int. Immunopharmacol. 2016, 38, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.D.; Palin, M.F.; Santos, G.T.; Benchaar, C.; Lima, L.C.R.; Chouinard, P.Y.; Petit, H.V. Effect of flax meal on the production performance and oxidative status of dairy cows infused with flax oil in the abomasum. Livest. Sci. 2014, 170, 53–62. [Google Scholar] [CrossRef]
- Mueed, A.; Ma, H.; Basit, A.; Madjirebaye, P.; Ali, A.; Khan, S.; Li, J.; Deng, Z.Y. Modulation of lipid profile by flaxseed cyclolinopeptides ameliorates lipid accumulation in obese mice liver and pancreas. Food Biosci. 2025, 71, 107236. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Moradnia, M.; Mohammadi, M.; Ebrahimpour, S.; Tabatabaei-Malazy, O.; Mirsadeghi, S.; Javar, H.A. Utilizing the healing power of flaxseed: A narrative review of its therapeutic applications. J. Diabetes Metab. Disord. 2025, 24, 128. [Google Scholar] [CrossRef]
- Langyan, S.; Khan, F.N.; Yadava, P.; Alhazmi, A.; Mahmoud, S.F.; Saleh, D.I.; Zuan, A.T.K. In silico proteolysis and analysis of bioactive peptides from sequences of fatty acid desaturase 3 (FAD3) of flaxseed protein. Saudi J. Bbiol. Sci. 2021, 28, 5480–5489. [Google Scholar] [CrossRef]
- Ji, D.; Udenigwe, C.C.; Agyei, D. Antioxidant peptides encrypted in flaxseed proteome: An in silico assessment. Food Sci. Hum. Wellness 2019, 8, 306–314. [Google Scholar] [CrossRef]
- Xu, L.; Wan, Y.; Liu, X.; Qin, Z.; Zhao, Y.; Fu, X.; Wei, C.; Liu, W. Insights on the binding mechanism between specified aldehydes and flaxseed protein using multispectral image and molecular docking. Food Chem. 2023, 422, 136256. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Huang, P.; Cai, Z.; Zhang, N.; Wang, Y.; Li, Y. The anti-inflammatory mechanism of flaxseed linusorbs on lipopolysaccharide-induced RAW 264.7 macrophages by modulating TLR4/NF-κB/MAPK pathway. Foods 2023, 12, 2398. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.J.; Tzen, J.T. The potential role of cyclopeptides from Pseudostellaria heterophylla, Linum usitatissimum and Drymaria diandra, and peptides derived from heterophyllin B as dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes: An in silico study. Metabolites 2022, 12, 387. [Google Scholar] [CrossRef]
- Harenčár, Ľ.; Ražná, K. In silico prediction of microRNA families involved in the biosynthesis of lignans and cyanogenic glycosides in flax (Linum usitatissimum L.). Plant Growth Regul. 2024, 104, 233–251. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Przybylski, R.; Rudzińska, M. Lipid components of flax, perilla, and chia seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 794–800. [Google Scholar] [CrossRef]
- da Silva Moura, M.; da Silva, C.A.M.; Braga, M.B. Flaxseed and avocado oil blends: Physical and physicochemical characterization, nutritional quality and oxidative stability. Appl. Food Res. 2023, 3, 100370. [Google Scholar] [CrossRef]
- Raczyk, M.; Popis, E.; Kruszewski, B.; Ratusz, K.; Rudzińska, M. Physicochemical quality and oxidative stability of linseed (Linum usitatissimum) and camelina (Camelina sativa) cold-pressed oils from retail outlets. Eur. J. Lipid Sci. Technol. 2016, 118, 834–839. [Google Scholar] [CrossRef]
- Aladedunye, F.; Sosinska, E.; Przybylski, R. Flaxseed cyclolinopeptides: Analysis and storage stability. J. Am. Oil Chem. Soc. 2013, 90, 419–428. [Google Scholar] [CrossRef]
- Cai, Z.Z.; Xu, C.X.; Song, Z.L.; Li, J.L.; Zhang, N.; Zhao, J.H.; Lee, Y.Y.; Reaney, M.J.T.; Huang, F.R.; Wang, Y. A two-step method of cyclolinopeptide (linusorb) preparation from flaxseed cake via dry-screening. Food Chem. 2024, 449, 139243. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xiao, T.; Ni, X.; Wei, T.; Liu, X.; Deng, Z.Y.; Li, J. The comparative analysis of different oil extraction methods based on the quality of flaxseed oil. J. Food Compost. Anal. 2022, 107, 104373. [Google Scholar] [CrossRef]
- Gui, B.; Shim, Y.Y.; Datla, R.S.; Covello, P.S.; Stone, S.L.; Reaney, M.J. Identification and quantification of cyclolinopeptides in five flaxseed cultivars. J. Agric. Food Chem. 2012, 60, 8571–8579. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Hano, C.; Corbin, C.; Fliniaux, O.; Lopez, T.; Montguillon, J.; Barakzoy, E.; Mesnard, F.; Lemblin, F.; Lainé, E. Cellulase-assisted release of secoisolariciresinol from extracts of flax (Linum usitatissimum) hulls and whole seeds. Food Chem. 2010, 122, 679–687. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Sun, P.; Su, W.; Qu, Z.; Dong, Y.; Du, S.; Yu, X. Effect of germination pretreatment on the physicochemical properties and lipid concomitants of flaxseed oil. RSC Adv. 2023, 13, 3306–3316. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Y.; Qiu, H.; Long, S.; Zhao, X.; Wang, Y.; Guo, X.; Baitelenova, A.; Qiu, C. Comparative Assessment of Lignan, Tocopherol, Tocotrienol and Carotenoids in 40 Selected Varieties of Flaxseed (Linum usitatissimum L.). Foods 2023, 12, 4250. [Google Scholar] [CrossRef]
- Bozan, B.; Temelli, F. Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresour. Technol. 2008, 99, 6354–6359. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Kaufmann, H.P.; Tobschirbel, A. Über ein oligopeptid aus leinsamen. Chem. Berichte 1959, 92, 2805–2809. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Luo, T.; Zheng, L.; Deng, Z. Effect of reduced flaxseed cyclic peptide [1–9-NαC]-linusorb B2 (CLB) and its oxidized form on the oxidative stability of flaxseed oil. Food Chem. 2025, 465, 142011. [Google Scholar] [CrossRef]
- Deng, D.; Li, J.; Yu, J.; Li, W.; Reaney, M.J.; Cai, Z.; Wang, Y. The changes of Trp-deficient linusorb during the oxidation and the influence of γ-tocopherol on their oxidative stability. Grain Oil Sci. Technol. 2024, 7, 219–228. [Google Scholar] [CrossRef]
- Brühl, L.; Bonte, A.; N’Diaye, K.; Matthäus, B. Oxidation of Cyclo-Lino Peptides in Linseed Oils during Storage. Eur. J. Lipid Sci. Technol. 2022, 124, 2200137. [Google Scholar] [CrossRef]
- Li, W.Z.; Song, Z.L.; Yu, J.H.; Deng, D.J.; Cai, X.Q.; Reaney, M.J.; Cai, Z.Z.; Wang, Y. Stability of tryptophan-containing LOs in flaxseed oil and their response towards γ-tocopherol. Food Chem. 2024, 448, 139026. [Google Scholar] [CrossRef]
- Zou, X.G.; Hu, J.N.; Zhu, X.M.; Wang, Y.F.; Deng, Z.Y. Methionine sulfone-containing orbitides, good indicators to evaluate oxidation process of flaxseed oil. Food Chem. 2018, 250, 204–212. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Luo, T.; Deng, Z. Flaxseed cyclic pep-tide [1-9-NαC]-linusorb B3 (CLA) improves oxidative stability of flaxseed oil by chelating metal ions and intermediate oxidative products. J. Agric. Food Chem. 2022, 70, 15776–15786. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Lee, Y.Y.; Li, K.Y.; Li, W.Z.; Du, Y.; Liu, X.; Li, G.Y.; Wang, Y. Status of linusorbs in cold-pressed flaxseed oil.during oxidation and their response toward antioxidants. Food Res. Int. 2022, 161, 111861. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Frank, O.; Lang, R.; Hofmann, T.; Behrens, M. Activation spectra of human bitter taste receptors stimulated with cyclolinopeptides corresponding to fresh and aged linseed oil. J. Agric. Food Chem. 2022, 70, 4382–4390. [Google Scholar] [CrossRef] [PubMed]
| Sample | Parameters | Reference | |||
|---|---|---|---|---|---|
| AOA | Value | BAS | Value | ||
| flaxseed | DPPH• FRAP | 35.68–66.76% 608.95–5031 μM Fe2+/g | TPC TFC | 69.34–84.13 mg GA/100 g 2.1–5.11 mg QE/100 g | [21] |
| flaxseed (4) | ORAC DPPH• FRAP | 38.43–88.16 μmol TE/g 3.25–8.40 μmol TE/g 32.22–61.65 μmol TE/g | TPC * SDG SECO α-linolenic acid | 235.15–389.65 mg GA/100 g 61.98–144.66 μg/g 76.54–243.69 μg/g 47.44–53.67% | [23] |
| flaxseed | DPPH• ORAC | 0.081–0.134 g/L (EC50) 0.36–1.07 mmol TE/g | SDG SECO | 5.12–77.98 mg/g 6.43–15.84 mg/g | [24] |
| flaxseed | DPPH• FRAP H2O2 NO | 39.07 ± 2.84 µg TE/g 64.57 ± 12.09 µg TE/g 14.33 ± 3.86 µg TE/g 57.72 ± 3.58 µg TE/g | TPC TFC | 95.18 mg GA/100 g 28.61 mg QE/100 g | [25] |
| flaxseed | DPPH• | 27.5–89.9% | chlorogenic acid methyl gallate gallic acid ellagic acid rutin coffeic acid coumaric acid vanillin cinnamic acid | 1932.20 µg/mL 284.31 µg/mL 155.10 µg/mL 120.86 µg/mL 32.81 µg/mL 32.78 µg/mL 17.02 µg/mL 16.45 µg/mL 8.84 µg/mL | [26] |
| flaxseed | DPPH• TAC | 0.25 ± 0.02 µmol TE/mg 0.21 ± 0.04 µmol TE/mg | α-linolenic acid | 73.2 ± 0.4% | [27] |
| flaxseed | TPC SDG | 85 ± 5 mg CE/g 333 ± 15 mg/g | [28] | ||
| flaxseed | DPPH• ALA | 42.2–87.5% 56.7–88.2% | TPC TFC | 1360–3260 mg GA/100 g 190–480 mg CE/100 g | [29] |
| flaxseed | DPPH• | 39.47–62.10% | TPC TFC | 11.5–15.5 mg/g 1.5–5.0 mg/g | [30] |
| flaxseed | DPPH• | 40–50% | TPC * TFC α-linolenic acid | 0.6–1.67 mg GA/100 g ~0.40–1.08 mg QE/g 52.40% | [31] |
| flaxseed (32) | DPPH• FRAP ABTS•+ | 32.56–46.22 mg TE/100 g 0.58–1.08 mg TE/g 14.22–36.14 mmol TE/g | TPC PSC SDG SECO | 109.93–246.88 mg/100 g 56.52–125.12 mg/g 11.37–21.25 mg/g 0.76–3.16 mg/g | [32] |
| flaxseed (5) | DPPH• | 9.42–13.14 mmol/kg | CaC TPC TFC α-linolenic acid | 0.14–0.66 μg/g 178.81–243.73 mg/100 g 255.71–424.29 mg/100 g 51.19–56.51% | [33] |
| flaxseed | ABTS•+ DPPH• FRAP ORAC | 0.90 mmol TE/g 0.12 mmol TE/g 0.13 mmol TE/g 7.6 µmol TE/g | TPC TFC α-linolenic acid | 1538.7 µg/g 1.7 mg/g 60.08% | [34] |
| flaxseed non-defatted | DPPH• FRAP | 25.7–76.3% 0.062 ± 0.007 mmol TE/g | TPC | 61.3 ± 0.02 mg CE/100 g | [35] |
| flaxseed defatted | DPPH• FRAP | 19.7–76.1% 0.058 ± 0.009 mmol TE/g | TPC | 98.8 ± 0.01 mg CE/100 g | [35] |
| flaxseed (5) | DPPH• ABTS•+ | 10.20–13.80 μmol TE/g 10.50–12.99 μmol TE/g | TPC * | 3.8–4.56 mg GA/100 g | [36] |
| flaxseed | DPPH• | 21.68–71.24% | TPC | 483–3315 mg GA/100 g | [37] |
| flaxseed (2) | ABTS•+ DPPH• FRAP | 3.38–3.70 mmol TE/g 1.16–1.56 mmol TE/g 0.33–0.76 mmol TE/g | α-linolenic acid | 396.56–544.85 mg/g | [38] |
| flaxseed (8) | DPPH• ALA | 63.06–86.58% 65.59–85.29% | TPC TFC SDG SECO | 2560–3286 mg GA/100 g 232.53–346.67 mg CE/100 g 14.78–124.27 mg/100 g 396.49–1518.2 mg/100 g | [39] |
| flaxseed (3) | ABTS•+ DPPH• FRAP CA | 0.46–5.06 mmol TE/g 0.06–0.51 g/L (EC50) 0.44–4.96 mmol FeS04/g 0.11–6.54 g/L (EC50) | SDG SECO | 9.55–107.37 mg/g 2.11–21.74 mg/g | [40] |
| flaxseed (15) | DPPH• ABTS•+ CA CUPRAC | 1.89–6.03 µg/mL (IC50) 0.61–3.21 µg/mL (IC50) 0.92–3.84 µg/mL (IC50) 6.34–11.78 µmol TE/mg | TPC TFC α-linolenic acid | 613.6–3164.6 mg GA/g 176.25–689.20 mg QE/g 39.21–54.2% | [41] |
| flaxseed | DPPH• FRAP | 57.2 ± 11.0 mg TE/100 g 8.5 ± 0.4 mmol FeS04/100 g | TPC TFC α-linolenic acid | 142.5 ± 7.2 mg GA/100 g 53.9 ± 2.4 mg epicatechin/100 g 56.75 ± 0.19% | [42] |
| Sample | Parameters | Reference | |||||
|---|---|---|---|---|---|---|---|
| AOA | Value | OS | Value | BAS | Value | ||
| FOC (5) | - | - | p-AnV PV AV DSC TOTOX | 0.65–0.94 1.21–6.9 meq O2/kg 0.4–1.6 mg KOH/g 37–51 min (120 °C) 3.17–14.74 | α-linolenic acid | 55.91–63.11% | [54] |
| FOC | ABTS•+ DPPH• FRAP | 17.9 ± 1.1 μmol TE/kg 9 ± 0.2 μmol TE/kg 169.9 ± 12.9 mmol Fe(II)/kg | OSI PV AV | 1.6 ± 0.06 h 17.5 ± 0.5 meq O2/kg 3.7 ± 0.2 mg KOH/g | TPC TTC CaC ChC α-linolenic acid | 15.4 ± 1.4 mg GA/kg 32.8 ± 1.6 mg/100 g 2.1 ± 0.02 mg/kg 0.61 ± 0.02 mg/kg 56.0% | [55] |
| FOCG | ABTS•+ DPPH• FRAP | 1612.1 ± 45.3 μmol TE/kg 609.9 ± 11.4 μmol TE/kg 3092.2 ± 85 mmol Fe(II)/kg | OSI PV AV | 6.2 ± 0.06 h 6 ± 0.4 meq O2/kg 37.2 ± 0.2 mg KOH/g | TPC TTC CaC ChC α-linolenic acid | 572.8 ± 13.1 mg GA/kg 43.1 ± 1.5 mg/100 g 15.7 ± 0.02 mg/kg 16.4 ± 0.02 mg/kg 55.0% | [55] |
| FOE | - | - | PV AV p-AnV OSI TOTOX | 0.29 ± 0.10 meq O2/kg 2.16 ± 0.23 mg KOH/g 0.740 ± 0.080 1.28 ± 0.16 h 1.32 ± 0.23 | TPC TTC TSC α-linolenic acid | 19.5 ± 0.49 mg GA/kg 447 ± 12 mg/kg 3510 ± 2.5 mg/kg 53.4 ± 1.4% | [56] |
| FOE (polar solvent) | FRAP ABTS•+ DPPH• | 10 ± 0.03 µmol/mL 43 ± 0.52% 80 ± 0.34% | AV PV | 0.80 ± 0.25 mg KOH/g 0.95 ± 0.19 meq O2/kg | TFC TPC α-linolenic acid | 402 ± 0.95 µg CE/mg 1975 ± 1.11 mg GA/100 g 53.29% | [57] |
| FOE (non- polar solvent) | FRAP ABTS•+ DPPH• | 11 ± 0.39 µmol/mL 44 ± 0.42% 82 ± 0.21% | AV PV | 0.84 ± 0.14 mg KOH/g 0.99 ± 0.21 meq O2/kg | TFC TPC α-linolenic acid | 441 ± 0.87 µg CE/mg 2120 ± 1.07 mg GA/100 g 57.35% | [57] |
| FOC, FOH (FOE, FOSC) | PCL | 0.6–1.2 μm TE/g | DSC | 105–163 °C (t onset of oxidation) | SDG | 20.19–51.72 mg/g | [58] |
| FOC | - | - | PV AV | 0.85 ± 0.04 meq O2/kg 1.59 ± 0.07 mg KOH/g | Vit E TPC α-linolenic acid | 380 mg/kg 118 mg GA/g 49.33–51.01 g/100 g | [59] |
| FOSE | - | - | PV AV | 0.75 ± 0.01 meq O2/kg 0.93 ± 0.03 mg KOH/g | Vit E TPC α-linolenic acid | 410 mg/kg 139 mg GA/g 48.14–50.95 g/100 g | [59] |
| FOC | - | - | PV | 2 ± 0.03 meq O2/kg | TPC TFC α-linolenic acid | 10 mg GA/100 g 5 mg rutin/100 g 46.03–47.15% | [60] |
| FOH | DPPH• | 64.7% | AV PV p-AnV CDs CTs OSI TOTOX COX | 1.5 ± 0.14 mg KOH/g 1.85 ± 0.08 meq O2/kg 1.10 ± 0.17 1.50 ± 0.10 0.24 ± 0.06 1.4 ± 0.28 h 4.8 ± 0.16 11.87 ± 0.20 | TFC TPC CaC ChC PLC α-linolenic acid | 18 ± 1.40 mg luteolin/100 g 84 ± 9.36 mg GA/100 g 7.82 ± 0.64 mg/kg 3.16 ± 0.28 mg/kg 2.27 ± 0.32% 47.19 ± 0.41% | [61] |
| FO | TAC DPPH• ALA | 65.44 ± 0.39% 45.75 ± 0.42% 28.49 ± 0.72% | OSI PV | 3.62 ± 0.02 h 0.23 ± 0.02 meq O2/kg | TSC * TPC * α-linolenic acid | 103.6 mg/100 g 145 GA/100 g 54.67 ± 0.07 mg/100 g | [64] |
| FOC | - | - | PV p-AnV AV CDs CTs | 2.04 ± 0.15 meq O2/kg 0.52 ± 0.03 1.49 ± 0.02 mg KOH/g 2.08 ± 0.03 0.02 ± 0.01 | ChC TTC CaC TPC TFC α-linolenic acid | 6.78 ± 0.01 mg pheophytin/kg 37.00 ± 0.02 mg/100 g 0.06 ± 0.00 mg/100 g 136.93 ± 1.36 mg GA/100 g 18.75 ± 0.36 mg luteolin/100 g 59.34 ± 1.34% | [65] |
| FOC | DPPH• | 1.58 ± 0.17 TE, mM/kg | AV PV p-AnV CDs CTs | 0.17 ± 0.07 mg KOH/g 0.60 ± 0.10 meq O2/kg 0.87 ± 0.57 1.94 ± 0.28%E 0.30 ± 0.27%E | α-linolenic acid | 51.2 ± 3.7% | [66] |
| FOC | FRAP DPPH• ABTS•+ | 78.63 ± 1.64 μmol TE/100 g 185.36 ± 7.62 μmol TE/100 g 1040.86 ± 41.69 μmol TE/100 g | PV p-AnV AV TOTOX COX OSI | 0.61 ± 0.02 meq O2/kg 0.39 ± 0.02 0.37 ± 0.01 mg KOH/g 1.61 13.14 4.87 ± 0.21 h | TTC * TPC TSC * α-linolenic acid | 44.04 ± 1.04 mg/100 g 2.93 ± 0.20 mg GA/100 g 335 ± 7 mg/100 g 52.12 ± 0.28% | [68] |
| FO | DPPH• | 74.7% | - | - | TPC TFC | 32.2 mg GA/100 g 22.82 mg QE/100 g | [69] |
| FOC (15) | DPPH• | 50.1–56.3% | PV AV p-AnV TOTOX OSI COX DSC | 1.23–4.50 meq O2/kg 0.53–3.15 mg KOH/g 0.07–1.43 3.11–9.07 2.85–4.96 h 12.03–15.40 56.48–125.19 min | CaC TPC ChC α-linolenic acid | 18.34–67.97 mg β-carotene/kg 60.25–115.12 mg ferulic acid/100 g 0.06–3.93 mg pheophytin/kg 44.90–64.62% | [70] |
| FOC (6) | DPPH• | 1.10–2.30 mM TE/kg | AV PV p-AnV CDs CTs | 0.52–1.74 mg KOH/g 0–1.17 meq O2/kg 0.27–0.73 1.51–1.89 µmol/g 0.16–0.28 µmol/g | TPC TTC ChC TSC α-linolenic acid | 55.8 mg GA/kg 588.7 mg/kg 0.79 mg/kg 5171.7 mg/kg 49.3–59.3% | [71] |
| FOEG | DPPH• | 51.4% | AV p-AnV PV CDs CTs COX | 2.48 ± 0.08 mg KOH/g 1.6 ± 0.25 2.4 ± 0.16 meq O2/kg 2.12 ± 0.10 0.44 ± 0.12 10.82 ± 0.32 | ChC CaC TFC TPC α-linolenic acid | 7.37 ± 0.28 mg/kg 6.27 ± 0.07 mg/kg 11.82 ± 1.20 mg luteolin/100 g 73.11 ± 4.29 mg ferulic acid/100 g 41.07–43.24% | [72] |
| Oil Type | Added Components | Parameters | Note | Application | Reference | |
|---|---|---|---|---|---|---|
| AOA | OS | |||||
| FOCD | Polar fractions from flaxseed oil | - | OSI | The addition of a polar fraction containing mainly cyclolinopeptide A led to ↑OSI. | Increasing the OS of FO. Studying the role of CLs in the mechanism of AOA. | [19] |
| FOCU | Butylated hydroxy anisole (BHA), blend (α-tocopherol, ascorbyl palmitate, citric and ascorbic acids, ethoxylated ethylene glycol) | - | PV/OSI/DSC/ TGA | A complex of methods made it possible to establish that a mixture of antioxidant protected flaxseed oil from oxidation more effectively than BHA. Meanwhile, in samples with antioxidants, ↓PV, ↑OSI, and ↑tonset,TGA/DSC were observed relative to pure oil. | DSC and TGA methods can be used to predict the OS of oils and evaluate the effectiveness of antioxidants. | [75] |
| FOCU | Rosemary and sage oleoresin, citric acid | - | PV/p-AnV/ CDs/CTs | The use of oleoresins extracted from rosemary and sage and citric acid leads to ↓PV, ↓p-AnV, ↓CDs, and ↓CTs. | Increasing the OS of flaxseed oil. The described model can be used to predict and optimize the effect of antioxidants on the oil’s properties. | [76] |
| FOD | Hemp extract, α-tocopherol | - | PV/VOCs | The use of hemp flower extract and α-tocopherol leads to ↓PV and reduces the formation of VOCs (by hexanal). | Slowing down oxidation processes in oils with a high degree of saturation. | [77] |
| FOC | Extract from spirulina and black elderberry, butylhydroxytoluene (BHT), GA | DPPH• | PV/p-AnV | The addition of natural extracts led to ↓PV, and the addition of BHT and GA led to ↑PV during 28 days of storage relative to the control. The value p-AnV↑ during 28 days. | There are advantages to using a mixture of natural antioxidants compared to synthetic ones when assessing the OS of oil. | [78] |
| FO | Pomegranate seed and peel powder, β-carotene, quercetin GA, tert-butylhydroquinone (TBHQ) | - | OSI | Addition of quercetin, β-carotene, GA, TBHQ (0.01%), and pomegranate seed and peel powder (over 0.1%) led to ↑OSI. | Development of new oils with increased OS. | [79] |
| FOCU | Vanillin, Hibiscus sabdariffa L. extract, α-tocopherol, BHT | - | PV/AV/CDs | The ability of additives to inhibit oxidation in flaxseed oil decreases as follows: Hibiscus sabdariffa L. extract, vanillin, BHT, α-tocopherol. | Flaxseed oil can be used as a test system for assessing AOA substances. | [80] |
| FOC | Rosemary extract | DPPH• | AV/PV/ TBARSs | The addition of rosemary extract did not affect the AV, PV, and TBARS values during storage. | The addition of rosemary extract affects secondary oxidation processes, which can be taken into account when storing oils. | [81] |
| FOC | CoQ, selenomethionine, cholecalciferol, α-tocopherol acetate, α-tocopherol, zeaxanthin, lutein, β-carotene, ascorbic acid esters | - | PV/OSI/AV/ p-AnV | Lutein, CoQ, β-carotene, zeaxanthin in flaxseed oil exhibited pro-oxidant effect (↑PV, p-AnV↑). Addition of α-tocopherol and α-tocopherol acetate (30–150 mg/100 g), and also vitamin D3 (50–150 µg/100 g), did not significantly change the OS of flaxseed oil. Ascorbic acid esters effectively inhibit lipid oxidation processes. | New functional food products based on flaxseed oil that are stable to oxidation. | [82] |
| FO | Tocopherol, tea polyphenol palmitate, rosemary extract, tea polyphenol extract, antioxidant of bamboo leaves, phytic acid, ascorbyl palmitate | - | PV/OSI/ TBARSs/ESR | The composition 80 mg/kg tocopherol + 40 mg/kg ascorbyl palmitate + 40 mg/kg phytic acid + 240 mg/kg tea polyphenol palmitate had the best antioxidant activity, which increased the shelf life of flaxseed oil by 3.22 times. | Flaxseed oil with improved OS. | [83] |
| FOC | Ferulic acid, 4-vinylguaiacol, dihydroferulic acid, vanillic acid | - | PV/AV/OSI/ p-AnV/CDs/CTs | Shelf life predictions at 20 °C showed that all tested phenolic additives can be considered effective antioxidants in FOC. However, their AOA depends on concentration (25–200 mg/100 g of oil) and processing temperature (60–110 °C). | Development of new oils with increased OS. | [84] |
| FOC | Tannic acid, TBHQ, eugenol, β-carotene, α-tocopherol, ascorbyl palmitate, quercetin, L-ascorbic acid, caffeic acid | DPPH•/ CA | PV/OSI/p-AnV | The antiradical activity among hydrophilic antioxidants decreased in the following order: tannic acid, caffeic acid, ascorbic acid; among hydrophobic antioxidants: α-tocopherol, eugenol, β-carotene; among antioxidants with intermediate polarity: quercetin, ascorbyl palmitate. The addition of all natural antioxidants, except α-tocopherol, led to ↓PV and ↑OSI. | Understanding the mechanism of antioxidant action in the presence of minor oil components. | [85] |
| FOC | Hexyl and palmitoyl esters of sinapic acid | DPPH•/BCB | CDs/CTs/OSI/PV | Palmitoyl sinapate showed the highest AOA. Encapsulation and addition of antioxidants resulted in ↓PV, CDs, CTs, and ↑OSI. | Stabilization of microencapsulated oil. | [86] |
| FOC | Mullein flower extract (Verbascum nigrum L.) | DPPH•/ABTS | TOTOX/PV/ AV/p-AnV/OSI | The addition of Mullein flower extract increased the oil’s ability to absorb ABTS and DPPH radicals, while increasing OSI, AV, PV (not significantly), p-AnV, and TOTOX. | Development of new oils with increased oxidative stability. | [87] |
| FOC | Alkyl esters of sinapic acid | DPPH•/BCB | PV/CDs/CTs/OSI | Palmitoyl sinapate showed the lowest PV, while oil stabilized with hexyl sinapate had higher values. It was found that sinapic acid conjugates inhibit the formation of primary oxidation products and slow down the formation of secondary oxidation products. | Development of new oils with increased oxidative stability. | [88] |
| FOC | Basil, fennel, oregano, rosemary, chili | DPPH• | PV/SA | The addition of spices and herbs led to ↑ antiradical activity and ↓PV. Flaxseed oil with chili had the highest total score (SA) during storage. | Development of new oils with increased oxidative stability. | [89] |
| FO | Nanofiber zein/basil seed gum/thyme essential oil and nanofiber zein/basil seed gum/encapsulated thyme essential oil | DPPH• | PV/OSI | The addition of nanofibers to oil resulted in ↓PV and ↑DPPH scavenging activity and OSI. | Increasing the shelf life of flaxseed oil. | [90] |
| FO | Ethylenediaminetetraacetic acid (EDTA), citric acid, rosmarinic acid, vitamin E | - | FR | Encapsulation significantly improved FO stability, both in terms of induction period and oxidation rate (i.e., the slope of the fluorescence-to-time ratio). The stability of the encapsulated oil was slightly improved by rosmarinic acid, while most antioxidants exhibited a pro-oxidant effect. | Incorporating oil into a powder with a high antioxidant content through encapsulation yields an easily added ingredient for enrichment. | [91] |
| FOC | BHT, TBHQ, 2,5-di-tert-butyl hydroquinone (DTBHQ), propyl gallate, ascorbyl stearate, ronoxan A, 2,20-methylene-bis-(4-methyl-6-tert-butylphenol) (AO-2246), mixed tocopherols 95, α-tocopherol, δ-tocopherol, ascorbyl palmitate, shredded beans, and soybeans | - | OSI/AV/PV/ p-AnV | The addition of all antioxidants studied, except for α-tocopherol, led to ↑OSI at 100 °C. The addition of ascorbyl palmitate (0.04%) to FOC increased OSI at 100 °C from 4.25 to 14.45 h, and at a concentration of 0.02%, it led to ↓PV and ↓p-AnV at room temperature. The addition of shredded beans and soybeans (0.8%) contributed to a decrease in oxidative indices (↓PV, AV, and p-AnV). | Development of new oils with increased OS. | [92] |
| FSO | SDG, tea polyphenol, resveratrol, caffeic acid, vitamin E, BHT, BHA, TBHQ | - | PV/VOCs | The tested antioxidants showed effective inhibition of oxidation processes in the following order: TBHQ > resveratrol > SDG > tea polyphenol > BHT > vitamin E > caffeic acid > BHA. The use of a combination of 0.01% SDG, 0.01% tea polyphenol, and 0.02% vitamin C inhibits oxidation processes better than 0.02% TBHQ, which increased the shelf life of FSO from 295 to 761 days. | Development of new oils with increased OS. | [93] |
| FOC | BHT, TBHQ, mixed tocopherols, DTBHQ, ronoxan A, n-propyl-3,4,5-trihydroxybenzene, α-tocopherol, δ-tocopherol, 6-O-palmitoyl-L-ascorbic acid, 2,2′-methylenebis (4-methyl-6-tert-butylphenol), 6-O-stearoyl-L-ascorbic acid | - | PV/AV/p-AnV/OSI | All antioxidants used, except for α-tocopherol, increased the induction period (↑OSI) at 100 °C. The addition of ascorbyl palmitate (0.02%) to flaxseed oil resulted in ↓PV and ↓p-AnV at room temperature. | Increasing the shelf life of flaxseed oil. | [94] |
| FOCU | Clove essential oil; ginger, allspice, and black pepper extracts; ascorbyl palmitate | - | PV/[ROOH]/ CDs/TBARSs/VOCs | Samples containing clove essential oil and ascorbyl palmitate showed ↓PV, [ROOH], TBARSs, CDs. Ginger, allspice, and black pepper extracts either had no effect on the intensity of lipid peroxidation or exhibited a pro-oxidant effect. All antioxidants led to ↓VOC levels. | Development of new oils with increased OS. | [95] |
| Sample | Methods | Reference | |||||
|---|---|---|---|---|---|---|---|
| AOA | Value | OS | Value | BAS | Value | ||
| DFM (Extracts) | ORAC DPPH• | 0.36–1.07 mmol TE/g 0.081–0.125 g/L | - | - | TPC | 7.7–106.5 mg/g | [24] |
| DFM | ORAC | 100.82–136.05 | - | - | - | - | [102] |
| FM (Extracts) | DPPH• ORAC | 35.6–63.5% 0.23–0.65 mmol TE/g | PV Aldehyde content | 1.6–2.3 mEq/kg 60–190 mmol/L | TPC TTC α-linolenic acid | 68.2–92.3 mg GA/g DW 98.2–100% 0.24–57.0% | [103] |
| FM (Extracts) | RP DPPH• BCB | 4240 μg/g 55.28% 73.52% | - | - | - | - | [104] |
| DFM (Extracts) | TAC RP DPPH• Scavenging of hydrogen peroxide Nitric oxide-scavenging activity | 54.44 ± 0.02% 0.0–0.17 30.16 ± 0.80% 25.52 ± 0.075% 24.41 ± 0.39% | - | - | TPC | 225 ± 0.025 µg/mg | [105] |
| FM (Extracts) | ABTS•+ DPPH• | 2.95–3.10 mg/mL 3.45–3.95 mg/mL | - | - | TPC * TFC | 2.66–2.80 mg GA/g DW 1.20–1.32 mg GA/g DW | [106] |
| FM (Extracts) | - | - | - | - | TFC * SDG * TPC * | 390–1130 μg/g DW 1615.27–3416.95 μg/g DW 4.96–9.85 mg GA/g | [107] |
| FM (Extracts) | DPPH• ABTS•+ FRAP CUPRAC PCL | 9.25 ± 0.68 mg TE/g 12.13 ± 0.61 mg TE/g 61.54 ± 4.98 mg TE/g 75.50 ± 8.02 mg TE/g 191.27 mg TE/g | - | - | TPC TFC | 3.80 ± 0.28 mg GA/g n/d | [108] |
| FM (Extracts) | - | - | - | - | TPC* | 14.38–1191.21 mg/kg | [109] |
| FM and Flaxseed Cake Flour | FRAP DPPH• | 59.00–62.00 µmol TE/g DW 11.65–12.28 µmol TE/g DW | - | - | TPC * TFC * | 2.64–3.50 mg GA/g 9.06–10.54 mg QE/g | [110] |
| Object | Methods | Reference | |||||
|---|---|---|---|---|---|---|---|
| AOA | Value | OS | Value | BAS | Value | ||
| FH | DPPH• | 52.74–78.55% | PV AV p-AnV CDs CTs COX TOTOX | 1.28–4.24 meq/kg 1.4–3.2 mgKOH/g 1.23–2.51 1.45–2.64 0.20–0.55 12.49–12.94 4.83–9.88 | ChC CaC TPC TFC Vitamin C α-linolenic acid | 2.34–65.71 mg/kg 7.52–47.15 mg/kg 62.4–128.3 mg GA/100 g 12.27–17.85 mg luteolin/100 g 1.30–3.20 mg/100 g 48.95–51.52% | [111] |
| FH (Extracts) | DPPH• | 4.95–8.23 g TE/kg | - | - | TPC SDG CouAG FeAG | 15.38–32.96 g ferulic acid/kg 16.4–33.9 g/kg 35.7–49.2 g/kg 5.1–15.2 g/kg | [112] |
| FH (Extracts) | ABTS•+ DPPH• DMPD•+ O2•− scavenging effects FRAP CUPRAC TAC CA | 25.67–27.72 μg/mL 49.50–53.30 μg/mL 24.75–28.88 μg/mL 24.75–49.50 μg/mL 0.489–0.525 μg/mL 0.172–0.437 μg/mL 73.95–87.23 μg/mL 8.88–9.24 μg/mL | - | - | TPC p-Hydroxybenzoic acid Vanillin p-Coumaric acid Ascorbic acid Ferulic acid Ellagic acid | 3.88–23.30 mg QE/g 120–779 mg/kg 0–8 mg/kg 30–192 mg/kg 9–57 mg/kg 0–71 mg/kg 13–85 mg/kg | [113] |
| Object | Methods | Reference | |||||
|---|---|---|---|---|---|---|---|
| AOA | Value | OS | Value | BAS | Value | ||
| FC | ABTS•+ DPPH• FRAP | 3.1 ± 0.1 mmol TE/g DW 4.2 ± 0.0 mg GA/g DW 28.3 ± 0.1 μg of ascorbic acid/g DW | CDs PV TBARSs | 15.8 ± 0.5 μmol/mg DW 109.2 ± 5.7 μg/kg DW 4.7 ± 0.6 mg MDA/kg DW | TTC * TPC CaC * ChC * α-linolenic acid | 2563.0 ± 209.2 μg 100/g DW 484.6 ± 76.1 mg GA 100/g DW 517.0 ± 9.4 μg 100/g DW 137.8 ± 7.5 μg 100/g DW 42.81 ± 0.69% | [114] |
| FC (Various Extraction Methods) | DPPH• ABTS•+ FRAP RP | 29.59–52.96% 52.30–83.10% 0.73–1.09 mmol FeSO4/g 0.27–0.57 | AV PV p-AnV CDs CTs | 1.66–2.06 mg KOH/g 5.27–95.25 mmol/kg 0.02–90.1 2.33–15.12 0.32–2.13 | TPC TFC | 32.00–78.01 mg GA/g 1.42–2.73 mg rutin/g DW | [115] |
| DFC | DPPH• FRAP | 7.24–22.54% 1.28–8.67 μmol Fe(II)/g FW | - | - | TFC TPC | 5.61–15.64 mg luteolin/100 g FW 475.4–1257.37 mg GA/100 g FW | [116] |
| DFC (Extracts) | DPPH• FRAP | 1.72–11.39% 0.03–1.48 μmol Fe (II)/g FW | - | - | TPC * TFC | 108.33–774.33 mg GA/100 g FW 0.08–9.18 mg luteolin/100 g FW | [117] |
| FC (Extracts) | ABTS•+ | 10.5% | - | - | - | - | [118] |
| FC (Extracts) | DPPH• | 12.28 µmol TE/g | - | - | TFC * TPC * | 10.54 ± 0.18 mg QE/g DW 3.50 ± 0.02 mg GA/g DW | [119] |
| DFC (Extracts) | DPPH• FRAP | 21.15–22.56% 8.50–8.85 µmol of Fe(II)/g FW | - | TPC TFC | 1089.68–1128.53 mg GA/100 g FW 11.25–13.80 mg luteolin/100 g FW | [120] | |
| DFC (Extracts) | DPPH• | 42.79–76.22% | - | - | TPC | 22.84–53.01 mg GA/g | [121] |
| DFC | - | AV PV FTIR NMR | 1.80–3.77 mg KOH/g 0.99–3.51 mEq/kg (depending on storage time) | α-linolenic acid | 52.01 ± 0.30% | [122] | |
| DFC (Extracts) | DPPH• | 41–73% | - | - | TPC SECO MATA LARI | 206–1115 mg GA/L 0.22–7.08 mg SECO/L 0.02–0.06 mg MATA/L 0.02–0.03 mg LARI/L | [123] |
| Sample | Object of Study | Dosage | Experiment Duration | Parameters | Results | Reference |
|---|---|---|---|---|---|---|
| Flaxseed | Wistar albino rats of both sexes | 5% and 10% (0.75 and 1.5 g/kg of body weight) | 14 days | CAT Peroxidase SOD LPO | Treatment of rats with CCl4 at a dose of 2.0 g/kg of body weight ↓ activity of CAT, SOD, and peroxidase by 35.6%, 47.76%, and 53.0% compared to the control, and the value of LPO ↑ by 1.2 times. The addition of 5% flaxseed to the diet followed by CCl4 treatment caused a recovery of CAT, SOD, and peroxidase by 39.7%, 181.42%, and 123.7%, respectively. The group receiving 10.0% flaxseed showed a recovery of 95.02%, 182.31%, and 136.0% of CAT, SOD, and peroxidase. In the group receiving the toxin without flaxseed, the levels of superoxide dismutase and catalase decreased by 91.4% and 55.33%, respectively. | [129] |
| Flaxseed with increased content of phenylpropanoid compounds and hydrolyzable tannin | White Giant rabbits | 100 g/kg | 10 weeks | TAS SOD | The dyslipidemic diet had a negative effect on the lipid profile in rabbits at the 10th week of feeding. Flaxseed of the W86 variety ↑ SOD and TAS activity compared to the group receiving Linola seeds. | [130] |
| Flaxseed | Female Huoyan geese | 5%, 10%, and 15% | 56 days | CAT SOD GPx MDA | With an increase in flaxseed concentration, the activity of goose liver enzymes ↑ (CAT, SOD, GPx). MDA content in the goose liver decreased proportionally with higher dietary flaxseed levels. Supplementation of up to 15% flaxseed in the maternal diet resulted in a dose-dependent improvement in the antioxidant status of offspring. | [131] |
| Flaxseed | Landrace pigs | 10% | From 3 to 6 weeks | TAS FRAP dROMs TAC CAT SOD GPx TBARSs | No significant differences in TAS, FRAP, dROMs, or TAC were observed. In the group that received flaxseed for 3 weeks, there was a decrease in SOD, CAT, and GPX activity in the heart compared to the control group. In the group that received flaxseed for 6 weeks, the activity of these enzymes began to increase compared to the 3-week group, but the values were lower than in the control group. | [132] |
| Flaxseed oil | Male Wistar rats | 1 mL/kg of body weight | 30 days | TBARSs CAT SOD GPx | The addition of flaxseed oil prevented oxidative damage to lipids and proteins. The oil improved enzymatic antioxidant protection and ↓ glutathione levels. | [133] |
| Flaxseed oil | Crossbred Hampshire boars (50% Hampshire and 50% Gunghroo) | 3% (90 mL) | 16 weeks | GPx MDA TAC CAT | The addition of flaxseed oil significantly (p < 0.01) ↑ the concentration of GPx and CAT in blood serum, while the concentration of MDA ↓. | [134] |
| Flaxseed oil | Wistar rats | 1, 2, 3 mL/kg, intraperitoneally | 21 days | TBARSs SOD CAT | A dose-dependent inhibitory effect on antioxidant enzymes in heart, liver, and kidney tissues has been shown. | [135] |
| Flaxseed oil | Teressa goats | 25 mL | 16 weeks in the rainy season (June to September) and 16 weeks in the dry summer season (December to March) | TAC SOD CAT MDA | It has been shown that adding flaxseed oil to the diet leads to a decrease in oxidative stress levels (MDA↓ and ↑ TAC, CAT, SOD). Meanwhile, high stress levels were observed during the summer season. | [136] |
| SDG | Male Wistar rats | 20 mg/kg of body weight | 30 days | TBARSs CAT SOD GPx | SDG prevented lipid oxidative damage and enhanced enzymatic antioxidant defense while increasing total polyphenol content. The study demonstrated that the antioxidant effects attributed to flaxseed are mainly due to its high lignan content, particularly SDG. | [133] |
| Flaxseed extract | Female BALB/c mice | 150 mg/kg, 300 mg/kg, 500 mg/kg | 7 days | MDA SOD GPx CAT | The effects of flaxseed extract in the treatment of inflammatory bowel disease (colitis) were studied. Intake of the extract ↓ MDA levels and enhanced antioxidant activity. | [137] |
| Flaxseed flour | Multiparous lactating Holstein cows fitted with ruminal cannulas | 124 g/kg | 21 days | TAC MDA TBARSs | The addition of 124 g/kg of flaxseed flour to the diet of dairy cows did not improve the oxidative stability of milk. More research is needed with higher levels of flaxseed flour to assess its potential for improving the oxidative status of cows and preventing milk and plasma lipoperoxidation. | [138] |
| CLs | Male C57BL/6J mice | 10 mg/kg 30 mg/kg | 10 weeks | MDA GPx GSH GSSG | CL administration significantly enhanced antioxidant capacity in both liver and pancreatic tissues by ↑ GPx and GSH levels, while reducing GSSG and MDA concentrations. | [139] |
| Parameter | Seeds | Oil | Meal/Cake/Hull | Reference |
|---|---|---|---|---|
| TPC, mg GA/100 g | 0.6–3315.0 | 1.5–2120.0 | 2.6–128.3 | [21,23,24,30,31,36,37,41,55,111,114] |
| TFC, mg luteolin/100g | 0.4–689.2 2 | 11.8–18.8 | 0.1–17.9 | [25,31,41,65,69,75,111,116,117,120] |
| TSC, mg/kg | n/d | 3350.0–5171.7 | n/d | [56,68,71] |
| ChC, mg/kg | n/d | 0.6–16.4 | 2.3–65.7 | [55,65,111,114] |
| α-linolenic acid, % | 39.2–58.2 | 44.9–80.7 | 49.0–54.9 | [23,41,42,82,111,114,147,148,149] |
| CLs, mg/kg | 188.6–623.8 | 229.3–631.4 | 385.6–1268.9 | [4,150,151,152,153] |
| SDG, mg/g | 0.15–333.0 | 20.2–51.7 | 1.6–3.4 | [24,28,39,40,58,106,107,112] |
| SECO, mg/g | 0.8–21.7 | n/d | 0.2–7.8 1 | [23,39,40,123,154] |
| CaC, mg/kg | 0.1–6.9 | 1.6–623.0 | 5.2–47.2 | [33,84,111,149,155,156] |
| TTC, mg/kg | 70.7–747.0 | 537.0–1065.2 | 25.6–48.5 | [42,72,82,84,147,148,156,157] |
| CLs | Methods | Results | Reference |
|---|---|---|---|
| Polar fraction containing a mixture of CLs (CLA, CLD, CLE, CLF, CLG) | OSI | The polar fraction containing a mixture of CLs improved the oxidative stability of the oil. Dose-dependent and time-dependent AOA of these peptides were identified. It was established that CLA can selectively interact with metals; consequently, it can inhibit the oxidation process by chelating metal ions. | [19] |
| CLB, CLC, CLK | AV PV p-AnV TBARSs Aldehyde Ketones Molecular docking | CLB inhibited the oxidation of flaxseed oil (containing Cu2+) at the initial stage of accelerated oxidation, whereas CLK accelerated oxidation. The AOA of CLB and its oxidized form are due to their reducing capacity, as well as their ability to bind with metal ions and intermediate products of fatty acid oxidation. | [160] |
| CLB, CLC, CLE, CLP, CLJ, CLK | PV p-AnV | CLs themselves exhibited a moderate antioxidant effect on PV, but a weaker effect on p-AnV in flaxseed oil. The methionine content of CLs, in particular CLP, showed a high correlation with the accumulation of primary oxidation products (PV) in the tested matrices, while methionine sulfoxide-containing CLs better reflected changes in secondary oxidation products (p-AnV). The observed consistent correlation between CLP and CLE with the oxidation index of the oil sample indicates their potential usefulness as reliable markers for assessing oil oxidation. | [161] |
| CLO, CLM, CLN, CLL, CLB | OSI PV | The oxidation of the original, non-oxidized CLs occurs earlier and more rapidly than the oxidation of γ-tocopherol and plastochromanol-8. It has been suggested that CLs provide a certain degree of protection for vitamin E-active compounds. It has been demonstrated that CLs are essentially ingredients for retarding the oxidation of flaxseed oil. | [162] |
| CLA, CLB, CLC, CLD, CLE, CLF, CLG, CLL, CLM, CLO, CLP | OSI PV p-AnV | CLs containing tryptophan (Trp) exhibit distinct oxidative behavior in the presence of γ-tocopherol. γ-Tocopherol inhibits the oxidation of Trp-containing CLs with methionine (Met) residues and facilitates the oxidation and decomposition of Trp-containing CLs with methionine sulfoxide (MetO) residues. | [163] |
| CLB, CLC, CLK, CLE, CLJ | AV PV p-AnV | Met-containing CLs oxidize more easily than γ-tocopherol and have specific antioxidant activity. A logarithmic correlation has been found between methionine sulfone-containing CLs and oxidation values. | [164] |
| CLA | AV PV p-AnV Molecular docking Fluorescence quenching | CLA increases the antioxidant stability of refined flaxseed oil and is capable of slowing down its oxidation by chelating metal ions and intermediate oxidation products. | [165] |
| CLP, CLB, CLL, CLM, CLO, CLD, CLE, CLC, CLF, CLG | PV AV Off-line MS/MS analysis | The number of Trp and Met residues is crucial for the oxidative stability of CLs. L-ascorbyl palmitate is effective in suppressing the oxidation of both Trp-containing and Trp-free CLs. | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolova, Y.; Sobolev, R.; Kochetkova, A. Antioxidant Activity and Oxidative Stability of Flaxseed and Its Processed Products: A Review. Sci 2025, 7, 155. https://doi.org/10.3390/sci7040155
Frolova Y, Sobolev R, Kochetkova A. Antioxidant Activity and Oxidative Stability of Flaxseed and Its Processed Products: A Review. Sci. 2025; 7(4):155. https://doi.org/10.3390/sci7040155
Chicago/Turabian StyleFrolova, Yuliya, Roman Sobolev, and Alla Kochetkova. 2025. "Antioxidant Activity and Oxidative Stability of Flaxseed and Its Processed Products: A Review" Sci 7, no. 4: 155. https://doi.org/10.3390/sci7040155
APA StyleFrolova, Y., Sobolev, R., & Kochetkova, A. (2025). Antioxidant Activity and Oxidative Stability of Flaxseed and Its Processed Products: A Review. Sci, 7(4), 155. https://doi.org/10.3390/sci7040155






