Abstract
Citrus fruit processing, mainly for fresh juice production in the food industry, generates significant amounts of residues and by-products enriched with bioactive components. Peels are the primary waste fraction of citrus fruits, along with discarded pulp and seeds. This study aimed to identify the most fast and sustainable extraction process for flavonoids on a laboratory scale by varying the solvent and extraction methodology, and comparing the yields in order to evaluate their influence on total and individual flavonoid content. A chromatographic analysis was also performed using ultrahigh-performance liquid chromatography (UHPLC) with a 10 min run time. Our focus was on selecting the most user-friendly and cost-effective methodology. Ultrasound- and microwave-assisted extraction equipment were used with green solvents (water and ethanol) and compared for their efficiency in recovering flavonoid compounds from a mixture of peel and pulp. For this study, two widely cultivated Mediterranean citrus varieties were selected: ‘Marsh’ seedless grapefruits (Citrus paradisi Macf.) and ‘Comun’ mandarins (C. deliciosa Ten.). Lab-scale extraction results showed that ultrasound-assisted extraction with a simple ultrasonic bath, using an ethanol–water mixture provided the highest total flavonoid recovery and improved the extraction of key flavanones such as hesperidin, narirutin, and naringin. All ethanol–water mixtures tested (1:1, 7:3, and 3:7) yielded higher flavonoid levels in grapefruit (approximately 2500 mg/100 g DW) and mandarin (approximately 1200 mg/100 g DW) wastes compared with water or ethanol alone. This method offers a scalable and green strategy for valorizing citrus residues.
Keywords:
circular economy; grapefruits; green chemistry; mandarins; peel; UHPLC-MS; ultrasound; waste 1. Introduction
Citrus fruits are among the most widely cultivated and traded crops worldwide, with an estimated global production of about 158 million tons. The leading production regions are Asia (51%), South America (18%), Europe (15%), and Africa (13%). The main species include orange (76.1 Mt), mandarin/tangerine (39.5 Mt), lemon (9.7 Mt), and grapefruit (2 Mt) [1,2]. The World Citrus Organization (WCO) published its annual northern hemisphere citrus forecast for the 2024–2025 season, estimating production could reach 30 million tons—a notable decrease compared to the average of the past four seasons [2]. Oranges, mandarins, lemons, and grapefruits are the most widely consumed citrus fruits due to their high nutritional value. These are also the main species processed industrially, being primarily traded and consumed as fresh fruit, juice, or concentrate [3,4,5,6]. Around a third of total fresh citrus harvest is destined for juice, generating significant waste materials each year. It is necessary to manage this waste through responsible and sustainable practices, potentially increasing the product’s ultimate cost. In recent years, the recycling of food waste and its value addition have become a significant area of research focused on sustainability [7]. Peels are the main waste fraction of citrus fruits, together with discarded pulp and seeds. They provide a cost-effective and sustainable basis for the production of novel innovative food ingredients and nutraceuticals [3,4,5,8,9]. Around 15 million tons of citrus by-products are generated worldwide each year [3]. Despite the extensive knowledge regarding the composition and nutraceutical properties of citrus peels, their potential is often underestimated and neglected, resulting in the waste of thousands of tons during juice processing [10]. There is growing interest in developing initiatives for the treatment of food by-products, aiming to recover valuable compounds from them. These initiatives offer a dual benefit: reducing waste volume and minimizing waste management costs, while also enabling the recovery or production of new marketable products with economic benefits. Due to the valuable functional constituents contained in citrus waste and the growing emphasis on valorization strategies, numerous studies have explored various uses for citrus residues, minimizing environmental damage caused by improper disposal [6].
Citrus processing residues, constitute an abundant source of bioactive molecules especially phenolic compounds with positive effects on human health. These compounds play key roles in biological systems and plant stress responses, offering health benefits such as antioxidant, anticancer, cardiovascular protective, and antiviral properties [4,8,11,12,13]. Over 8000 phenolic compounds have been reported in fruits, making them one of the most important antioxidant components in fresh produce. As a result, substantial efforts have been made to quantify them in common plant species. The phenolic profile of citrus fruits has been extensively studied, with flavonoids, the principal class of polyphenols, being distributed in nearly all tissues. Their content and profile vary significantly between species and are largely influenced by genetic and environmental factors [11,14,15,16,17]. Flavanones represent the predominant class of flavonoids in citrus with characteristic compounds such as hesperidin and naringin. Moreover, citrus peels are particularly rich in polymethoxyflavones like sinensetin, nobiletin, and tangeretin [11,16].
The recovery of bioactive compounds using conventional solvents is referred to as traditional extraction and includes methods such as maceration and hot continuous extraction [11]. These methods often require large amounts of energy and organic solvents like methanol, which pose toxicity and environmental risks, as well as long processing times, limiting their practical use [18,19]. The selection of solvent depends on the chemical properties of the compounds of interest. Water–alcohol mixtures are commonly used due to their effectiveness: water facilitates the hydration of plant cells, whereas alcohol dissolves active whereas more efficiently [20]. In recent years, efforts have been made to develop alternative extraction methods that are environmentally sustainable and economically viable. These aim to recover phenolic compounds from food by-products through innovative “green” technologies, enabling the reuse of agri-food waste to obtain high-value organic compounds. It is essential that these techniques are safe, non-toxic, and environmentally friendly—aligned with green chemistry principles [21]. Extraction processes can also be supported by green technologies including ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) [10]. Many researchers have focused on extracting biologically valuable compounds from citrus waste using non-conventional techniques like UAE and MAE [6,11,18,19,22,23,24,25,26]. These approaches enhance yields, shorten extraction times, and also improve energy efficiency compared to conventional methods. This represents a sustainable alternative within the framework of the circular economy [10].
This study aimed to identify the most fast and sustainable extraction process for flavonoids on a laboratory scale. Chromatographic analysis was carried out using ultrahigh-performance liquid chromatography (UHPLC) in a short time. The experimental procedure used green solvents—water and/or ethanol—and focused on determining optimal temperature, time, and solvent conditions for microwave- and ultrasound-assisted extraction of flavonoids from citrus waste. Our objective was also to identify the easiest and most cost-effective methodology. For this purpose, several low-cost methods were developed as alternatives to traditional ones, using technologies such as ultrasound and microwaves, which yield high extraction efficiency and are economically viable. These phenolic compounds have potential applications in the food, cosmetic, and pharmaceutical industries. Grapefruit was selected for this study as it generates a large amount of peel rich in flavonoids, and mandarin, one of the most abundant citrus fruits in the Mediterranean region and widely used in the juice industry. Both produce significant amounts of waste, which, if properly managed, could yield high-value by-products.
2. Materials and Methods
2.1. Raw Materials: Plant Material and Sampling
Fruits were taken from healthy adult trees of ‘Marsh’ seedless grapefruit (Citrus paradisi Macf.) grafted onto Troyer citrange [C. sinensis (L.) Osb. × Poncirus trifoliata (L.) Raf.] rootstock, and ‘Comun’ mandarin (C. deliciosa Ten) grafted onto Carrizo citrange [C. sinensis (L.) Osb. × P. trifoliata (L.) Raf.] rootstock, from the Field Collection of Citrus Germoplasm Bank held at Instituto Valenciano de Investigaciones Agrarias (IVIA) located at Moncada (Valencia), Spain (39°33′ N; 24°24′ W). Soil typical of a Mediterranean citrus cultivation area, and the same microclimatic conditions, soil and standard cultural practices with drip irrigation. Representative samples were collected within a single harvest season between 2023 and 2024, from 14 November to 21 February for ‘Marsh’ seedless grapefruit and from 4 December to 15 January for ‘Comun’ mandarin. For each variety, fruits were harvested from three different trees, with around 20 fruits in total, and processed into four independent biological replicates. After each harvest time, fruits were transported to the Center of Citriculture and Plant Production where physicochemical and biocomponents analyses were carried out.
Fruits were randomly selected at commercial maturity and squeezed using an electric juice extractor with a rotating head (Lomi®, Model 4, Lorenzo Miguel, S.L., Madrid, Spain), and a part of the discarded pulp was collected along with the peel, and dried, powdered and kept at −80 °C until extraction and analysis (grapefruit waste, and mandarin waste). In addition, a portion of grapefruit peel was also collected separately, dried, pulverized and stored at −80 °C until analysis (grapefruit peel). Tissue homogenization was carried out using a Polytrom 3100 (Kinematica AG, Malters, Switzerland) and a vortex. Fruit quality data: juice yield, total soluble solids contents (TSS) and titratable acidity (TA), are reported in Table 1.

Table 1.
Fruit quality data for grapefruit and mandarin harvested during 2023/2024 season *.
Four samples of six fruit were used to determine the physicochemical parameters. The juice yield was measured and expressed as a percentage, calculated by dividing the volume of juice by the total fruit weight. The TSS content in juice of each sample was measured twice with a digital refractometer (model PR1, Atago, Tokyo, Japan) and data were expressed as °Brix. The determination of TA was carried out by titration with a 0.1 N sodium hydroxide solution using phenolphthalein as the indicator and expressed as g of citric acid/L of juice. The maturity index (MI) was calculated as the TSS/TA ratio.
2.2. Extraction Procedures
2.2.1. Pre-Selection of Extraction Procedures
For the pre-selection of the extraction procedures, grapefruit peel and grapefruit waste samples were used. A dried and powdered one-gram sample was dissolved in 100 mL of extraction solvent, shaken, and extracted. Briefly, one gram of sample dried and powdered was dissolved in 100 mL of extraction solvent, shaken, and extracted. To determinate the most suitable extraction, four different methods were preliminarily tested: ultrasonic extraction using an ultrasound cleaner (US) or an ultrasound-assisted extraction system (UAE), and microwave extraction using a domestic microwave oven (DM) or a high-performance microwave digestion system (MAE). Each method was evaluated in combination with two solvents (water and ethanol). Specific extraction conditions applied for each method, including time, temperature, power settings and pulse programs, are detailed in Table 2.

Table 2.
Pre-selection of extraction procedures (grapefruit peels and wastes).
Mixtures resulting were centrifuged at 4 °C for 30 min at 12,000 rpm (Centrifuge 5810R, Eppendorf Iberica, Madrid, Spain), and the supernatants were filtered through a 0.45 µm nylon filter, passed to the chromatography vial and were stored at −20 °C until analysis. In view of the results obtained, ultrasonic extraction was the best-selected technique for the following section (see below Section 2.2.2, Ultrasound-assisted extraction procedures).
2.2.2. Ultrasound-Assisted Extraction Procedures
Ultrasound-assisted extraction procedures with different solvent ratios were performed with samples of grapefruit waste and mandarin waste. A dried and powdered 100 mg sample was dissolved in 10 mL of extraction solvent, shaken, and extracted under ultrasounds during 20 min at 50 °C, according to the following ethanol-water ratios: 0:1, 1:0, 1:1, 7:3 and 3:7 (v/v). Mixtures resulting were centrifuged at 4 °C for 30 min at 12.000 rpm, and the supernatants were filtered through a 0.45 µm nylon filter, passed to the chromatography vial and were stored at −20 °C until analysis.
2.3. Flavonoid UHPLC-MS Analysis
Individual flavonoid compounds were analyzed according to the methodology and equipment described by Morales et al. (2025) [27] for apricots and by Giménez-Sanchis et al. (2024) [28] for blood oranges. An Ultra-High Performance Liquid Chromatography (UHPLC) system equipped with Vanquish separation modules, a diode array detector, autosampler and pump modules, and a column compartment, coupled to a TSQ Fortis Triple-Quadrupole Mass Spectrometer (UHPLC-QqQ-MS, Thermo Fisher Scientific, Madrid, Spain) were applied, using an Acquity Premier HSS T3 C18 (100 × 2.1 mm, 1.8 µm, Waters) column. The column temperature was maintained at 35 °C, the autosampler at 10 °C, and the injection volume was 1 µL. The mobile phase consisted of acetonitrile (A) and water with 0.1% formic acid (B) in a linear gradient of 10 min at 0.3 mL/min, starting with 10% A, ramped to 100% A at 4 min, returned to 10% A at 5 min, then held at 10% A until 10 min (total run time was 10 min). Chromatograms were recorded from 210 to 400 nm absorbance, and mass analysis was performed in a full scan from 100 to 850 m/z, with an electrospray ionization source in positive and negative modes. Chromeleon, 7.3 chromatography data system software was used for data treatment.
Compounds were identified based by comparing their retention times, UV–Vis spectra and mass spectral data with authentic standards purchased from Cymit química S.L. (Barcelona, Spain) and quantified using an external calibration curve with them (see Supplementary Figure S1: UHPLC standards chromatograms). Concentrations were determined using an external calibration curve with eriocitrin, neoeriocitrin, narirutin, naringin, hesperidin, neohesperidin, didymin, poncirin, sinensetin, nobiletin and tangeretin (Table 3). Total flavonoid content was determined by summing the major individual flavonoids quantified by UHPLC-MS, as the minor compounds were detected only at trace levels and thus excluded from the calculation.

Table 3.
Chromatographic, spectral characteristics, and mass data of flavonoids found in grapefruit and mandarin wastes.
The calibration range was adjusted based on the expected phenolics concentrations in the samples. Linearity was assessed via linear regression analysis, with correlation coefficients (R2) of 0.990 or higher, demonstrating good linearity. Furthermore, low relative deviation percentages indicated satisfactory precision in analyses conducted. All the solvents used were of UHPLC-MS grade. Three replicates per sample were analyzed and the results were expressed as mg/100 g DW. Four concentrations were prepared to carry out the external calibration curve: 0.1; 0.05; 0.02; and 0.01 mg/mL. Stock solutions of each compound at a concentration of 0.5 mg/mL were prepared using dimethyl sulfoxide-methanol (1:1). All standards and samples were filled into UHPLC brown glass vials and sealed properly to protect the solutions from light and evaporation. Standards were run daily with samples for validation.
2.4. Statistical Analysis
Statistical procedures were performed using the Statgraphics Centurion 18 software application (Manugistics Inc., Rockville, MD, USA). All the data were subjected to an analysis of variance, and means were compared by an LSD test at p ≤ 0.05.
3. Results and Discussion
3.1. Selection of Optimal Extraction Procedure
We know that industry can rely on complex and highly effective systems for waste management, but these are often expensive and technologically challenging to implement. As mentioned in the introduction, the aim of this study was to identify the fastest, most cost-effective, and environmentally friendly process for extracting citrus flavonoids at a laboratory scale, along with a chromatographic analysis conducted within a short timeframe. To this end, we developed various low-cost extraction methods as alternatives to conventional ones, employing technologies such as ultrasound and microwaves in different solvents and with different types of equipment. These methods showed high yields and demonstrated economic viability, considering the broad application of phenolic compounds in the food, cosmetic, and pharmaceutical industries. For the pre-selection of extraction procedures, we considered both ultrasound and microwave techniques. Specifically, we used an ultrasonic bath typically employed as a cleaner (US) and a domestic microwave oven (DM), both of which are inexpensive and easy to operate. In addition, we used more advanced ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) systems, which are more expensive and require specialized handling.
Over the years, various technologies and solvents have been established for the extraction of phenolic compounds. Several advanced and efficient MAE methods are available, including solvent-free microwave-assisted extraction and pressurized microwave-assisted extraction [20]. MAE offers many advantages over traditional techniques: it is faster, uses less solvent, and has higher extraction rates. Similarly, UAE is widely used for the extraction of bioactive compounds. One of its key benefits is the preservation of compounds that degrade at high temperatures. The shorter processing times and lower temperatures are particularly advantageous for thermo-labile phytochemicals like phenolics. UAE also enables faster and more reproducible extractions [22].
The effectiveness of the different extraction methods and solvents was evaluated based on the total flavonoid content obtained from grapefruit peel and waste. The results are summarized in Figure 1.
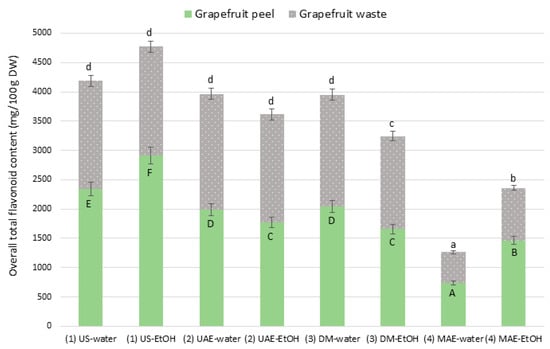
Figure 1.
Overall total flavonoid content (mg/100 g DW) from grapefruit peel and waste under different extraction procedures *: (1) US: ultrasound cleaner; (2) UAE: ultrasound-assisted extraction system; (3) DM: domestic microwave oven; (4) MAE: microwave-assisted extraction. * Data are expressed as average 3 replicates (grapefruit peel: dried peel; grapefruit waste: dried mixture of peel and discarded pulp); different letters in the same sample-type (uppercase for peels and lowercase for wastes) indicate significantly different values among extraction procedures.
Overall, US proved to be the most effective technique. In grapefruit peel, US–ethanol achieved the highest flavonoid yield, followed by US–water. In grapefruit waste, however, no significant differences were observed among the ultrasound treatments regardless of solvent, and their performance did not differ statistically from DM–water, indicating comparable efficiency for these three methods in this matrix. Thus, according to the data shown in Figure 1, in grapefruit peel, ultrasonic extraction (US and UAE) using either water or ethanol, yielded the best results (between 1800 mg/100 g DW and 3000 mg/100 g DW), compared to microwave-based methods. The only exception was microwave extraction with domestic microwave oven (DM) in water (approx. 2000 mg/100 g DW), which did not show significant differences from UAE in water, although its yield was significantly lower than ultrasonic extraction with ultrasound cleaner (US) in both solvents. Regarding grapefruit waste samples, ultrasonic extraction (US and UAE), again in both water and ethanol, produced the highest yields (1800–2000 mg/100 g DW), with the only exception being DM in water (approx. 1900 mg/100 g DW), which also did not differ significantly from either US or UAE in either solvent. In both grapefruit peel and waste samples, the least efficient methods were the advanced MAEs. Yields ranged from 500 to 700 mg/100 g DW in water, and from 900 to 1400 mg/100 g DW in ethanol.
The observed differences in extraction efficiency between grapefruit peel and waste are best explained by their distinct matrix compositions. Grapefruit peel—comprising flavedo and albedo layers—contains high amounts of cellulose, hemicellulose, lignin, and pectin (approximately 18–25% cellulose, 10–20% hemicellulose, and 15–30% lignin) [29]. Its rigid structure forms a dense barrier that limits solvent penetration and requires more aggressive or tailored extraction conditions. In contrast, citrus processing waste consists mainly of fragmented albedo, membranes, and pulp residues, which exhibit higher porosity and reduced structural integrity, facilitating solvent access and reducing sensitivity to extraction method or solvent polarity. Both UAE and DM methods showed intermediate performance, following the different trend as US in grapefruit peel since water as solvent led to higher flavonoid recovery than ethanol. In contrast, MAE was the least effective technique, particularly when combined with water. The MAE–water treatment yielded the lowest flavonoid content in both peel and waste. Although ethanol slightly improved MAE performance, the values remained significantly lower than those obtained with US, UAE, or DM. This low performance may be attributed to degradation of thermo-sensitive flavonoids under prolonged or intense microwave exposure, despite moderate temperatures being used (60 °C) [30]. These findings are consistent with literature showing that ultrasonic extraction offers gentler, more reproducible extraction of heat-sensitive bioactives compared to microwave methods [31]. Moreover, acoustic cavitation in ultrasound aids cell disruption while avoiding excessive heat, making it well-suited for extracting flavonoids from citrus peels [32].
Based on these results, we observed that ultrasonic extraction was more efficient than microwave extraction, regardless of the complexity of the equipment. Once this hypothesis was confirmed, we optimized the extraction conditions using an ultrasonic cleaner for 20 min at 50 °C and 180 W. This setup was inexpensive and easy to use.
3.2. Effect of Ethanol-Water Ratio on Flavonoid Extraction Efficiency
Following the comparative evaluation of extraction methods, US was selected for further study, as it consistently yielded the highest flavonoid contents across grapefruit peel and waste. Having identified the extraction technique, the next step focused on optimizing the solvent system to further enhance extraction efficiency while maintaining environmental and operational sustainability. For this purpose, we selected grapefruit and mandarins, two abundant citrus fruits in the Mediterranean region that are widely used in the juice industry and therefore generate significant amounts of waste. The grapefruit and mandarin waste samples consisted of dried mixtures of peel and discarded pulp. The results are summarized in Figure 2.
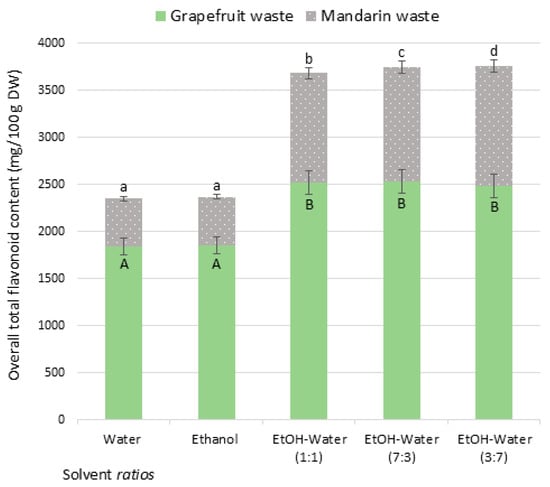
Figure 2.
Overall total flavonoid content (mg/100 g DW) from grapefruit and mandarin waste under different solvent ratios with ultrasound-assisted extraction *. * Data are expressed as average 3 replicates (grapefruit and mandarin wastes: dried mixture of peel and discarded pulp); extraction carried out under different solvent conditions; different letters in the same sample-type (uppercase for grapefruit and lowercase for mandarin wastes) indicate significantly different values among solvent ratio conditions.
Among the commonly used green solvents, ethanol stands out for its low toxicity, biodegradability, and renewable origin [33]. Additionally, combining ethanol with water has been shown to improve solvent polarity and matrix permeability, resulting in enhanced extraction efficiency [22]. Thus, in this section, pure water, pure ethanol, and ethanol–water mixtures at various ratios were evaluated to determine the most efficient solvent composition under US conditions. Figure 2 shows the total flavonoid content under different ethanol–water ratios: 0:1, 1:0, 1:1, 7:3, and 3:7 (v/v). Data indicate that all ethanol–water mixtures tested (1:1, 7:3, and 3:7) yielded higher flavonoid contents in both grapefruit (approx. 2500 mg/100 g DW) and mandarin (approx. 1200 mg/100 g DW) waste samples than did either pure water or pure ethanol alone. These findings are consistent with previous studies [20,22,34], which reported that ethanol–water mixtures are generally more effective for extracting flavonoids from plant material than either solvent alone [6,8,34]. The presence of water enhances mass transfer between solid and liquid phases by increasing the permeability of the plant matrix, thus improving extraction efficiency [22].
Regarding mandarin waste, among the mixtures tested, the 3:7 ethanol–water ratio achieved the highest flavonoid content, followed by 7:3 and 1:1. This trend suggests that a greater imbalance between water and ethanol, particularly when water is the dominant component, enhances extraction efficiency. Such mixtures benefit from a synergistic effect: ethanol improves the solubility of the solute and water contributes in its desorption from the matrix [35]. Moreover, ethanol–water systems with high water content exhibit lower viscosity and surface tension, improving mass transfer and acoustic cavitation during ultrasound-assisted extraction [36]. These physicochemical properties increase solvent accessibility to intracellular compartments, especially in soft or partially disrupted matrices like mandarin waste. Similar observations have been reported in citrus waste extractions, where intermediate-to-high water ratios maximized flavonoid yields due to enhanced solvent–matrix interactions [37]. In grapefruit waste, however, no significant differences were observed among the ethanol–water mixtures (1:1, 7:3, 3:7), all yielding comparable flavonoid contents. This matrix-insensitivity may stem from the structural properties of grapefruit waste, which, unlike the denser peel, already facilitates solvent diffusion due to its higher porosity and fragmented nature.
Another notable result is that grapefruit waste consistently yielded higher flavonoid contents than mandarin waste across all treatments, including water, ethanol, and all ethanol–water combinations. This is likely attributable to a higher intrinsic flavonoid content or more favorable extractability in grapefruit residues, consistent with previous literature reporting variability in phenolic distribution among citrus species and tissues [38].
3.3. The Profile of Flavonoid Compounds
In-depth profiling of individual flavonoids across citrus waste tissues is of paramount importance for both scientific and practical reasons. Different citrus species and tissues (peel vs. pulp) exhibit distinct flavonoid compositions, not only in terms of total content but also in the relative abundance of specific compounds such as naringin, hesperidin, or narirutin [39]. This tissue- and species-specific distribution directly impacts the bioactivity, extraction efficiency, and potential application of the extracts. For instance, naringin, abundant in grapefruit flavedo, has demonstrated strong antioxidant and anti-inflammatory properties, while hesperidin, more prevalent in mandarin peel, exhibits vasoprotective effects [40]. Understanding this variability enables more targeted valorization of citrus waste streams, guiding their optimal use in nutraceuticals, food fortification, or pharmaceutical formulations.
Thus, in addition to the aforementioned differences in total flavonoid content obtained using various solvent ratios with ultrasound-assisted extraction (Figure 2), we also observed notable differences in the profiles of major individual flavonoids (mg/100 g DW) from grapefruit and mandarin wastes extracted using the same technique (Table 4).

Table 4.
Major individual flavonoid profile (mg/100 g DW) from grapefruit and mandarin wastes with ultrasound-assisted extraction procedures *1.
In ‘Marsh’ grapefruit (C. paradisi Macf.), several studies have reported that the predominant flavanone is naringin, followed by narirutin and poncirin, with smaller amounts of neohesperidin and other compounds such as eriocitrin. Flavonoid composition and content vary among cultivars, and previous reports have highlighted that the pulp contains relatively higher concentrations of phenolic compounds compared to the peel [11,16]. In our study, the most abundant flavanone glycosides identified in grapefruit waste were naringin and neohesperidin, which were present at the highest concentrations, followed by narirutin, hesperidin, neoeriocitrin, and poncirin. Eriocitrin and didymin were detected in much lower amounts. The levels of these flavanone glycosides varied depending on the ethanol–water ratios used: 0:1, 1:0, 1:1, 7:3, and 3:7 (v/v), although it can be observed that the concentration ranges obtained are consistent with data previously reported for grapefruit. For naringin, we observed around 940 mg/100 g DW in extracts obtained using either ethanol or water alone, and around 1650 mg/100 g DW in extracts obtained using ethanol–water mixtures. Notably, this pattern aligns with the trend previously observed for total flavonoid content in mandarin waste, where the 30:70 ethanol–water ratio yielded the highest flavonoid recovery, followed by 70:30, and finally 50:50 mixtures. In addition to naringin, the extraction of didymin, a minor flavonoid glycoside, was also enhanced by ethanol–water combinations, indicating that intermediate solvent polarities favor the recovery of a broader spectrum of compounds, even those present at lower concentrations.
However, a contrasting trend was observed for neohesperidin, the second most abundant flavonoid in grapefruit waste. Its extraction was less efficient with ethanol–water mixtures compared to pure ethanol or pure water, suggesting that this compound may have a different solubility profile or stronger matrix interactions that are disrupted in the presence of mixed solvents. Wang et al. (2023) [41] pointed out that neohesperidin is difficult to extract due to its hydrophobicity, molecular conformation, or hydrogen-bonding capacity. Due to that, it is plausible that these same properties could also limit its release from the plant matrix under ultrasonic conditions. This behavior may partly explain why, despite the improvement in naringin and didymin extraction, no significant differences were previously observed in the total flavonoid content of grapefruit waste across mixed solvent treatments. A similar trend was reported by Palaigiannis et al. (2023) [42] in Cistus creticus extraction, where while a 50% ethanol–water mixture achieved total phenolic yields comparable to pure ethanol, it produced a significantly different profile of individual flavonoids without altering the total flavonoid content.
In ‘Comun’ mandarin (C. deliciosa Ten.), hesperidin has been reported as the major flavanone, followed by narirutin and didymin, among others [11,15]. Similarly, in our study, hesperidin was the most abundant flavanone glycoside in mandarin waste, detected at the highest concentrations, followed by narirutin, didymin, and eriocitrin. As observed with grapefruit, the flavanone glycoside content varied according to the ethanol–water ratios used. In all cases, significantly lower flavanone concentrations were found in extracts obtained using ethanol or water alone, compared to those using ethanol–water mixtures. For example, hesperidin concentrations were approximately 330 mg/100 g DW in extracts from pure solvents, and about 850 mg/100 g DW in those from ethanol–water mixtures. These findings align with previously published data for mandarin, and are consistent with the extraction pattern observed for total flavonoid content in mandarin waste, where the 30:70 ethanol–water mixture yielded the highest recovery, followed by 70:30, and finally 50:50. This trend was particularly clear for hesperidin, and also for narirutin, although the latter showed no significant differences between the 70:30 and 50:50 mixtures.
Regarding the content of polymethoxyflavones (PMFs), concentrations are not shown in Table 4 because the extraction conditions employed did not yield significant amounts compared to the flavanones discussed above. In grapefruit waste, two PMFs were detected: nobiletin and tangeretin. Nobiletin was present at approximately 10.00 mg/100 g DW in all samples except those extracted with water alone, in which it was not detected. Tangeretin was only found in the ethanol-only extract, at approximately 0.6 mg/100 g DW. In mandarin waste, three PMFs were identified: nobiletin (25.00–80.00 mg/100 g DW in all samples), tangeretin (approximately 20.00 mg/100 g DW, except in the water extract, which contained about 1.00 mg/100 g DW), and sinensetin (approximately 5.00 mg/100 g DW in all samples except the water extract). The low PMF yield may be attributed to their naturally low concentration in the discarded grapefruit and mandarin residues used—primarily pulp and albedo. Additionally, PMFs are moderately lipophilic, so extraction with polar solvents such as ethanol, water, or their mixtures—even when assisted by ultrasound—may be inefficient without further optimization. Other factors, such as varietal differences in flavonoid composition and potential degradation due to light, heat, or air exposure during processing, could also contribute to the low recovery.
4. Conclusions
Citrus fruits are widely consumed worldwide and generate substantial amounts of by-products rich in bioactive compounds. This study evaluated the recovery of flavonoids from grapefruit and mandarin residues, demonstrating that food-grade solvent extraction—particularly ethanol–water mixtures—provides an efficient and sustainable approach for their valorization. Ultrasound-assisted extraction, even with simple laboratory equipment, proved to be a green, low-cost, and effective alternative to conventional methods, yielding high recovery without specialized instrumentation.
Our results also emphasize the importance of considering tissue-specific differences, since peel and heterogeneous residues (pulp and albedo) respond differently to extraction methods. Notably, the 30:70 ethanol–water mixture enhanced the recovery of key flavanones such as hesperidin, narirutin, and naringin, underscoring the need to tailor solvent polarity and parameters to the matrix.
This work demonstrates the potential of sustainable extraction approaches to valorize citrus by-products as a source of bioactive compounds, particularly flavonoids, and highlighting the value of profiling individual flavonoids rather than relying solely on total content. A simple and cost-effective methodology was identified at the laboratory scale, supported by UHPLC-MS analysis, which offers a robust and reproducible strategy aligned with circular bioeconomy principles. The findings highlight the feasibility of developing innovative industrial processes for phenolic recovery, with direct relevance to the Valencian agricultural sector and possible extension to other fruits such as persimmon and pomegranate. Comparisons for their effectiveness in flavonoid recovery from citrus waste, via ultrasound- and microwave-extraction techniques using green solvents, further reinforce the practicality and sustainability of the proposed approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sci7040156/s1, Supplementary Figure S1: UHPLC standards chromatograms.
Author Contributions
J.M. and A.M. carried out the formal analysis, validation and investigation. A.B. wrote the main manuscript text including writing—original draft, and carried out the supervision, conceptualization, review & editing, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been financially supported by the project IVIA-GVA 52201 from Instituto Valenciano de Investigaciones Agrarias (project co-financed by the European Union through the ERDF Program 2021–2027 Comunitat Valenciana).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DW | Dry Weight |
| DM | Microwave extraction using a domestic microwave oven |
| MAE | Microwave extraction using a high-performance microwave digestion system |
| UAE | Ultrasonic extraction using an ultrasound-assisted extraction system |
| US | Ultrasonic extraction using an ultrasound cleaner |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
References
- Dhuique-Mayer, C.; Servent, A. An overview of the nutritional quality and health benefits linked to the world diversity of citrus fruits/juices. J. Food Sci. 2025, 90, e17576. [Google Scholar] [CrossRef]
- World Citrus Organization. Citrus World Statistics. Available online: https://worldcitrusorganisation.org/activities/citrus-world-statistics) (accessed on 5 May 2025).
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus by-products: Valuable source of bioactive compounds for food applications. Antioxidants 2023, 12, 38. [Google Scholar] [CrossRef]
- Sanches, V.L.; Cunha, T.A.; Viganó, J.; de Souza Mesquita, L.M.; Faccioli, L.H.; Breitkreitz, M.C.; Rostagno, M.A. Comprehensive analysis of phenolics compounds in citrus fruits peels by UPLC-PDA and UPLC-Q/TOF MS using a fused-core column. Food Chem. X 2022, 14, 100262. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Kumar, H.; Guleria, S.; Kimta, N.; Nepovimova, E.; Dhanjal, D.S.; Sethi, N.; Suthar, T.; Shaikh, A.M.; Bela, K.; Harsányi, E. Applications of citrus peels valorisation in circular bioeconomy. J. Agric. Food Res. 2025, 20, 101780. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Farga, A. Citrus waste as source of bioactive compounds: Extraction and utilization in health and food industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Roselli, V.; Pugliese, G.; Leuci, R.; Brunetti, L.; Gambacorta, L.; Tufarelli, V.; Piemontese, L. Green methods to recover bioactive compounds from food industry waste: A sustainable practice from the perspective of the circular economy. Molecules 2024, 29, 2682. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from citrus fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Vianello, F.; Correa, C.R.; Campos, R.A.d.; Borguini, M.G. Polyphenols in fruits and vegetables and its effect on human health. Food Nutri. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High biological value compounds extraction from citrus waste with non-conventional methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Gattuso, A.; Piscopo, A.; Romeo, R.; De Bruno, A.; Poiana, M. Recovery of bioactive compounds from calabrian bergamot citrus waste: Selection of best green extraction. Agriculture 2023, 13, 1095. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Rostagno, M.A. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits by-products–A source of valuable active principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Shpigelman, A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Chen, J.B.; Zhao, C.N.; Liu, X.J.; Chen, Y.Y.; Liang, J.J.; Sun, C.D. Advances in extraction and purification of citrus flavonoids. Food Front. 2023, 4, 750–781. [Google Scholar] [CrossRef]
- Morales, J.; Gómez-Martínez, H.; Bermejo, A. Phenolic profiles of different apricot varieties grown in Spain. Discrimination among cultivars during the harvest season. Agronomy 2025, 15, 1652. [Google Scholar] [CrossRef]
- Giménez-Sanchis, A.; Bermejo, A.; Besada, C. Changes in the sugars and volatile compounds profiles associated with anthocyanin accumulation in oranges: Blood vs. blond varieties, and slightly pigmented vs. intensely pigmented blood fruit. Food Res. Int. 2024, 197, 115199. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Vo, T.P.; Nguyen, N.T.U.; Le, V.H.; Phan, T.H.; Nguyen, T.H.Y.; Nguyen, D.Q. Optimizing ultrasonic-assisted and microwave-assisted extraction processes to recover phenolics and flavonoids from passion fruit peels. ACS Omega 2023, 8, 33870–33882. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, M.A.; Psakis, G.; Attard, K.; Lia, F.; Gatt, R. A comprehensive analysis of non-thermal ultrasonic-assisted extraction of bioactive compounds from citrus peel waste through a one-factor-at-a-time approach. Molecules 2025, 30, 648. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q.V. Impact of different solvents on the recovery of bioactive compounds and antioxidant properties from lemon (Citrus limon L.) pomace waste. Food Sci. Biotechnol. 2016, 25, 971–977. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Elkhatim, K.A.; Elagib, R.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Bermejo, A. Influence of rootstock and cultivar on bioactive compounds in citrus peels. J. Sci. Food Agric. 2011, 91, 1702–1711. [Google Scholar] [CrossRef]
- Deng, M.; Dong, L.; Jia, X.; Huang, F.; Chi, J.; Muhammad, Z.; Zhang, R. The flavonoid profiles in the pulp of different pomelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars and their in vitro bioactivity. Food Chem. X 2022, 15, 100368. [Google Scholar] [CrossRef]
- Wang, C.; Xia, N.; Yu, M.; Zhu, S. Physicochemical properties and mechanism of solubilised neohesperidin system based on inclusion complex of hydroxypropyl-β-cyclodextrin. Int. J. Food Sci. Technol. 2023, 58, 107–115. [Google Scholar] [CrossRef]
- Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Successive solvent extraction of polyphenols and flavonoids from Cistus criticus L. leaves. Oxygen 2023, 3, 274–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).