Abstract
Background: Obesity is a chronic and complex disease; by its nature, it represents an enormous challenge to be solved and managed. For that matter, several guidelines have been published, but there is still a long way to go until concrete scaled results can be presented. Adults with obesity, and especially severe obesity, need to have access to treatment programs, but they are not available for the vast majority of the population. Objective: The aim of this study is to evaluate the effectiveness of a multidisciplinary treatment program for obesity (MTPO) offered to adults (ages 18 to 50 years old) with a BMI over 30 kg/m2. Methods: Participants were invited through media ads, resulting in 404 participants for the first phase of that study, from whom the risk profile was assessed. After that, 180 participants (82.8% with severe obesity) concluded the MTPO, which consisted of 48 sessions of exercises and the same number of professional orientations about a healthy lifestyle, including the importance of being physically active, how to improve their eating habits, and how to control their emotions. Results: For the analysis of results, participants were grouped according to their weight loss in terciles, with the first, tercile presenting an average weight loss of 7.6%, which is considered clinically significant. In the same way, the average percental variations were even higher in this group for body fat (12.7%) and the lean mass to fat mass ratio (LM/FM), which increased by 14.3%. The homeostatic model assessment for insulin resistance, HOMA-IR, was around 3 times the variation of body mass, whereas the triglycerides (TG) and the hemoglobin A1C (H1Ac) were around twice that rate. Conclusions: These results made clear the effectiveness of the MTPO, which needs to be tested in public health services.
1. Introduction
Over the past three decades, obesity has emerged as a pervasive global health crisis [1]. Once considered primarily a concern in high-income nations, the most significant rise and highest prevalence are now observed in low- and middle-income countries [2]. Data from Brazil’s VIGITEL surveillance system, operational since 2006, indicate that over 60% of Brazilian adults were overweight in 2023. The prevalence is higher among men (63.4%) than women (59.6%), and a worsening trend in both overweight and obesity has been consistently observed in adults since the system’s inception [3]. The adoption of unhealthy lifestyle habits, including low levels of physical activity and poor dietary choices, has led to numerous population-level consequences, most notably the increasing prevalence of obesity [4].
Despite the establishment of national health improvement targets, such as Brazil’s Strategic Action Plan to Combat Chronic Noncommunicable Diseases (2011–2022 and 2021–2030), favorable progress has not materialized. In fact, the trend has moved in the opposite direction. The adult obesity prevalence, which was 15.1% in 2010 and was targeted for stabilization by 2022, rose to 18.9% in 2015, 20.3% in 2019, and, according to the most recent 2023 data, now stands at 24.3% [5].
Although global recognition of rising obesity rates and their profound health implications, as well as efforts by authorities to set and monitor targets, consistent progress remains elusive [2]. This should come as no surprise, given that most efforts have historically focused on persons living with obesity, while the societal norms and conditions shaping lifestyles have received comparatively little attention [6].
The management of obesity is notably complex and has historically received insufficient attention [7,8]. It is important to emphasize that significant challenges remain in establishing objective criteria for diagnosis and clinical decision-making, including prioritization of therapeutic interventions and public health strategies. These were highlighted by The Lancet Diabetes & Endocrinology Commission on Clinical Obesity [9].
Given that obesity is a complex, chronic, and multifactorial disease, initiating treatment in its earlier stages is crucial, especially as weight-related alterations are increasingly prevalent in adults [10,11]. There is a clear need to develop comprehensive strategies and define measurable goals for behavioral change programs aimed at improving health and quality of life. Ideally, these should take the form of multidisciplinary intervention programs (MIPs), encompassing physical exercise, nutritional and psychological counseling, and consistent clinical follow-up [12,13,14]. Evidence supports the effectiveness of MIPs, even among patients with severe obesity [15,16,17].
However, there are still several unresolved issues related to implementing these intervention models in public health settings [18,19,20].
One of the most urgent questions concerns the effectiveness of these MIPs when applied in real-world environments, such as public health services, where conditions often differ substantially from those of randomized controlled trials (RCTs) [21,22]. In this context, pragmatic clinical trials (PCTs) are the most appropriate, as their findings can be translated to both public and private healthcare settings [23,24]. Although this issue remains largely unresolved, it has at least been recognized by some researchers [25,26]. While relatively few PCTs have been conducted in this field, their number is increasing across various countries [27,28].
These studies reinforce the need to use real-world settings, such as health units, sports centers, public parks, or even stadiums, to develop programs that address real problems. In this case, success depends on the sustainability of new behaviors; in other words, the promotion of a healthy lifestyle [29].
The purpose of this study is to verify the effectiveness of an MIP in promoting clinically significant weight loss (defined as 5% or more) in adults with obesity classes I to IV. Notably, the majority (82%) of the participants presented severe obesity [30].
2. Materials and Methods
2.1. Study Design
This is a pragmatic clinical trial of intervention, carried out and described based on the CONSORT 2010 framework [31]. Pragmatic clinical trials are very important because they seek to answer relevant questions that are applicable to everyday clinical practice. In PCT, the external validity is maximized by having few exclusion criteria and by allowing flexibility in the interpretation of the intervention and management decisions, since its goal is to test interventions, devices, or therapeutic resources that could be applied in real-world settings [32]. Therefore, they are the cornerstone of the comparative effectiveness agenda [33].
This study is part of the integrated project entitled “Effectiveness of a multiprofessional program in the evaluation of cardiometabolic risk factors and treatment of abdominal obesity in two municipalities in northwestern Parana”, which was funded by the Fundação Araucária in the call PPSUS/2016. It had an average score of 4.2 using the PRECIS-2 tool, which is a scale whose maximum score is 5. Therefore, indicating a high degree of pragmatism [34].
2.2. Participants
Participants were invited to voluntarily participate in the research based on disclosure in the local media (television, radio, and newspaper) and electronic media (website, institutional e-mail, and Facebook). The following inclusion criteria were adopted: (1) age between 18 and 50 years; (2) being overweight or obese; (3) residing in Maringá or Paranavaí, both in Parana state, Brazil; (4) being available to fully participate in the interventions; (5) not having undergone bariatric surgery; (6) not undergoing any other treatment for obesity (therapies, medications, regular physical exercises); and (7) signing the Free and Informed Consent Form.
Those interested in participating in the Multidisciplinary Treatment Program of Obesity previously went through Phase 1 of this study, Pre-inclusion Assessment, divided into Step 1, in which the eligibility conditions to participate in this study were verified when 774 people were in one of the 14 initial pre-inclusion meetings held between December 2017 and March 2018 and between June and July 2019 in the municipality of Maringá held at the Regional University Hospital of Maringá (HUM). And between December 2019 and January 2020 and February 2022 in Paranavaí at the Regional Meeting Centre, and once more in August 2022 in the municipality of Maringá held at HUM, where they responded to an anamnesis to collect data about their socioeconomic and health status and performed a preliminary assessment to measure body mass, height, BMI, waist circumference (WC), and blood pressure (BP). More details can be found in Westphal-Nardo et al. [29].
The phase 2 of this study, called the Multidisciplinary Treatment Program of Obesity, can be classified as an intensive intervention in which the participants enter the treatment program with physical exercise interventions and theoretical classes of healthy eating, psychology, and physical activity to guide and help the participants make changes in their lifestyle. A total of 404 participants, 85 males and 319 females, were considered eligible to participate in the MTPO for 16 weeks, as shown in the flowchart (Figure 1). From those numbers, 166 participants withdrew: 12 due to pregnancy, 46 due to work schedule, 51 due to family or emotional problems (reports of anxiety, depression, and lack of willpower), 33 due to lack of transportation, and 24 with scheduling problems, and the other 58 had no time to be involved in the program as required, and for that reason they did not start it.
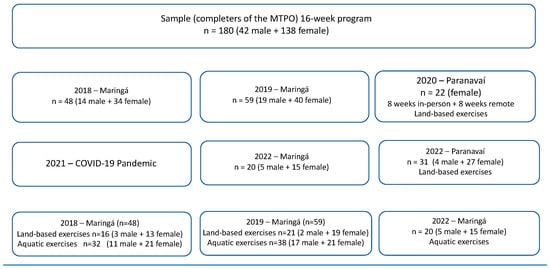
Figure 1.
Flowchart of MTPO procedures and exercises.
A total of 180 participants completed the 16-week MTPO: 42 males and 138 females. In Maringá, 48 participants (14 males and 34 females) concluded in 2018, subdivided into 32 participants in the aquatic exercise (11 males and 21 females) and 16 participants for the terrestrial exercise (3 males and 13 females). In 2019, 59 participants completed the MTPO (19 males and 40 females), subdivided into 38 participants for aquatic exercise (17 males and 21 females) and 21 participants for land exercise (2 males and 19 females). And in 2020, in Paranavaí, with theoretical interventions remotely and with land exercise due to the COVID-19 pandemic, 22 female participants completed the MTPO. And to finish in 2022 in Paranavaí, 31 participants completed the MTPO with land exercise (4 males and 27 females), and in Maringá, 20 participants completed it with aquatic exercise (5 males and 15 females) (Figure 1).
The MTPO’s exercise modalities were established based on facility accessibility during the intervention schedule. Participants in Maringá had access to both land-based and aquatic exercises (water aerobics), whereas those in Paranavaí were limited to land-based exercises due to swimming pool unavailability. Land-based exercise sessions occurred simultaneously. For logistical efficiency, the land-based group was divided, dedicating approximately 30 min to weight training and another 30 min to aerobic exercises. All land-based activities maintained a moderate intensity, targeting 40–59% of the individual’s RHR or a modified Borg RPE of 5–6. Aquatic exercise groups adhered to identical frequency, intensity, and duration parameters. Their water-based routine comprised a 5 min warm-up with escalating intensity, followed by a 50 min conditioning phase, and concluded with a cool-down session that incorporated stretching, deep breathing, and relaxation techniques [35,36].
For the control group (CG), 30 participants were evaluated (11 males and 19 females), and after 16 weeks, they were reassessed. Participants who were unable to attend (have no schedule) for the MTPO or those who withdrew from the intervention within the first month were given the opportunity for reassessment, since they did not engage in any other training or weight loss program.
2.3. Ethical Approval
This study has obtained approval from the Research Ethics Committee in accordance with Resolutions 466/2012 and 510/2016 under the registration number CAAE: 56721016.7.1001.0104, approved under no 2.655.268. Additionally, it has been registered with the Brazilian Registry of Clinical Trials (REBEC) under the registration number RBR2yzs76. Furthermore, this study adheres to the ethical principles outlined in the Helsinki Declaration for medical research involving human subjects.
2.4. Statistical Analysis
For the data analyses, a Microsoft Excel 2020 spreadsheet was used, and the statistical tests were performed using the SPSS statistical package version 20.0 [37]. To analyze data distribution, the Shapiro–Wilk test was used to verify normality when the sample was less than 50. For samples larger than 50, the Kolmogorov–Smirnov test was adopted. The data that did not present a normal distribution were normalized by a logarithmic function when necessary; if the majority of the sample presented parametric data, they were presented using descriptive statistics by mean and standard deviation. Data that did not present a normal distribution, considered non-parametric data, were presented by median and interquartile range (percentile). Levene’s test was used to analyze the homogeneity of variances. The Mauchly test was used for variables in which sphericity was violated, and the analyses were adjusted using the Greenhouse–Geisser correction. The unpaired t-test was used to compare variables according to gender and group. To compare variables according to age group and level of obesity, the one-way ANOVA test was used, with Post Hoc Bonferroni for multiple comparisons, to indicate which groups had differences. The Pearson correlation coefficient was used to correlate the variables. The effect size was computed as partial eta-squared values (ηp2; small: ≥0.01, medium: ≥0.06, large: ≥0.14). A significance level of p < 0.05 was adopted for all analyses [38,39,40].
3. Results
The participants in this study were enrolled in seven MPTO groups offered between 2018 and 2022. They shared common characteristics, including the number of sessions and duration (48 sessions over 16 weeks), as well as the session structure and length. Consequently, they were analyzed collectively.
The dropout rates were relatively high, attributable to various reasons outlined in the methods section, resulting in an average dropout rate of 55.45%, with a minimum of 15.8% and a maximum of 75.9%.
Considering the completers or intervention group (IG) (n = 180), there were 138 females and 42 males. In the control group, there were 11 males and 19 females (n = 30).
Table 1 shows that they have an average age of 39.20 (±7.54) for the CG and 39.27 (±8.82) for the IG. There is no significant difference in age between the groups. The CG had an average body mass of 114.27 (±21.51), and in the IG, it was 109.98 (±23.46), which was a significant difference between the groups at the baseline (p < 0.01). The same was observed for BMI, body fat (%), absolute body fat (kg), lean mass/fat mass ratio, waist circumference, abdomen circumference, hip circumference, and waist/height ratio, all with a significant difference between the groups at the baseline (p < 0.01).

Table 1.
General effect of intervention comparing the intervention group (IG n = 180) with the control group (CG n = 30).
When comparing the CG and the IG at baseline, Table 1 reveals significant differences in hemodynamic and health-related physical fitness variables, specifically in Diastolic Blood Pressure (DBP) and dynamic lower limb muscular endurance (p < 0.01).
Regarding the biochemical parameters, there are also significant differences at baseline in insulin, HOMA-IR, total cholesterol, and LDL-c (low-density lipoprotein cholesterol) (p < 0.01). This information suggests that there were notable distinctions between the CG and IG participants in these variables at the outset of this study.
Comparing the baseline values of the indexes derived from the biochemical and anthropometric parameters, Table 1 shows significant differences in the percentile of BMI, MetS-Z WC (metabolic syndrome severity z-score waist circumference), percentile of WC and in TyG-BMI (Triglyceride-Glucose Index) with (p < 0.01).
When comparing the different time points (baseline vs. after MTPO or 16 weeks), Table 1 reveals significant differences between the groups in various parameters such as Body Mass: In the CG, there was a significant increase (p < 0.01) in body mass, while in the IG, the opposite occurred, with a significant decrease (p < 0.01). Concerning BMI, the IG demonstrated a significant reduction (p < 0.01), which was not observed in the CG. The same happened for Body Fat (%), Absolute Body Fat (kg), and Neck Circumference with reductions just for IG with (p < 0.01). Opposite responses were observed for Waist circumference (cm) and Waist to Height Ratio (cm) with IG showing significant reductions (p < 0.01), whereas the CG presented significant increments (p < 0.05). The same behavior was observed for the variable lean mass to fat mass ratio for CG and IG, both showing significant increments related to that variable (p < 0.01).
Indeed, these findings suggest that the intervention had a significant impact on multiple aspects of body composition and anthropometric measures in the IG when compared to the CG over the course of this study.
Throughout this study, significant differences were observed in hemodynamic and health-related physical fitness parameters between the groups. In the IG, there were notable reductions in both systolic blood pressure (SBP) and DBP, all of which were statistically significant (p < 0.01). The same was not observed in the CG.
Furthermore, the IG exhibited substantial improvements in various components of health-related physical fitness. There was a significant increase in static abdominal muscle endurance. Dynamic lower limb muscle endurance also showed significant improvements. Flexibility demonstrated a significant increase, all with (p < 0.01). Conversely, the CG experienced a significant reduction (p < 0.01) in the distance covered during the 6-Minute Walk Test (6MWT).
These findings underscore the positive impact of the intervention on hemodynamic parameters and multiple components of physical fitness in the IG, whereas the CG primarily showed a decline in 6MWT performance.
Related to the biochemical parameters within IG (pre vs. pos), there were significant reductions in glycemia, insulin, HOMA-IR, HOMA-B, glycated hemoglobin, total cholesterol, LDL-c, VLDL-c (Very-low-density lipoprotein cholesterol), TG, and H1Ac (p < 0.01).
Considering the indexes derived from the biochemical and anthropometric parameters within IG, there were significant reductions in AIP (Atherogenic index of plasma), MetS-Z BMI, Percentile BMI, MetS-Z WC, Percentile WC, TyG, TyG-BMI, and TyG-WC (p < 0.01).
Table 2 brings the results stratified by terciles according to the percentual of weight loss. The first tercile is the one whose weight loss was more prominent, with an average percentual weight loss of −7.6%, with the second tercile presenting −2.4%, whereas the third tercile had an average percentual gain of 1.5% (Figure 2). There are significant differences in group 1 (first tercile) compared to the other two groups (p < 0.01). The same happened to BMI, Lean Body Mass, Skeletal Muscle Mass, Body Fat %, Absolute Body Fat, LM/FM ratio, WC, Abdominal Circumference, Hip Circumference, WHtR (Waist/Height Ratio), with group 1 (first tercile) showing great reductions compared to the other two groups.

Table 2.
Relative (%) responses in the intervention group (IG) stratified by the percentual of weight loss in terciles (n = 180).
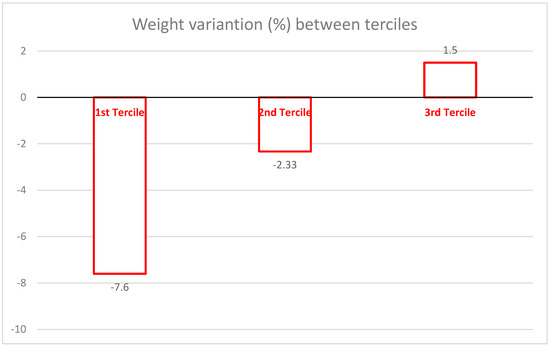
Figure 2.
Mean percentage of weight loss according to tercile groups.
Related to the hemodynamic and health-related physical fitness variables, only the 6MWT showed significant difference between the first tercile and the third tercile (p < 0.01). In the group of biochemical parameters, there were significant differences between the first tercile against and the tercile in VLDL-c and TG, and also between the second tercile and the first one (p < 0.01).
Related to the indices derived from the biochemical and anthropometric parameters, Table 2 shows significant differences between the first tercile and the third tercile for AIP, TyG-BMI, and TyG-WC. Whereas the differences were also significant between the second and third terciles and the first and second terciles only for TYG-BMI (p < 0.01).
4. Discussion
The main objective of this study was to analyze the responses promoted by an MTPO in adults, most of whom had severe obesity. In fact, 82% of the participants fell into this category, with an average BMI over 40 kg/m2 [30,41].
Considering the primary outcome, clinically significant weight loss, it was shown that at least one-third of the participants achieved a weight reduction over 5%, as illustrated in Figure 2. This is a notable outcome, especially since the MTPO was implemented under conditions similar to those found in public health services. The professionals involved in the intervention—psychologist, dietitian, and physical activity or exercise specialist—are commonly available in Brazil’s public health system. Using this type of strategy could make similar programs affordable enough to be broadly adopted within Brazil’s public health framework and possibly could be implemented in other developing countries too. Although program costs were not formally assessed, interventions delivered by non-medical professionals are generally less expensive than those led by general practitioners or nurses. Furthermore, the availability of existing institutional facilities helps to further reduce implementation costs. This suggests that the main challenge lies in formulating effective policies to facilitate the execution of such programs.
The results are consistent with findings in the literature regarding the proportion of participants who achieve clinically significant weight loss. Behavioral programs typically result in about 20% of participants meeting this target. In our study, over 25% achieved it [42]. More intensive interventions, generally conducted as RCTs under ideal conditions, tend to report higher success rates. For example, studies in Norway by Berge et al. and Gjevestad et al. demonstrated a higher proportion of participants reaching the 5% weight loss goal. In one such study, participants with higher cardiorespiratory fitness achieved an average weight loss of 7.6%, comparable to the average in the first tercile of our study. However, in the Norwegian study, this goal was achieved in just three months through a 6-hour-per-day program, three times per week for 12 weeks (216 h total). The sessions included two supervised training periods (60–90 min each), followed by lectures on nutrition, physical activity, and motivation. This highlights the significant role that intervention intensity plays and provides an important basis for comparison between efficacy and effectiveness trials [17,43]. In contrast, our study’s total intervention time was 96 h over 16 weeks (48 h of physical exercises and 48 h of theoretical classes). These classes addressed behavioral aspects such as eating habits, reducing sedentary behavior, and implementing sustainable increases in habitual physical activity.
It is very important to note that efficacy trials are typically developed under ideal conditions, requiring extensive infrastructure and personnel. Consequently, these programs are more intensive and often involve inpatient treatment, making them significantly more costly. Such approaches are usually feasible only in high-income countries.
Beyond weight loss, our study demonstrated that several other health parameters improved significantly—some even more than the weight loss itself. Notably, parameters related to glucose metabolism, such as insulin, HOMA-IR, and HOMA-Beta, showed improvements of approximately 20%. These findings highlight the multiple health benefits of such programs and the importance of addressing obesity-related comorbidities [15,44].
One challenge is that MTPO offered by hospitals and universities are less frequently accessed by the public than commercial programs. However, they often include components not commonly found in commercial programs [22].
While the weight loss outcomes met expectations, it is possible that some participants might not perceive the results as sufficient. Some studies suggest that a weight loss of over 30% is considered ideal, 25% acceptable, and anything below 17% disappointing [45]. Thus, educating participants on the clinical relevance of a 5% weight loss is critical for managing expectations and avoiding frustration when meaningful health benefits, like those seen in this study, are achieved [13]. Although our MTPO included both land-based and aquatic exercise programs, this variation likely had minimal impact. Both programs shared similar structures in terms of frequency (three times per week), intensity (moderate), and session duration (60 min). Additionally, participants were continuously encouraged to increase physical activity and reduce sedentary time.
Metabolic abnormalities are frequently observed in individuals with obesity [46], underscoring the importance of public health programs aimed at reducing these risks. While individual biomarkers are useful for health monitoring, composite indices such as the TyG index and AIP have been shown to be even stronger predictors of cardiovascular disease [47]. Our study demonstrated substantial improvements in both individual biochemical markers and these composite indices.
Obesity is closely linked with insulin resistance (IR), a relationship that was clearly evident in our study. Recently, TyG-related markers, which combine obesity indicators with the TyG index, have shown promise in evaluating IR in adults with obesity [48]. We assessed these markers as alternative indicators of IR, in line with current studies [48,49].
The findings not only revealed variations in these parameters but also showed their responsiveness to the MTPO. Interestingly, their response patterns mirrored those of the anthropometric measurements, underscoring the interconnectedness between obesity, IR, and the potential benefits of MTPOs.
Our results align with those of the Diabetes Prevention Program (DPP), which reported an average weight loss of around 7%. Even among participants in the other two terciles—who did not achieve substantial weight loss—there were still notable improvements in metabolic risk and physical fitness [50].
These findings emphasize the importance of evaluating not just weight loss but also other health improvements when assessing the effectiveness of obesity interventions [51]. A more comprehensive evaluation that includes various health parameters provides a holistic perspective on the overall benefits. Based on our experience with MTPO for adolescents, our group has also proposed a criterion for success that can be extended to adults [52].
A significant limitation of this study is the high dropout rate. Attrition is particularly problematic in weight management interventions, as participants who do not see progress are more likely to drop out. Studies show that trials with attrition rates above 20% tend to report less weight loss [26]. High dropout rates can reduce program effectiveness, compromise data integrity, and introduce bias into study results [53].
Despite this, attrition in weight management programs is a well-documented issue, with rates reaching up to 80% [54]. Our ~50% attrition rate is consistent with the literature, as many obesity intervention programs report similarly high dropout rates. Moreover, dropout tends to increase over time—ranging from 20–30% in the first 4 months to 85% after 36 months [54]. It is also important to highlight that the literature on attrition is highly heterogeneous, with ranges from 10% to 80% depending on the setting and the type of program [55].
Another limitation is the lack of a follow-up phase to assess the long-term sustainability of the results. Also, the absence of sensitivity analyses (e.g., intention-to-treat or imputation for missing data). Future studies should address this in order to better evaluate the lasting impact of such interventions. Moreover, a major strength of this study is that it was conducted in a developing country, where conducting such research is particularly challenging due to limited funding and resources. Additionally, this study was implemented in two different cities using the same protocol. This allowed us to generalize the results, despite differences such as exercise modality, and reflects the real-world variability professionals may face when implementing similar programs.
5. Conclusions
These results reinforce the evidence supporting the effectiveness of lifestyle intervention in promoting substantial weight loss and enhancements in cardiometabolic risk factors among a considerable proportion of individuals with obesity or severe obesity. They also confirm that pragmatic trials conducted in a developing country can yield meaningful outcomes, even for participants who did not achieve the clinically significant weight loss. These findings should be replicated in other developing countries under similar conditions, and future studies must include follow-up periods to assess the long-term sustainability of the results.
Author Contributions
Conceptualization, G.W.-N., J.-P.C. and N.N.J.; methodology, G.W.-N., A.S.M.M., P.C.F., M.J.P.d.S., G.N.R., I.C.C., A.H.-S., F.M.F.G., J.-P.C. and N.N.J.; software, G.W.-N.; validation, G.W.-N. and N.N.J.; formal analysis, G.W.-N., J.-P.C. and N.N.J.; investigation G.W.-N., A.S.M.M., P.C.F., M.J.P.d.S., G.N.R., I.C.C., A.H.-S., F.M.F.G., J.-P.C. and N.N.J.; resources, G.W.-N., J.-P.C. and N.N.J.; data curation, G.W.-N. and N.N.J.; writing—original draft preparation, G.W.-N., J.-P.C. and N.N.J.; writing—review and editing, G.W.-N., A.H.-S., J.-P.C. and N.N.J.; visualization, J.-P.C. and N.N.J.; supervision, J.-P.C. and N.N.J.; project administration, G.W.-N. and N.N.J.; funding acquisition, N.N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Effectiveness of a multidisciplinary program in the assessment of cardiometabolic risk factors and treatment of abdominal obesity in two municipalities in northwestern Paraná, funded by the Araucaria Foundation and the Ministry of Health through the public notice CP 01/2016 Research Program for the Unified Health System: Shared Management in Health—PPSUS 2015 Edition Araucaria Foundation-PR/SESA-PR/CNPq/MS-Decit and Coordination for the Improvement of Higher Education Personnel—CAPES Brazil for the Sandwich Doctorate Scholarship Abroad.
Institutional Review Board Statement
This study has obtained approval from the Research Ethics Committee in accordance with Resolutions 466/2012 and 510/2016 under the registration number (CAAE: 56721016.7.1001.0104, approved under no 2.655.268). Additionally, it has been registered with the Brazilian Registry of Clinical Trials (REBEC), under the registration number RBR2yzs76. Furthermore, this study adheres to the ethical principles outlined in the Helsinki Declaration for medical research involving human subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
The Coordination for the Improvement of Higher Education Personnel—CAPES Brazil for the Sandwich Doctorate Scholarship Abroad and the Healthy Active Living and Obesity Research Group—HALO, Children’s Hospital of Eastern Ontario Research Institute—CHEO, Ottawa, Canada, for the opportunity to carry out a doctoral internship.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MTPO | multidisciplinary treatment program of obesity |
| BMI | Body mass index |
| LM/FM | lean mass to fat mass ratio |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| HOMA-B | homeostatic model assessment for beta |
| TG | triglycerides |
| H1Ac | hemoglobin A1C |
| PCT | pragmatic clinical trial |
| RCT | randomized controlled trials |
| WC | waist circumference |
| BP | blood pressure |
| IG | intervention group |
| CG | control group |
| DBP | diastolic blood pressure |
| LDL-c | low-density lipoprotein cholesterol |
| MetS-Z WC | metabolic syndrome severity z-score waist circumference |
| TyG | Triglyceride-Glucose Index |
| SBP | systolic blood pressure |
| 6MWT | 6-Minute Walk Test |
| VLDL-c | very-low-density lipoprotein cholesterol |
| AIP | atherogenic index of plasma |
References
- Kaplan, L.M.; Apovian, C.M.; Ard, J.D.; Allison, D.B.; Aronne, L.J.; Batterham, R.L.; Busetto, L.; Dicker, D.; Horn, D.B.; Kelly, A.S.; et al. Assessing the state of obesity care: Quality, access, guidelines, and standards. Obes. Sci. Pract. 2024, 10, e765. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. Obesity: Missing the 2025 Global Targets; World Obesity Federation: London, UK, 2020; pp. 1–242. [Google Scholar]
- Brasil. Vigitel Barsil 2023; Ministério da Saúde. Secretaria de Vigilância em Saúde e Ambiente; Departamento de Análise Epidemiológica e Vigilância de Doenças Não Transmissíveis; Vigitel Brazil 2023; Ministério da Saúde: Brasilia, Brasil, 2023. [Google Scholar]
- Brasil. Vigitel Brasil 2018: Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquerito Telefônico; G. Estatística e Informação em Saúde; Ministério da Saúde: Brasilia, Brasil, 2019; 131p. [Google Scholar]
- Brasil. Ministério da Saúde. Plano de Ações Estratégicas para o Enfrentamento Das Doenças Crônicas E Agravos; Ministério da Saúde: Brasília, Brasil, 2021; Volume 1, 121p. [Google Scholar]
- WHO (WHA75/2022/REC/1). Acceleration Plan to Support Member States in Implementing the Recommendations for the Prevention and Management of Obesity over the Life Course—ANNEX 7—Seventy-Fifth World Health Assembly. 2022. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA75/A75_10Add4-en.pdf (accessed on 12 March 2025).
- Montesi, L.; Ghoch Mel Brodosi, L.; Calugi, S.; Marchesini, G.; Grave, R.D. Long-term weight loss maintenance for obesity: A multidisciplinary approach. Diabetes Metab. Syndr. Obes. 2016, 9, 37–46. [Google Scholar]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Rubino, F.; E Cummings, D.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; A Brown, W.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Kahan, S.; Manson, J.E. Obesity Treatment, Beyond the Guidelines. JAMA 2019, 321, 1349. [Google Scholar] [CrossRef]
- Bim, R.H.; Westphal, G.; Thon, R.A.; Alisson, I.; Pereira, S.; Castilho, M.M.; Oltramari, K.; Martins, F.M.; Nardo, N., Jr. Prevalência de fatores de risco cardiometabólico em adultos com obesidade. RBONE Rev. Bras. Obesidade Nutr. E Emagrecimento 2022, 2, 1270–1282. [Google Scholar]
- Kahan, S. Overweight and obesity management strategies. Am. J. Manag. Care. 2016, 22 (Suppl. S7), s186–s196. [Google Scholar]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts. 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.; Kushner, R.F. Lifestyle Medicine. In Lifestyle Medicine: A Masnual for Clinical Practice, 1st ed; Mechanick, J.I., Kushner, R.F., Eds.; Springer International Publishing: New York, NY, USA, 2016; pp. 1–367. [Google Scholar]
- Ryan, D.H. Nonsurgical Weight Loss for Extreme Obesity in Primary Care Settings. Arch. Intern. Med. 2010, 170, 146. [Google Scholar] [CrossRef]
- Martins, C.; Strømmen, M.; Stavne, O.A.; Nossum, R.; Mårvik, R.; Kulseng, B. Bariatric Surgery versus Lifestyle Interventions for Morbid Obesity—Changes in Body Weight, Risk Factors and Comorbidities at 1 Year. Obes. Surg. 2011, 21, 841–849. [Google Scholar] [CrossRef]
- Berge, J.; Støren, Ø.; Hertel, J.K.; Gjevestad, E.; Småstuen, M.C.; Hjelmesæth, J. Associations between cardiorespiratory fitness and weight loss in patients with severe obesity undergoing an intensive lifestyle intervention program: Retrospective cohort study. BMC Endocr. Disord. 2019, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Richards, R.; Jones, R.A.; Whittle, F.; Woolston, J.; Stubbings, M.; Sharp, S.J.; Griffin, S.J.; Bostock, J.; Hughes, C.A.; et al. Supporting Weight Management during COVID-19 (SWiM-C): Twelve-month follow-up of a randomised controlled trial of a web-based, ACT-based, guided self-help intervention. Int. J. Obes. 2023, 47, 51–59. [Google Scholar] [CrossRef]
- Turicchi, J.; O’driscoll, R.; Finlayson, G.; Duarte, C.; Hopkins, M.; Martins, N.; Michalowska, J.; Larsen, T.M.; A van Baak, M.; Astrup, A.; et al. Associations between the proportion of fat-free mass loss during weight loss, changes in appetite, and subsequent weight change: Results from a randomized 2-stage dietary intervention trial. Am. J. Clin. Nutr. 2020, 111, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Lugones-Sanchez, C.; I Recio-Rodriguez, J.; Agudo-Conde, C.; Repiso-Gento, I.; Adalia, E.G.; Ramirez-Manent, J.I.; Sanchez-Calavera, M.A.; Rodriguez-Sanchez, E.; A Gomez-Marcos, M.; Garcia-Ortiz, L. Long-term Effectiveness of a Smartphone App Combined With a Smart Band on Weight Loss, Physical Activity, and Caloric Intake in a Population With Overweight and Obesity (Evident 3 Study): Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e30416. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Martin, C.K.; Newton, R.L.; Apolzan, J.W.; Arnold, C.L.; Davis, T.C.; Price-Haywood, E.G.; Denstel, K.D.; Mire, E.F.; Thethi, T.K.; et al. Weight Loss in Underserved Patients—A Cluster-Randomized Trial. N. Engl. J. Med. 2020, 383, 909–918. [Google Scholar] [CrossRef]
- Mullin, G.E.; Cheskin, L.J.; Matarese, L.E. Integrative Weight Management A Guide for Clinicians; Humana Press: Totowa, NJ, USA, 2014; 523p. [Google Scholar]
- Befort, C.A.; Kurz, D.; Vanwormer, J.J.; Ellerbeck, E.F. Recruitment and reach in a pragmatic behavioral weight loss randomized controlled trial: Implications for real-world primary care practice. BMC Fam. Pract. 2020, 21, 47. [Google Scholar] [CrossRef]
- Singal, A.G.; Higgins, P.D.R.; Waljee, A.K. A Primer on Effectiveness and Efficacy Trials. Clin. Transl. Gastroenterol. 2014, 5, e45. [Google Scholar] [CrossRef]
- Anderson, A.S.; Chong, H.Y.; Craigie, A.M.; Donnan, P.T.; Gallant, S.; Hickman, A.; McAdam, C.; McKell, J.; McNamee, P.; Macaskill, E.J.; et al. A novel approach to increasing community capacity for weight management a volunteer-delivered programme (ActWELL) initiated within breast screening clinics: A randomised controlled trial. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Madigan, C.D.; E Graham, H.; Sturgiss, E.; E Kettle, V.; Gokal, K.; Biddle, G.; Taylor, G.M.J.; Daley, A.J. Effectiveness of weight management interventions for adults delivered in primary care: Systematic review and meta-analysis of randomised controlled trials. BMJ 2022, 377, e069719. [Google Scholar] [CrossRef]
- de Sousa, T.R.; Alexandrino, W.G.d.S.; Souza, A.; Faundez-Casanova, C.; Pascoini, M.J.d.S.; Awada, M.A.M.; De Paula, R.; Grizzo, F.M.F.; Oltramari, K.; Westphal-Nardo, G.; et al. Effects of physical activity in adults with severe obesity: A systematic review. Retos 2024, 53, 671–680. [Google Scholar] [CrossRef]
- Westphal-Nardo, G.; Magnani Branco, B.H.; Chaput, J.P.; Nardo Junior, N. Efficacy and Effectiveness of Clinical Trials Applied to the Treatment of Obesity: A Systematic Review. Retos 2024, 53, 628–635. [Google Scholar] [CrossRef]
- Westphal-Nardo, G.; Chaput, J.-P.; Faúndez-Casanova, C.; Fernandes, C.A.M.; Gonçalves, E.C.d.A.; Utrila, R.T.; Oltramari, K.; Grizzo, F.M.F.; Nardo-Junior, N. Exploring New Tools for Risk Classification among Adults with Several Degrees of Obesity. Int. J. Environ. Res. Public. Health 2023, 20, 6263. [Google Scholar] [CrossRef] [PubMed]
- Longhi, R.; Santos, A.S.e.A.d.C.; López-Yerena, A.; Rodrigues, A.P.S.; de Oliveira, C.; Silveira, E.A. The Effectiveness of Extra Virgin Olive Oil and the Traditional Brazilian Diet in Reducing the Inflammatory Profile of Individuals with Severe Obesity: A Randomized Clinical Trial. Nutrients 2021, 13, 4139. [Google Scholar] [CrossRef]
- Schulz, K.F. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 2010, 152, 726. [Google Scholar] [CrossRef]
- Godwin, M.; Ruhland, L.; Casson, I.; MacDonald, S.; Delva, D.; Birtwhistle, R.; Lam, M.; Seguin, R. Pragmatic controlled clinical trials in primary care: The struggle between external and internal validity. BMC Med. Res. Methodol. 2003, 3, 28. [Google Scholar] [CrossRef]
- Williams, H.C.; Burden-Teh, E.; Nunn, A.J. What Is a Pragmatic Clinical Trial? J. Investig. Dermatol. 2015, 135, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ré, R.; Janiaud, P.; Ioannidis, J.P.A. Real-world evidence: How pragmatic are randomized controlled trials labeled as pragmatic? BMC Med. 2018, 16, 1–6. [Google Scholar] [CrossRef]
- Hossein Abadi, F. Weight Loss through Aquatic Exercise. In Body Mass Index—Overweight, Normal Weight, Underweight; IntechOpen: London, UK, 2023. [Google Scholar]
- Bunæs-Næss, H.; Kvæl, L.A.H.; Nilsson, B.B.; Heywood, S.; Heiberg, K.E. Aquatic high-intensity interval training (HIIT) may be similarly effective to land-based HIIT in improving exercise capacity in people with chronic conditions: A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2023, 9, e001639. [Google Scholar] [CrossRef]
- Andy Field. Discovering Statistics Using SPSS Statistics; SAGE Publications: London, UK, 2009; Volume 66, p. 822. Available online: http://www.amazon.com/Discovering-Statistics-using-IBM-SPSS/dp/1446249182 (accessed on 12 March 2025).
- Cohen, H.W. P Values: Use and Misuse in Medical Literature. Am. J. Hypertens. 2011, 24, 18–23. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, H.Y.; Jung, W.S.; Lim, K. Effects of Twenty-Four Weeks of Resistance Exercise Training on Body Composition, Bone Mineral Density, Functional Fitness and Isokinetic Muscle Strength in Obese Older Women: A Randomized Controlled Trial. Int. J. Environ. Res. Public. Health. 2022, 19, 14554. [Google Scholar] [CrossRef]
- Lindenau, J.; Guimarães, L. Calculando o tamanho de efeito no SPSS. Rev. HCPA 2012, 32, 365. [Google Scholar]
- Kral, J.G.; Kava, R.A.; Catalano, P.M.; Moore, B.J. Severe Obesity: The Neglected Epidemic. Obes. Facts. 2012, 5, 254–269. [Google Scholar] [CrossRef]
- Al-ruwaili, H.; WLe-Roux, C. Treating Obesity as a Disease. Acad. Lett. 2021. [Google Scholar] [CrossRef]
- Gjevestad, E.; Karlsen, T.I.; Røislien, J.; Mæhlum, S.; Hjelmesæth, J. The effectiveness of secondary and tertiary care lifestyle intervention in morbidly obese patients: A 1-year non-randomized controlled pragmatic clinical trial. Clin. Obes. 2013, 3, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; DeLany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of Diet and PA intervention on WT loss and Cardiometabolic risk factor in very obese adults. JAMA 2010, 304, 1795–1802. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Flint, A.J.; de Gonzalez, A.B.; Bernstein, L.; Brotzman, M.; MacInnis, R.J.; Moore, S.C.; Robien, K.; Rosenberg, P.S.; Singh, P.N.; et al. Association between Class III Obesity (BMI of 40–59 kg/m2) and Mortality: A Pooled Analysis of 20 Prospective Studies. PLoS Med. 2014, 11, e1001673. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Lotfaliany, M.; Sathish, T.; Thankappan, K.R.; Thomas, N.; Furler, J.; Oldenburg, B.; Tapp, R.J. Prevalence of normal weight obesity and its associated cardio-metabolic risk factors—Results from the baseline data of the Kerala Diabetes Prevention Program (KDPP). PLoS ONE 2020, 15, e0237974. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J.; Koo, S.H.; Kwon, G.C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 2019, 14, e0212963. [Google Scholar] [CrossRef]
- Er, L.-K.; Wu, S.; Chou, H.-H.; Hsu, L.-A.; Teng, M.-S.; Sun, Y.-C.; Ko, Y.-L. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS ONE 2016, 11, e0149731. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Robert, F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation 2014, 129 (Suppl. S1), 102–138. [Google Scholar] [CrossRef] [PubMed]
- Unick, J.L.; Beavers, D.; Bond, D.S.; Clark, J.M.; Jakicic, J.M.; Kitabchi, A.E.; Knowler, W.C.; Wadden, T.A.; Wagenknecht, L.E.; Wing, R.R. The Long-term Effectiveness of a Lifestyle Intervention in Severely Obese Individuals. Am. J. Med. 2013, 126, 236–242.e2. [Google Scholar] [CrossRef] [PubMed]
- Nardo Junior, N.; Bianchini, J.A.A.; da Silva, D.F.; Ferraro, Z.M.; Lopera, C.A.; Antonini, V.D.S. Building a response criterion for pediatric multidisciplinary obesity intervention success based on combined benefits. Eur. J. Pediatr. 2018, 177, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goode, R.W.; Ye, L.; Sereika, S.M.; Zheng, Y.; Mattos, M.; Acharya, S.D.; Ewing, L.J.; Danford, C.; Hu, L.; Imes, C.C.; et al. Socio-demographic, anthropometric, and psychosocial predictors of attrition across behavioral weight-loss trials. Eat. Behav. 2016, 20, 27–33. [Google Scholar] [CrossRef]
- Ponzo, V.; Scumaci, E.; Goitre, I.; Beccuti, G.; Benso, A.; Belcastro, S.; Crespi, C.; De Michieli, F.; Pellegrini, M.; Scuntero, P.; et al. Predictors of attrition from a weight loss program. A study of adult patients with obesity in a community setting. Eat. Weight. Disord. —Stud. Anorex. Bulim. Obes. 2021, 26, 1729–1736. [Google Scholar] [CrossRef]
- Colombo, O.; Ferretti, V.V.V.; Ferraris, C.; Trentani, C.; Vinai, P.; Villani, S.; Tagliabue, A. Is drop-out from obesity treatment a predictable and preventable event? Nutr. J. 2014, 13, 13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).