Abstract
Background: Dietary advice for Paralympic athletes (PAs) with a spinal cord injury (PAs-SCI) requires particular attention and has been widely studied. However, currently, no particular attention has been addressed to nutritional guidelines for athletes with an amputation (PAs-AMP). This study aimed at filling up this gap, at least partially, and compared veteran PAs-SCI with PAs-AMP. Methods: A sample of 25 male PAs (12 with SCI and 13 with AMP), recruited during two training camps, was submitted to the following questionnaires: allergy questionnaire for athletes (AQUA), Nordic Musculoskeletal Questionnaire (NMQ), Starvation Symptom Inventory (SSI), neurogenic bowel dysfunction (NBD), orthorexia (ORTO-15/ORTO-7), alcohol use disorders identification test (AUDIT), and Mediterranean diet adherence (MDS). The PAs were also submitted to the following measurements: dietary Oxygen Radical Absorbance Capacity (ORAC) and intakes, body composition, handgrip strength (HGS), basal energy expenditure (BEE), peak oxygen uptake (VO2peak), peak power, peak heart rate (HR), post-exercise ketosis, and antioxidant response after a cardiopulmonary exercise test (CPET) to voluntary fatigue. Results: Compared to PAs-AMP, PAs-SCI had higher NBD and lower VO2peak (p < 0.05), peak power, peak HR, peak lactate, phase angle (PhA) of the dominant leg (p < 0.05), and ORTO15 (p < 0.05). The latter was related to NBD (r = −0.453), MDS (r = −0.638), and ORAC (r = −0.529), whereas ORTO7 correlated with PhA of the dominant leg (r = 0.485). Significant differences between PAs-AMP and PAs-SCI were not found in the antioxidant response, glucose, and ketone levels after CPET, nor in dietary intake, AUDIT, AQUA, NMQ, SSI, BEE, HGS, and FM%. Conclusions: The present study showed that PAs-SCI and PAs-AMP display similar characteristics in relation to lifestyle, energy intake, basal energy expenditure, and metabolic response to CPET. Based on both the similarities with PAs-SCI and the consequences of the limb deficiency impairment, PAs-AMP and PAs-SCI require personalized nutritional advice.
1. Introduction
The International Paralympic Committee, the global governing body of the movement of the athletes with an impairment, organizes, every four years and “parallel” to the Olympic Games, some of the largest sportive events in the world named Summer and Winter Paralympic Games (PGs) [1]. In the last Summer PGs (Paris 2024), about 4400 athletes participated competing in 22 sports: Para archery, Para athletics, Blind 5-a-side soccer (for athletes with a visual impairment), Boccia, Para canoe, Para cycling, Para equestrian, Goalball, Para judo, Para powerlifting, Para rowing, Shooting Para sport, Sitting volleyball, Para fencing, Para swimming, Para table tennis, Para triathlon, Wheelchair basketball, Wheelchair rugby, Wheelchair tennis, Para badminton, and Para taekwondo. The latter substituted Parasailing and 7-a-side soccer (for athletes with cerebral palsy), which were present at the Paralympic level up to Rio 2016. In the Winter PGs, athletes compete in six sports: Para alpine skiing, Para biathlon, Para cross-country skiing, Para ice hockey, Para snowboard, and Wheelchair curling [1].
To be eligible to compete in Para sports, an athlete must have a diagnosis that leads to a permanent impairment [2]. This impairment must fall within one of the three main eligible impairment types: vision, intellectual, and physical/motor impairments [3]. The latter type includes eight impairments: impaired muscle power, impaired passive range of movement, limb deficiency, leg length difference, short stature, hypertonia, ataxia, and athetosis [2,3]. Physical impairments are divided into two subgroups: neurological and musculoskeletal impairments [3]. Among the Paralympic athletes (PAs), the most common health conditions that lead to neurological and musculoskeletal physical impairments are spinal cord injury (SCI) and amputation (AMP), respectively [3].
Apart from the cardiovascular-related physiopathology determined by the health conditions, several differences exist between PAs with SCI (PAs-SCI) and those with AMP (PAs-AMP). It is known for example that from a cardiovascular point of view, short- and long-term adaptations to sport/exercise occur in PAs-SCI [4], leading often to a reduced oxygen uptake peak (VO2peak), the measurement of cardiorespiratory fitness, which is inversely related to cardiovascular risk [5] and is higher in PAs-AMP compared to those with SCI [5]. During the Paralympic Games, PAs with neurological health conditions, such as SCI, lost more days per year due to infections and gastrointestinal problems compared with PAs with other health conditions [6].
On the other hand, regardless of the health condition, it has been suggested that pain occurs in wheelchair users because of incorrect posture, chronic overuse, and obesity [7]. The latter is a common condition in individuals with SCI [8] and AMP [9], and it is one of the most prevalent cardiometabolic risk factors [5,8,9]. Phase angle (PhA), measured by bioelectrical impedance analysis (BIA) and reflecting the extra- and intracellular water content/balance, has been suggested as a discriminator of sarcopenia in chronic musculoskeletal pain patients [10] and as a marker of oxidative stress [11].
Compared to healthy individuals, those with SCI have lower levels of exogenous antioxidants, but a similar antioxidant response to exercise [12]. In a previous study, we observed that post-exercise ketosis was associated with a reduced antioxidant response after a simulated wheelchair basketball match, with no differences in Wheelchair Basketball Athletes (WBAs) with different health conditions [13].
Although concerns about carbohydrate (CHO) intake and glycogen stores have been raised in athletes with SCI [14], limb deficiency determining muscle asymmetry should affect glycogen stores. Muscle asymmetry is related to pain in individuals with AMP [15]. It has been suggested that the Mediterranean diet (Med-D) [16,17] and plant-based diets [18,19] reduce musculoskeletal pain. Moreover, to reduce cardiometabolic risk, the Med-D has been suggested for individuals with SCI [20] and with lower limb amputation (LLA) [21]. A prospective intervention (calorie-restricted Med-D and circuit resistance training) in a cohort study involving 20 individuals with SCI reduced body mass index (BMI) and total fat mass, and improved glucose regulation, insulin sensitivity, and lipid profiles [22]. Additionally, resting energy expenditure (REE), fat oxidation, cardiorespiratory fitness, and dynamic strength increased [22].
Despite the potential of the Med-D and plant-based diets to reduce both the cardiovascular risk and musculoskeletal pain, the influence of the health conditions on digestive functions is among the determinants of the dietary behaviors of wheelchair users with both SCI and LLA [23].
It has been stated that energy balance, a fundamental health related parameter in PAs, is extremely difficult to be assessed [24] due to the measurements of both energy expenditure for the wide ranges of types, intensities, and durations of practiced sports [25,26] and activities of daily living [27] and energy intake for the physio-pathological characteristics of some PAs, such as those with SCI [28]. Moreover, high variability in resting metabolic rate exists in PAs, as well as dietary restrictions related to the health condition [28]. In particular, individuals with restrictions on eating due to gastrointestinal symptoms require an evaluation for orthorexia [29].
It is known that dietary advice for individuals and PAs-SCI requires particular attention [30,31,32], whereas an unresolved question is whether PAs-AMP should follow nutritional advice as able-bodied athletes. Indeed, at our best knowledge only two studies have specifically evaluated PAs-AMP [33,34]. In these papers, the PAs of both the Brazilian Wheelchair Women’s Basketball Team [33] and the male Brazilian Amputee Soccer Team [34] had lower CHO intake compared to the CHO recommendations for able-bodied athletes. On the other hand, Scaramella et al. [35] did not give conclusive recommendations for PAs-AMP. Indeed, from one point of view, they [35] suggested that appropriate CHO intake should be based on the lower end limit of the CHO range of able-bodied athletes for PAs with less active muscle mass (i.e., those with SCI or with double leg amputees) [35]. However, from another point of view, PAs-AMP should increase their CHO needs, because the possible inefficiency of movement of ambulant athletes with AMP may increase glycogen utilization [35].
This study aimed to deepen the knowledge on the appropriate nutritional regimen of PAs-AMP, comparing PAs-SCI with PAs-AMP using a multidimensional approach. To accomplish this aim, a descriptive cross-sectional observational study was conducted [36,37] with a mixed quantitative methodology (questionnaires, fitness and clinical measurements) [36] on veteran PAs of the “Gruppo Sportivo Paralimpico della Difesa” (GSPD) [38]. We hypothesize that, despite the expected differences in cardiorespiratory fitness, gastrointestinal symptoms, and eating behaviors, both PAs-SCI and PAs-AMP can have similar dietary habits and post-exercise glucose and ketone responses. Therefore PAs-AMP would necessitate a tailored nutrition plan [36,37] based on recommendations similar to those for PAs-SCI [14,30,31,32,35] rather than those for able-bodied athletes.
2. Materials and Methods
2.1. Recruitment, Study Design, and Characteristics of the Athletes
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Italian Army Medical Hospital. All participants read and signed the informed consent form, and they knew that they could withdraw at any time. The presence of SCI or AMP was applied as inclusion criteria.
Recruitment was carried out among PAs participating in two training camps (May and September 2022, Jesolo, Italy) of the Italian Veterans. Thirty-nine eligible PAs (19 PAs-SCI and 20 PAs-AMP), participated in the two camps, among these 25 PAs (13 PAs-AMP and 12 PAs-SCI) signed the informed consent form to participate in the study. In this cross-sectional observational study, each PA followed the study setting described in Figure 1.
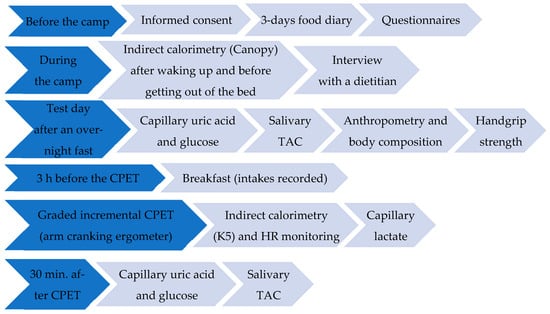
Figure 1.
Schematic diagram of the study setting.
Data were collected through questionnaires (Figure 1), including the Starvation Symptom Inventory (SSI), neurogenic bowel dysfunction (NBD) score, and allergy questionnaire for athletes (AQUA); all of them have been previously used in wheelchair basketball players [39]. The health condition of each athlete was provided by the Joint Veteran Defence Center, Scientific Department, Army Medical Center, Rome, Italy. Wheelers used manual wheelchairs with propulsion-assist devices in their leisure time.
2.2. Anthropometric Measurements, Reported Pain, and Handgrip Strength
Body mass (BM, kg) was measured on an electronic wheelchair scale with an accuracy of 0.01 kg (Wunder RW 02, Trezzo sull’ Adda (MI), Italy). BM was calculated by subtracting the mass of the wheelchair and clothes (measured separately) from the total mass. Arm and waist circumferences were measured with a constant tension, non-elastic measuring tape meter (accuracy ± 1.0 mm). Arm span measurements were taken from the tip of the middle finger of one arm to the tip of the middle finger of the other arm with the arms outstretched at right angles of 180° to the body, with extended elbow and wrist, and the palms facing directly forward. Supine length was determined using a portable stadiometer (SECA 217, Gallarate, Italy, accuracy ± 0.5 mm). BMI was calculated (mass in kilograms divided by the square of height in meters) for PAs with bilateral LLA arm spam [40], and the amputation-adjusted BMI was calculated as previously described [41] with the calculator Amputee Coalition BMI Calculator Widget 2021 (amputee-coalition.org). After this normalization for amputation level, a BMI over 25 was considered the cut-off for overweight/obesity for PAs-AMP [42]. On the other hand, the “Guidelines for Identification and Management of Cardiometabolic Risk after Spinal Cord Injury” of the Consortium for Spinal Cord Medicine expert panel suggested a cutoff of BMI ≥ 22 kg/m2, given that waist circumference is not a validated proxy for obesity in individuals with SCI [43]. Therefore, we used this cut-off for the overweight/obesity prevalence evaluation in PAs-SCI. The Nordic Musculoskeletal Questionnaire (NMQ) [44] was administered to evaluate the reported pain. A dynamometer (DINAMOMETRO SMEDLEY—SA.NI.MEDICAL S.R.L., Rome, Italy) was used to measure the handgrip strength (HGS, as kilograms to the nearest 0.1 kg).
2.3. Body Composition and Phase Angle
Fat mass percentage (FM%) was calculated by 4 skinfolds (biceps, triceps, subscapular, and suprailiac ±0.2 mm) [45], measured with a skinfold caliper (Holtain Tanner/Whitehouse skinfold caliper 610 ND, Holtain Limited®, UK). Body density equations by Durnin and Womersley [46], converted to %FM using the Siri equation [47], were used. Phase angle (PhA) was measured using the BIA 101 BIVA® PRO (Akern, Italy). The regional BIA assessment of single anatomical regions was performed using the tetrapolar technique that is useful to evaluate muscle asymmetries [48]. At the start of the measurement, participants were tested for at least 4 min in a supine position. For PAs-AMP, the electrodes were placed according to the literature [48].
2.4. Dietary Habits
Breakfast intake (buffet option, 3 h before the exercise test) was recorded on the day of the cardiopulmonary exercise test (CPET) (Figure 1). On the other hand, considering the circumstance/opportunity pattern [49] for dietary habits during a camp (full board) that could be a bias for the evaluation of differences in the habitual diet between study groups, questionnaires, food diaries, and interviews have been used to evaluate habitual dietary intake (Figure 1). In particular, dietary intake was evaluated through a 24 h food diary completed for 3 days prior to the camp (covering two consecutive weekdays and one weekend day) [50,51], followed by an interview with an expert dietitian to identify additional information considering the variation that is usually present in the diets [13,39]. The energy intake (EnI), the nutritional composition of the diet, and the dietary antioxidant capacity, expressed as Oxygen Radical Absorbance Capacity (ORAC), were calculated by the software Metadieta 3.0.1 (Meteda srl, Italy). Adherence to the Med-D was assessed with two different scores: the Mediterranean Diet Score (MDS, range 0–14: 14 items, each 0–1 score) and the Mediterranean Score (MEDScore, range 0–55: 11 items, each score range 0–5) [39]. The alcohol use disorders identification test (AUDIT) was used to assess alcohol-related problems (a score of 8 or more is considered to indicate hazardous or harmful alcohol use) [52]. The ORTO-15 and the ORTO-7, a shorter version of the ORTO-15 questionnaire, were used [53]. The ORTO-7 is based on items (1, 3, 4, 7, 9, 11 and 13) that mostly highlight the presence of orthorexia nervosa [53].
2.5. Basal and Maximal Oxygen Consumption
Basal oxygen uptake (VO2) and carbon dioxide production (VCO2) were measured following standard conditions (after waking up and before getting out of the bed) [54] by indirect calorimetry using a new generation of portable metabolimeter (Q-NRG plus, Cosmed, Italy) with a Canopy hood. After having reached the steady state conditions, measurements were continued for 10 min. Basal Respiratory Exchange Ratio (RER) and Basal Energy Expenditure (BEE) were therefore appropriately calculated (Omnia Software 2.2, Cosmed, Italy).
Two PAs with upper limb amputation (ULA, without prosthesis) and one with above-knee amputation (AKA, with pacemaker) did not perform the CPET. Therefore, 22 PAs (12 with SCI and 10 with AMP) carried out a CPET to volitional exhaustion to determine the VO2peak [4]. The protocol consisted of a graded incremental (ramp) exercise test carried out with an arm cranking ergometer (ACE). The test started with a warm-up with no resistance (0 watt [W]) for 1 min and then continued with 2 min stages. The selected power for each stage depended on the health condition and the level of lesion of the athlete [26]. The protocols were designed to complete the test in about 10 min [45,55]. The cardiorespiratory measurements (heart rate—HR, pulmonary ventilation—VE, VO2, and VCO2) were obtained using the wearable K5 breath-by-breath metabolic cart (COSMED, Italy). The file was used to assess and quantify VO2peak, peak power, and HRpeak (Omnia Software, Cosmed, Italy).
2.6. Capillary Markers and Salivary Total Antioxidant Capacity
Before and after the CPET, lactate was measured from capillary blood samples (Figure 1) taken at the earlobe through a portable instrument (Lactate Pro2 LT-1730—Arkray, Japan) at rest, at the end of the CPET test, and during recovery. The multi-parametric Fora 6 (METER S.r.l., Italy) was used to evaluate capillary blood ketone, uric acid, and glucose concentrations, after an overnight fast (pre) and 30 min after the CPET (post) (Figure 1). At the same time periods (pre and 30 min. post CPET), the salivary Total Antioxidant Capacity (TAC) was measured (Figure 1) using the SAT test with the MiniSat instrument (H&D srl, Italy), according to the manufacturer’s instructions. Absolute changes in the concentrations of capillary markers and salivary TAC following the CPET were calculated as changes versus baseline concentrations.
2.7. Statistical Analysis
The significance of differences in categorical variables among groups was assessed by the Chi-square test, and the data were expressed as percentages. Continuous variables were expressed as means and standard errors of the means (SEMs) for results passing the normality test (Shapiro–Wilk test); otherwise, the data were expressed as medians (25–75% ranges). The t-test was used for normally distributed data, otherwise the Kruskal–Wallis test was performed. Spearman’s correlations (r = coefficient of correlation) were evaluated among variables. The level of significance was set at p < 0.05.
3. Results
3.1. Characteristics of the Athletes
Among the medal winners in Veteran National competitions in 2022 (seven PAs-AMP, five PAs-SCI and two with other health conditions), all but one participated in the study. A total of twenty medals were awarded in these Games by the recruited Veteran PAs across six disciplines, with nine gold, four silver, and seven bronze medals earned in Athletics, Swimming, Para-Badminton, Open Water Swimming, Archery, and Sailing. The prevalence of medalists in the two groups of PAs was: seven medalists out of thirteen recruited PAs-AMP and four medalists out of twelve recruited PAs-SCI.
The main characteristics of the PAs are described in Table 1.

Table 1.
Characteristics of the athletes.
All PAs-SCI and 23% of the PAs-AMP were wheelchair users (Table 1). No significant differences were found between groups in the time spent on different daily activities, as well as in sporting activities and in the number of body regions with reported pain (Table 1). However, 96% of the athletes reported pain in at least one part of the body. In both groups, the prevalence of shoulder pain (64% in PAs-AMP—71% in PAs-SCI) was above 60%, whereas the prevalence of pain in other body regions was below 50% (hip/thighs 45% in PAs-AMP and 14% in PAs-SCI, ankles/feet 27% in PAs-AMP and 14% in PAs-SCI, wrists/hands 18% in PAs-AMP and 29% in PAs-SCI). Differences among the percentages of athletes who reported trunk pain (27% of PAs-AMP and 57% of PAs-SCI) or neck pain (73% of PAs-AMP and 43% of PAs-SCI) did not reach statistical significance, whereas PAs-AMP had a higher (p < 0.05) prevalence of knee/s (54% of PAs-AMP—0% of PAs-SCI) and lumbar pain (83% of PAs-AMP—29% of PAs-SCI). Furthermore, a higher (p < 0.05) percentage of PAs-AMP used anti-inflammatory drugs (73%) compared to PAs-SCI (25%), while the use of analgesics was comparable (63.6% in PAs-AMP and 50.0% in PAs-SCI).
Half of the PAs-SCI had a neurogenic bladder (treatment with oxybutynin or solifenacin). Both the AQUA and SSI scores did not differ between groups, whereas the NBD score was obviously significantly higher in PAs-SCI compared to PAs-AMP (Table 1).
PAs-SCI had lower PhA values of the dominant leg than PAs-AMP, whereas no significant differences were observed in the PhA of the dominant arm, other anthropometric measures, and HGS (Table 2). Similar prevalences of overweight/obesity and smoking habits were found in PAs-SCI (75% and 14%) and PAs-AMP (69% and 10%).

Table 2.
Anthropometry and handgrip strength.
3.2. Dietary Habits, Basal Metabolism, and Energy Intake
As described in Table 3, no significant differences were found in Med-D adherence and AUDIT scores. Median AUDIT scores (Table 3) were below those indicating hazardous or harmful alcohol use (cut-off of eight points for men) and none of the PAs-SCI or PAs-AMP showed hazardous alcohol use. ORTO-15 (but not ORTO-7) was significantly lower in PAs-SCI compared to PAs-AMP (Table 3). The prevalence of orthorexia was 9.1% and 33.3% in PAs-AMP and PAs-SCI, respectively. The normalized mean BEE expressed both as kcal/day or VO2/minute did not differ significantly between the two groups (Table 3). EnI and En% for all macronutrients, alcohol, and fiber were also similar in PAs-AMP and PAs-SCI (Table 3). ORAC and micronutrients of habitual diet did not differ significantly between groups (Table 4).

Table 3.
Dietary habits, basal metabolism, and energy intake.

Table 4.
Dietary antioxidant capacity and intake of micronutrients.
The day of the exercise test, PAs consumed the breakfast “ad libitum” (about 3 h before the CPET), with no differences between groups in EnI (327 ± 65 kcal in PAs-AMP vs. 347 ± 96 kcal in PAs-SCI), CHO %EnI (57 ± 7 in PAs-AMP, 49 ± 7 in PAs-SCI), fat %EnI (29 ± 5 in PAs-AMP, 35 ± 4 in PAs-SCI), dietary ORAC, and micronutrients (Table 4).
3.3. Response to the Cardiopulmonary Exercise Test (CPET)
VO2peak, HRpeak, power, and peak lactate levels were lower in PAs-SCI compared to PAs-AMP (Table 5), whereas no significant differences were found in basal VO2 (Table 3).

Table 5.
Responses to the cardiopulmonary exercise test (CPET).
Although two PAs-AMP had ketone levels above 0.6 mM, no significant differences were found in the variations in glucose (decrease) and ketone (increase) levels post CPET between groups (Table 5). Capillary uric acid levels and the salivary TAC increased after CPET with no differences between the two groups of PAs (Table 5).
3.4. Spearman’s Correlations
VO2peak correlated with peak power (r = 0.834), peak HR (r = 0.783), peak lactate level (r = 0.472), BEE (r = 0.491), and PhA of the dominant leg (r = 0.587). Additionally, peak power correlated with HGS (r = 0.580) and PhA of the dominant arm (r = 0.419). The latter correlated with EnI (r = 0.636), protein intake (r = 0.636), and ORAC (r = 0.591).
On the other hand, capillary uric acid levels correlated with the salivary TAC (r = 0.575). Post-exercise ketosis was inversely related to both CHO %EnI (r = −0.530) and the increase in uric acid levels (r = −0.516, p < 0.05), whereas it positively correlated with SSI (r = 0.423).
Fat %EnI was inversely related to VO2peak (r = −0.503), peak power (r = −0.665), PhA of the dominant arm (r = −0.482), Med-D adherence (MEDscore r = −0.817, MDS r = −0.626) and fiber intake (r = −0.532).
The ORTO15 and the ORTO7 (reverse scores: high values indicate low orthorexia) were related to both EnI and PhA of the dominant arm (Table 6). ORTO15 was related to NBD, MDS, ORAC, proteins in g/kg, and fiber intakes (Table 6). Moreover, higher values of ORTO15 (less orthorexia) correlated with FM%, BMI, and fat %EnI (Table 6).

Table 6.
Spearman’s correlations for orthorexia.
4. Discussion
The multidimensional evaluation through questionnaires and clinical and functional measurements carried out in the present study in veteran athletes with physical (motor) impairments determined by different health conditions (SCI and AMP) showed a wide range of responses and a great overlap of results. Although the PAs in the present study presented the expected differences in cardiorespiratory fitness (lower in PAs-SCI than PAs-AMP) and gastrointestinal symptoms (NBD scores higher in PAs-SCI than PAs-AMP), adherence to the Med-D, lifestyle characteristics (sedentary behavior and sleeping time), and the number of painful sites were not different. Moreover, similar values were found for the nutritional status and dietary intake, basal metabolism, and glucose and ketone levels after CPET, and body composition in the parts of the body without impairment. Therefore, we verified the hypothesis that athletes with an impairment need a tailored nutrition plan based on a comprehensive clinical and functional assessment that also includes and evaluation of eating behavior (e.g., eating habits, preferences, and orthorexia).
The Paralyzed Veterans of America (PVA) dietary criteria include a nutrition plan similar to the Med-D (high levels of whole grains, fruits, vegetables, legumes, and low-fat dairy products, and low levels of red meat and sugar) [56]. In the present study, adherence to the Med-D was equal to about 50%, comparable to that observed in international-level WBAs [39] but with lower values of dietary ORAC, similar to those reported in obese individuals with low adherence to the Med-D [57] and definitively lower than those found in professional cyclists during training [58]. A significant negative correlation between the Med-D adherence and the ORTO-15 score has been previously reported in both professional (r = −0.365) and recreational (r = −0.309) able-bodied athletes [59]. Accordingly, the already quoted previous study of ours revealed through Spearman’s correlation data on WBAs indicated that WBAs with a low ORTO-15 score (high orthorexia) had a high MEDScore (r = −0.539) [39]. In the present study, we confirmed that Spearman’s correlations showed that PAs with high orthorexia, assessed through ORTO-15, had high adherence to the Med-D. ORTO-15 was also related to NBD scores and ORAC. It has been suggested that ORTO-15 may be related to a commitment to wellness in WBAs [39]. Contrary to ORTO-15, which can reveal positive healthy characteristics, in university students, the ORTO-7 was more specific than ORTO-15 in assessing orthorexia nervosa, being independent from confounding variables such as body image concerns, distress, appearance, fitness, and health orientation [53]. In our study, both ORTO-15 and ORTO-7 inversely correlated with EnI (ORTO-15 r = −0.542, ORTO-7 r = −0.624) and PhA of the dominant arm (ORTO-15 r = −0.579; ORTO-7 r = −0.500). Using ORTO-7, the negative correlation between ORTO-15 score and Med-D adherence was not found. Accordingly, Med-D adherence was not related to ORTO-7 in gym attendees with an FM% below 17 [39]. In the present study, the differences between groups in FM% did not reach statistical significance, but the FM% of PAs-SCI was found to be higher than previously reported data for PAs-SCI [60]. On the other hand, the FM% of PAs-AMP was comparable to previously reported data found in PAs with above-knee amputation (AKA) or below-knee amputation (BKA) [61]. We must stress the fact that not only the PAs-SCI but also the PAs-AMP displayed FM% higher than alpine and Nordic skiers and Para ice hockey players [45]. Indeed, the PAs in the present study, regardless of the health conditions, had an FM% similar to the Para Curlers who had an FM% equal to 26.2 ± 7.74% [45].
A recent position statement based on expert consensus suggests that the health and fitness evaluation of the preparticipation screening should include nutritional and body composition assessments and a daily energy expenditure (DEE) evaluation [24]. The energy expenditure of individuals with a locomotor impairment in both physical exercise and Paralympic sports vary widely depending on the type of physical activity, the actual intensity, and the duration of both exercise and sports [25,26]. Mean energy expenditure, for example, in sitting intermittent sports (aerobic and anaerobic mixed metabolism), such as wheelchair fencing, wheelchair tennis and wheelchair basketball, and endurance sports, such as sitting Nordic skiing and long-distance wheelchair racing, ranges between 7.21 and 11.7 metabolic equivalents of tasks (Mets), respectively [25]. The assessment of DEE becomes even more difficult during sport training in mixed metabolism sports, such as wheelchair basketball (circumstance sports) and during technical training. A DEE assessment in alpine sports training can also vary widely. Furthermore, the assessment of DEE is also dependent on the activities of daily living, which are difficult to assess [27]. Due to the difficulty in the evaluation of energy balance [24], a strict periodic evaluation should be carried out, addressing the point of tailored nutrition advice.
In the present study, the fat %EnI was higher than the reported one in high-performance PAs [62], whereas the observed low intake of CHO is consistent with the previous literature related to other PAs [13,63,64]. In a review on CHO consideration for PAs, it has been pointed out that the CHO oxidation rate was lower in PAs-SCI compared to able-bodied athletes, and that they have a greater dependency on CHO timing, due to the reduced glycogen storage capacity [14]. Accordingly, we proposed that elite WBAs with relatively low CHO intake could be at risk of both malnutrition and post-exercise ketosis [13]. Furthermore, it has been suggested that able-bodied individuals, following a session of aerobic exercise, presented increased TAC and uric acid levels as a protective reaction against oxidative stress [65]. In the present study, after CPET, both salivary TAC and blood uric acid levels increased, with no differences between PAs-AMP and PAs-SCI in the antioxidant response to CPET, as well as in glucose and ketone levels after CPET.
Positive correlations between TAC and PhA have been observed in patients with chronic kidney disease and older women [11]. In individuals with chronic musculoskeletal pain, the proposed cut-off value for PhA discriminating sarcopenia was 5.1 for men [10]. In the present study, the mean PhA value was below the cut-off for sarcopenia [10] only in the lower limbs of PAs-SCI.
On the other hand, the mean intake of proteins normalized for BM (mean values ranging from 1.2 to 1.4 g/kg BM) was adequate for PAs (1.2–1.7 g/kg BM) [20]. Although a high-protein intake diet might have a negative effect on kidney function in individuals with SCI, malnutrition can increase the risk of pressure ulcers [32]. The latter increases protein needs in sedentary individuals with SCI (at least 1.25 g/kg BM) for wound healing [32]. Furthermore, because stump ulcers are common problems in amputees using prosthetic limbs [66], a diet rich in protein would be advisable.
In veterans with unilateral BKA (97.9% with a prosthetic limb), a high prevalence of pain was reported (stump pain 84.2%, low back pain 78.1%, and knee pain 54.7%) and low back pain was higher in amputees with stump pain [67]. Comparing reported pain, we observed a high prevalence of both lumbar pain and use of anti-inflammatory drugs in PAs-AMP compared to PAs-SCI, whereas the use of analgesic drugs was comparable. Chronic pain is common (80%) in individuals with SCI [68] and it is known that neuropathic pain in both lower trunk and legs and spasticity can be observed in individuals with SCI [69]. In a previous study, the prevalence of neuropathic pain was 51% and 38% of patients with cervical and thoracic SCI, respectively, and 85% of patients with thoracic SCI and 87% of those with cervical SCI experienced spasticity [69]. Overall, in our study the prevalence of musculoskeletal pain at any location was higher (96%) than that previously reported in wheelchair users (50%) [7]. In both groups, we found the well-documented high prevalence of shoulder pain in wheelchair athletes (68%) and nonathletic wheelchair users (67%) [70]. Although it has been suggested that shoulder pain has a significant impact on the range of motion, leading to functional limitations [7], the absence of relationship between number of regions with reported pain and VO2peak suggested that pain did not affect the execution of CPET on the arm cranking ergometer.
Health conditions, the type of impairment, and level of lesion impact VO2peak, and therefore the central (heart) and peripheral (vascular and muscular systems) short- [4] and long-term [71] adaptations to exercise differ when comparing PAs-AMP and PAs-SCI [4,71]. For this reason, VO2peak, which has an impact on performance in endurance, power, and intermittent (aerobic-anaerobic alternated) sports [45], shows also an inverse relationship with cardiovascular risk factors [5]. VO2peak is therefore a fundamental component of physical fitness to be assessed in PAs competing in different sports [72]. In our study, cardiorespiratory fitness (VO2peak and peak power) was higher in PAs-AMP compared to PAs-SCI. PAs-AMP had VO2peak values consistent with the values of the Italian National WBAs [13,26]. Because the intensity and energy expenditure of a sport discipline has a strict relationship with VO2peak [26], PAs competing in different sports display different VO2peak values [26,45,72]. Therefore, the major limitation of our study is that the limited sample size prevented us from pooling athletes in sport discipline groups.
The first point of strength of the present paper is that we collected parameters with non-invasive (VO2, HR, and saliva) or minimally invasive (capillary blood) methods, making the study easily transferable on a large-scale during training camps. The second point of strength is that performing the test during training camps allowed us to measure BEE after waking up and before getting out of bed by indirect calorimetry, according to the best practice suggested in the literature [30,54]. In the present study, differences in both BEE and FM% between groups did not reach significance, suggesting that limb muscle asymmetry due to amputation generates similar effects to those observed in PAs-SCI with different upper and lower limb PhA. However, the limited sample size prevented us from subgrouping athletes for type and level of injury (i.e., PAs with AKA–BKA, unilateral–bilateral AMP, and paraplegia versus tetraplegia). Moreover, the selected population recruited, veteran PAs from the GSPD [30], could reduce the generalization of the results to other PAs. From that, further studies are needed to give specific and definitive recommendations.
5. Conclusions
Nutrient requirements for PAs are influenced by numerous intrinsic (health condition, impairment, and body composition) and extrinsic factors (lifestyle and type and intensity of the practiced sport). The present experimental study, aimed at clarifying the appropriate methodology to prescribe the best diet for PAs-AMP, utilizes fundamental and typically used measurements and questionnaires and introduces new (for PAs) measurements, such as metabolic responses to CPET and lifestyle questions. The results of the study showed substantial similarities between PAs-SCI and PAs-AMP, but with a wide range of responses. Indeed, reported pain, starvation symptoms, as well as antioxidant and glucose responses to CPET did not differ between the two groups of PAs. Therefore, the nutritional consideration for CHO intake due to the high risk of diabetes in PAs-SCI [5,14] should be made also for PAs-AMP, also considering that PAs with LLA showed higher blood GLU levels compared to PAs-SCI in our previous study [73]. Furthermore, Med-D adherence and dietary intakes of the PAs with different health conditions were also comparable, despite NBD and ORTO-15 scores being significantly different between groups. A high prevalence of orthorexia nervosa has been found in able-bodied individuals focused on sports performance or body composition (34.5%) [74], whereas in PAs it was related to gastrointestinal symptoms [39]. These data demonstrate that PAs with a locomotor impairment are, in some ways, fragile individuals, despite being athletes, and therefore they should be also evaluated from a clinical point of view using also laboratory measurements. In conclusion, confirming our hypothesis (PAs-AMP should follow the nutritional advice for PAs-SCI rather than those typical of able-bodied athletes), both PAs-SCI and PAs-AMP require detailed clinical and functional evaluations and assessments to facilitate the development of personalized nutritional advice. From this, the “single case experimental design” recently suggested for PAs in each feature of the clinical and sport life [75] could be the best practice to manage nutrition in Paralympians with a motor impairment.
Author Contributions
Conceptualization, I.P., T.S., and M.B.; formal analysis, I.P., A.R. (Anna Raguzzini), and E.T.; investigation, I.P., A.R. (Anna Raguzzini), E.T., G.B., R.F., D.M., P.R.B., and T.S.; data curation, I.P., A.R. (Anna Raguzzini), and E.T.; data interpretation: I.P., A.R. (Anna Raguzzini), E.T., R.F., V.C., and M.B.; writing, I.P., A.R. (Anna Raguzzini), E.T., and M.B.; review and editing, G.B., A.R. (Alberto Rainoldi), V.C., C.M., and T.S.; supervision, I.P. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINISTERO DELLA DIFESA grant number M_D E13985 REG2021 0032116 28-07-2021 (Project AMAMP).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Italian Army Medical Hospital, Rome, Italy. (protocol code CE/2021u/03/a-31/03/2021-09.a and 31 March 2021).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data is unavailable due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AKA | above-knee amputation |
| AMP | amputation |
| AQUA | allergy questionnaire for athletes |
| AUDIT | alcohol use disorders identification |
| BEE | basal energy expenditure |
| BIA | bioelectrical impedance analysis |
| BKA | below-knee amputation |
| BM | body mass |
| BMI | body mass index |
| CPET | cardiopulmonary exercise test |
| EnI | energy intake |
| FM%: | fat mass percentage |
| HGS | handgrip strength |
| HR | heart rate |
| LLA | lower limb amputation |
| MDS | Mediterranean diet score |
| Med-D | Mediterranean diet |
| MEDScore | Mediterranean score |
| NBD | neurogenic bowel dysfunction |
| NMQ | Nordic Musculoskeletal Questionnaire |
| ORAC | Oxygen Radical Absorbance Capacity |
| ORTO | score for orthorexia |
| PA | Paralympic athletes |
| PhA | phase angle |
| PVA | Paralyzed Veterans of America |
| R | coefficient of correlation |
| RER | Respiratory Exchange Ratio |
| SCI | spinal cord injury |
| SSI | Starvation Symptom Inventory |
| TAC | Total Antioxidant Capacity |
| ULA | upper limb amputation |
| VO2 peak | peak oxygen uptake |
| WBAs | Wheelchair Basketball Athletes |
References
- International Paralimpic Committe. Available online: https://www.paralympic.org/sports (accessed on 21 February 2025).
- International Paralimpic Committee. Available online: https://www.paralympic.org/classification (accessed on 21 February 2025).
- Derman, W.; Badenhorst, M.; Blauwet, C.; Emery, C.A.; Fagher, K.; Lee, Y.H.; Kissick, J.; Lexell, J.; Miller, I.S.; Pluim, B.M.; et al. Para sport translation of the IOC consensus on recording and reporting of data for injury and illness in sport. Br. J. Sports Med. 2021, 55, 1068–1076. [Google Scholar] [CrossRef]
- Bernardi, M.; Guerra, E.; Rodio, A.; Dante, D.; Castellano, V.; Peluso, I.; Schena, F.; Bhambhani, Y. Assessment of Exercise Stroke Volume and Its Prediction From Oxygen Pulse in Paralympic Athletes With Locomotor Impairments: Cardiac Long-Term Adaptations Are Possible. Front Physiol. 2020, 10, 1451. [Google Scholar] [CrossRef]
- Bernardi, M.; Romano, S.; Squeo, M.R.; Guerra, E.; Adami, P.E.; Alviti, F.; Mattei, A.; Corsi, L.; Lanzano, R.; Curatulo, P.G.; et al. Aerobic fitness is a potential crucial factor in protecting paralympic athletes with locomotor impairments from atherosclerotic cardiovascular risk. Sport Sci. Health 2021, 17, 363–374. [Google Scholar] [CrossRef]
- Steffen, K.; Clarsen, B.; Gjelsvik, H.; Haugvad, L.; Koivisto-Mørk, A.; Bahr, R.; Berge, H.M. Illness and injury among Norwegian Para athletes over five consecutive Paralympic Summer and Winter Games cycles: Prevailing high illness burden on the road from 2012 to 2020. Br. J. Sports Med. 2022, 56, 204–212. [Google Scholar] [CrossRef]
- Liampas, A.; Neophytou, P.; Sokratous, M.; Varrassi, G.; Ioannou, C.; Hadjigeorgiou, G.M.; Zis, P. Musculoskeletal Pain Due to Wheelchair Use: A Systematic Review and Meta-Analysis. Pain Ther. 2021, 10, 973–984. [Google Scholar] [CrossRef]
- McMillan, D.W.; Bigford, G.E.; Farkas, G.J. The Physiology of Neurogenic Obesity: Lessons from Spinal Cord Injury Research. Obes. Facts 2023, 16, 313–325. [Google Scholar] [CrossRef]
- Ladlow, P.; Nightingale, T.E.; McGuigan, M.P.; Bennett, A.N.; Koumanov, F.; Phillip, R.; Bilzon, J.L.J. Influence of traumatic lower-limb amputation on physical activity, body composition, and cardiometabolic risks: A descriptive preliminary study. PM R 2023, 15, 413–425. [Google Scholar] [CrossRef]
- Tsuji, H.; Tetsunaga, T.; Misawa, H.; Nishida, K.; Ozaki, T. Association of phase angle with sarcopenia in chronic musculoskeletal pain patients: A retrospective study. J. Orthop. Surg. Res. 2023, 18, 87. [Google Scholar] [CrossRef]
- da Silva, B.R.; Gonzalez, M.C.; Cereda, E.; Prado, C.M. Exploring the potential role of phase angle as a marker of oxidative stress: A narrative review. Nutrition 2022, 93, 111493. [Google Scholar] [CrossRef]
- Wouda, M.F.; Slettahjell, H.B.; Lundgaard, E.; Bastani, N.E.; Raastad, T.; Blomhoff, R.; Kostovski, E. Acute changes in antioxidants and oxidative stress to vigorous arm exercise: An intervention trial in persons with spinal cord injury and healthy controls. Spinal Cord Ser. Cases 2023, 9, 32. [Google Scholar] [CrossRef]
- Raguzzini, A.; Toti, E.; Bernardi, M.; Castellucci, F.; Cavedon, V.; Fedullo, A.L.; Milanese, C.; Sciarra, T.; Peluso, I. Post-Exercise Ketosis, Salivary Uric Acid and Interleukin-6 after a Simulated Wheelchair Basketball Match. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2055–2062. [Google Scholar] [CrossRef]
- Ruettimann, B.; Perret, C.; Parnell, J.A.; Flueck, J.L. Carbohydrate Considerations for Athletes with a Spinal Cord Injury. Nutrients 2021, 13, 2177. [Google Scholar] [CrossRef]
- Kowal, M.; Winiarski, S.; Gieysztor, E.; Kołcz, A.; Dumas, I.; Paprocka-Borowicz, M. Symmetry Function: The Differences between Active and Non-Active Above-the-Knee Amputees. Sensors 2022, 22, 5933. [Google Scholar] [CrossRef]
- Mendonça, C.R.; Noll, M.; Castro, M.C.R.; Silveira, E.A. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients 2020, 12, 3075. [Google Scholar] [CrossRef]
- Cuevas-Cervera, M.; Perez-Montilla, J.J.; Gonzalez-Muñoz, A.; Garcia-Rios, M.C.; Navarro-Ledesma, S. The Effectiveness of Intermittent Fasting, Time Restricted Feeding, Caloric Restriction, a Ketogenic Diet and the Mediterranean Diet as Part of the Treatment Plan to Improve Health and Chronic Musculoskeletal Pain: A Systematic Review. Int. J. Environ. Res. Public Health. 2022, 19, 6698. [Google Scholar] [CrossRef]
- Mendonça, C.R.; Noll, M.; Cardoso, C.K.S.; Santos, A.S.A.C.; Rodrigues, A.P.D.S.; Silveira, E.A. Reduction in Pain and Pain Intensity with Nonpharmacological Treatment in Severely Obese Patients: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 11112. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.; Deliens, T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A. Do Nutritional Factors Interact with Chronic Musculoskeletal Pain? A Systematic Review. J. Clin. Med. 2020, 9, 702. [Google Scholar] [CrossRef]
- Bernardi, M.; Fedullo, A.L.; Bernardi, E.; Munzi, D.; Peluso, I.; Myers, J.; Lista, F.R.; Sciarra, T. Diet in neurogenic bowel management: A viewpoint on spinal cord injury. World J. Gastroenterol. 2020, 26, 2479–2497. [Google Scholar] [CrossRef]
- Köroğlu, Ö.; Tel Adıgüzel, K. Cardiometabolic risk parameters of individuals with lower extremity amputation: What is the effect of adherence to DASH diet and Mediterranean diet? Turk. J. Phys. Med. Rehabil. 2020, 66, 291–298. [Google Scholar] [CrossRef]
- Bigford, G.E.; Lehmann, D.A.; Betancourt, L.F.; Maher, J.L.; Mendez, A.J.; Nash, M.S. Modification of the Diabetes Prevention Program Lifestyle Intervention in Persons with Spinal Cord Injury: Efficacy for Reducing Major Cardiometabolic Risks, Increased Fitness, and Improved Health-Related Quality of Life. J. Spine Res. Surg. 2024, 6, 6–25. [Google Scholar] [CrossRef]
- Holla, J.F.M.; van den Akker, L.E.; Dadema, T.; de Groot, S.; Tieland, M.; Weijs, P.J.M.; Deutekom, M.; WHEELS-Study Group. Determinants of dietary behaviour in wheelchair users with spinal cord injury or lower limb amputation: Perspectives of rehabilitation professionals wheelchair users. PLoS ONE 2020, 15, e0228465. [Google Scholar] [CrossRef]
- Pinheiro, L.; Verhagen, E.; Ocarino, J.; Fagher, K.; Ahmed, O.H.; Dalton, K.; Mann, D.L.; Weiler, R.; Akinyi Okoth, C.; Blauwet, C.A.; et al. Periodic health evaluation in Para athletes: A position statement based on expert consensus. BMJ Open Sport Exerc. Med. 2024, 10, e001946. [Google Scholar] [CrossRef]
- Price, M. Energy expenditure and metabolism during exercise in persons with a spinal cord injury. Sports Med. 2010, 40, 681–696. [Google Scholar] [CrossRef]
- Bernardi, M.; Guerra, E.; Di Giacinto, B.; Di Cesare, A.; Castellano, V.; Bhambhani, Y. Field evaluation of paralympic athletes in selected sports: Implications for training. Med. Sci. Sports Exerc. 2010, 42, 1200–1208. [Google Scholar] [CrossRef]
- Nightingale, T.E.; Rouse, P.C.; Thompson, D.; Bilzon, J.L.J. Measurement of Physical Activity and Energy Expenditure in Wheelchair Users: Methods, Considerations and Future Directions. Sports Med. Open 2017, 3, 10. [Google Scholar] [CrossRef]
- Jonvik, K.L.; Vardardottir, B.; Broad, E. How Do We Assess Energy Availability and RED-S Risk Factors in Para Athletes? Nutrients 2022, 14, 1068. [Google Scholar] [CrossRef]
- Tuck, C.J.; Sultan, N.; Tonkovic, M.; Biesiekierski, J.R. Orthorexia nervosa is a concern in gastroenterology: A scoping review. Neurogastroenterol. Motil. 2022, 34, e14427. [Google Scholar] [CrossRef]
- Wilson, J.; Brochetti, A.; Shermon, S.; Twist, E. Recent Updates in Nutrition After Spinal Cord Injury: 2015 Through 2021. Curr. Phys. Med. Rehabil. Rep. 2022, 10, 282–290. [Google Scholar] [CrossRef]
- Flueck, J.L. Nutritional Considerations for Para-Cycling Athletes: A Narrative Review. Sports 2021, 9, 154. [Google Scholar] [CrossRef]
- Flueck, J.L.; Parnell, J.A. Protein Considerations for Athletes With a Spinal Cord Injury. Front. Nutr. 2021, 8, 652441. [Google Scholar] [CrossRef]
- Eskici, G.; Ersoy, G. An evaluation of wheelchair basketball players’ nutritional status and nutritional knowledge levels. J. Sports Med. Phys. Fit. 2016, 56, 259–268. [Google Scholar] [PubMed]
- Innocencio da Silva Gomes, A.; Gonçalves Ribeiro, B.; de Abreu Soares, E. Nutritional profile of the Brazilian Amputee Soccer Team during the precompetition period for the world championship. Nutrition 2006, 22, 989–995. [Google Scholar] [CrossRef]
- Scaramella, J.; Kirihennedige, N.; Broad, E. Key Nutritional Strategies to Optimize Performance in Para Athletes. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 283–298. [Google Scholar] [CrossRef]
- Ranganathan, P.; Aggarwal, R. Study designs: Part 1—An overview and classification. Perspect. Clin. Res. 2018, 9, 184–186. [Google Scholar] [CrossRef]
- Boushey, C.; Harris, J.; Bruemmer, B.; Archer, S.L.; Van Horn, L. Publishing nutrition research: A review of study design, statistical analyses, and other key elements of manuscript preparation, Part 1. J. Am. Diet. Assoc. 2006, 106, 89–96. [Google Scholar] [CrossRef]
- Sport Paralimpici Militari. Available online: https://www.difesa.it/smd/approfondimenti/sportparalimpicimilitari/index.html (accessed on 21 February 2025).
- Toti, E.; Cavedon, V.; Raguzzini, A.; Fedullo, A.L.; Milanese, C.; Bernardi, E.; Bellito, S.; Bernardi, M.; Sciarra, T.; Peluso, I. Dietary Intakes and Food Habits of Wheelchair Basketball Athletes Compared to Gym Attendees and Individuals who do not Practice Sport Activity. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 38–48. [Google Scholar] [CrossRef]
- Miller, M.; Wong, W.K.; Wu, J.; Cavenett, S.; Daniels, L.; Crotty, M. Upper-arm anthropometry: An alternative indicator of nutritional health to body mass index in unilateral lower-extremity amputees? Arch. Phys. Med. Rehabil. 2008, 89, 2031–2033. [Google Scholar] [CrossRef]
- Wong, C.K.; Wong, R.J. Standard and Amputation-Adjusted Body Mass Index Measures: Comparison and Relevance to Functional Measures, Weight-Related Comorbidities, and Dieting. Am. J. Phys. Med. Rehabil. 2017, 96, 912–915. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 21 February 2025).[Green Version]
- Nash, M.S.; Groah, S.L.; Gater, D.R., Jr.; Dyson-Hudson, T.A.; Lieberman, J.A.; Myers, J.; Sabharwal, S.; Taylor, A.J.; Consortium for Spinal Cord Medicine. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury: Clinical Practice Guideline for Health Care Providers. Top. Spinal Cord Inj. Rehabil. 2018, 24, 379–423. [Google Scholar] [CrossRef]
- Karabay, D.; Yildiz, M.; Caliskan, N.; Ozer Kaya, D. Comparisons and associations of psychological factors and the number of painful sites in wheelchair basketball athletes with and without shoulder pain: A cross-sectional case-control study. J. Spinal Cord Med. 2024, Oct 14, 1–12. [Google Scholar] [CrossRef]
- Bernardi, M.; Carucci, S.; Faiola, F.; Egidi, F.; Marini, C.; Castellano, V.; Faina, M. Physical fitness evaluation of paralympic winter sports sitting athletes. Clin. J. Sport Med. 2012, 22, 26–30. [Google Scholar] [CrossRef]
- Durnin, J.V.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef]
- Siri, W.E. (Ed.) Body Composition from Fluid Spaces and Density: Analysis of Method; National Academy of Sciences: Washington, DC, USA, 1961. [Google Scholar][Green Version]
- Choi, H.J.; Ko, C.Y.; Chang, Y.; Kim, G.S.; Choi, K.; Kim, C.H. Development and validation of bioimpedance prediction equations for fat-free mass in unilateral male amputees. PeerJ 2021, 9, e10970. [Google Scholar] [CrossRef]
- Hamlin, R. The Relative Merits of Observational and Experimental Research: Four Key Principles for Optimising Observational Research Designs. Nutrients 2022, 14, 4649. [Google Scholar] [CrossRef]
- Green, T.J.; Allen, O.B.; O’Connor, D.L. A three-day weighed food record and a semiquantitative food-frequency questionnaire are valid measures for assessing the folate and vitamin B-12 intakes of women aged 16 to 19 years. J. Nutr. 1998, 128, 1665–1671. [Google Scholar] [CrossRef]
- Bailey, R.L. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr. Opin. Biotechnol. 2021, 70, 91–96. [Google Scholar] [CrossRef]
- Wood, E.; Pan, J.; Cui, Z.; Bach, P.; Dennis, B.; Nolan, S.; Socias, M.E. Does This Patient Have Alcohol Use Disorder?: The Rational Clinical Examination Systematic Review. JAMA 2024, 331, 1215–1224. [Google Scholar] [CrossRef]
- Aiello, P.; Toti, E.; Villaño, D.; Raguzzini, A.; Peluso, I. Overlap of orthorexia, eating attitude and psychological distress in some Italian and Spanish university students. World J. Psychiatry 2022, 12, 1298–1312. [Google Scholar] [CrossRef]
- Alazzam, A.M.; Alrubaye, M.W.; Goldsmith, J.A.; Gorgey, A.S. Trends in measuring BMR and RMR after spinal cord injury: A comprehensive review. Br. J. Nutr. 2023, 130, 1720–1731. [Google Scholar] [CrossRef]
- van der Woude, L.H.; Bouten, C.; Veeger, H.E.; Gwinn, T. Aerobic work capacity in elite wheelchair athletes: A cross-sectional analysis. Am. J. Phys. Med. Rehabil. 2002, 81, 261–271. [Google Scholar] [CrossRef]
- Farkas, G.J.; Sneij, A.; McMillan, D.W.; Tiozzo, E.; Nash, M.S.; Gater, D.R., Jr. Energy expenditure and nutrient intake after spinal cord injury: A comprehensive review and practical recommendations. Br. J. Nutr. 2022, 128, 863–887. [Google Scholar] [CrossRef]
- Kolomvotsou, A.I.; Rallidis, L.S.; Mountzouris, K.C.; Lekakis, J.; Koutelidakis, A.; Efstathiou, S.; Nana-Anastasiou, M.; Zampelas, A. Adherence to Mediterranean diet and close dietetic supervision increase total dietary antioxidant intake and plasma antioxidant capacity in subjects with abdominal obesity. Eur. J. Nutr. 2013, 52, 37–48. [Google Scholar] [CrossRef]
- Leonardo-Mendonça, R.C.; Concepción-Huertas, M.; Guerra-Hernández, E.; Zabala, M.; Escames, G.; Acuña-Castroviejo, D. Redox status and antioxidant response in professional cyclists during training. Eur. J. Sport Sci. 2014, 14, 830–838. [Google Scholar] [CrossRef]
- Martinovic, D.; Tokic, D.; Martinovic, L.; Vilovic, M.; Vrdoljak, J.; Kumric, M.; Bukic, J.; Ticinovic Kurir, T.; Tavra, M.; Bozic, J. Adherence to Mediterranean Diet and Tendency to Orthorexia Nervosa in Professional Athletes. Nutrients 2022, 14, 237. [Google Scholar] [CrossRef]
- Cavedon, V.; Sandri, M.; Peluso, I.; Zancanaro, C.; Milanese, C. Body composition and bone mineral density in athletes with a physical impairment. PeerJ 2021, 9, e11296. [Google Scholar] [CrossRef]
- Cavedon, V.; Sandri, M.; Peluso, I.; Zancanaro, C.; Milanese, C. Sporting activity does not fully prevent bone demineralization at the impaired hip in athletes with amputation. Front. Physiol. 2022, 13, 934622. [Google Scholar] [CrossRef]
- Duarte Junior, M.A.; Enriquez-Martinez, O.G.; Brisola, K.M.; Oliveira, J.; Molina, M.D.C.B.; Trakman, G.L.; de Mello, M.T.; Longhi, R. Nutritional intake in high-performance para athletes. Nutrition 2023, 116, 112168. [Google Scholar] [CrossRef]
- Ferro, A.; Garrido, G.; Villacieros, J.; Pérez, J.; Grams, L. Nutritional Habits and Performance in Male Elite Wheelchair Basketball Players During a Precompetitive Period. Adapt. Phys. Activ. Q 2017, 34, 295–310. [Google Scholar] [CrossRef]
- Weijer, V.C.R.; Jonvik, K.L.; van Dam, L.; Risvang, L.; Plasqui, G.; Sandbakk, Ø.; Raastad, T.; van Loon, L.J.C.; van Dijk, J.W. Energy Requirements of Paralympic Athletes: Insights from the Doubly Labeled Water Approach. Med. Sci. Sports Exerc. 2024, 56, 963–971. [Google Scholar] [CrossRef]
- González, D.; Marquina, R.; Rondón, N.; Rodriguez-Malaver, A.J.; Reyes, R. Effects of aerobic exercise on uric acid, total antioxidant activity, oxidative stress, and nitric oxide in human saliva. Res. Sports Med. 2008, 16, 128–137. [Google Scholar] [CrossRef]
- Salawu, A.; Middleton, C.; Gilbertson, A.; Kodavali, K.; Neumann, V. Stump ulcers and continued prosthetic limb use. Prosthet. Orthot. Int. 2006, 30, 279–285. [Google Scholar] [CrossRef]
- Allami, M.; Faraji, E.; Mohammadzadeh, F.; Soroush, M.R. Chronic musculoskeletal pain, phantom sensation, phantom and stump pain in veterans with unilateral below-knee amputation. Scand J. Pain. 2019, 19, 779–787. [Google Scholar] [CrossRef]
- Koukoulithras, I.; Alkhazi, A.; Gkampenis, A.; Stamouli, A.; Plexousakis, M.; Drousia, G.; Xanthi, E.; Roussos, C.; Kolokotsios, S. A Systematic Review of the Interventions for Management of Pain in Patients After Spinal Cord Injury. Cureus 2023, 15, e42657. [Google Scholar] [CrossRef]
- Skoog, B.; Jakobsson, K.E. Prevalence of Spasticity and Below-Level Neuropathic Pain Related to Spinal Cord Injury Level and Damage to the Lower Spinal Segments. J. Rehabil. Med. Clin. Commun. 2020, 3, 1000039. [Google Scholar] [CrossRef]
- Soo Hoo, J.A.; Kim, H.; Fram, J.; Lin, Y.S.; Page, C.; Easthausen, I.; Jayabalan, P. Shoulder pain and ultrasound findings: A comparison study of wheelchair athletes, nonathletic wheelchair users, and nonwheelchair users. PM R 2022, 14, 551–560. [Google Scholar] [CrossRef]
- Pelliccia, A.; Quattrini, F.M.; Cavarretta, E.; Squeo, M.R.; Adami, P.E.; Di Paolo, F.M.; Spataro, A.; Bernardi, M. Physiologic and Clinical Features of the Paralympic Athlete’s Heart. JAMA Cardiol. 2021, 6, 30–39. [Google Scholar] [CrossRef]
- Baumgart, J.K.; Brurok, B.; Sandbakk, Ø. Peak oxygen uptake in Paralympic sitting sports: Asystematic literature review, meta- and pooled-data analysis. PLoS ONE 2018, 13, e0192903. [Google Scholar] [CrossRef]
- Bernardi, M.; Fedullo, A.L.; Di Giacinto, B.; Squeo, M.R.; Aiello, P.; Dante, D.; Romano, S.; Magaudda, L.; Peluso, I.; Palmery, M.; et al. Cardiovascular Risk Factors and Haematological Indexes of Inflammation in Paralympic Athletes with Different Motor Impairments. Oxid. Med. Cell. Longev. 2019, 2019, 6798140. [Google Scholar] [CrossRef]
- López-Gil, J.F.; Tárraga-López, P.J.; Soledad Hershey, M.; López-Bueno, R.; Gutiérrez-Espinoza, H.; Soler-Marín, A.; Fernández-Montero, A.; Victoria-Montesinos, D. Overall proportion of orthorexia nervosa symptoms: A systematic review and meta-analysis including 30 476 individuals from 18 countries. J. Glob. Health 2023, 13, 04087. [Google Scholar] [CrossRef]
- Tweedy, S.; Dutia, I.M.; Cairney, J.; Beckman, E. Single case experimental design: A rigorous method for addressing inequity and enhancing precision within Para sport and exercise medicine research. Br. J. Sports Med. 2024, 58, 1242–1243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).