Thermodynamic Jump from Prebiotic Microsystems to Primary Living Cells
Abstract
1. Introduction
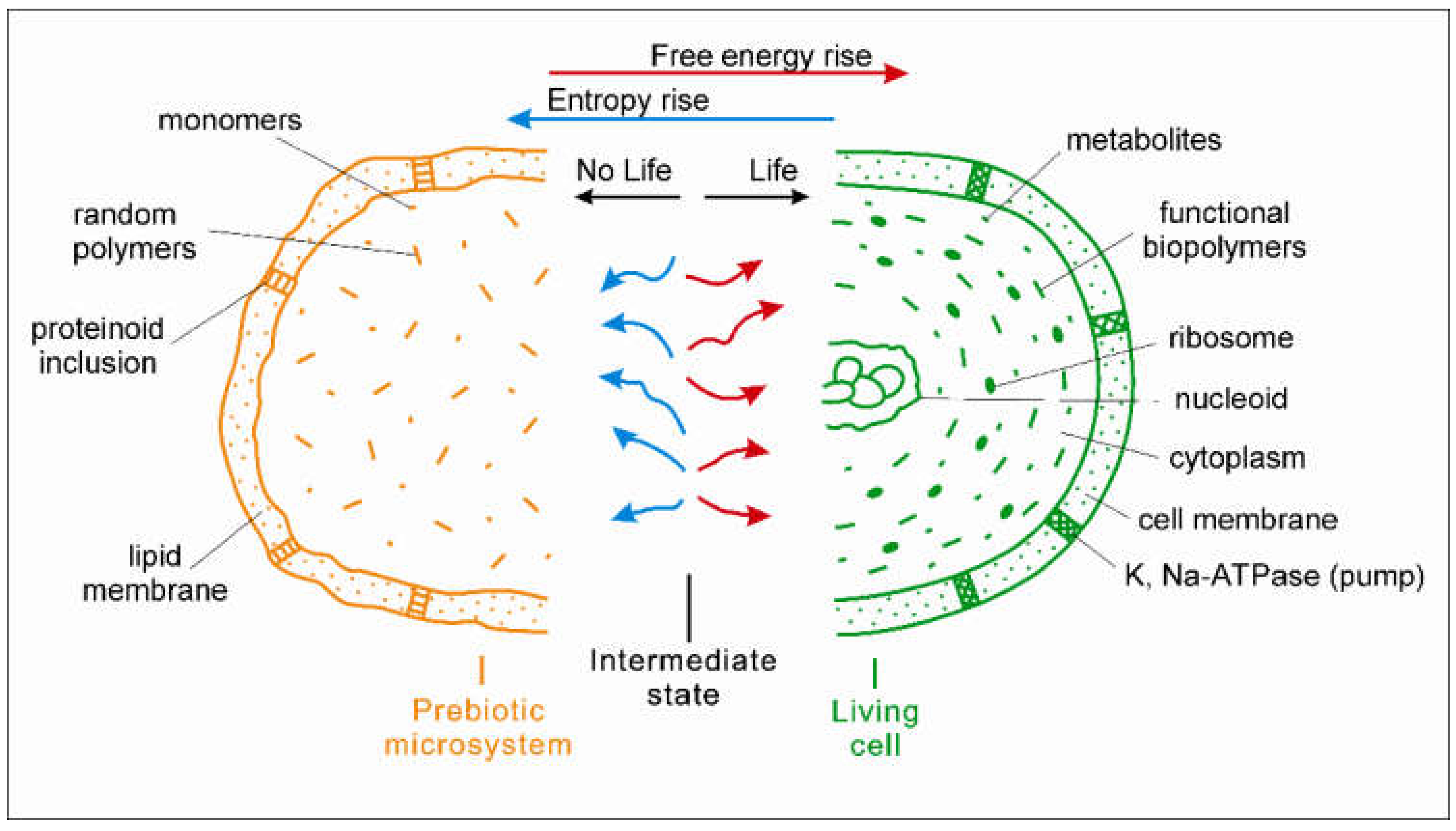
2. Thermodynamic Difference between Living and Non-Living Systems
3. Moment of the Origin of Life
4. Approaches to Experimental Verification of the Proposed Concept
5. Conclusions
Funding
Conflicts of Interest
References
- Schrodinger, E. What is Life? The Physical Aspect of the Living Cell; Lectures at the Trinity College: Dublin, Ireland, 1944. [Google Scholar]
- Kensuke, K.; Mieko, T.; Koh-ichiroh, S.; Taro, T.; Kentaro, S.; Tadashi, S. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem. 2011, 3, 775–781. [Google Scholar]
- Sugawara, T.; Kurihara, K.; Suzuki, K. Constructive approach toward protocells. In Engineering of Chemical Complexity; Mikhailov, A., Ed.; World Scientific Publishing Co.: Hackensack, NJ, USA, 2012; pp. 1–17. [Google Scholar]
- Jheeta, S. Life: Probable chemistry rather than improbable one. In Proceedings of the Abstracts of 4th NoR HST & LUCA Conference, Athens, Greece, 4–6 November 2018; p. 32. Available online: http://www.nor-cel.com/2018-conference.html (accessed on 3 July 2019).
- Kompanichenko, V.N. Thermodynamic Inversion: Origin of Living Systems; Springer International Publishing: Cham, Switzerland, 2017; Available online: https://link.springer.com/book/10.1007/978-3-319-53512-8 (accessed on 3 July 2019).
- Kompanichenko, V. The Rise of a Habitable Planet: Four Required Conditions for the Origin of Life in the Universe. Geosciences 2019, 9, 92. [Google Scholar] [CrossRef]
- Prigogine, I.; Stengers, I. Order out of Chaos; Bantam: New York, NY, USA, 1984. [Google Scholar]
- Feistel, R.; Ebeling, W. Physics of Self-organization and Evolution; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Georgiou, C. The molecular biology of the elites is replaced by an environmentally interactive biology of social equality. Critique 2019, 47, 89–121. [Google Scholar] [CrossRef]
- Kompanichenko, V. Arising of the Functional Sequences and Their Horizontal Transfer at the Moment of the Origin of Life. In Proceedings of the Abstracts of 4th NoR HST & LUCA Conference, Athens, Greece, 4–6 November 2018; pp. 26–27. Available online: http://www.nor-cel.com/2018-conference.html (accessed on 3 July 2019).
- Levchenko, V.F. Biosphere: stages of life; ISVOE: St.Petersburg, Russia, 2012. (in Russian) [Google Scholar]
- Yokoyama, S.; Koyama, A.; Nemoto, A.; Honda, H.; Imai, E.-I.; Hatori, K.; Matsuno, K. Amplification of diverse catalytic properties of evolving molecules in a simulated hydrothermal environment. Orig. Life Evol. Biosph. 2003, 33, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, S.D. Kinetic models of the prebiological evolution of macromolecules. Mendeleev Commun. 2007, 17, 7–9. [Google Scholar] [CrossRef]
- Ross, D.S.; Deamer, D. Dry/wet cycling and the thermodynamics and kinetics of prebiotic polymer synthesis. Life 2016, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.; Aubrey, A.D.; Bada, J.L. An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig. Life Evol. Biosph. 2008, 39, 109–126. [Google Scholar] [CrossRef]
- Osipovitch, D.C.; Barratt, C.; Schwartz, P.M. Systems chemistry and Parrondo’s paradox: Computational models of thermal cycling. New J. Chem. 2009, 33, 2022–2027. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kompanichenko, V. Thermodynamic Jump from Prebiotic Microsystems to Primary Living Cells. Sci 2020, 2, 14. https://doi.org/10.3390/sci2010014
Kompanichenko V. Thermodynamic Jump from Prebiotic Microsystems to Primary Living Cells. Sci. 2020; 2(1):14. https://doi.org/10.3390/sci2010014
Chicago/Turabian StyleKompanichenko, Vladimir. 2020. "Thermodynamic Jump from Prebiotic Microsystems to Primary Living Cells" Sci 2, no. 1: 14. https://doi.org/10.3390/sci2010014
APA StyleKompanichenko, V. (2020). Thermodynamic Jump from Prebiotic Microsystems to Primary Living Cells. Sci, 2(1), 14. https://doi.org/10.3390/sci2010014




