Abstract
In this work, we have studied the microstructure and unusual ferromagnetic behavior in epitaxial tin dioxide (SnO2) films implanted with 40 keV Co+ ions to a high fluence of 1.0 × 1017 ions/cm2 at room or elevated substrate temperatures. The aim was to comprehensively understand the interplay between cobalt implant distribution, crystal defects (such as oxygen vacancies), and magnetic properties of Co-implanted SnO2 films, which have potential applications in spintronics. We have utilized scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), vibrating sample magnetometry (VSM), differential thermomagnetic analysis (DTMA), and ferromagnetic resonance (FMR) to investigate Co-implanted epitaxial SnO2 films. The comprehensive experimental investigation shows that the Co ion implantation with high cobalt concentration induces significant changes in the microstructure of SnO2 films, leading to the appearance of ferromagnetism with the Curie temperature significantly above the room temperature. We also established a strong influence of implantation temperature and subsequent high-temperature annealing in air or under vacuum on the magnetic properties of Co-implanted SnO2 films. In addition, we report a strong chemical effect of ethanol on the FMR spectra. The obtained results are discussed within the model of two magnetic layers, with different concentrations and valence states of the implanted cobalt, and with a high content of oxygen vacancies.
1. Introduction
Semiconducting tin dioxide (SnO2) doped with a magnetic 3D elements is a promising material for optoelectronic, photovoltaic, and spintronic applications [1,2]. Although the room temperature ferromagnetism (FM) in transition metal (TM)-doped SnO2 was reported earlier ([2,3] and references therein), the origin and the mechanism of long-range ferromagnetic ordering are still under scrutiny. It is often indicated that the FM strongly depends on the fabrication method of TM-doped SnO2. Moreover, the presence of oxygen vacancies was systematically related to the observed ferromagnetic state [4,5,6]. Consequently, the undoped SnO2 films may also exhibit ferromagnetic response at room temperature [7,8,9,10]. The ferromagnetism is originated by the tin or oxygen defects on the films’ surface and the positively charged monovalent oxygen vacancies in the films’ volume [8]. However, in most cases, this defect-induced magnetism in SnO2 films and nanoparticles is weak, and is characterized by small values of the coercivity field, although unusually high magnetization (up to 100 emu/cm3) in undoped SnO2 films with a thickness less than 100 nm was recently reported [11].
The ion-beam implantation has been widely used in microelectronics over time as an effective method of doping classical semiconductors (Si, Ge, GaAs, etc.) with a donor or acceptor impurity [12]. Therefore, it is interesting to investigate the possibilities of ion-beam implantation for doping wide-gap oxide semiconductor SnO2 with a magnetic dopant, i.e., with cobalt, in order to create a material for semiconductor spintronics. Moreover, this technique allows not only doping materials with any impurity in a wide range of concentrations, but also generates a large number of oxygen vacancies in oxide structure during the ion irradiation process [12]. The last is important, because the oxygen vacancies may play a crucial role in affecting the ferromagnetism in Co-doped SnO2, as previously mentioned.
Ion implantation has already been utilized for doping SnO2 films with cobalt impurity in the concentration range of 1–7 at.% to create an oxide-based dilute magnetic semiconductor [13,14,15]. However, up until now, a systematical study of the effect of Co ion implantation on the structure and magnetic properties of SnO2 thin films has been missing. In this work, Co ions were implanted into epitaxial SnO2 films at high concentrations of the implanted region (more than 12 at.%). Additionally, the impact of the temperature of SnO2 during the high-fluence implantation with cobalt ions on the manifestation of ferromagnetism was also investigated.
2. Materials and Methods
The experimental samples were obtained in two stages. At the first stage, thin SnO2−δ films with the thickness of 140–150 nm were grown on C-cut Al2O3 single crystalline substrates (15.0 × 15.0 × 1.0 mm3 in sizes) by the reactive (Ar + O2) magnetron sputtering of the elemental tin target (Sn, Purity: 99.915%). Sputtering was conducted in a BESTEC high-vacuum chamber (Bestec GmbH, Berlin, Germany) at a residual vacuum of 6 × 10−3 mbar. The substrate was rotated at 5 rpm and heated to the temperature of 873 K during the deposition process. The subsequent annealing in air at the temperature of 1323 K for 120 min was performed to recover the oxygen stoichiometry in the films. As a result, epitaxial SnO2 films were obtained. At the second stage, 40 keV Co+ ions were implanted into epitaxial SnO2 films to the fluence of 1.0 × 1017 ion/cm2, with an ion current density of 2–3 μA/cm2. The implantation was performed using an ILU-3 ion-beam accelerator (ZPTI FRC KSC of RAS, Russia) at different temperatures of sample during ion irradiation: either at room temperature (sample CoSO-1) or at the elevated temperature of 750 K (“hot” implantation, sample CoSO-2h). Additionally, an additional SnO2 epitaxial film was implanted with 40 keV ions of inert gas (argon) to the same fluence at the elevated substrate temperature (sample ArSO-3h). The last sample was found to be useful to establish microstructure changes, without inducing ferromagnetism, to determine the nature of ferromagnetism in the Co-ions implanted samples. To consider the SnO2 film surface sputtering during intensive ion irradiation, the steps between the implanted and non-irradiated regions of the films were measured using the DektactXT profilometer (Bruker Nano GmbH, Berlin, Germany). The sputtering coefficients were calculated as α ≈ 3.78 and 5.16 atoms/ion for the CoSO-1 and CoSO-2h samples, respectively, considering the step heights of about 45 nm and 62 nm. The small pieces (approximately 3.0 × 3.0 × 1.0 mm3 in sizes) of both CoSO-1 and CoSO-2h samples were annealed first at the temperature of Tann. = 873 K for 30 min in air atmosphere. These samples are designated as CoSO-1a and CoSO-2ha. After measuring their magnetic properties, these samples were annealed for the second time in high vacuum (10−6 Torr) at the same temperature of 873 K for 30 min. These double-annealed samples are marked by the suffix “av”, as CoSO-1_av and CoSO-2h_av, respectively.
The phase composition and crystal structure of SnO2 films before and after Co-ions implantation were investigated using the X-ray diffraction (XRD) method. The measurements were carried out using a Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with the Cu-Kα X-ray tube (λ = 0.15418 nm). Additionally, the surface morphology and elemental composition of both the as-prepared and implanted SnO2 films were studied using an EVO 50 XVP scanning electron microscope (SEM) (Carl Zeiss AG, Oberkochen, Germany), equipped with an energy-dispersive X-ray (EDX) spectrometer (Oxford Inca Energy-350, Oxford Instruments, Abingdon, England).
The valence state of the elements at various depths of the samples were investigated using X-ray photoelectron spectroscopy (XPS), employing an ion etching technique. The step-by-step etching process was conducted utilizing a 2 keV Ar+ ion beam. The Bruker DektakXT profilometer was utilized to control the etching depth. After each etching session, XPS spectra were recorded using a Phoibos 150 hemispherical energy analyzer in an ultra-high vacuum (UHV) chamber (base pressure 5 × 10−10 mbar) equipped with an Mg Kα X-ray source operated at 12.5 kV and 250 W (SPECS GmbH, Berlin, Germany). The obtained results were analyzed using CasaXPS (ver. 2.3.26) software [16].
Magnetic properties were studied using the vibrating sample magnetometry (VSM), ferromagnetic resonance spectroscopy (FMR), and the differential thermomagnetic analysis (DTMA) methods. The magnetization curves were recorded using a homemade rotating sample magnetometer [17] in a magnetic field up to 5 kOe at room temperature. The diamagnetic contribution of the Al2O3 substrate was subtracted from the data after the magnetic measurements. The magnetization values were normalized per implanted cobalt ion.
Ferromagnetic resonance (FMR) spectra were measured on a Bruker ElexsysE580 (Bruker GmbH, Berlin, Germany) X-band spectrometer (9.64 GHz) at room temperature. The angular dependences of the FMR spectra were recorded by rotating of the applied DC magnetic field in a plane perpendicular to the sample plane (“out-of-plane” geometry). As usual, the first field-derivative of microwave power absorption (dP/dB) was recorded as a function of the static magnetic field (B). The value of the resonant field (Bres.) was determined by the intersection point of the magnetic resonance curve with the baseline of the empty resonator.
The thermomagnetic curves were measured using the Faraday balance technique [18] by heating the samples from 300 to 1000 K with the rate of 100 K/min in air in an applied magnetic field of 500 mT.
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analysis
The results of the X-ray diffraction studies are presented in Figure 1. The θ–2θ scan of the epitaxial SnO2 film grown on a C-cut Al2O3 substrate is shown in Figure 1a by the green curve. The sharp diffraction peaks observed at 2θ angles of ~41.9° and ~91° may be unambiguously attributed to the (0006) and (000 12) reflections from the C-cut Al2O3 substrate. Two other peaks at the 2θ angles of ~37.9° and ~80.6° were assigned to (200) and (400) reflections from the SnO2 film. The last proves an out-of-plane epitaxial relationship for SnO2 film on the C-cut Al2O3 substrate as SnO2(200) || Al2O3(0006).
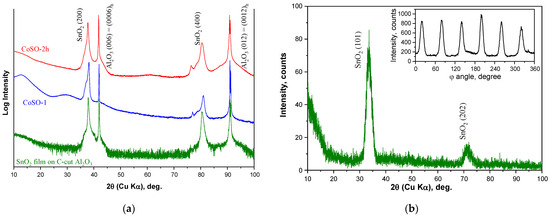
Figure 1.
XRD patterns of the epitaxial SnO2 film on C-cut Al2O3 substrate before (green curve) and after (blue and red curves) Co-ion implantation with the fluence of 1 × 1017 ion/cm2. Left panel (a) shows θ–2θ scans taken in normal Bragg–Brentano parafocusing geometry for as-prepared SnO2 film, CoSO-1, and CoSO-2h samples, respectively, and right panel shows (b) θ–2θ scan taken under fixed tilt angles of ψ = 56.2° and φ = 79.9° for as-prepared SnO2 film before implantation. The inset in (b) shows the φ-scan of the (101) reflections for the as-grown film at 2θ = 33.7° and ψ = 56.2°. The φ-scans for CoSO-1 and CoSO-2h samples are shown in the Suplementary Materials.
To confirm the epitaxy of the synthesized SnO2 films, the φ-scan of the (101) reflection was recorded under the tilt angles of ψ = 56.2° and 2θ = 33.7° (see inset in Figure 1b). In this pattern, the six-fold symmetry was observed, which contradicts the tetragonal rutile structure, since a two-fold symmetry should be characteristic for (101) reflections. This six-fold symmetry may be associated with the formation of three equivalent domains during the growth of the SnO2 films. Such a result has been previously reported [19]. In Figure 1b, the θ–2θ scan of the pristine SnO2 film under fixed angles of ψ = 56.2° and φ = 79.9° further confirms the phase purity and the epitaxy of the synthesized SnO2 films.
The XRD pattern of the CoSO-1 sample implanted with Co ions at room substrate temperature is depicted in Figure 1a by the blue curve. It is observed that that the positions of the SnO2-related reflexes are slightly shifted toward higher 2θ angles. Furthermore, distinctive shoulders appeared in the lower angle sides of the (200) and (400) reflexes. A qualitatively similar XRD pattern (red curve in Figure 1a) was observed for the CoSO-2h sample implanted with Co ions at the elevated substrate temperature of 750 K. The more detailed XRD profiles of the investigated samples are shown in Figures S1–S3 in the Supplementary Materials.
The following model is proposed to explain the observed XRD patterns. Under the high-fluence implantation, initially uniform SnO2 films were separated into two layers. The highest concentration of the implanted cobalt is found in the first layer, located closer to the film surface. This is confirmed by the analysis of XPS depth profiles of cobalt concentration, as shown below. A notable portion of the implanted cobalt atoms forms precipitates in this layer, leading to possible stretching of the SnO2 matrix and an expected enhancement in lattice spacings. Thus, the shoulder-like reflexes in the lower angle sides of the SnO2 reflexes may be originated by the cobalt precipitates. In the second deeper layer, cobalt impurity is found to be in the 2+ valence ionic state, according to the XPS results. These Co2+ ions may substitute Sn4+ cations in the SnO2 structure in the low-spin state [20,21]. Note that the ionic radius of the low-spin Co2+ ion is smaller than that of the Sn4+ ion in the six-fold oxygen environment (0.65 Å and 0.69 Å, respectively [22]). Therefore, such substitution should decrease the lattice parameters and reduce the lattice spacings. This leads to the shift of the XRD reflexes towards higher angles. On the other hand, a decrease in lattice parameters may be caused by a decrease in film thickness due to surface sputtering during ion implantation. Indeed, a decrease in lattice parameters with decreasing film thickness has already been reported for epitaxial SnO2 films on C-cut Al2O3 [19].
3.2. Scanning Electron Microscopy (SEM)
The surface topography and elemental composition of both the as-prepared and the implanted SnO2 films were studied in detail by SEM methods. The SEM analysis reveals significant changes in the morphology of the SnO2 films upon cobalt (or argon) ion implantation. The as-prepared SnO2 films have a smooth and compact surface (see Figure S4 in the Supplementary Materials). In the contrast, the cobalt-implanted films exhibit the presence of neoplasms scattered uniformly on the surface. As demonstrated in Figure 2, these neoplasms have a semi-spherical form, with sizes ranging from 40–120 nm. It is important to note that the formation of neoplasms cannot be attributed to the precipitation of cobalt impurities on the surface of Co-implanted samples, since the exact same neoplasms are observed on the surface of SnO2 film implanted with argon ions (see Figure S5 in the Supplementary Materials). Furthermore, this cannot be attributed to metallic tin nanoparticles. Both XPS analysis and Mössbauer studies show that the tin atoms in the SnO2 films are in a 4+ valence state before and after ion implantation (Figures S7 and S8 in the Supplementary Materials). Moreover, EDX element mapping at a submicron scale (see Figure S4 in the Supplementary Materials) demonstrates that both cobalt implants and tin are homogenously distributed over the surface of the implanted SnO2 films, without any regions exhibiting a higher content of either chemical element. Consequently, the observed morphological changes have been associated with radiation defects and structural distortions in the SnO2 lattice, leading to microscale swelling of the surface. The formation of a developed relief on the surface of oxide materials as a result of high-fluence implantation is a characteristic occurrence [23], often associated with the processes of releasing gaseous elements (specifically oxygen) from the surface region of the irradiated material [12]. The same changes in the surface morphology as a result of Co ions implantation were also detected in the atomic force microscopy images (see Figure S6 in the Supplementary Materials).

Figure 2.
SEM image of the surface of SnO2 film after implantation of Co ions with the fluence of 1 × 1017 ion/cm2 (a), and SEM image of same film at the 70° position (b).
The chemical element content in the studied SnO2 samples was estimated from the EDX microanalysis data, and their values are given in Table 1. It is important to note that Table 1 shows the mean values of the atomic concentration of each chemical element, obtained by averaging the thickness of the layer in which the characteristic X-ray radiation is generated during EDX measurements. Based on our calculations within the CASINO program [24], for a 5 keV electron beam, the thickness of the generation layer of the K-lines (Al and O) and L-lines (Sn and Co) is approximately 110–120 nm. This value is close to the thickness of the implanted SnO2 films (~100 nm) taking into account the film sputtering during ion implantation.

Table 1.
Chemical element content in both the as-prepared SnO2 film and SnO2 films implanted with Co (or Ar) ions at the fluence of 1 × 1017 ion/cm2. Here, Timp and Tann in the second column show the values of the implantation temperature and temperature of subsequent double annealing in air and then under vacuum, respectively.
EDX elemental microanalysis confirms the successful implantation of cobalt into the SnO2 films, with an average cobalt concentration of 12–14 at.%. The chemical formula of Co-ion implanted samples can be written as Sn(1 − x)CoxO2 − δ. The concentration ratio of cobalt to tin (column Co/Sn) allows for the determination of the cobalt dopant content (x) in the films, as x ≅ 0.38 in the sample implanted at room temperature and x ≅ 0.33 in sample implanted at a high temperature of 750 K. As follows from the Table data, the content of the structure-forming elements, tin (Sn) and oxygen (O), is significantly reduced in Co- or Ar-ion-implanted SnO2 films when compared with the as-prepared SnO2 film. These data confirm the occurrence of film sputtering during ion implantation. The sputtering process of SnO2 film during ion irradiation is also indicated by a significant decrease in the concentration ratio of tin to aluminum (column Sn/Al). The Sn/Al ratio decreases from 36.0 in as-prepared film to 7.4 in Co-implanted films and 9.8 in the Ar-implanted sample. Here, the EDX signal from Al comes from the corundum substrate (Al2O3) used for SnO2 film growth. Moreover, the Sn/Al ratio continues to decrease in the samples after high-temperature annealing. The last result indicates the evaporation of the implanted SnO2 material after double annealing.
3.3. X-ray Photoelectron Spectroscopy (XPS)
The results of the XPS measurements of SnO2 films implanted with cobalt ions at room temperature (CoSO-1 sample) or the elevated substrate temperature (CoSO-2h sample) are illustrated in Figure 3. The experimental depth profiles of the cobalt concentration and XPS spectra taken at the different depths are shown in the upper (a,b) and lower (c,d) panels, respectively. The dashed lines in (a,b) are the theoretical profiles of cobalt concentration calculated by using the SRIM algorithm [25] and considering the different values of the film sputtering during the ion irradiation [26]. The calculated SRIM profiles exhibit a half-bell-shaped distribution, with concentration maxima (~26 or 19 at.%) on the surface. The experimental profile of the cobalt concentration in the CoSO-1 sample (Figure 3a) exhibits almost the same depth distribution, differing only by a shift in depth (4 nm) of the concentration maximum and its higher (~32 at.%) value when compared to the SRIM profile. In contrast, “hot” implantation (CoSO-2h sample, Figure 3b) leads to much wider depth distribution (up to 60 nm) of cobalt impurity and a notably lower peak concentration (~14 at.%). We associate these experimental findings with diffusion blurring of the depth distribution profile, causing the diffusion of the Co implant in the film bulk during implantation at high substrate temperatures.
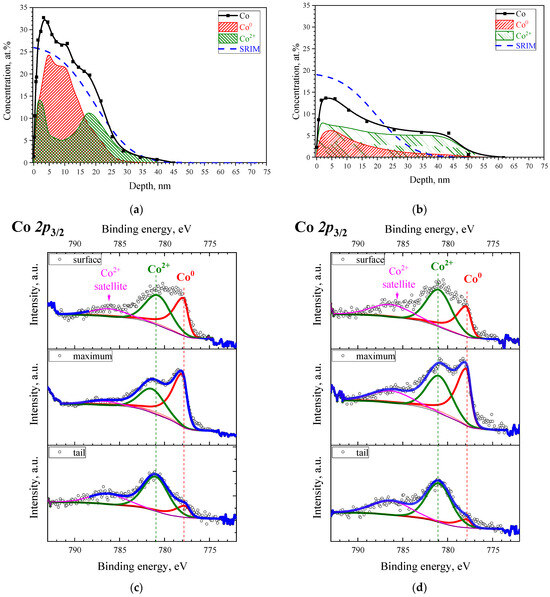
Figure 3.
Depth distribution profiles of the cobalt implant (a,b) and high-resolution XPS spectra (c,d) taken at the different depths of analysis for the CoSO-1 (a,c) and CoSO-2h (b,d) samples, respectively. The dashed lines in (a,b) show the theoretical profiles of the implant distribution, calculated using the SRIM algorithm [25], and the substrate sputtering during the ion irradiation [26]. The circles in (c,d) correspond to experimental data, whereas color lines—to the fitting curves (blue lines) and spectral components (green, red, and magenta lines).
Figure 3c,d shows the Co 2p3/2 binding energy regions of the Co 2p XPS spectra taken at the sample surface, as well as at the maximum and the tail of the depth profile of cobalt distribution. The experimental XPS spectra can be decomposed into two components corresponding to different valence states of the implant. The values of the binding energies of the components are typical: 777.9 eV for the metallic state of Co0 and 781.1 for the ionic state of Co2+ [27,28]. Moreover, the observation of a satellite line in the spectra of Co 2p at ~786 eV allows for the unambiguous determination of the oxidation state of cobalt as 2+. A comprehensive analysis of all XPS data recorded at different depths allows for the establishment of the depth distributions of both cobalt components, as shown by the red and green shaded areas in Figure 3a,b. It is clear that the implanted cobalt is represented by a mixture of metallic and oxidized divalent states in the CoSO-1 sample. The metallic Co0 component dominates at the maximum (~5 nm) cobalt distribution. Consequently, herein, the implanted impurity obviously occurs in the form of nanoparticles of metallic cobalt due to the high atomic concentration of cobalt. Conversely, a significant part of the cobalt is in the oxidized divalent state in the tail of the impurity distribution. In the case of “hot” implantation (Figure 3b), the Co2+ component is dominant throughout the entire depth of the impurity distribution. Thus, the main portion of cobalt in the CoSO-2h sample can be found in the form of a solid solution of Co2+ ions, which substitute Sn4+ sites in the SnO2 matrix.
It should be noted that according to both the Sn 3d3/2 XPS spectra and our 119Sn Mössbauer results, tin atoms preserve their 4+ state in the SnO2 film after implantation (see Figures S7 and S8 in the Supplementary Materials). The XPS spectra of oxygen are also presented in Figure S8 of the Supplementary Materials. The analysis of XPS data shows a broadening of the O1s XPS peak and the appearance of a distinctive shoulder on the higher energy side of the spectrum in the Co-implanted samples compared to the spectrum of the as-prepared SnO2 film. The latter can be attributed to oxygen vacancies formed in the oxide material as a result of ion irradiation [11].
3.4. Magnetic Properties
Magnetic measurements of as-prepared epitaxial SnO2 films reveal diamagnetic behavior at room temperature, as shown in Figure S9 of the Supplementary Materials. However, the implantation of cobalt in the SnO2 films results in an appearance of ferromagnetism with the Curie temperature significantly above room temperature. Figure 4a shows the magnetic hysteresis loops recorded at room temperature for the CoSO-1 and CoSO-2h samples implanted with cobalt ions at different substrate temperatures. It is clear that both samples show a pronounced ferromagnetic response. On the other hand, the SnO2 film implanted with Ar ions at the same conditions (ArSO-3h sample) exhibits only a paramagnetic slope in the field dependence of the magnetic moment (black curve in Figure 4a). Thus, the Ar+ ions exhibit a mass close to that of the Co+ ions, and the implantation with inert argon results in a comparable radiation damage to that of the SnO2 lattice [12]. Therefore, radiation defects (including oxygen vacancies) cannot explain the observed room temperature ferromagnetic response, and Co implantation is the main source of the ferromagnetism in the Co-ion-implanted SnO2 films.
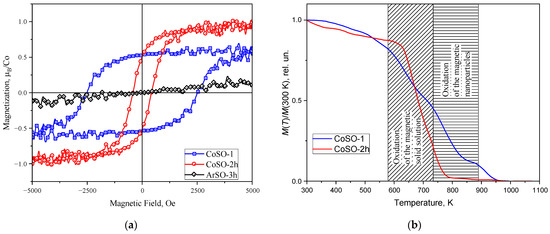
Figure 4.
(a) Room temperature ferromagnetic hysteresis loops for SnO2 films implanted with Co ions, with the substrates at room temperature (CoSO-1 sample, blue squares) and elevated temperature (CoSO-2h sample, red circles). The paramagnetic curve of the SnO2 film implanted with Ar ions at elevated substrate temperature is shown by black rhombs (AeSO_3h sample). (b) DTMA curves for the same Co-ion-implanted samples. Patterned regions in (b) show the specific oxidation conditions for the various sources of ferromagnetism established in the studied samples.
However, there are notable differences in the parameters of the loops for SnO2 films implanted with Co ions at different substrate temperatures. The saturation magnetization value of the CoSO2-2h sample obtained at the elevated substrate temperature is higher than for the another CoSO-1 sample implanted at room temperature (~0.95 μB per implanted Co ion (μB/Co) vs. ~0.55 μB/Co). At the same time, the coercivity behaves differently, with its value being lower in the case of the Co implantation into the SnO2 film at the elevated substrate temperature than in the case of the implantation at room temperature (~400 Oe and ~2500 Oe, respectively). The coercivity for the SnO2 film implanted with Co ions at room temperature is very high, and to our knowledge, similar values have not yet been reported for Co-doped SnO2 systems. We speculate that this unexpectedly large coercivity is due to the formation of oriented metallic Co nanoparticles, and possibly due to the shape anisotropy of these particles. However, high-resolution transmission electron microscopy studies are required to confirm our assumption. In addition, the hysteresis loops recorded in both the in-plane and out-of-plane geometries of the magnetic field sweep are shown in Figure S10 in the Supplementary Material. The strong difference in the shapes of the loops associated with the strong easy-plane magnetic anisotropy is clearly manifested in the studied samples. This magnetic anisotropy was also observed in the ferromagnetic resonance studies, which will be discussed in the next section.
In Figure 4b, the thermomagnetic curves for both samples are shown. It is evident that the thermal behavior of the samples differs significantly. In the curve for the CoSO-1 sample implanted with Co ions at room temperature (the blue curve), two steps of the “magnetization removal” can be observed in the regions of ~570–730 K and ~730–900 K. Meanwhile, for the second CoSO-2h sample, there is only a single step, located mainly in the first temperature region.
Air and vacuum thermal annealing of the Co-implanted SnO2 samples were performed to investigate the role of oxygen vacancies (VO) in the manifestation of ferromagnetism. Due to the low formation energy of oxygen vacancies [29], their number in the sample can be easily controlled by changing the annealing environment. It is well known (see e.g., [30]) that the oxidizing atmosphere of air compensated the oxygen vacancies in the SnO2-based nanomaterials, while the vacuum annealing resulted in the increase in the concentration of oxygen vacancies. Figure 5 shows the magnetization curves of both as-implanted samples (CoSO-1 and CoSO-2h) and the same samples annealed in air and subsequently, under vacuum. The annealing in air at 873 K almost totally suppresses ferromagnetism in both samples. At the same time, the ferromagnetism may be recovered in these samples after the subsequent second annealing under vacuum at 873 K. However, the magnetic characteristic of the recovered ferromagnetic loop for the CoSO-1 samples implanted with cobalt with the substrate at room temperature is strongly different when compared with those observed in the initial state, i.e., the coercivity is ~70 Oe after the vacuum annealing as compared to the ~2500 Oe in the initial state. Considering the CoSO-2h sample implanted at the elevated substrate temperature, the magnetic characteristics of this sample after vacuum annealing are almost comparable with the values in its initial state. Thus, the above results of our magnetic measurements indicate the crucial role of oxygen vacancies in producing ferromagnetism in Co-implanted SnO2 films.
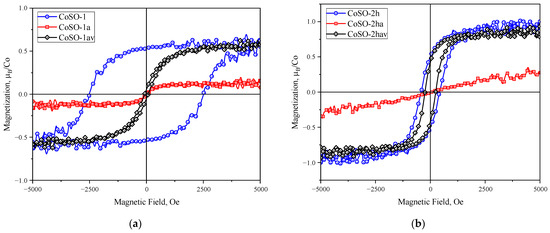
Figure 5.
Room temperature ferromagnetic hysteresis loops for CoSO-1 (a) and CoSO-2h (b) samples, recorded in the initial as-implanted state of the samples (blue curves) after annealing at 873 K in air (red curves) and after subsequent vacuum annealing at 873 K (black curves).
3.5. Ferromagnetic Resonance (FMR)
3.5.1. FMR Spectra
Figure 6a,b show the magnetic resonance spectra recorded at room temperature and at different values of the polar angle θ in the CoSO-1 and CoSO-2h samples, respectively. Three FMR signals are clearly observed in the magnetic resonance spectra at the limiting value of θ = 0° when the magnetic field is perpendicular to the sample plane. The first two FMR signals, marked with a blue triangle and a red circle in Figure 6a,b, reveal a strong out-of-plane angular dependence of the FMR signal positions. Namely, these two signals quickly shift from the high-field portion to the low-field portion of the magnetic spectrum and broaden when the value of polar angle θ is increased. On the other hands, the third signal marked with a black square has a weak angular dependence in the CoSO-2h sample implanted with Co ions at the elevated substrate temperature. The angular dependence of the third signal is totally absent in the CoSO-1 sample, as seen in Figure 6c.
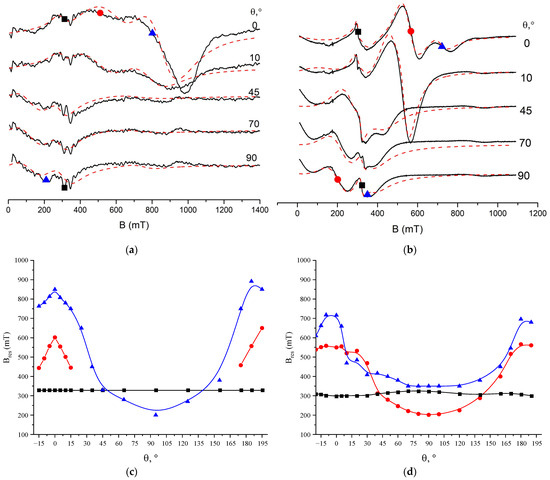
Figure 6.
Upper panel—room temperature FMR spectra for various orientations of the magnetic field in CoSO-1 (a) and CoSO-2h (b) samples. Here, θ is polar angle between a magnetic field and the normal to sample plane (“out-of-plane” geometry). The black and the red dashed lines show experimental and simulation spectra, respectively. Three FMR signals observed in the spectra marked with blue triangles, red circles and black squares. Lower panel—angular dependences of resonance fields (Bres) for three FMR signals in CoSO-1 (c) and CoSO-2h (d) samples, respectively. The experimental data are shown by symbols, and the color solid lines used as guide for eyes.
The angular dependences were analyzed by fitting the experimental spectra with the sum of three independent FMR lines. The observed lines were described by the Dyson shape line [31]. To fit the experimental spectra, a program was written in Python using the SciPy library [32]. Through fitting the FMR spectra, resonance field values were obtained for three lines at different orientations of the samples in the magnetic field. The angular dependencies of the resonance fields are presented in Figure 6c,d for the Co-implanted SnO2 films implanted with Co+ ions at room and high temperatures, respectively. A detailed analysis of the observed angular and temperature dependences of the ferromagnetic resonance spectra, as well the determination of the spectroscopic and magnetic parameters of the samples, such as the values of the g-factor, magnetization, and magnetic anisotropy constants, is the subject of a separate article. At this stage, however, it can be noted that the angular dependences of the resonant field of the first two FMR signals presented in Figure 6c,d are typical for thin granular magnetic films with strong magnetic anisotropy [33,34]. In contrast, the weak angular dependence of the third signal is characteristic of isotropic bulk ferromagnetic materials. Thus, FMR studies indicate the formation of two different magnetic phases in the Co-ion-implanted SnO2 films.
3.5.2. Chemical Effect of Ethanol on FMR Spectra
In this study, the strong influence of ethanol media on the FMR was observed for the first time. This chemical effect of ethanol is observed in the example of the influence on the FMR spectrum of storage of the Co-implanted SnO2 film in ethanol media. It should be noted that this effect was observed for samples subjected to double annealing, first in air and then under vacuum, at a temperature of 873 K for 30 min. The FMR spectrum of the CoSO-2h_av sample is shown in Figure 7. For this spectrum (in Figure 7, labeled as “Initially”), an angular dependence was observed similar to that observed in the as-implanted CoSO-2h sample (Figure 6b). After maintaining the sample in ethanol for more than 2 h, the FMR spectrum totally disappeared (in Figure 7, labeled as “After Ethanol”). Then, when the CoSO-2h_av sample was annealed under vacuum again, the FMR spectrum was recovered (in Figure 7, labeled as “Recovery”), for which an angular dependence was also observed. However, the “Recovered” spectrum differs from the “Initial” example.
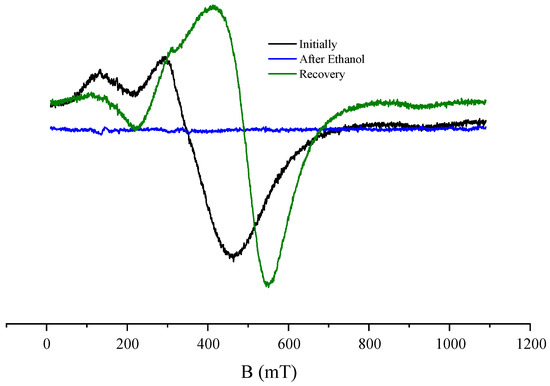
Figure 7.
Room temperature FMR spectra of CoSO-2h_av sample for the orientation of the magnetic field perpendicular to the sample plane, recorded before (black curve) and after (blue curve) storage of the sample in ethanol medium. The green curve represents the FMR spectrum after the vacuum annealing of the sample stored in ethanol.
3.6. Two Magnetic Phases in Co-Ions Heavily Implanted SnO2 Films
Based on the abovementioned peculiarities in the magnetic properties of Co-ion-implanted SnO2 films at various temperatures of substrates, along with the XRD and the XPS results, we propose the following explanation of the experimental data. The difference in the magnetic properties of the CoSO-1 and CoSO-2h samples is due to the various sources of ferromagnetism in each. Indeed, according to the XPS results, after the implantation at room temperature, the cobalt atoms exist predominantly in the metallic state, i.e., they form metallic nanoparticles, which are responsible for the room temperature ferromagnetism, with large coercivity, in CoSO-1 sample.
At the same time, after the implantation at the elevated temperature, the content of metallic Co atoms is sufficiently lower, and the implanted cobalt ions mainly occur in the 2+ valence state. We assume that these ions form a magnetically ordered solid solution in the SnO2 host, and the room temperature ferromagnetism of the corresponding CoSO-2h sample is mainly originated by this solid solution. Since all our samples are in a highly insulating state (surface resistance more than 50 MΩ), the mechanism of the magnetic ordering of the Co2+ ions in this solid solution cannot be associated with carrier-mediated exchange, such as the RKKY–Zener type interaction [35]. For insulating rutile-type oxide materials, such as SnO2 and TiO2, the F-center-mediated (FC) exchange between paramagnetic 3D metal ions is often discussed to explain the high Curie temperature ferromagnetism [4,5,14,36,37]. In the FC exchange model, the localized spins of the magnetic dopants (e.g., Co2+ ions in a low spin state with S = 1/2) interact with each other by an indirect ferromagnetic exchange through electrons trapped by neighboring oxygen vacancies. Thus, the presence of a certain concentration of oxygen vacancies plays a crucial role in producing ferromagnetism, according to FC exchange mechanism.
The above-suggested model of two magnetic phases in Co-ion heavily implanted SnO2 films can explain the influence of the annealing environment on the magnetic properties of the samples. When annealed in air, the Co2+-based magnetic solid solution is oxidized at temperatures around 570–730 K, but this state can be recovered by subsequent annealing under vacuum. Thus, the initial state may be almost completely restored, even after the ferromagnetic response has been suppressed by annealing in air, in the case of SnO2 film implanted with Co ions at elevated temperature (CoSO-2h sample). The magnetic Co nanoparticles are oxidized at higher temperatures, approximately 730–900 K, but they cannot be recovered under vacuum annealing. Therefore, in the case of the implantation with Co ions at room temperature, the ferromagnetism in the CoSO-1 sample after the annealing in air cannot be fully restored because it is impossible to recover the oxidized Co nanoparticles under the experimental conditions of vacuum annealing.
The chemical effect of alcohol on FMR spectra, as we suppose, is due to the high content of atomic oxygen in chemical molecular solutions such as alcohol, toluene, and acetone [38]. When a sample is kept in alcohol, oxygen atoms from the solution diffuse into the implanted SnO2 film and fill oxygen vacancies in the crystal lattice. Consequently, ferromagnetism is suppressed, since oxygen vacancies act as mediators of indirect exchange between Co2+ ions and play a dominant role in establishing long-range magnetic order, according to the FC exchange model. It should be noted that subsequent pumping of the sample in a vacuum chamber does not restore ferromagnetism. This means that oxygen atoms from the alcohol media, when penetrating the SnO2 film, interact with tin atoms and form a chemical bond with them. Ferromagnetism in the Co-implanted film can only be recovered by heating the sample in a vacuum at temperatures above 450 °C (720 K). We hypothesize that under these conditions, the chemical bond of oxygen with tin is loosened, and the oxygen atoms may be removed from the matrix by back diffusion from the film into the vacuum.
4. Conclusions
In summary, epitaxial SnO2 thin films with a rutile structure were grown on C-cut Al2O3 substrates using reactive magnetron sputtering and subsequent high-temperature annealing in air. Then, 40 keV Co+ ions were implanted into SnO2 films, at different substrate temperatures, with a high concentration reaching a mean level of 12–14 at.%. The microstructure and magnetic properties of the Co-ions heavily-implanted SnO2 films were investigated in detail. The Co implant was found to be in the mixed valence state, both in the form of metal Co0 atoms and Co2+ ions; they are distributed non-homogenously in the implanted region of the SnO2 films. All Co-implanted SnO2 films reveal room-temperature ferromagnetism, with clearly defined magnetic hysteresis loops at in-plane geometry. It was shown that both the saturation magnetization (Ms) and the coercive field (Hc) strongly depend on the substrate temperature during implantation. In particular, unusually wide magnetic hysteresis loops for Ms ~ 0.55 μB/Co and Hc ~ 2.5 kOe were observed in the SnO2 films implanted with cobalt when using the substrate at the room temperature. To the best of our knowledge, such values of Hc have not yet been reported for Co-doped SnO2 systems. Moreover, the disappearance and then recovery of ferromagnetism are observed as a result of the sequential annealing of Co-implanted films in air and then under vacuum. In addition, the strong chemical effect of ethanol on the FMR spectra were shown for the first time. These observations indicate a crucial role of oxygen vacancies in the establishment of ferromagnetism in the Co-implanted SnO2 films. A model of the formation of two magnetic phases (Co nanoparticles and a magnetically isotropic solid solution of Co2+ ions), located at different depths in the Co-ions heavily-implanted SnO2 films, was proposed to explain the complex of the observed magnetic phenomena. However, additional investigations using high-resolution transmission electron microscopy are needed to verify the proposed two-phase model of ferromagnetism in Co-implanted SnO2 films.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/condmat9020027/s1, Figure S1: The θ–2θ scan of the SnO2 film before implantation under fixed angles and the φ-scan of the (101) reflex; Figure S2: The θ–2θ scan for the CoSO-1 sample under fixed angles and the φ-scans of the (110) and (101) reflexes; Figure S3: The θ–2θ scan for the CoSO-2h sample under fixed angles and the φ-scans of the (110) and (101) reflexes; Figure S4: SEM images and EDX spectra of the surface of the SnO2 film, before and after implantation of Co ions, with the fluence of 1.0 × 1017 ion/cm2; Figure S5: SEM image of the surface of the SnO2 film after implantation of Ar ions with the fluence of 1.0 × 1017 ion/cm2; Figure S6: AFM images of the surface of the SnO2 film, before and after implantation of Co ions, with the fluence of 1.0 × 1017 ion/cm2; Figure S7: 119Sn Mössbauer spectra of the epitaxial SnO2 film on the C-cut Al2O3 substrate, before and after the implantation with 40 keV Co+ ions, with the fluence of 1.0 × 1017 ion/cm2; Figure S8: High-resolution XPS spectra of Sn3d, O1s, and Co2p binding energy regions for both as-prepared and Co ions implanted SnO2 films; Figure S9: The room temperature magnetization curves of as-prepared epitaxial SnO2 films, as well the same films after annealing in high vacuum or after Ar+ ions implantation; Figure S10: The room temperature magnetic hysteresis loops recorded in the in-plane and out-of-plane geometries for the CoSO-1 and CoSO-2h samples.
Author Contributions
Conceptualization, R.I.K. and A.L.Z.; methodology, R.I.K. and A.L.Z.; validation, R.I.K. and A.L.Z.; formal analysis, R.I.K. and A.L.Z.; investigation, R.I.K., A.I.G., I.R.V., A.A.S., N.M.L., A.G.K., D.M.K., V.V.B. and A.L.Z.; resources, R.I.K.; data curation, R.I.K.; writing—original draft preparation, R.I.K. and A.L.Z.; visualization, R.I.K., A.I.G., I.R.V. and A.L.Z.; supervision, R.I.K.; project administration, R.I.K.; funding acquisition, R.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation (project number 22-19-00712, https://rscf.ru/project/22-19-00712/).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors gratefully acknowledge V.I. Nuzhdin and V.F. Valeev (from Zavoisky Physical-Technical Institute, FRC Kazan Scientific Center of RAS, Russia) for help in the ion implantation experiments, as well as Alexey Rogov (from Kazan Federal University, Russia) for carrying out of the AFM measurements. Magnetron sputtering and XPS measurements were carried out under the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dalapati, G.K.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Sai Krishna, A.M.; Mukhopadhyay, S.; Dey, A.; et al. Tin Oxide for Optoelectronic, Photovoltaic and Energy Storage Devices: A Review. J. Mater. Chem. A 2021, 9, 16621–16684. [Google Scholar] [CrossRef]
- Worku, Y.; Sahu, D.R.; Srinivasu, V.V. Ferromagnetism in SnO2 Doped with Transition Metals (Fe, Mn and Ni) for Spintronics Application: A Review of Experimental Status. In Magnetic Materials and Magnetic Levitation; Sahu, D.R., Stavrou, V.N., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 7; pp. 1–14. [Google Scholar] [CrossRef][Green Version]
- Fitzgerald, C.B.; Venkatesan, M.; Dorneles, L.S.; Gunning, R.; Stamenov, P.; Coey, J.M.D.; Stampe, P.A.; Kennedy, R.J.; Moreira, E.C.; Sias, U.S. Magnetism in Dilute Magnetic Oxide Thin Films Based on SnO2. Phys. Rev. B 2006, 74, 115307. [Google Scholar] [CrossRef]
- Liu, X.F.; Sun, Y.; Yu, R.H. Role of Oxygen Vacancies in Tuning Magnetic Properties of Co-Doped SnO2 Insulating Films. J. Appl. Phys. 2007, 101, 123907. [Google Scholar] [CrossRef]
- Zuo, Y.; Ge, S.; Zhao, Y.; Zhou, X.; Xiao, Y.; Zhang, L. Room Temperature Ferromagnetism of Sn1−xCoxO2−δ Films Fabricated by Sol-Gel Method. J. Appl. Phys. 2008, 104, 023905. [Google Scholar] [CrossRef]
- Borges, P.D.; Scolfaro, L.M.R.; Alves, H.W.L.; da Silva, E.F., Jr.; Assali, L.V.C. Study of the Oxygen Vacancy Influence on Magnetic Properties of Fe- and Co-Doped SnO2 Diluted Alloys. Nanoscale Res. Lett. 2012, 7, 540. [Google Scholar] [CrossRef]
- Hong, N.H.; Poirot, N.; Sakai, J. Ferromagnetism Observed in Pristine SnO2 Thin Films. Phys. Rev. B 2008, 77, 033205. [Google Scholar] [CrossRef]
- Li, J.; Bai, G.; Jiang, Y.; Du, Y.; Wu, C.; Yan, M. Origin of Room Temperature Ferromagnetism in SnO2 Films. J. Magn. Magn. Mater. 2017, 426, 545–549. [Google Scholar] [CrossRef]
- Rasaili, P.; Sharma, N.K.; Bhattarai, A. Comparison of Ferromagnetic Materials: Past Work, Recent Trends, and Applications. Condens. Matter 2022, 7, 12. [Google Scholar] [CrossRef]
- Manikandan, D.; Murugan, R. Genesis and Tuning of Ferromagnetism in SnO2 Semiconductor Nanostructures: Comprehensive Review on Size, Morphology, Magnetic Properties and DFT Investigations. Prog. Mater. Sci. 2022, 130, 100970. [Google Scholar] [CrossRef]
- Hong, N.H.; Friák, M.; Pazourek, P.; Pham, N.S.; Nhu, T.Q.; Kiaba, M.; Gazdová, K.; Pavlů, J. 2D Nature of Magnetic States at SnO2 Surfaces: A Combined Experimental and Theoretical Study. RSC Adv. 2024, 14, 13583–13590. [Google Scholar] [CrossRef]
- Ryssel, H.; Ruge, I. Ionenimplantation; Teubner: Stuttgart, Germany, 1978; 366p. [Google Scholar]
- Heo, Y.W.; Kelly, J.; Norton, D.P.; Hebard, A.F.; Pearton, S.J.; Zavada, J.M.; Boatner, L.A. Effects of High Dose Ni, Fe, Co, and Mn Implantation into SnO2. Electrochem. Solid-State Lett. 2004, 7, G309. [Google Scholar] [CrossRef]
- Schoenes, J.; Pelzer, U.; Menzel, D.; Franke, K.; Ludwig, F.; Schilling, M. Ferromagnetism in Fe and Co-implanted SnO2 Films. Phys. Status Solidi C 2006, 3, 4115–4118. [Google Scholar] [CrossRef]
- Menzel, D.; Awada, A.; Dierke, H.; Schoenes, J.; Ludwig, F.; Schilling, M. Free-Carrier Compensation in Ferromagnetic Ion-Implanted SnO2:Co. J. Appl. Phys. 2008, 103, 07D106. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and Collaborative Approach to Problem Solving Using X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Iassonov, P.G.; Nourgaliev, D.K.; Bourov, B.V.; Heller, F. A modernized coercivity spectrometer. Geol. Carpathica 1998, 49, 224–226. [Google Scholar]
- Kosareva, L.R.; Shcherbakov, V.P.; Nurgaliev, D.K.; Nurgalieva, N.G.; Sycheva, N.K.; Antonenko, V.V.; Kuzina, D.M.; Evtyugin, V.G. Periodization of Holocene Climatic Cycles Based on Synchronous Variations in the Magnetic and Geochemical Parameters of the Sediments of Lake Bolshoe Yarovoe (Southwestern Siberia). Russ. Geol. Geophys. 2020, 61, 723–737. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, M.; Li, M.; Zhang, Q.; Lu, Y.; Chen, J.; Li, M.; Dai, J.; Chen, C.; He, Y. SnO2 Epitaxial Films with Varying Thickness on C-Sapphire: Structure Evolution and Optical Band Gap Modulation. Appl. Surf. Sci. 2017, 423, 611–618. [Google Scholar] [CrossRef]
- Liu, H.-G.; Zheng, W.-C.; He, L. EPR g Factors and Defect Structures for Co2+ Ions at the Substitutional and Interstitial Sites of SnO2 Lattice. Radiat. Eff. Defects Solids 2008, 163, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Y.; Mohammed, Y.S.; Du, X.; Li, K.; Jin, H. First-Principle Study of Magnetism in Co-Doped SnO2. J. Magn. Magn. Mater. 2009, 321, 337–342. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zinnatullin, A.L.; Petrov, A.V.; Yusupov, R.V.; Valeev, V.F.; Khaibullin, R.I.; Vagizov, F.G. Unusual Compositions of Fe-Nb Alloy Precipitates in Iron-Implanted LiNbO3. Magnetochemistry 2023, 9, 121. [Google Scholar] [CrossRef]
- Drouin, D.; Couture, A.R.; Joly, D.; Tastet, X.; Aimez, V.; Gauvin, R. CASINO V2.42—A Fast and Easy-to-use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users. Scanning 2007, 29, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The Stopping and Range of Ions in Matter. Nucl. Instrum. Methods Phys.Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Achkeev, A.A.; Khaibullin, R.I.; Tagirov, L.R.; Mackova, A.; Hnatowicz, V.; Cherkashin, N. Specific Features of Depth Distribution Profiles of Implanted Cobalt Ions in Rutile TiO2. Phys. Solid State 2011, 53, 543–553. [Google Scholar] [CrossRef]
- Powell, C. X-ray Photoelectron Spectroscopy Database XPS, Version 4.1; NIST Standard Reference Database 20; NIST: Gaithersburg, MD, USA, 1989. [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Kılıç, Ç.; Zunger, A. Origins of Coexistence of Conductivity and Transparency in SnO2. Phys. Rev. Lett. 2002, 88, 095501. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lin, Y.; Wang, X.; Zhao, Y.; Tian, J. Defect Engineering on SnO2 Nanomaterials for Enhanced Gas Sensing Performances. Adv. Powder Mater. 2022, 1, 100033. [Google Scholar] [CrossRef]
- Joshi, J.P.; Bhat, S.V. On the Analysis of Broad Dysonian Electron Paramagnetic Resonance Spectra. J. Magn. Reson. 2004, 168, 284–287. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Okay, C.; Vakhitov, I.R.; Valeev, V.F.; Khaibullin, R.I.; Rameev, B. Magnetic Resonance Study of Fe-Implanted TiO2 Rutile. Appl. Magn. Reson. 2017, 48, 347–360. [Google Scholar] [CrossRef]
- Kazan, S.; Mikailzade, F.A.; Şale, A.G.; Maksutoğlu, M.; Acikgoz, M.; Khaibullin, R.I.; Khalitov, N.I.; Gatiiatova, J.I.; Valeev, V.F. Magnetic Properties of Co-Implanted BaTiO3 Perovskite Crystal. Phys. Rev. B 2010, 82, 054402. [Google Scholar] [CrossRef]
- Zener, C. Interaction Between The d Shells in the Transition Metals. Phys. Rev. 1951, 81, 440–444. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Douvalis, A.P.; Fitzgerald, C.B.; Venkatesan, M. Ferromagnetism in Fe-Doped SnO2 Thin Films. Appl. Phys. Lett. 2004, 84, 1332–1334. [Google Scholar] [CrossRef]
- Khaibullin, R.I.; Ibragimov, S.Z.; Tagirov, L.R.; Popok, V.N.; Khaibullin, I.B. Formation of Anisotropic Ferromagnetic Response in Rutile (TiO2) Implanted with Cobalt Ions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 257, 369–373. [Google Scholar] [CrossRef]
- Shchukarev, S.A.; Tolmacheva, T.A. Solubility of Oxygen in Ethanol-Water Mixtures. J. Struct. Chem. 1968, 9, 16–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).