Sports Massage and Blood Flow Restriction Combined with Cold Therapy Accelerate Muscle Recovery After Fatigue in Mixed Martial Arts Athletes: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
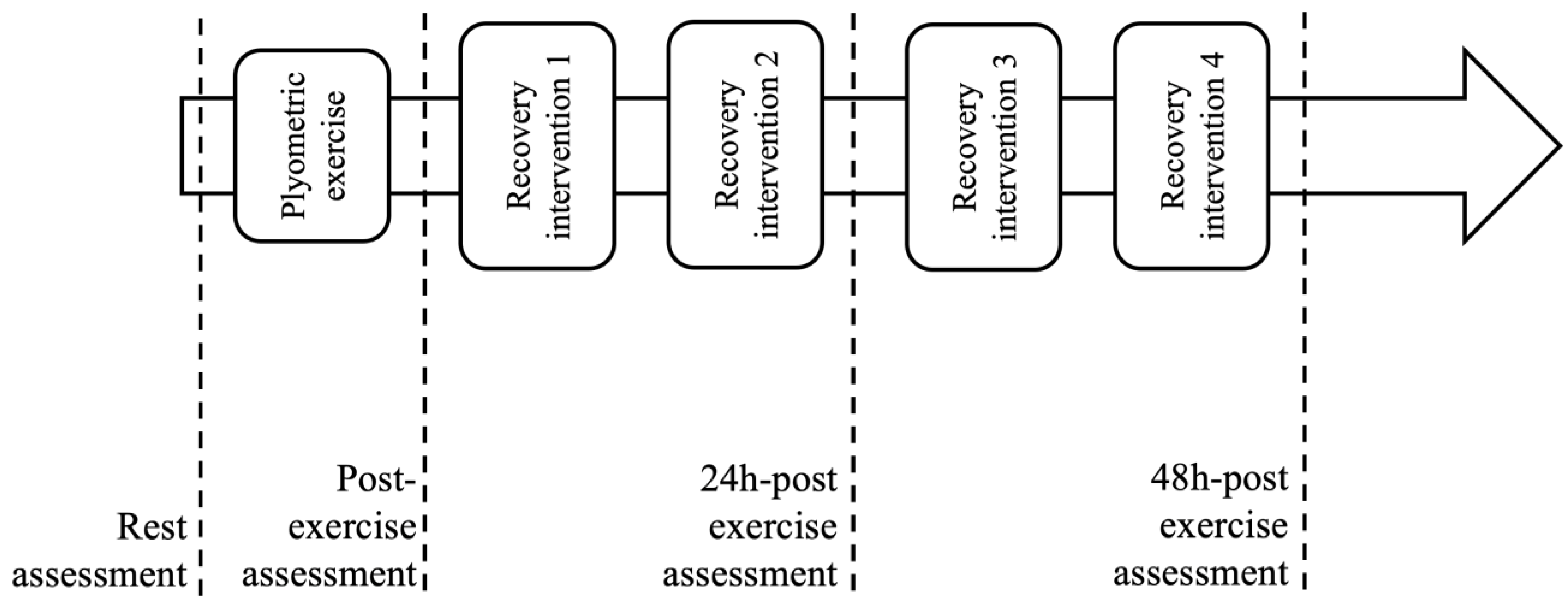
2.1. Study Design
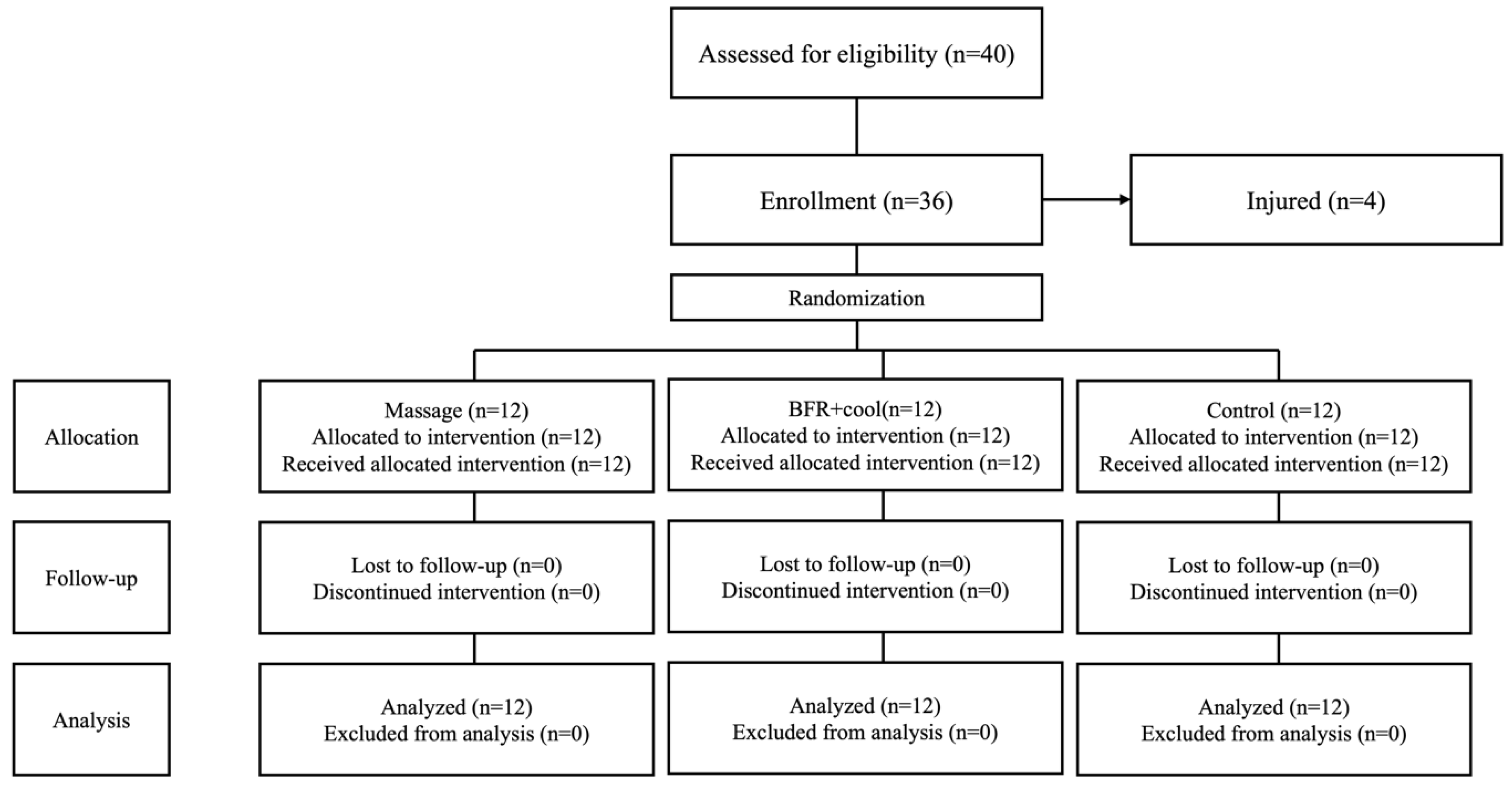
2.2. Participants
2.3. Muscle Fatigue Protocol
2.4. Recovery Intervention
2.5. Outcomes
2.5.1. Tissue Perfusion (PU)
2.5.2. Biomechanical Properties—Elasticity (E [arb: Relative Arbitrary Unit])
2.5.3. Pressure Pain Threshold (PPT [N/cm])
2.5.4. Reactive Strength Index (RSI[m·s−1])
2.5.5. The Total Quality of Recovery (TQR)
2.6. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TQR | Total quality recovery |
| PPT | Pressure pain threshold |
| BFR | Blood flow restriction |
| RSI | Reactive strength index |
References
- Zebrowska, A.; Trybulski, R.; Roczniok, R.; Marcol, W. Effect of Physical Methods of Lymphatic Drainage on Postexercise Recovery of Mixed Martial Arts Athletes. Clin. J. Sport Med. 2019, 29, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.C.A.; Faro, H.; Lenetsky, S.; Gonçalves, A.F.; Dias, S.B.C.D.; Ribeiro, A.L.B.; da Silva, B.V.C.; Filho, C.A.C.; de Vasconcelos, B.M.; Serrão, J.C.; et al. Exploratory Systematic Review of Mixed Martial Arts: An Overview of Performance of Importance Factors with over 20,000 Athletes. Sports 2022, 10, 80. [Google Scholar] [CrossRef]
- Miarka, B.; Coswig, V.; Brito, J.C.; Slimani, M.; Amtmann, J.; Del Vecchio, F.B. Comparison of Combat Outcomes: Technical and Tactical Analysis of Female MMA. Int. J. Perform. Anal. Sport 2016, 16, 539–552. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.A.; Maxwell, L. Delayed Onset Muscle Soreness: Treatment Strategies and Performance Factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Trybulski, R.; Kużdżał, A.; Stanula, A.; Muracki, J.; Kawczyński, A.; Kuczmik, W.; Wang, H.K. Acute Effects of Cold, Heat and Contrast Pressure Therapy on Forearm Muscles Regeneration in Combat Sports Athletes: A Randomized Clinical Trial. Sci. Rep. 2024, 14, 22410. [Google Scholar] [CrossRef] [PubMed]
- Kużdżał, A.; Clemente, F.M.; Kawczyński, A.; Ryszkiel, I.; Trybulski, R. Comparing The Effects of Compression Contrast Therapy and Dry Needling on Muscle Functionality, Pressure Pain Threshold, and Perfusion after Isometric Fatigue in Forearm Muscles of Combat Sports Athletes: A Single-Blind Randomized Controlled Trial. J. Sports Sci. Med. 2024, 23, 548–558. [Google Scholar] [CrossRef]
- Slimani, M.; Davis, P.; Franchini, E.; Moalla, W. Rating of Perceived Exertion for Quantification of Training and Combat Loads during Combat Sport-Specific Activities: A Short Review. J. Strength Cond. Res. 2017, 31, 2889–2902. [Google Scholar] [CrossRef]
- Trybulski, R.; Vovkanych, A.; Bas, O.; Tyravska, O. The Low-Temperature Effect on Sports Regeneration. Fisioter. Movimento 2023, 36, e36204. [Google Scholar] [CrossRef]
- Yoshida, R.; Nakamura, M.; Ikegami, R. The Effect of Single Bout Treatment of Heat or Cold Intervention on Delayed Onset Muscle Soreness Induced by Eccentric Contraction. Healthcare 2022, 10, 2556. [Google Scholar] [CrossRef]
- Trybulski, R.; Żebrowska, A.; Bichowska-Pawęska, M.; Kużdżał, A.; Ryszkiel, I.; Silva, R.M.; Muracki, J.; Kawczyński, A. The Effects of Combined Contrast Heat Cold Pressure Therapy on Post-Exercise Muscle Recovery in MMA Fighters: A Randomized Controlled Trial. J. Hum. Kinet. 2024, 94, 127–146. [Google Scholar] [CrossRef]
- Weerapong, P.; Hume, P.A.; Kolt, G.S. The Mechanisms of Massage and Effects on Performance, Muscle Recovery and Injury Prevention. Sports Med. 2005, 35, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Best, T.M.; Hunter, R.; Wilcox, A.; Haq, F. Effectiveness of Sports Massage for Recovery of Skeletal Muscle from Strenuous Exercise. Clin. J. Sport Med. 2008, 18, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Butterfield, T.A.; Abshire, S.; Zhao, Y.; Zhang, X.; Jarjoura, D.; Best, T.M. Massage Timing Affects Postexercise Muscle Recovery and Inflammation in a Rabbit Model. Med. Sci. Sports Exerc. 2013, 45, 1105–1112. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Gong, Y.; Zhu, R.; Xu, J.; Zou, J.; Chen, X. Massage Alleviates Delayed Onset Muscle Soreness after Strenuous Exercise: A Systematic Review and Meta-Analysis. Front. Physiol. 2017, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Folhes, O.; Reis, V.M.; Marques, D.L.; Neiva, H.P.; Marques, M.C. Maximum Isometric and Dynamic Strength of Mixed Martial Arts Athletes According to Weight Class and Competitive Level. Int. J. Environ. Res. Public Health 2022, 19, 8741. [Google Scholar] [CrossRef]
- Trybulski, R.; Stanula, A.; Żebrowska, A.; Podleśny, M.; Hall, B. Acute Effects of the Dry Needling Session on Gastrocnemius Muscle Biomechanical Properties, and Perfusion with Latent Trigger Points—A Single-Blind Randomized Controlled Trial in Mixed Martial Arts Athletes. J. Sports Sci. Med. 2024, 23, 136–146. [Google Scholar] [CrossRef]
- Draper, S.N.; Kullman, E.L.; Sparks, K.E.; Little, K.; Thoman, J. Effects of Intermittent Pneumatic Compression on Delayed Onset Muscle Soreness (DOMS) in Long Distance Runners. Int. J. Exerc. Sci. 2020, 13, 75–86. [Google Scholar] [CrossRef]
- Brunt, V.E.; Howard, M.J.; Francisco, M.A.; Ely, B.R.; Minson, C.T. Passive Heat Therapy Improves Endothelial Function, Arterial Stiffness and Blood Pressure in Sedentary Humans. J. Physiol. 2016, 594, 5329–5342. [Google Scholar] [CrossRef]
- Ouergui, I.; Hammouda, O.; Chtourou, H.; Gmada, N.; Franchini, E. Effects of Recovery Type after a Kickboxing Match on Blood Lactate and Performance in Anaerobic Tests. Asian J. Sports Med. 2014, 5, 99–107. [Google Scholar]
- Cabak, A.; Deca, S. Massage in Biological Regeneration—A Review of Current Literature. Pol. J. Sports Med. 2023, 39, 59–66. [Google Scholar] [CrossRef]
- Howatson, G.; Gaze, D.; Van Someren, K.A. The Efficacy of Ice Massage in the Treatment of Exercise-Induced Muscle Damage. Scand. J. Med. Sci. Sports 2005, 15, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Gasibat, Q.; Rafieda, A.E.; Aween, M.M. The Influence of Therapeutic Massage on Muscle Recovery, Physiological, Psychological and Performance in Sport: A Systematic Review. Sport Mont. 2024, 22, 147–161. [Google Scholar] [CrossRef]
- Gholami, Z.; Faezi, S.T.; Letafatkar, A.; Madreseh, E. Pain Neuroscience Education, Blended Exercises and Booster Sessions as an Effective Therapy for Pain, Functional and Psychological Factors in Patients with Knee Osteoarthritis: A Study Protocol for a Single-Blind Randomised Controlled Trial with 2 2 Factorial Design during 6-Month Follow-Up. BMJ Open 2023, 13, e070336. [Google Scholar] [CrossRef]
- Jones, M.T.; Aguiar, E.J.; Winchester, L.J. Proposed Mechanisms of Blood Flow Restriction Exercise for the Improvement of Type 1 Diabetes Pathologies. Diabetology 2021, 2, 176–189. [Google Scholar] [CrossRef]
- Oliva-Lozano, J.M.; Patterson, S.D.; Chiampas, G.; Maybury, E.; Cost, R. Blood Flow Restriction as a Post-Exercise Recovery Strategy: A Systematic Review of the Current Status of the Literature. Biol. Sport 2024, 41, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, J.; Gaweł, D.; Krzysztofik, M.; Zając, A.; Tsoukos, A.; Bogdanis, G.C.; Wilk, M. Effects of Blood Flow Restriction on Mechanical Properties of the Rectus Femoris Muscle at Rest. Front. Physiol. 2023, 14, 1244376. [Google Scholar] [CrossRef]
- Wong, V.; Spitz, R.W.; Song, J.S.; Yamada, Y.; Kataoka, R.; Hammert, W.B.; Kang, A.; Seffrin, A.; Bell, Z.W.; Loenneke, J.P. Blood Flow Restriction Augments the Cross-Education Effect of Isometric Handgrip Training. Eur. J. Appl. Physiol. 2024, 124, 1575–1585. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, E.J.; Lane, T.; Cannon, J. Effect of an Acute Bout of Plyometric Exercise on Neuromuscular Fatigue and Recovery in Recreational Athletes. J. Strength Cond. Res. 2009, 23, 1181–1186. [Google Scholar] [CrossRef]
- Dakić, M.; Ilić, V.; Toskić, L.; Duric, S.; Šimenko, J.; Marković, M.; Dopsaj, M.; Cuk, I. Acute Effects of Short-Term Massage Procedures on Neuromechanical Contractile Properties of Rectus Femoris Muscle. Medicina 2024, 60, 125. [Google Scholar] [CrossRef]
- Dakić, M.; Toskić, L.; Ilić, V.; Đurić, S.; Dopsaj, M.; Šimenko, J. The Effects of Massage Therapy on Sport and Exercise Performance: A Systematic Review. Sports 2023, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Liana, R.; Chudański, M.; Ponikowska, I. Standarisation of Laser Doppler Flowmetry-Own Standards. Clin. Diabetol. 2009, 10, 58–64. [Google Scholar]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-Frequency Oscillations of the Laser Doppler Perfusion Signal in Human Skin. Microvasc. Res. 2006, 72, 120–127. [Google Scholar] [CrossRef]
- Rajan, V.; Varghese, B.; Van Leeuwen, T.G.; Steenbergen, W. Review of Methodological Developments in Laser Doppler Flowmetry. Lasers Med. Sci. 2009, 24, 269–283. [Google Scholar] [CrossRef]
- Nguyen, A.P.; Detrembleur, C.; Fisette, P.; Selves, C.; Mahaudens, P. MyotonPro Is a Valid Device for Assessing Wrist Biomechanical Stiffness in Healthy Young Adults. Front. Sports Act. Living 2022, 4, 797975. [Google Scholar] [CrossRef]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.F.; Bauermeister, W.; Klingler, W.; Schleip, R. Assessing Reliability and Validity of Different Stiffness Measurement Tools on a Multi-Layered Phantom Tissue Model. Sci. Rep. 2023, 13, 815. [Google Scholar] [CrossRef]
- Trybulski, R.; Kużdżał, A.; Wilk, M.; Więckowski, J.; Fostiak, K.; Muracki, J. Reliability of MyotonPro in Measuring the Biomechanical Properties of the Quadriceps Femoris Muscle in People with Different Levels and Types of Motor Preparation. Front. Sports Act. Living 2024, 6, 1453730. [Google Scholar] [CrossRef]
- Seo, H.; Kim, J.; Yu, C.; Lim, H. Intra-Rater and Inter-Rater Reliability Analysis of Muscle-Tone Evaluation Using a Myotonometer for Children with Developmental Disabilities. Healthcare 2023, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, C.W.; Park, S.B.; Kim, M.J.; Jang, S.H. Reliability and Usefulness of the Pressure Pain Threshold Measurement in Patients with Myofascial Pain. Ann. Rehabil. Med. 2011, 35, 412. [Google Scholar] [CrossRef]
- Markwick, W.J.; Bird, S.P.; Tufano, J.J.; Seitz, L.B.; Haff, G.G. The Intraday Reliability of the Reactive Strength Index Calculated from a Drop Jump in Professional Men’s Basketball. Int. J. Sports Physiol. Perform. 2015, 10, 482–488. [Google Scholar] [CrossRef]
- Healy, R.; Kenny, I.C.; Harrison, A.J. Reactive Strength Index: A Poor Indicator of Reactive Strength? Int. J. Sports Physiol. Perform. 2018, 13, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; Ebben, W.P.; Jensen, R.L. Reliability of the Reactive Strength Index and Time to Stabilization during Depth Jumps. J. Strength Cond. Res. 2008, 22, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Wiewelhove, T.; Schneider, C.; Kellmann, M.; Pfeiffer, M.; Meyer, T.; Ferrauti, A. Recovery Management in Sport: Overview and Outcomes of a Nine-Year Multicenter Research Program. Int. J. Sports Sci. Coach. 2024, 19, 1223–1233. [Google Scholar] [CrossRef]
- Miranda-Mendoza, J.; Hernández-Cruz, G.; Reynoso-Sánchez, L.F.; González-Fimbres, R.A.; Hernández, B.A.C. Control de La Recuperación Utilizando La Escala de La Calidad de Recuperación Total (TQR) Durante Cuatro Microciclos de Acumulación y Su Relación Con Factores Fisiológicos (Control of Recovery Using the Total Quality Recovery (TQR) Scale during Four Accumulation Microcycles and Its Relationship to Physiological Factors). Retos 2023, 50, 1155–1162. [Google Scholar] [CrossRef]
- Tiidus, P.; Shoemaker, J. Effleurage Massage, Muscle Blood Flow and Long-Term Post-Exercise Strength Recovery. Int. J. Sports Med. 1995, 16, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Eriksson Crommert, M.; Lacourpaille, L.; Heales, L.J.; Tucker, K.; Hug, F. Massage Induces an Immediate, Albeit Short-term, Reduction in Muscle Stiffness. Scand. J. Med. Sci. Sports 2015, 25, e490–e496. [Google Scholar] [CrossRef]
- Rossi, F.E.; de Freitas, M.C.; Zanchi, N.E.; Lira, F.S.; Cholewa, J.M. The Role of Inflammation and Immune Cells in Blood Flow Restriction Training Adaptation: A Review. Front. Physiol. 2018, 9, 1376. [Google Scholar] [CrossRef]
- Maasri, R.E.; Jarvie, J.R.; Karski, J.S.; Smith, L.J.; Malek, M.H. The Influence of Cold Therapy on the Physical Working Capacity at the Electromyographic Threshold for Consecutive Exercise Sessions. Bioengineering 2024, 11, 292. [Google Scholar] [CrossRef]
- Lambert, B.S.; Hedt, C.; Moreno, M.; Harris, J.D.; McCulloch, P. Blood Flow Restriction Therapy for Stimulating Skeletal Muscle Growth: Practical Considerations for Maximizing Recovery in Clinical Rehabilitation Settings. Tech. Orthop. 2018, 33, 89–97. [Google Scholar] [CrossRef]
- Mustalampi, S.; Ylinen, J.; Kautiainen, H.; Weir, A.; Häkkinen, A. Acute Effects of Cold Pack on Mechanical Properties of the Quadriceps Muscle in Healthy Subjects. Phys. Ther. Sport 2012, 13, 265–269. [Google Scholar] [CrossRef]
- Dupuy, O.; Douzi, W.; Theurot, D.; Bosquet, L.; Dugué, B. An Evidence-Based Approach for Choosing Post-Exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review With Meta-Analysis. Front. Physiol. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.S.; Bender, P.U.; de Menezes, F.S.; Yamashitafuji, I.; Vargas, V.Z.; Wageck, B. Massage Therapy Decreases Pain and Perceived Fatigue after Long-Distance Ironman Triathlon: A Randomised Trial. J. Physiother. 2016, 62, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Trybulski, R.; Stanula, A.; Vovkanych, A.; Muracki, J.; Wang, H.-K.; Kużdżał, A. Immediate Effect of Ice and Dry Massage during Rest Breaks on Recovery in MMA Fighters: A Randomized Crossover Clinical Trial Study. Sci. Rep. 2025, 15, 12323. [Google Scholar] [CrossRef] [PubMed]
| Group n= 36 | |||
|---|---|---|---|
| Massage n = 12 | BFR/Cool n = 12 | Control n = 12 | |
| M ± SD | |||
| Age | 28 ± 3 | 29 ± 4 | 28 ± 5 |
| Height [cm] | 176.3 ± 11.4 | 175.8 ± 10.1 | 175.6 ± 10.4 |
| Weight [kg] | 77.8 ± 12.6 | 78.1 ± 16.1 | 79.0 ± 17.0 |
| Training experience (years) | 10 ± 2 | 11 ± 4 | 12 ± 5 |
| Body mass index (kg/m2) | 24.40 ± 1.73 | 24.99 ± 2.33 | 25.53 ± 2.66 |
| Sex | Group | Totals | ||
|---|---|---|---|---|
| Massage | BFR/Cool | Control | ||
| m | 10 | 9 | 9 | 28 |
| % | 83.33% | 75.00% | 75.00% | |
| f | 2 | 3 | 3 | 8 |
| % | 16.67% | 25.00% | 25.00% | |
| summary | 12 | 12 | 12 | 36 |
| χ2 = 0.33; df = 2; p = 0.85 | ||||
| Variables | Group | Main Effects and Interaction for MANOVA Repeated Measures F/p/η2 Or χ2; p | ||
|---|---|---|---|---|
| Massage (n = 12) | BFR/Cool (n = 12) | Control (n = 12) | ||
| M ± SD (95%;95%CI) | M ± SD (95%;95%CI) | M ± SD (95%;95%CI) | ||
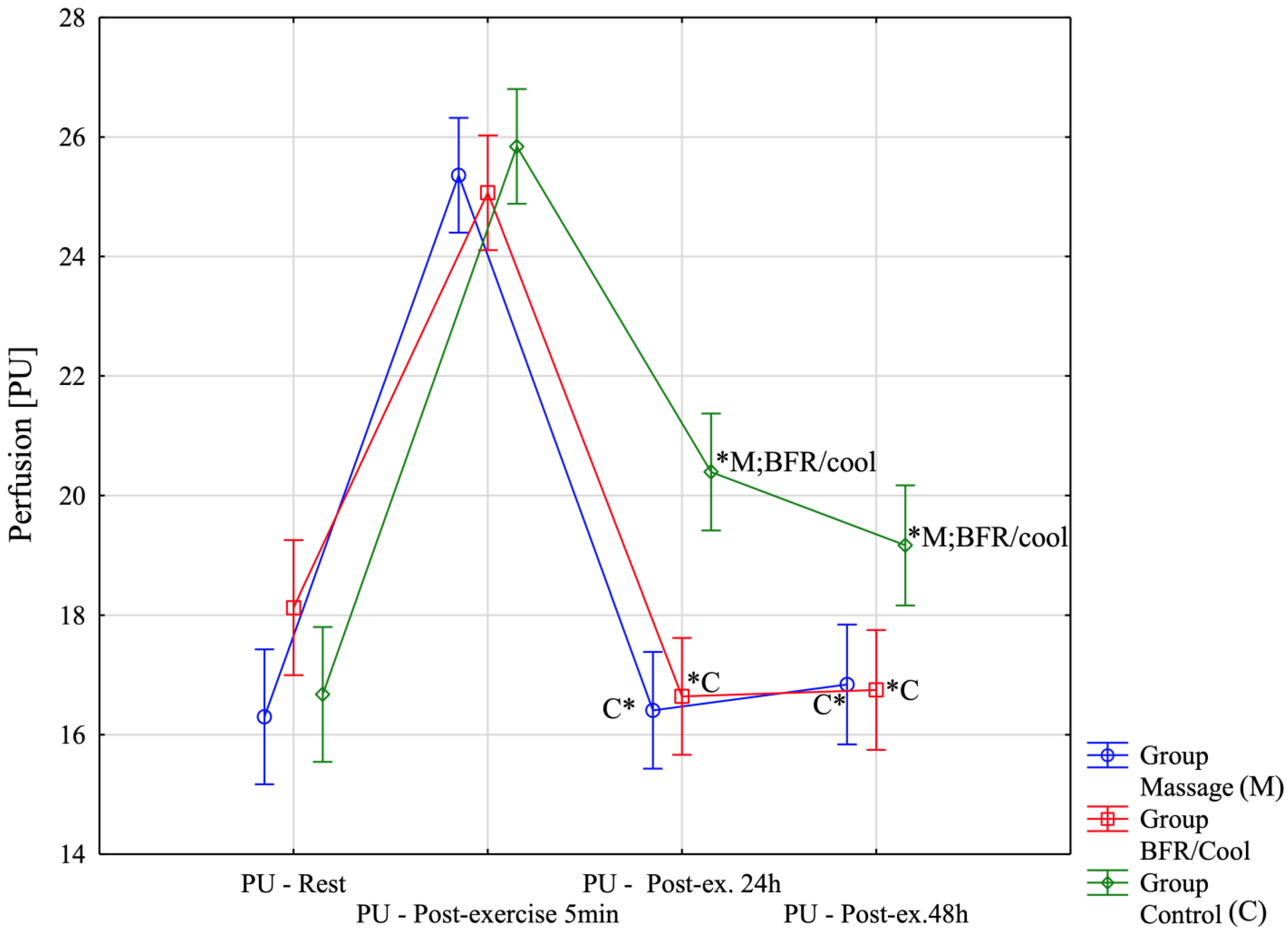
| PU-Rest | 16.30 ± 2.28 (14.85;17.75) | 18.13 ± 1.92 (16.90;19.35) | 16.68 ± 1.47 (15.74;17.61) | Massage: χ2 χ2 = 23.50; p = 0.00003 BFR/Cool χ2 = 24.70; p = 0.00002 Control χ2 = 28.00; p < 0.00001 |
| PU-Post-exercise 5 min | 25.36 ± 2.04 (24.06;26.65) | 25.07 ± 1.43 (24.16;25.97) | 25.84 ± 1.35 (24.98;26.70) | |
| PU-Post-ex. 24 h [2 session recovery] | 16.41 ± 1.76 (15.29;17.52) | 16.64 ± 1.44 (15.72;17.56) | 20.39 ± 1.77 (19.27;21.52) | |
| PU-Post-ex. 48 h [4 session] | 16.84 ± 1.91 (15.63;18.06) | 16.75 ± 1.32 (15.91;17.59) | 19.17 ± 1.83 (18.00;20.33) | |
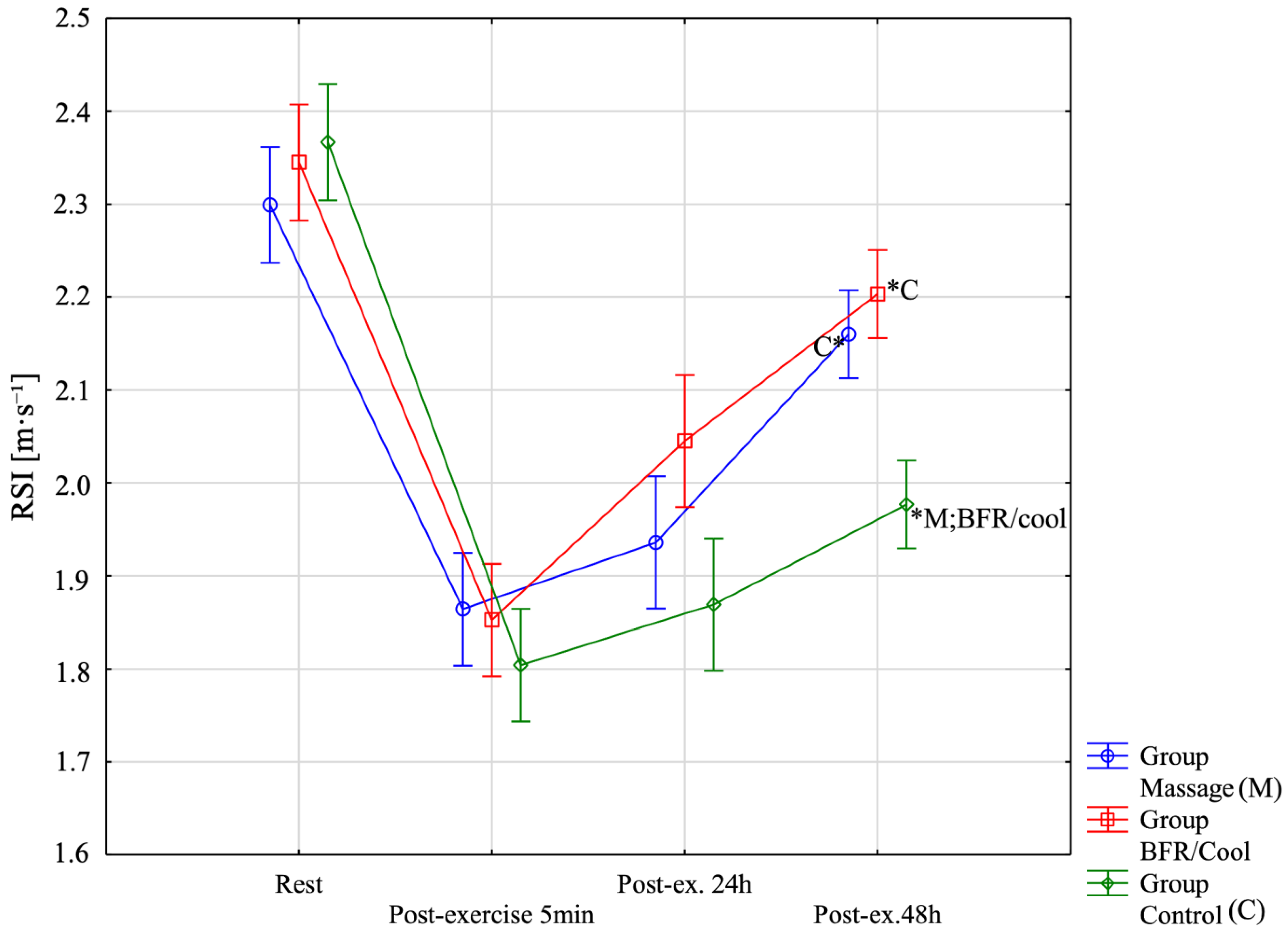
| RSI [m s−1]-Rest | 2.30 ± 0.11 (2.23;2.37) | 2.35 ± 0.11 (2.28;2.41) | 2.37 ± 0.10 (2.30;2.43) | Group: F = 6.78; p = 0.0034; η2 = 0.29 Before-After: F = 223.27; p < 0.0001; η2 = 0.87 Group x Before-After: F = 6.90; p < 0.0001; η2 = 0.30 |
| RSI [m s−1]-Post-exercise 5 min | 1.86 ± 0.14 (1.77;1.95) | 1.85 ± 0.04 (1.82;1.88) | 1.80 ± 0.10 (1.74;1.87) | |
| RSI [m s−1]-Post-ex. 24 h [2 session recovery] | 1.94 ± 0.13 (1.85;2.02) | 2.05 ± 0.06 (2.00;2.09) | 1.87 ± 0.15 (1.77;1.97) | |
| RSI [m s−1]-Post-ex. 48 h [4 session] | 2.16 ± 0.11 (2.09;2.23 | 2.20 ± 0.07 (2.16;2.24) | 1.98 ± 0.05 (1.94;2.01) | |
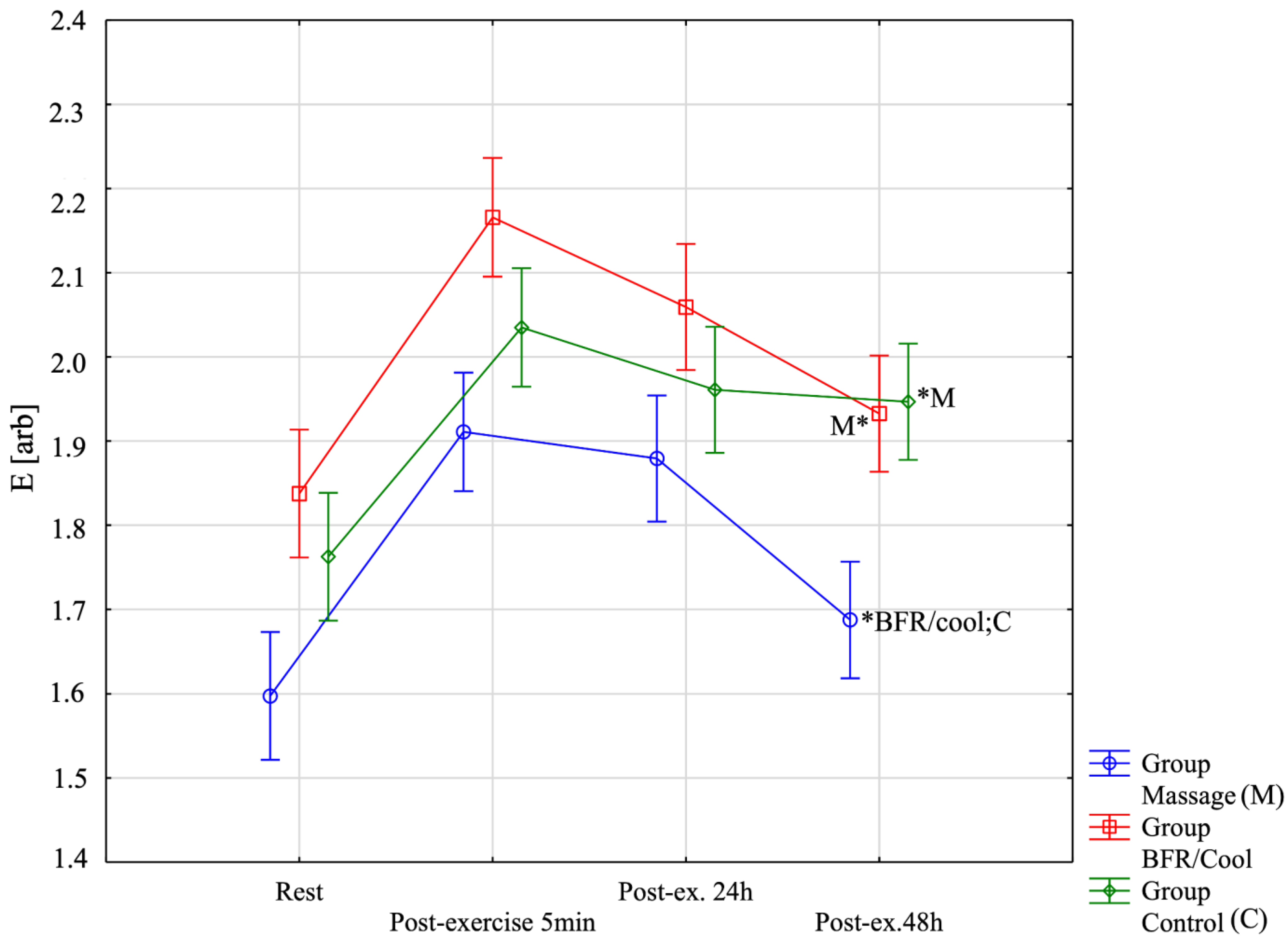
| E [arb]-Rest | 1.60 ± 0.12 (1.52;1.67) | 1.84 ± 0.17 (1.73;1.94) | 1.76 ± 0.09 (1.71;1.82) | Group: F = 13.24; p = 0.0001; η2 = 0.45 Before-After: F = 171.92; p < 0.0001; η2 = 0.84 Group x Before-After: F = 5.79; p < 0.0001; η2 = 0.26 |
| E [arb]-Post-exercise 5 min | 1.91 ± 0.12 (1.84;1.98) | 2.17 ± 0.14 (2.08;2.25) | 2.04 ± 0.11 (1.97;2.10) | |
| E [arb]-Post-ex. 24 h [2 session recovery] | 1.88 ± 0.12 (1.80;1.96) | 2.06 ± 0.13 (1.98;2.14) | 1.96 ± 0.13 (1.88;2.04) | |
| E [arb]-Post-ex. 48 h [4 session] | 1.69 ± 0,14 (1.60; 1.78) | 1.93 ± 0.11 (1.86; 2.00) | 1.95 ± 0.10 (1.88;2.01) | |
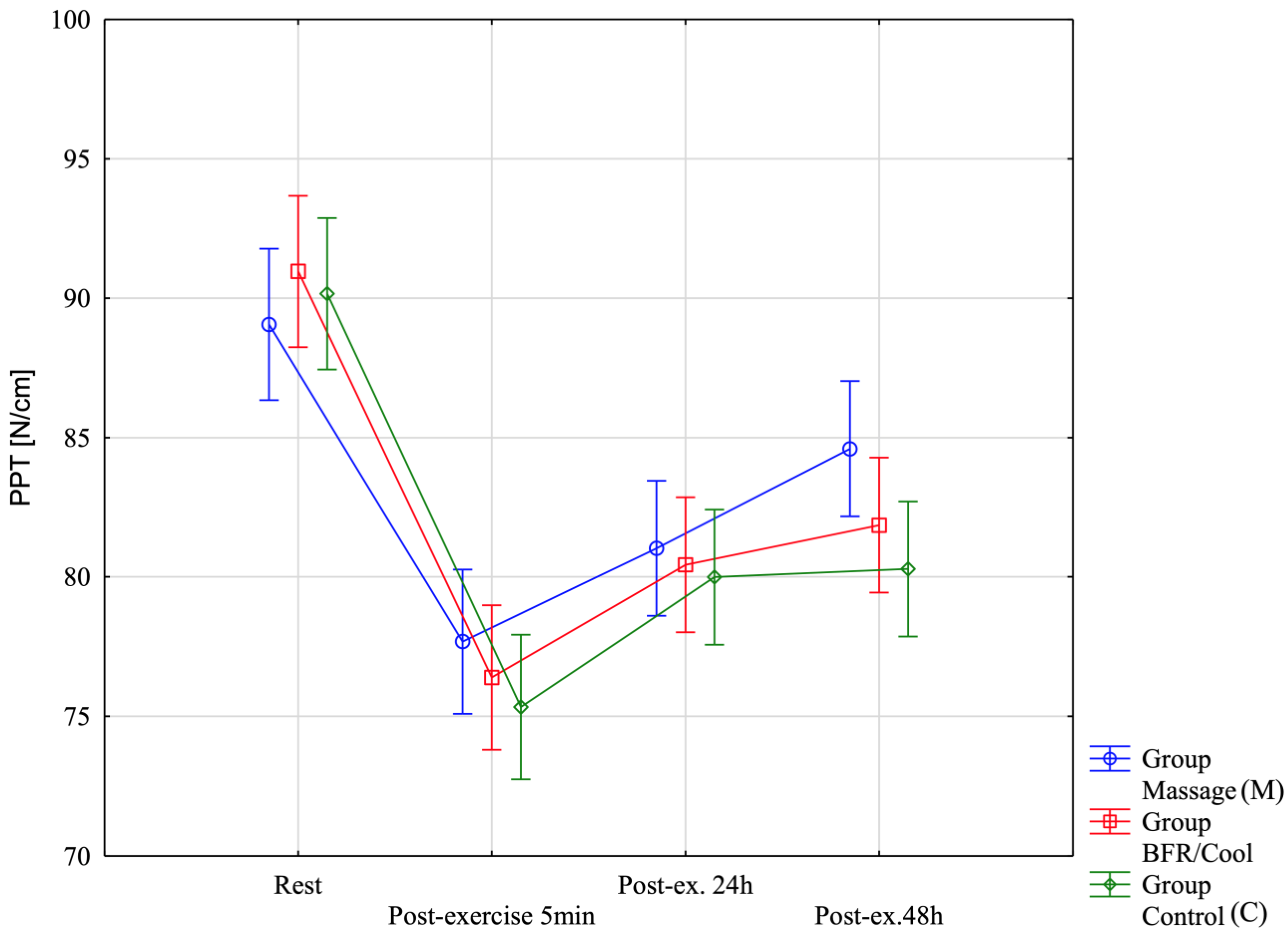
| PPT [N/cm]-Rest | 89.06 ± 5.77 (85.39;92.72) | 90.96 ± 4.03 (88.40;93.52) | 90.16 ± 3.82 (87.73;92.58) | Group: F = 1.67; p = 0.20; η2 = 0.091 Before-After: F = 63.67; p < 0.0001; η2 = 0.66 Group x Before-After: F = 1.00; p = 0.43; η2 = 0.057 |
| PPT [N/cm]-Post-exercise 5 min | 77.68 ± 5.56 (74.14;81.21) | 76.39 ± 2.73 (74.65;78.13) | 75.33 ± 4.45 (72.51;78.16) | |
| PPT [N/cm]-Post-ex. 24 h [2 session recovery] | 81.03 ± 3.37 (78.88;83.17) | 80.43 ± 4.16 (77.79;83.08) | 79.99 ± 4.75 (76.98;83.01) | |
| PPT [N/cm]-Post-ex. 48 h [4 session] | 84.60 ± 3.41 (82.43;86.77) | 81.86 ± 3.80 (79.44;84.28) | 80.28 ± 5.01 (77.10;83.47) | |
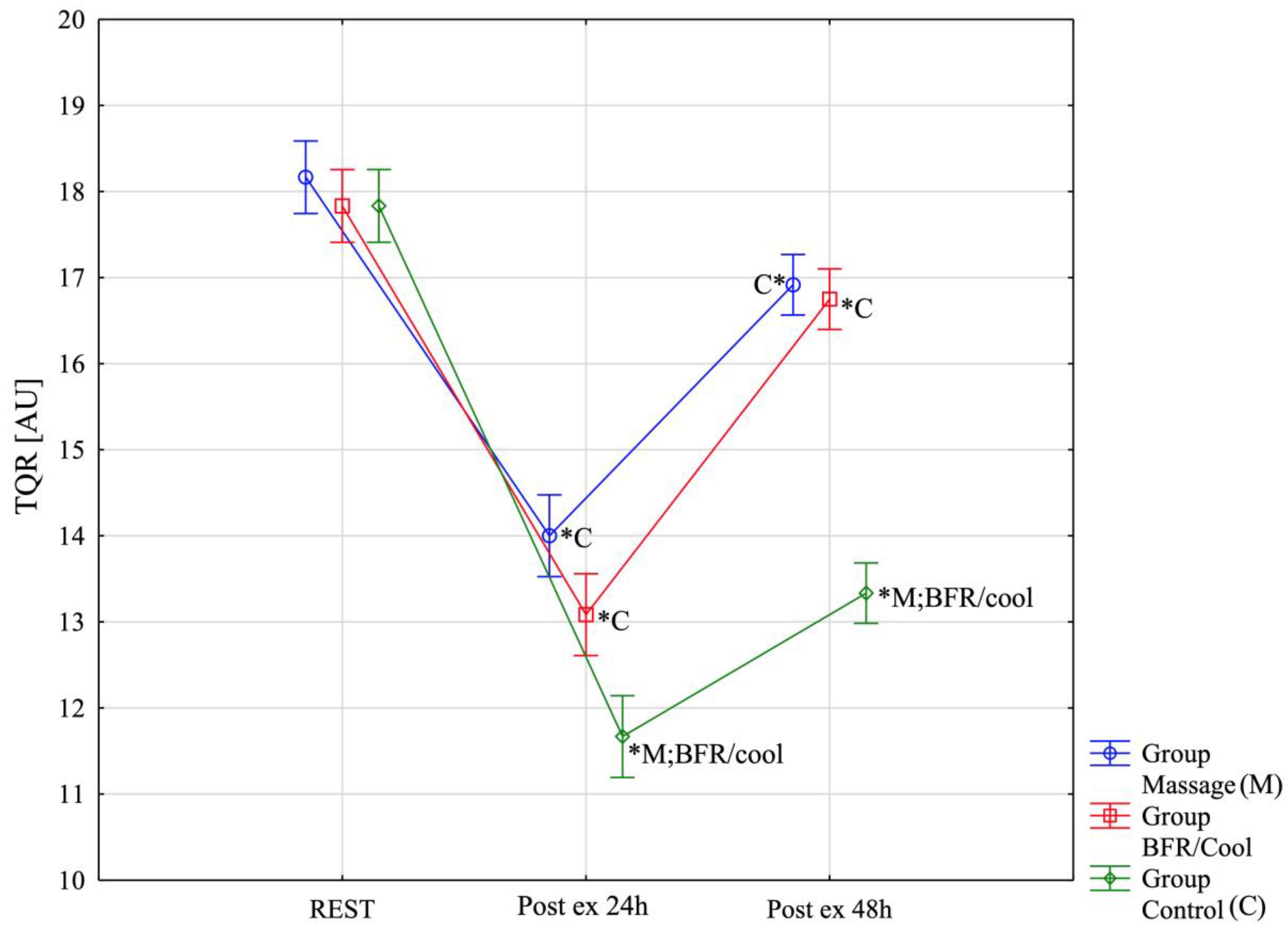
| TQR-REST | 18.17 ± 0.72 (17.71;18.62) | 17.83 ± 0.72 (17.38;18.29) | 17.83 ± 0.72 (17.38;18.29) | Massage: χ2 χ2 = 23.13; p < 0.0001 BFR/Cool χ2 = 23.13; p < 0.0001 Control χ2 = 23.53; p < 0.0001 |
| TQR-Post ex-5 min | 14.00 ± 0.85 (13.46;14.54) | 13.08 ± 0.51 (12.76;13.41) | 11.67 ± 0.98 (11.04;12.29) | |
| TQR-Post ex, 48 h | 16.92 ± 0.51 (16.59;17.24) | 16.75 ± 0.45 (16.46;17.04) | 13.33 ± 0.78 (12.84;13.83) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trybulski, R.; Roczniok, R.; Olaniszyn, G.; Svyshch, Y.; Vovkanych, A.; Wilk, M. Sports Massage and Blood Flow Restriction Combined with Cold Therapy Accelerate Muscle Recovery After Fatigue in Mixed Martial Arts Athletes: A Randomized Controlled Trial. J. Funct. Morphol. Kinesiol. 2025, 10, 194. https://doi.org/10.3390/jfmk10020194
Trybulski R, Roczniok R, Olaniszyn G, Svyshch Y, Vovkanych A, Wilk M. Sports Massage and Blood Flow Restriction Combined with Cold Therapy Accelerate Muscle Recovery After Fatigue in Mixed Martial Arts Athletes: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology. 2025; 10(2):194. https://doi.org/10.3390/jfmk10020194
Chicago/Turabian StyleTrybulski, Robert, Robert Roczniok, Gracjan Olaniszyn, Yaroslav Svyshch, Andryi Vovkanych, and Michał Wilk. 2025. "Sports Massage and Blood Flow Restriction Combined with Cold Therapy Accelerate Muscle Recovery After Fatigue in Mixed Martial Arts Athletes: A Randomized Controlled Trial" Journal of Functional Morphology and Kinesiology 10, no. 2: 194. https://doi.org/10.3390/jfmk10020194
APA StyleTrybulski, R., Roczniok, R., Olaniszyn, G., Svyshch, Y., Vovkanych, A., & Wilk, M. (2025). Sports Massage and Blood Flow Restriction Combined with Cold Therapy Accelerate Muscle Recovery After Fatigue in Mixed Martial Arts Athletes: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology, 10(2), 194. https://doi.org/10.3390/jfmk10020194







