Abstract
Healthcare workers across the globe rely on medical gloves to prevent the transfer of harmful bacteria and viruses between themselves and their patients. Unfortunately, due to the lack of an in-use durability standard for medical gloves by the American Society for Testing and Materials, many of these gloves are of low quality and are easily torn or punctured, exposing wearers and patients to potentially deadly diseases. To solve this problem, a device that automatically detects material failures the size of a pinhole during active testing was invented. The device consists of a prosthetic hand, vacuum pump, mobile textured roller, pressure sensor, and liquid spray system. It works by creating a vacuum inside the glove and repeatedly moving the textured roller into contact with the fingertips, which, on the prosthetic hand, are porous. When a glove perforates, the vacuum is broken, pressure within the hand rapidly increases, and the operator is alerted on a touchscreen that the glove has failed. In addition, the liquid spray system allows the user to test gloves in “real world” conditions, because healthcare workers often come into contact with liquids that may alter glove durability. As a preliminary test of the device’s accuracy, five nitrile and five latex exam gloves were tested using the system’s default settings. Natural latex is known to be the highest performing glove material, so the nitrile gloves were expected to fail more quickly than the latex gloves. The test results concur with this expected order of failure: nitrile first, with an average failure time of 300 s and 42 average number of roller touches, followed by natural latex, with an average failure time of 2206 s and 300 average number of roller touches. These results provide evidence that the device accurately ranks glove durability, and therefore could be used to develop an ASTM durability standard and improve the quality of gloves made from different polymers.
1. Introduction
Medical gloves are an essential piece of personal protective equipment (PPE) because they act as a protective barrier, preventing the transfer of bacteria, viruses, and toxins from healthcare worker to patient and vice versa. With the emergence and spread of COVID-19 in early 2020, the demand for medical gloves doubled, resulting in many healthcare facilities facing a shortage of these necessary supplies [1,2]. Despite the important role of gloves in disease prevention, the American Society for Testing and Materials (ASTM) standards for rubber surgical gloves (D3577-19) and rubber examination gloves (D3578-19) lack a specification for in-use glove durability once the gloves are removed from their packaging by the consumer [3,4]. Manufacturing and polymer differences mean that some types of gloves are more prone to breakage under stress than others. Unfortunately, the current ASTM standard for the detection of holes in medical gloves only measures the incidence of gloves with holes before their actual use, and this protocol entails merely filling a glove with tap water and observing it for leaks [5].
The need for a standardized medical glove assessment device is most evident when natural and synthetic gloves are compared. A 2011 study in which natural latex and latex-free gloves were examined for perforations following surgical use found that latex was perforated only 34.3% of the time, compared to 80% for synthetic polyisoprene [6]. An additional study in which gloves of a variety of materials made by different manufacturers were perforated using a standardized procedure found that bacterial passage through medical examination gloves occurred at a 10-fold higher rate in nitrile and neoprene gloves compared to natural latex [7]. In addition, the size of the hole left in the glove after a needle stick puncture was smaller in natural latex films than in nitrile films [8]. These results demonstrate that not all gloves provide the same level of protection, and that a uniform durability standard would ensure that all gloves provide a minimum level of protection regardless of material or manufacturer.
The combination of high glove demand and lack of a durability standard led to our development of a medical glove durability assessment machine. The device closely simulates real-world glove use because a glove is placed on a prosthetic hand, which comes into repeated contact with a roughened surface until a hole is detected in the glove. This device could ensure that all gloves meet a basic standard of durability while being worn and forms the basis for a new ASTM standard for medical and surgical glove durability.
2. Materials and Methods
2.1. Capstone Glove Assessment Device (C-GAD)
The glove testing prototype previously described [9] was modified in collaboration with the Center for Design and Manufacturing Excellence (CDME) at the Ohio State University, Columbus, Ohio, USA, due to expired software. Although the basic components of the C-GAD remained the same, an ESP32 Expressif Systems microcontroller (Expressif, Shanghai, China) with a Nextion Touch Display LED (light emitting diode) touchscreen (Nextion, Shenzhen, China) was wired to the motor for improved ease of use. The touchscreen displayed the elapsed time, test number, speed, and force of the motor. The hand was also attached to an aluminum rail structure, and the entire system was mounted on a piece of plywood for added stability (Figure 1). The major drawback of this design was the need to visually inspect the gloves for holes, which entailed frequent inspection stoppages and approximately a 5 s or more delay in break detection [6]. Therefore, a new iteration of this glove testing device was designed, with the goal of increasing precision of glove failure data, improving ease of use, and making a machined version which could be readily manufactured.
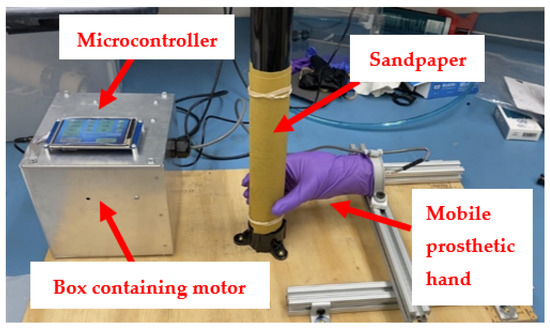
Figure 1.
Modified setup of the original glove testing device.
2.2. New Glove Assessment Device (N-GAD)
2.2.1. Touchscreen Display
The N-GAD was designed to automatically detect holes in medical gloves, eliminating the need for manual glove inspection. Like the C-GAD, the N-GAD also utilizes an ESP32 Expressif Systems microcontroller and a Nextion Touch Display LED touchscreen (Appendix A), however, the N-GAD was designed with increased functionality. The homepage of the LED touchscreen (Figure 2) displays the number of touches completed, the speed of the drum movement in mm/s, the force of the drum in mN, and the number of sprays between touches, and all settings are adjustable (Table 1 and Table 2).
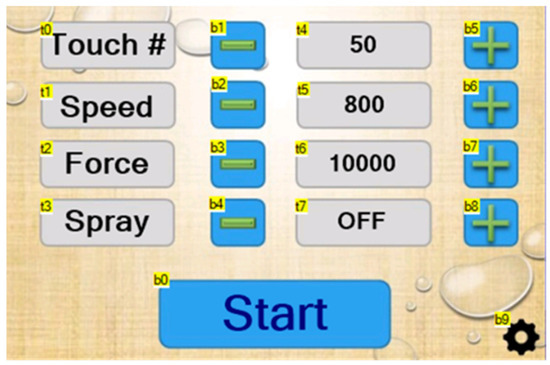
Figure 2.
Homepage of LED touchscreen.

Table 1.
Description of each text field found on the homepage.

Table 2.
Description of each button found on the homepage.
The depressurization failure page appears on the touchscreen if the machine cannot depressurize before a test has begun (Figure 3). This screen has only one text field and one button (Table 3 and Table 4).
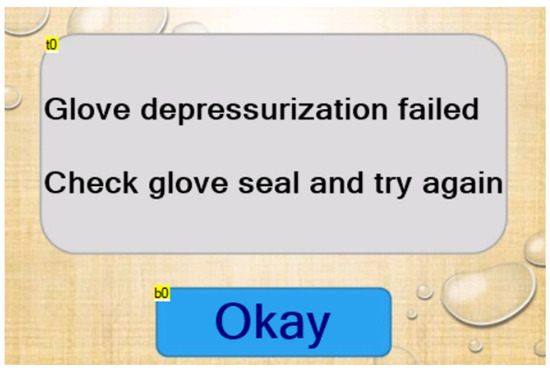
Figure 3.
Depressurization failure page.

Table 3.
Description of the text field found on the glove depressurization page.

Table 4.
Description of the button found on the glove depressurization page.
The test page appears after the glove has successfully been depressurized and the drum has begun to move. It displays the current elapsed time as well as the touch number that the machine is currently executing (Figure 4). It should be noted that the elapsed time is paused during the process of depressurization. A test can be aborted at any time by pressing the “Abort” button. Otherwise, the machine automatically switches to the “test complete” page once the glove has failed (Table 5 and Table 6).
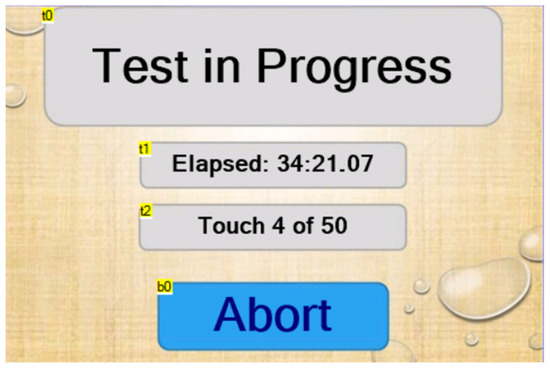
Figure 4.
Test page as it appears during a test.

Table 5.
Description of each text field found on the test page.

Table 6.
Description of the button found on the test page.
The test abort page is displayed after the “Abort” button has been selected (Figure 5). There are no buttons on this page, as its purpose is to inhibit user input until the machine has returned to the pre-test configuration (Table 7). The most time-consuming part of that process is the movement of the drum into the home position. Once the machine returns to the pre-test configuration, the display automatically switches to the home page.

Figure 5.
Test abort page.

Table 7.
Description of the button found on the test page.
The glove failure page is displayed when a pressure differential is detected by the sensor, indicating that the glove has been punctured. The elapsed time and number of touches are paused so that the user can note them down (Figure 6). Pressing the “Okay” button returns the user to the homepage (Table 8 and Table 9).

Figure 6.
Glove failure page with paused elapsed time and touch number.

Table 8.
Description of each text field found on the glove failure page.

Table 9.
Description of the button found on the glove failure page.
When the number of touches reaches the pre-set value without the glove failing, the “test complete” page is displayed (Figure 7). The elapsed time is paused so that the user can note it down. The machine returns to the home page when the “Okay” button is pressed (Table 10 and Table 11).
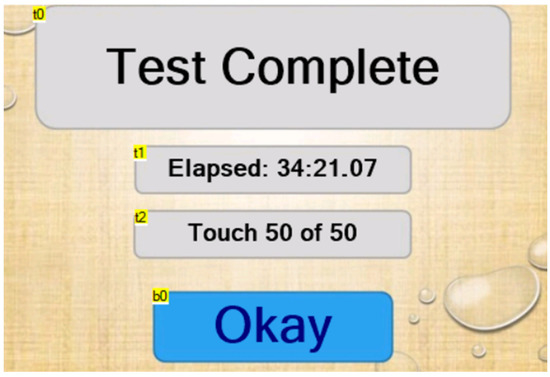
Figure 7.
“Test complete” page.

Table 10.
Description of each text field found on the “test complete” page.

Table 11.
Description of the button found on the “test complete” page.
2.2.2. System Design
Figure 8 shows a conceptual drawing of the basic components of the device. Notably, the new system is encased in plexiglass and contains a pump and reservoir system for testing gloves in wet conditions with various solvents. Another notable difference from the C-GAD is the stationary prosthetic hand and mobile drum. This design choice was made to allow the prosthetic hand to be easily interchanged with other prosthetic hands of various sizes.
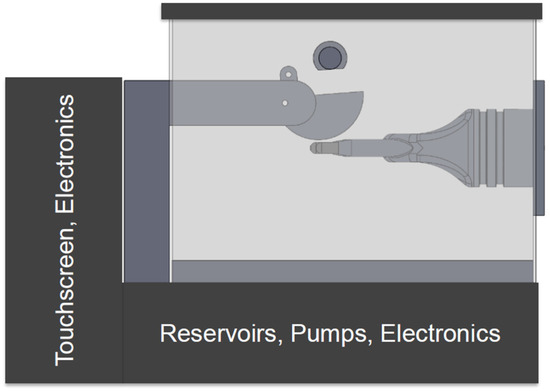
Figure 8.
Conceptual AutoCAD model of the new glove testing device.
In general, the device works by removing the air from within the base of the prosthetic hand via a vacuum pump after the hand is fitted with a glove. The textured drum then moves into position and comes into contact with the porous fingertips of the hand until a tear occurs in the glove. When this happens, a pressure differential is sensed by a pressure sensor located within the base of the hand, and the device stops, thereby eliminating manual inspection.
2.2.3. Hand and Vacuum Pump
For this prototype, a plastic prosthetic hand was 3D printed using the dimensions of an average adult male’s hand [10]. To improve the ease of glove application, the hand was designed without a thumb (Figure 9). The fingertips of the hand are sintered polyethylene mufflers, which contain intricate networks of open-celled, omni-directional pores that allow for airflow. After being applied to the hand, the glove is held in place with a silicone O-ring, and air is removed from within the base via a 12/24 VDC vacuum pump with a 2-way isolation/exhaust valve (Figure 9). However, when a puncture occurs in the glove, the air is free to flow through the porous fingertips, causing a positive pressure differential that is detected by an Adafruit pressure sensor located within the base of the hand (Figure 9). Prior to installation, the pressure sensor was manually calibrated to ensure accurate results.
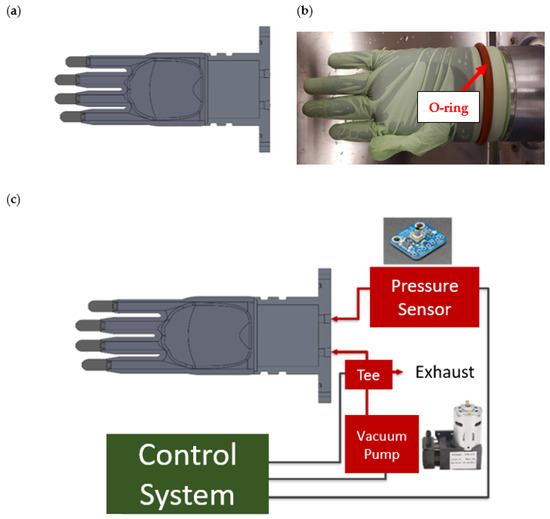
Figure 9.
(a) AutoCAD model of 3D printed prosthetic hand. (b) Silicone O-ring applied to the base of the prosthetic hand. (c) Diagram of the connections between the prosthetic hand, vacuum pump, pressure sensor, and control system.
2.2.4. Drum Motion
The plastic wedge-shaped drum was designed for easy application of sandpaper, which was held in place using two clamps, one on either end of the drum (Figure 10). A stepper motor controlled the movement of the drum by applying a horizontal force to an attached metal rod. The force caused the drum to pivot around a center pin, creating perpendicular contact with the fingertips of the hand. In order to control the force with which the drum contacts the hand, an in-line 10 kg load cell was calibrated using a second load cell and installed on the same rod that is moved by the stepper motor (Figure 10). Both the stepper motor and the load cell were electronically wired to the control center (Figure 11).
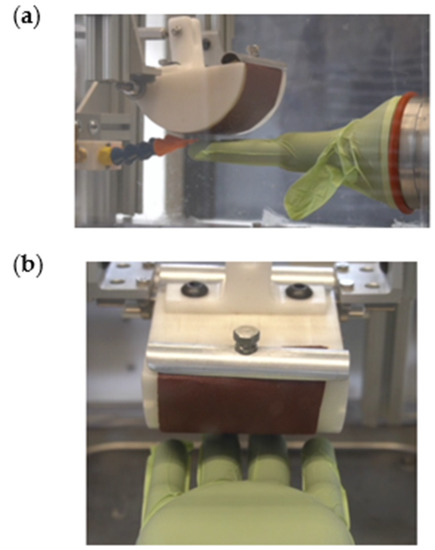
Figure 10.
(a) Side view of drum with sandpaper and clamps, and (b) top view of drum with sandpaper and clamps.
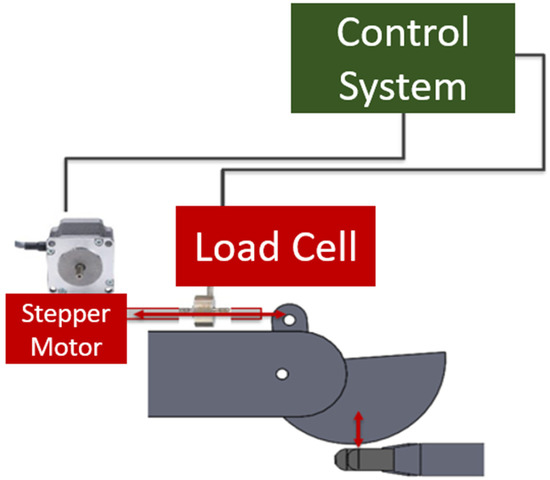
Figure 11.
Diagram of the connections between the drum, motor, and control system.
2.2.5. Liquid System
The liquid system allows the user to test the durability of medical gloves after the gloves are exposed to various solvents. The hand, drum, and spray nozzles were encased in a plexiglass testing chamber to keep the liquid contained during testing. The two spray nozzles were aligned to point at the fingertips of the hand (Figure 10a). The reservoirs were placed within the base of the device, and they consisted of two large plastic bottles with lids containing tubing that connect to either the pump or the liquid-collection pan (Figure 12). A 12/24 VDC peristaltic pump was chosen for the liquid system, and it was connected to the control system so that the user can adjust the number of liquid sprays between touches (Figure 12). The liquid stream was designed to leave the nozzle as a “drip” rather than a spray. This design choice was made to ensure user safety when performing tests with liquids that are toxic when aerosolized, such as ethanol.
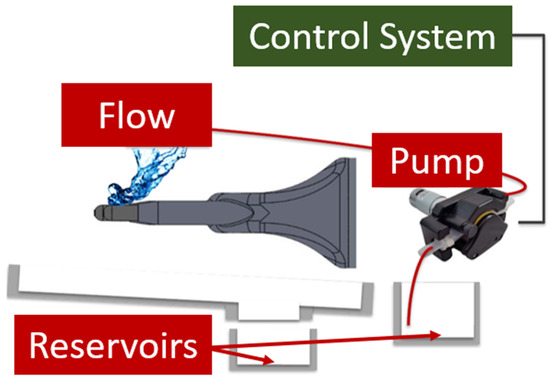
Figure 12.
Diagram of the connections between the hand, liquid pump, and control system.
In the final assembly of the device, the pumps and reservoir system are located underneath the testing chamber, and the electronic components are located behind the touchscreen to the left of the testing chamber (Figure 13). A complete list of replaceable parts is found in Appendix A. The specifications for the power supply, stepper motor, and pressure are found in Appendix B.
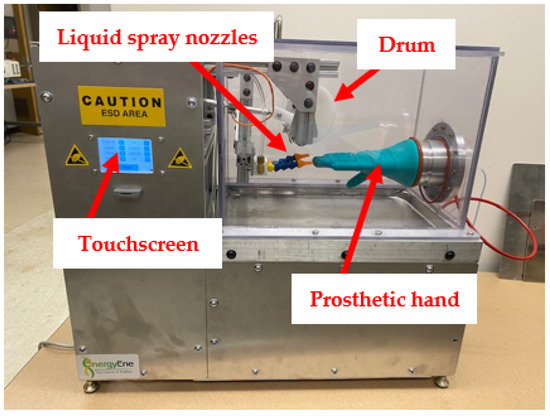
Figure 13.
Final assembly of the new glove testing device.
2.3. Experimental Design
The accuracy of the NGTD was verified by comparing the failure rates of two different exam glove types: nitrile and latex. A previous study found that the failure times of these two glove types were significantly different from each other, with nitrile having the statistically shorter failure time [11]. Thus, if the NGTD produced failure times that were consistent with these results, the device could be assumed to be accurate. For each trial, the selected glove was carefully placed onto the prosthetic hand through the lid at the top of the testing chamber. The two clamps on either end of the semicircular drum were loosened and a strip of 120-grit sandpaper was clamped in place, one end at a time.
The settings on the touchscreen remained at their default values (Table 12). If a glove did not fail by the end of the 50 touches, the time and number of touches were recorded, and a new test was run. When the glove did fail, the values for elapsed time and number of touches were totaled and recorded. After failure, the glove was removed from the hand and discarded along with the sandpaper. A new glove and piece of sandpaper were used for each trial.

Table 12.
Touchscreen settings input for data collection. Note that these are the default settings.
3. Results and Discussion
A one-way analysis of variance test was performed for glove material versus time and glove material versus number of touches (Table 13 and Table 14). Both of the analyses show that nitrile gloves break more easily than natural latex gloves (p = 0.0029 and p = 0.0031, respectively). Average failure times and average number of touches to failure are represented visually as a bar graph (Figure 14). It should be noted that the time and number of touches to failure data for latex has a large variation due to manufacturing inconsistencies, which further supports the need for a universal glove durability standard. The order of glove failure, nitrile followed by latex, concurs with the results of previous studies, and provides evidence that the N-GAD’s measurements are accurate [11].

Table 13.
One-way analysis of variance of glove material versus break time in seconds.

Table 14.
One-way analysis of variance of glove material versus number of sandpaper touches to failure.
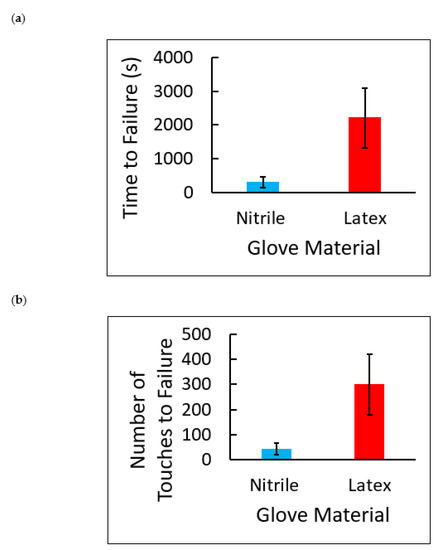
Figure 14.
(a) Failure times in seconds for nitrile and latex examination gloves. The average failure time for nitrile gloves was 300 ± 164 s, and the average failure time for latex gloves was 2206 ± 888 s. Each value is the mean of 5 ± SD. (b) Number of sandpaper touches to failure for nitrile and latex examination gloves. The average number of touches for nitrile glove failure was 42 ± 23 touches, and the average number of touches for latex gloves was 300 ± 121. Each value is the mean of 5 ± SD.
The rise in prevalence of synthetic gloves, especially in the examination glove market, was due to the widespread incidence of Type I latex allergies in the 1990s caused by sensitizing levels of soluble proteins in natural latex gloves. These proteins resulted from a manufacturing failure due to the rapid increase in glove demand in response to the AIDS epidemic. Many new manufacturers sprang up in southeast Asia, and their inexperience led to the elimination of the in-line glove washing process that had previously been the norm. Afterall, latex gloves look exactly the same whether washed or unwashed. After only a few exposures to unbleached high protein gloves, a patient can develop a permanent Type I latex allergy. However, since this manufacturing failure was characterized and found to be the cause of the Type I latex allergy “epidemic”, reputable manufacturers reinstated the inline washing process, and natural latex gloves again became safe for those not previously sensitized by high protein products. Only a few products remain still with high protein levels, such as some brands of dental dams [12]. When an unwashed dental dam is placed inside a patient’s mouth, the soluble proteins are extracted by saliva in sufficient quantity to sometimes induce sensitization, and certainly enough to cause an allergic reaction in a previously sensitized person [12].
Since medical workers may not be aware of the significant disparity in glove quality, implementing a post-packaging durability assessment as a standard part of the glove manufacturing quality assurance process will ensure that all medical gloves meet a minimum level of durability, protecting the consumer from low-quality gloves. Investing in high-quality gloves may also save healthcare facilities thousands of dollars per year on more expensive life-saving resources. In other words, higher quality PPE protects more people from diseases and hospitalization, can save lives, and reduce medical malpractice claims.
Finally, as the name suggests, natural latex is produced naturally by plants such as Hevea brasiliensis, or the Brazilian rubber tree. Plant-based materials are desirable because they are more environmentally sustainable than synthetic materials, which are synthesized from petroleum. Unlike natural latex products, synthetic rubber products are not biodegradable and contribute to the pollution of the environment. The rise in demand due to COVID-19 has also increased interest in alternative natural latex sources, such as Parthenium argentatum (guayule) and Taraxacum kok-saghyz (rubber dandelion). Guayule latex films outperform all other known natural and synthetic latices, including glove durability [11], puncture size [8], contain very little protein, and have no proteins which cross react with Type I latex allergy [13]. Rubber dandelion latex appears more similar to rubber tree latex and does contain cross-reactive proteins [14]. The introduction of new natural latices makes our device even more relevant to the medical glove industry and their customers.
4. Conclusions
The key findings of this study are as follows:
- The average failure time for nitrile gloves was 300 s, and the average number of roller touches to failure was 42 touches.
- The average failure time for latex gloves was 2206 s, and the average number of roller touches to failure was 300 touches.
- One-way ANOVA tests showed that nitrile failure time and number of touches to failure were significantly different from latex failure time and number of touches to failure (p = 0.0029 and p = 0.0031, respectively).
- The order of glove failure, nitrile followed by latex, concurs with the results of previous studies, and provides evidence that the N-GAD’s measurements are accurate.
Healthcare workers rely on medical gloves for protection against potentially deadly bacteria, viruses, and toxins, but the gloves they use may not actually be providing the level of protection they expect. The medical glove durability assessment device discussed in this paper has the potential to standardize the quality of medical gloves, no matter the material or manufacturer. Regardless of whether the glove is made of synthetic or natural materials, glove manufacturers have a responsibility to produce high-quality gloves with consistent in-use durability. Therefore, future work will include collecting failure data for a wider variety of natural and synthetic gloves produced by different manufacturers and presenting the N-GAD to ASTM as a solution to the lack of durability standard.
Author Contributions
Conceptualization, A.V., M.P., W.V., Y.W. and K.C.; methodology, A.V., M.P., W.V., Y.W. and K.C.; software, Y.W.; validation, A.V.; formal analysis, A.V.; investigation, A.V.; data curation, A.V.; writing—original draft preparation, A.V.; writing—review and editing, K.C.; funding acquisition, K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the USDA SBIR Phase II awarded to EnergyEne Inc., grant number 1024332.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was made possible through the USDA-NIFA Hatch Project OHO01417 and the engineers and students at the OSU Center for Design and Manufacturing Excellence.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
List of replaceable parts for the N-GAD.
Table A1.
List of replaceable parts for the N-GAD.
| Item | Description | Supplier | Part Number |
|---|---|---|---|
| Liquid Pump | Peristaltic Pump | Simply Pumps | PMP200 |
| Vacuum Pump | Mini Vacuum Pump, 5 L/min, 12 V DC | Amazon | 753,874,419,842 |
| Valves | Stainless Steel Solenoid Valve, 12 V DC, 1/8 NPT | McMaster-Carr | 5077T114 |
| Motor | Step Motor Hybrid Linear Actuator, 12 V DC | Digi-Key | 1568-1189-ND |
| Load Cell | Mini Tension and Compression Load Cell, 0–10 kg | Amazon | DYMH-103 |
| Pressure Sensor | MPRLS Ported Pressure Sensor, 0–25 PSI | Adafruit | 3965 |
| Sandpaper | Water Resistant Sanding Sheets | McMaster-Carr | 6835A48 |
| O-rings | Soft Silicone O-ring, 1/8, Dash Number 245 | McMaster-Carr | 1173N484 |
| O-rings | Soft Silicone O-ring, 1/4, Dash Number 411 | McMaster-Carr | 1173N607 |
| O-rings | Soft Silicone O-ring, 1/4, Dash Number 412 | McMaster-Carr | 1173N608 |
| O-rings | Soft Silicone O-ring, 1/4, Dash Number 413 | McMaster-Carr | 1173N609 |
| O-rings | Soft Silicone O-ring, 1/4, Dash Number 414 | McMaster-Carr | 1173N611 |
| Fingertips | NPT Male Polyethylene Plastic Fitting | McMaster-Carr | 4427K82 |
| LED Screen | Nextion Touch Display | Mouser | 713–104,990,604 |
| Stylus | LCD Touch Panels Stylus | Mouser | 817-N010-0557-T011 |
Appendix B

Table A2.
Power supply characteristics.
Table A2.
Power supply characteristics.
| Typical | Maximum Range | |
|---|---|---|
| Input Voltage * | 120 VAC or 230 VAC | 85–132 VAC or 170–264 VAC |
| Input Current | 0.2 A | 0.1 A–1.0 A |
| Fuse | 5 A, 250 V fast blow glass fuse | |
* Overvoltage category III protection.

Table A3.
Stepper motor characteristics.
Table A3.
Stepper motor characteristics.
| Maximum Speed | Maximum Force |
|---|---|
| 10 mm/s | 45 N |

Table A4.
Pressure characteristics.
Table A4.
Pressure characteristics.
| Depressurization Target | Working Range | Typical Depressurization Time |
|---|---|---|
| 40 MPa | 40–80 MPa | 23 s |
References
- Market Research Reports. Available online: https://www.researchandmarkets.com/reports/5403544/medical-gloves-market-global-outlook-and?utm_source=BW&%3Butm_medium=PressRelease&%3Butm_code=mbrq64&%3Butm_campaign=1578882%2B-%2BGlobal%2B%2418.56%2BBn%2BMedical%2BGloves%2BMarket%2BOutlook%2B%26%2BForecast%2Bto%2B2026%2Bwith%2BCOVID-19%2BImplication%2BAnalysis&%3Butm_exec=chdo54prd (accessed on 11 April 2022).
- U.S. Food and Drug Administration. Medical Device Shortages During the COVID-19 Public Health Emergency. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/medical-device-shortages-during-covid-19-public-health-emergency (accessed on 11 April 2022).
- D3577-19; Standard Specification for Rubber Surgical Gloves. ASTM International: West Conshohocken, PA, USA, 2019.
- D3578-19; Standard Specification for Rubber Examination Gloves. ASTM International: West Conshohocken, PA, USA, 2019.
- D5151-19; Standard Test Method for Detection of Holes in Medical Gloves. ASTM International: West Conshohocken, PA, USA, 2019.
- Thomas, S.; Aldlyami, E.; Gupta, S.; Reed, M.R.; Muller, S.D.; Partington, P.F. Unsuitability and high perforation rate of latex-free gloves in Arthroplasty: A cause for concern. Arch. Orthop. Trauma Surg. 2010, 131, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Bardorf, M.H.; Jäger, B.; Boeckmans, E.; Kramer, A.; Assadian, O. Influence of material properties on gloves’ bacterial barrier efficacy in the presence of microperforation. Am. J. Infect. Control. 2016, 44, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.; Brichta, J.L.; Yu, P.; Wood, D.F.; McGlothlin, M.W.; Martin, J.A. Guayule latex provides a solution for the critical demands of the non-allergenic medical products market. Agro-Food-Ind. Hi-Tech 2001, 12, 27–31. [Google Scholar]
- Michel, R.; Cornish, K. Comparative Durability and Abrasion Resistance of Natural and Synthetic Latex Gloves. In Proceedings of the International Latex Conference, Fairlawn, OH, USA, 11–12 August 2015; pp. 1–18. Available online: https://www.rubbernews.com/assets/PDF/RN10078185.PDF (accessed on 12 April 2022).
- NASA. 3 ANTHROPOMETRY AND BIOMECHANICS. Available online: https://msis.jsc.nasa.gov/sections/section03.htm (accessed on 11 April 2022).
- Michel, R.; Cornish, K. Comparing Natural, Synthetic Latex Gloves. Rubber Plast. News 2016, 10–13. Available online: https://s3-prod.rubbernews.com/s3fs-public/RN10326818.PDF (accessed on 12 April 2022).
- Cornish, K.; Bates, G.M.; Slutzky, J.L.; Meleshchuk, A.; Xie, W.; Sellers, K.; Mathias, R.; Boyd, M.; Castaneda, R.; Wright, M.; et al. Extractable protein levels in latex products and their associated risks, emphasizing American dentistry. Biol. Med. 2019, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Cornish, K. Assessment of the risk of Type I latex allergy sensitization or reaction during use of products made from latex derived from guayule and other alternate rubber producing species. Rubber Sci. 2012, 25, 139–155. [Google Scholar]
- Cornish, K.; Xie, W.; Kostyal, D.; Shintani, D.; Hamilton, R.G. Immunological analysis of the alternate natural rubber crop Taraxacum kok-saghyz indicates multiple proteins cross-reactive with Hevea brasiliensis latex allergens. J. Biotechnol. Biomater. 2015, 5, 201–207. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).