Abstract
Since 2012, layered compounds containing Bi-Ch (Ch: S and Se) layers have been extensively studied in the field of superconductivity. The most-studied system is BiS2-based superconductors with two-layer-type conducting layers. Recently, superconductivity was observed in La2O2M2S6 (M = metals), which contains four-layer-type conducting layers. The four-layer-type Bi-based superconductors are new systems in the family of Bi-based superconductors; we can expect further development of Bi-based layered superconductors. In this review article, we summarize the progress of synthesis, structural analysis, investigations on superconducting properties, and material design of the four-layer-type Bi-based superconductors. In-plane chemical pressure is the factor essential for the emergence of bulk superconductivity in the system. The highest Tc of 4.1 K was observed in Rare Earth elements (RE) substituted La2-xRExO2Bi3Ag0.6Sn0.4S6.
1. Introduction
Many new superconductors with a layered structure have been synthesized since the discovery of cuprate high-Tc (Tc: transition temperature) superconductor [1]. One of the big discoveries of a layered superconductor system is the discovery of Fe-based superconductors [2]. In addition, the BiS2-based layered superconductors are a recent example of layered superconductors [3,4]. The BiS2-based layered superconductors have tremendously attracted the condensed matter physics scientific community since 2012 [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. The crystal structure of the BiS2-based compounds is generally composed of the BiS2 layers as superconducting layers and insulating (blocking) layers like LaO, which is analogous to the structure of high-Tc cuprates and Fe-based superconductors [1,2]. The first BiS2-based compound was Bi4O4S3 with Tc (onset) = 8.6 K, later on, LnO1−xFxBiS2 (Ln = La, Ce, Nd, Yb, Pr), Sr1−xLaxFBiS2, and EuFBiS2 with Tc of 2–11 K have been discovered [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Mainly, the crystal structure of Ln(O,F)BiS2 consists of blocking layers (LnO) and the conducting layers (BiS2); the underlying crystal structure has been shown in Figure 1 [4]. Other materials similar to the BiS2 -based layered compounds were developed by replacing S by Se. Therefore, these compounds have been called BiCh2-based compounds (Ch = S, Se) [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. After the successful growth of single crystals of LnO1−xFxBiS2 phase, an important feature has been discussed in the crystal structure of the BiS2-based compound [46,47,48]. The single crystals of LnO1−xFxBiS2 can be easily exfoliated (layer by layer) because of the plate-like shape and the existence of a van der Waals gap between the BiS planes [46,47,48]. Notably, the van der Waals gap between two BiS planes became the essential feature when designing four-layer-type La2O2M4S6 (M: metals) compounds [49,50,51,52], which is the central issue of this review article.
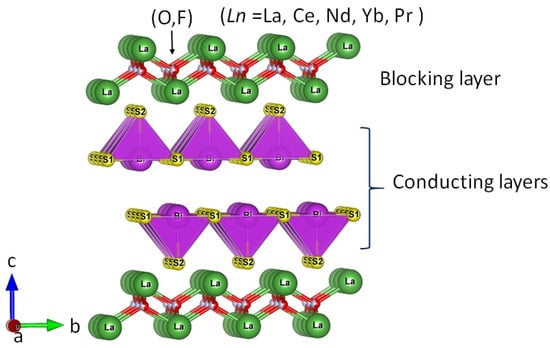
Figure 1.
The crystal structure of the BiS2-based Ln(O,F)BiS2 superconducting systems, the two BiS2 layers are conducting layers (CL), and LnO layers are Blocking layers (BL) [4].
The concept of designing new BiS2-based compounds with a thicker (four-layer-type) superconducting layer can be related to the case of the high-Tc cuprates [1,53,54,55,56,57]. The thickness of the superconducting layer was essential to achieve a higher Tc [53,54,55,56,57]. Tc above 100 K has been achieved by increasing the number of CuO2 (conducting) layers in the unit cell of the crystal structure. We have assumed the same concept to develop La2O2M4S6 four-layer type BiS2-based superconductors.
The first cuprate superconductor, La2-xBaxCuO4 (LBCO) with Tc = 30 K was discovered in 1986 [1]. The Tc has increased up to 40 K for the La2-xSrxCuO4 (LSCO) superconductor [54]. The structure of LBCO has been classified as K2NiF4 (214)-type tetragonal. Soon after the discovery of the LBCO superconductors, the YBa2Cu3O7−δ (YBCO) superconductor with Tc = 93 K has been discovered [53]. In the crystal structure of YBCO, there are two CuO2 planes [53]. The superconductivity in a more complex system Bi2Sr2Ca2Cu3Ox (BSCCO-2223) and Bi2Sr2CaCu2Ox (BSCCO-2212) was discovered in 1988 with Tc = 110 K and 96 K, respectively [55,56,57,58]. The common component in all the cuprates is the CuO2 planes. The LSCO, YBCO, and BSCCO-2223 compounds can be regarded as one-layer-type, two-layer-type, and three-layer-type structure among the cuprate family. It seems that (see Figure 2), the Tc of cuprates has been increased by increasing numbers of the CuO2 layers in the unit cell. This trend in cuprates may be expected for other layered superconductors.
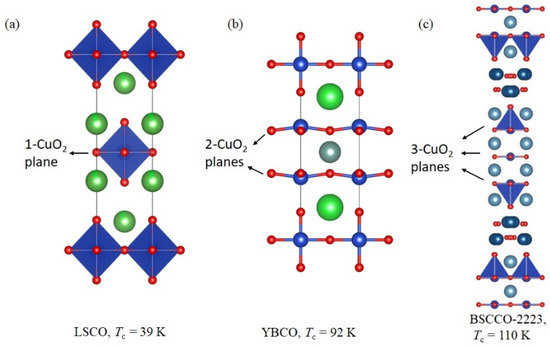
Figure 2.
The crystal structure of High Temperature Superconductors (HTSC) family. (a) La2-xSrxCuO4 (LSCO) [52], (b) YBa2Cu3O7−δ (YBCO) [51], and (c) Bi2Sr2Ca2Cu3Ox (BSCCO-2223) [55].
Since this review is focused on the four-layer-type BiS2-based compounds, a brief summary of the Bi-based compound is important to understand the four-layer-type compounds. Superconductivity in Bi-based compounds has been studied extensively. The highest Tc ever achieved for the Bi-2223 and 2212 compounds, the Tc most likely depends on the number of CuO2 layers in the crystal structure of these compounds [55,56,57,58]. The pure Bi is superconducting under pressure because, at low temperature, the formation of high-pressure metallic phases of Bi and the Tc of these phases are 3.9-8.3 K [59,60,61,62,63]. At ambient pressure, Bi shows the superconductivity in amorphous Bi-thin films, Bi granular composite, and Bi thin films on the Ni substrates and Tc around 6.0 K, 5.5 K, and 4.0 K respectively [64,65,66,67,68]. In addition, superconductivity has been revealed in the Bi-based compounds like; (Ba/Sr)Bi3 and BiNi3, and the Tc was nearly 5-6 K [69,70]. More recently, BiS2-based compounds have been discussed all-inclusive [3,4,5,6,7,8,9,10,11,12].
The continuous development of BiS2-based compounds has encouraged us to study the four-layer-type compounds [49,50,51,52]. Initially, the first four-layers-type LaOBiPbS3 (equivalently described as La2O2Bi2Pb2S6 to cover one unit cell) compound has been reported as a semiconductor and thermoelectric material [49]. Later on, the metal (M) site of La2O2M4S6 has been modified as M = Bi3Ag, and the resultant compound was La2O2Bi3AgS6 [51]. This newly designed oxychalcogenide La2O2Bi3AgS6 showed metallic conductivity and superconductivity at Tc = 0.5 K [52]. Previous studies on the two-layer-type (BiS2-based LnO1−xFxBiS2) phase has suggested that the doping effect at the various sites of a compound induced bulk superconductivity or improve the superconducting properties [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,68,69,70,71,72], which has been understood by in-plane chemical pressure effects. Therefore, we could assume that the superconducting properties of La2O2Bi3AgS6 may be improved by element substitutions. Then, we doped Sn for the Ag site and found that Tc increases up to 2.5 K in La2O2Bi3Ag0.6Sn0.4S6 [73]. The in-plane chemical pressure (chemical pressure effect along ab-plane) was not sufficient in La2O2Bi3Ag0.6Sn0.4S6, and Se substitution at the S site achieved bulk superconductivity with a Tc of 3.5 K in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3 [73]. Furthermore, substitutions in the block layer of BiS2-based compounds could generate in-plane chemical pressure and induce bulk superconductivity as well [71]. Therefore, in-plane chemical pressure by Rare Earth (RE) ion substitution in the blocking layer of La2O2Bi3Ag0.6Sn0.4S6 has been examined [74,75]. Bulk superconductivity has been induced by RE elements smaller than La, and Tc increased up to 4.0 K for the Eu-doped La2-xEuxO2Bi3Ag0.6Sn0.4S6 (x = 0.4) compound [74]. Furthermore, we have doped Sm at the La site as well and observed bulk superconductivity at Tc = 4.1 K in the La2-xSmxO2Bi3Ag0.6Sn0.4S6. The trend on the correlation between Tc and crystal structure in REO1−xFxBiS2 superconductors has been found, and the importance of in-plane chemical pressure has been confirmed.
Recently, superconductivity in Bi3O2S2Cl at Tc = 2.8 K has been reported by Ruan et al. As shown in Figure 3, this compound can be regarded as a one-layer-type Bi-based superconductor [76]. As mentioned earlier, the BiCh2-based systems like REO1−xFxBiS2 have two BiS2 layers in a unit cell [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Therefore, the BiCh2-based system can be regarded as two-layer-type. Then, according to the categorization of conducting layer structure, we regard the structure of La2O2M4S6 as four-layer-type as summarized in Figure 3. A similar strategy of cuprates, as mentioned in Figure 2, can be applied for the Bi-based compounds by changing constituent elements and the number of conducting layers to develop high Tc Bi-based superconductors.
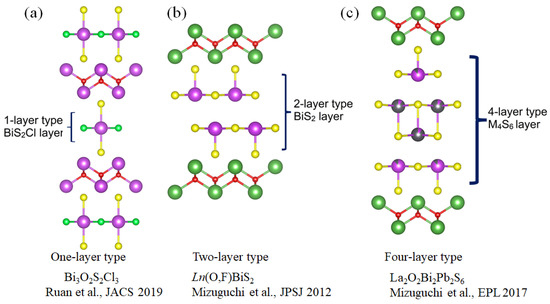
Figure 3.
The crystal structure of Bi-based compounds (a) 1-layer type Bi3O2S2Cl3 [76], (b) 2-layers type Ln(O, F)BiS2 superconductors [4], and (c) 4-layers type La2O2Bi2Pb2S6 [51].
The in-plane chemical pressure appears in the two-layers BiCh2-based, and four-layers type compounds can be regarded as a change in the lattice parameter a. Figure 4 shows the lattice parameter a dependence of Tc for 1-layer type Bi3O2S2Cl3 [76], 2-layers type Ln(O, F)BiS/Se2 superconductors, and 4-layers type La2O2M4S6 compounds. With replacing Ln (La to Ce, Pr Nd) in Ln(O, F)BiS2, the considerable change in lattice parameter a has been observed, which is changing the Tc largely of 2-layers type superconductors [3,4,5,6,7,8,9,10,11,12]. We notice that a slight change in the lattice parameter a is creating a large effect on the superconductivity on 4-layers-type La2O2M4S6 compounds [52,73,74,75].
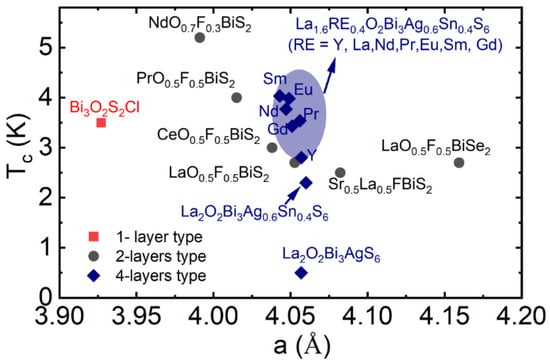
Figure 4.
The Tc vs. lattice parameter a, for 1-layer type, 2-layers type, and 4-layers type crystal structure of Bi-based compounds the data extract from references [3,4,5,6,7,8,9,10,11,12,52,73,74,75,76,77,78].
In this review article, we summarize the evolution of Tc in Table 1. Table 1 contains the change in Tc with the change of doping elements on various sites RE, M, and S of La2O2M4S6, four-layers-type BiS2-based compounds [52,73,74,75,76,77,78].

Table 1.
Four-layer-type superconductors with Tc and references.
2. The Crystal Structure of La2O2M4S6 Type Four-Layer Compounds
2.1. La2O2Bi2Pb2S6
Primarily, the La2O2Bi2Pb2S6 compound was synthesized and reported as thermoelectric material by Sun et al. [49]. La2O2Bi2Pb2S6 is composed of Bi2Pb2S6 conducting layers and LaO block layers. La2O2Bi2Pb2S6 has a tetragonal structure with space group P4/nmm, as shown in Figure 5. We further investigated the site selectivity at the M (=Bi2Pb2) site and performed band calculations [50]. From synchrotron X-ray and neutron diffraction, we revealed that the M4S6 layers were almost ordered into Bi- and Pb-rich sites. According to our study, the structure could be rather regarded as stacks of LaOBiS2-type and rock-salt-type (PbS-type) layers; a schematic image of the crystal structure of La2O2Bi2Pb2S6 is shown in Figure 5.
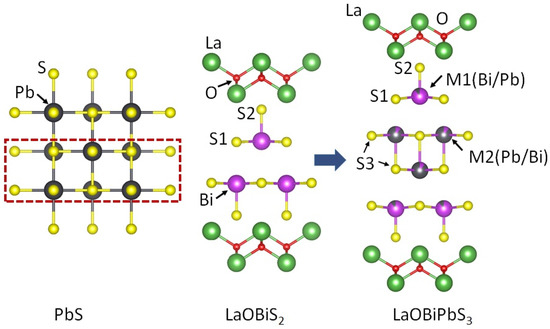
Figure 5.
The schematic unit cell of La2O2Bi2Pb2S6 with the relationship of the crystal structure between PbS (rock-salt-type), LaOBiS2, and LaOBiPbS3 [50].
2.2. La2O2Bi3AgS6
The concept of structural (stacking) in La2O2Bi2Pb2S6 was taken to synthesize new phase La2O2Bi3AgS6, which was initially reported as a non-superconducting compound [51]. In La2O2Bi3AgS6, the (Ag, Bi)S layer was inserted in between two BiS2 layers of the LaOBiS2 in place of the PbS layer in La2O2Bi2Pb2S6 [51]. It is worth noting that the interlayer distance M2-S1 is clearly different between M = Bi2Pb2 and M = Bi3Ag. The structural difference affects physical properties in those compounds, which will be shown in the following parts.
2.3. Available Elements for RE, M, and S Sites of La2O2M4S6
Figure 6a shows the crystal structure for La2O2Bi2Pb2S6. The substitution effect has been studied for the La2O2Bi2Pb2S6 compound, and different metals like Cd, Sn, and Sb have doped at the Pb (M2) site. The Se can partially substitute at the S (S1 and S3) site of La2O2Bi2Pb2S6.
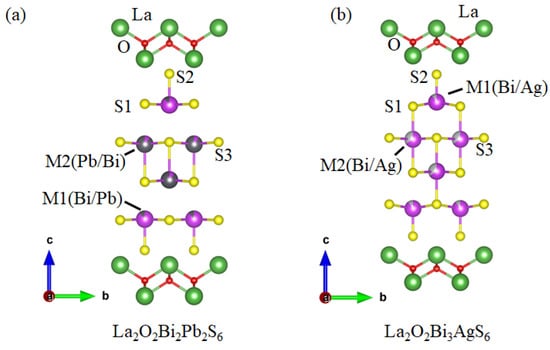
Figure 6.
The schematic unit cell of (a) La2O2Bi2Pb2S6; (b) La2O2Bi3AgS6 [50,51].
Figure 6b displays the crystal structure of La2O2Bi3AgS6. La2O2Bi3AgS6 is a superconductor of Tc = 0.5 K [52]. To increase the Tc and induce the bulk superconductivity, various sites of La2O2Bi3AgS6 can be doped. The available elements for La sites are different REs like Eu, Sm, Pr, Nd, Y, and Gd. The In and Sn can be doped at the Ag (M2) site of La2O2Bi3AgS6. In addition, the S (S1 and S3) site can be partially substituted by Se of La2O2Bi3AgS6.
To summarize, so far, we could use M = Bi, Pb, Ag, Sn, Cd, Sb, and In, RE = La, Pr, Nd, Sm, Eu, and Gd, and Ch = S and Se to synthesize the new four-layer-type Bi-based compounds.
3. Physical Properties of La2O2M4S6 (M = Pb, Ag)
3.1. La2O2Bi2Pb2S6; Doping Effect of Cd, Sn, and Sb at Pb Site
Since the data for La2O2Bi2Pb2-xCdxS6, La2O2Bi2Pb2-xSnxS6, and La2O2Bi2Pb2-xSbxS6 have not been published in other journals, and has appeared in this paper for the first time, we summarized experimental details in the final section of this article.
The La2O2Bi2Pb2S6 compound has been reported as a narrow-gap semiconductor with an activation energy of ~17 meV, confirmed by electrical resistivity and Hall effect measurements [49]. Moreover, the band calculations predicted that La2O2Bi2Pb2S6 was close to a zero-gap semiconductor (or metal), which is a clear difference from the electronic structure of the parent phase of the BiCh2-based (two-layer-type) systems [3]. Experimentally obtained samples of La2O2Bi2Pb2S6, however, showed insulating behavior in the electrical resistivity measurements at low temperatures [49,50]. Therefore, we have investigated the substitution effects of the Pb site with Cd, Sn, and Sb in La2O2Bi2Pb2S6. Our motivation of the Pb-site substitution was to enhance the charge carriers and/or charge mobility by the substitution effects, but all the Pb-site substitutions resulted in insulating transport properties, while the insulating behavior has been somehow suppressed by the substitutions. Figure 7a–c shows the temperature dependences of electrical resistivity ρ(T) for the Cd-, Sn-, and Sb-doped La2O2Bi2Pb2S6; insets are the log ρ vs. T. Unfortunately, we did not observe superconductivity by doping with Cd, Sn, and Sb.
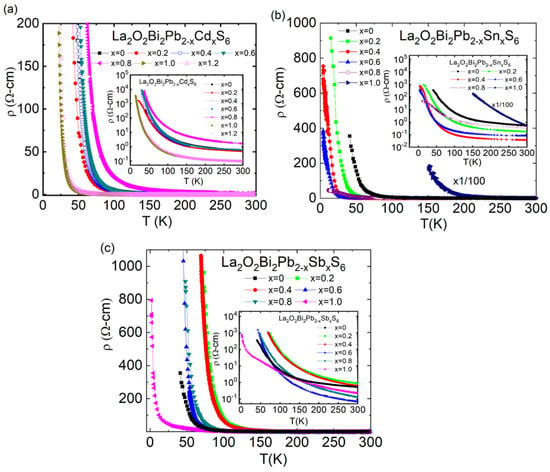
Figure 7.
The temperature dependence of the electrical resistivity ρ(T) for (a) the La2O2Bi2Pb2-xCdxS6, inset is the log ρ vs. T for the La2O2Bi2Pb2-xCdxS6. (b) the La2O2Bi2Pb2-xSnxS6 and inset is the log ρ vs. T for the La2O2Bi2Pb2-xSnxS6. (c) the La2O2Bi2Pb2-xSbxS6 and inset is the log ρ vs. T for La2O2Bi2Pb2-xSbxS6.
3.2. Superconductivity in Se-Doped La2O2Bi2Pb2S6-xSex
Superconductivity has been observed for Se-substitution La2O2Bi2Pb2S6-xSex [77]. Undoped La2O2Bi2Pb2S6 shows insulating behavior at low temperatures, but a partial substitution of S by Se induces metallicity in La2O2Bi2Pb2S6-xSex [77]. We have confirmed that the lattice parameter a slightly expended by the substitution effects of Se for the S site in La2O2Bi2Pb2S6-xSex, which is an indication of the presence of in-plane chemical pressure effects in La2O2Bi2Pb2S6-xSex due to Se substitutions. We observed superconductivity in La2O2Bi2Pb2S6-xSex.The estimated transition temperatures are Tc = 1.15 K for x = 0.5 and Tc = 1.9 K for x = 1.0 [77].
3.3. Superconductivity in La2O2Bi3AgS6
Initially, La2O2Bi3AgS6 was synthesized and reported as a non-superconducting compound [51]. Through optimization of synthesis condition to get a high quality La2O2Bi3AgS6 sample, we obtained a sample which shows metallic conductivity and superconductivity at 0.5 K (see Figure 8a,b) [52]. La2O2Bi3AgS6 shows a charge density wave (CDW)-like transition at temperature (T*) in the ρ(T) curve below 180 K, which is similar to the case of EuFBiS2 [15]. We measured the ρ(T) by using an adiabatic demagnetization refrigerators (ADR) system down to the T = 0.1 K. Superconductivity at Tc (onset) = 0.66 K has been observed in La2O2Bi3AgS6 [52]. La2O2Bi3AgS6 was the first superconductor among La2O2M4S6-type (four-layer-type) Bi-based layered compounds.
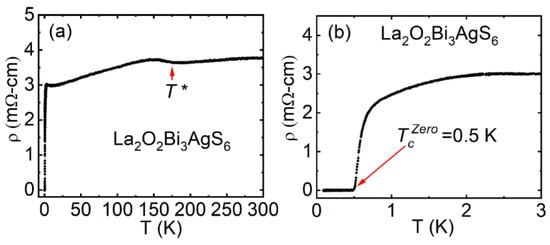
Figure 8.
(color online) (a) The ρ(T) from 300-0.1K for the La2O2Bi3AgS6 compound, hump in the ρ(T) curve denoted as T*, Inset is the schematic unit cell of La2O2Bi3AgS6. (b) The zoomed view of the ρ(T) curve near Tc in the temperature range 3.0–0.1 K. Reproduced from J. Phys. Soc. Jpn. 87, 083704 (2018) [52], copyrighted by the Physical Society of Japan.
3.3.1. Effect of In Doping in La2O2Bi3Ag1-xInxS6
Initially, we had tried to modify the conducting layers of the La2O2Bi3AgS6 compound by substituting Ag by In [69]. The ρ(T) curve for La2O2Bi3Ag1-xInxS6 from 300 – 0.4 K has been shown in Figure 9a; expanded view near the superconducting transition temperature range is shown in Figure 9b. The In-doped La2O2Bi3Ag1-xInxS6 shows that the Tc slightly decreases with increasing In doping level. In contrast, the anomaly temperature of the CDW-like transition T* did not change, although it was expected that the CDW-like transition is suppress by doping carrier. Figure 9c exhibits the T vs. x for La2O2Bi3Ag1-xInxS6, i.e., x-T phase diagram, which shows the change in superconducting transition Tc (zero) as a function of In doping level [78]. T* does not change with increasing x. The Tc slightly decreases with increasing x in La2O2Bi3Ag1-xInxS6. The decrease in Tc for the In-doped samples might be caused by the introduced disorder at the M site and the robustness of the CDW-like ordering (T*) against the In doping. A comparatively similar situation has been reported for the NbSe2−xTex; the Tc was decreasing by doping of Te at the Se site [79]. The structural analysis showed that the lattice parameters increasing with increasing In doping, which suggests the in-plane interaction is somehow decreasing.
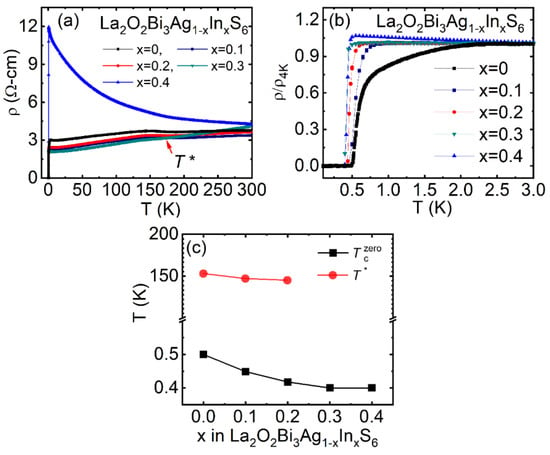
Figure 9.
(color online) (a) The ρ(T) from 300–0.1 K for the La2O2Bi3Ag1-xInxS6 (x = 0–0.4) compounds. (b) The normalized ρ(T)/ ρ(4 K) curve in the temperature range 3.0–0.1 K. (c) The x dependence of Tc for La2O2Bi3Ag1-xInxS6 (x = 0–0.4) compounds and T * scaled on the x-T phase diagram for La2O2Bi3Ag1-xInxS6. CDW and SC symbolize as charge density wave and superconductivity, respectively. Reproduced from J. Phys.: Conf. Ser. 1293, 012001 (2019) [78], copyrighted by the IOP Publishing Ltd.
3.3.2. Sn-Substitution Effect in La2O2Bi3Ag1-xSnxS6
We have substituted Ag at the M2 site of La2O2Bi3AgS6 by Sn [73]. The Sn substitution improved superconducting properties, and the maximum Tc was 2.5 K for the x = 0.4 in La2O2Bi3Ag1-xSnxS6 [73]. Figure 10a–c shows the superconducting transitions in the temperature dependences of magnetization χ(T) and resistivity ρ(T). Figure 10d is the phase diagram for La2O2Bi3Ag1-xSnxS6: Tc and T* are plotted as a function of Sn concentration x. In La2O2Bi3AgS6, anomalies have been observed in the ρ(T). Although In doping could not suppress the anomaly, the anomaly temperature T* shifted to a lower temperature by Sn substitution. Finally, the anomaly disappeared at x = 0.3. Generally, CDW ordering can be expected to be suppressed by an increase in charge carriers or introduction of disorder in the lattice. As a result, Tc increases by the suppression of CDW temperature in materials in which superconductivity and CDW ordering coexist [80]. The magnitude of the Seebeck coefficient slightly decreased by Sn substitution, i.e., amount of electron charge carriers increased. Therefore, increased charge carrier would have suppressed anomaly temperature T*. At the same time, Sn substitution should increase randomness at the M2 site. Therefore, the increase in Tc in the present phase La2O2Bi3Ag1-xSnxS6 would be achieved by an increase in electron carriers and the introduced disorder at the M2 site.
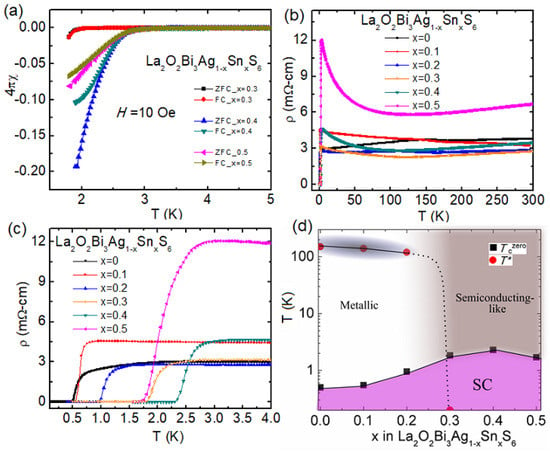
Figure 10.
(a) Temperature (T) dependences of magnetic susceptibility (χ) for La2O2Bi3Ag1-xSnxS6 (x = 0.3, 0.4, and 0.5) compounds measured in Zero Field Cool (ZFC) and Field Cool (FC) protocol at the applied magnetic field 1 mT. (b) The ρ(T) from 300–0.1 K for the La2O2Bi3Ag1-xSnxS6 (x = 0–0.5) compounds. (c) The ρ(T) curve in the temperature range 4.0–0.1 K. (d) The x dependence of Tc for La2O2Bi3Ag1-xSnxS6 (x = 0–0.5) compounds and T* scaled on the x-T phase diagram for La2O2Bi3Ag1-xSnxS6. Reproduced from Sci. Rep. 9, 13346 (2019) [73], copyrighted by the Springer Nature Limited.
3.3.3. Se-Substitution Effect in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3
To induce bulk superconductivity and to increase Tc, in-plane chemical pressure effects (by chemical substitution or high-pressure annealing) are essential in the two-layer-type BiCh2-based systems [27,28,81,82,83]. For example, in LnO0.5F0.5BiS2, lattice shrinkage by RE site substitution in blocking layers or Se substitution for the S site in conducting layers achieve generation of in-plane chemical pressure effect and induce bulk superconductivity. We had applied the same strategy to improve superconducting properties of four-layers-type La2O2Bi3Ag0.6Sn0.4S6 as well. Small amount (5%) of Se was substituted for the S site. Tc increased by Se substitution in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3 [73]. A large shielding volume fraction was observed as shown in Figure 11a, which suggested the emergence of bulk superconductivity in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3. As we discussed above, the Sn substitution improved the superconducting properties of La2O2Bi3Ag1-xSnxS, and the highest Tc of 2.5 K was observed for x = 0.4. However, the shielding volume fraction for x = 0.4 was clearly lower than 100% shielding. We have discussed the possible origin of an increase in Tc by Sn substitution. It was clear from the structural analysis that the essential parameter in-plane chemical pressure was not sufficient in La2O2Bi3Ag0.6Sn0.4S6. Since the shielding property has been improved by Se substitution, we consider that partial Se substitution for the S site in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3 generated in-plane chemical pressure effects as observed in two-layer-type LnO0.5F0.5BiS2.
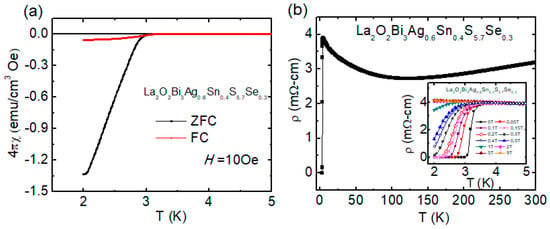
Figure 11.
(a) Temperature dependence of magnetic susceptibility for La2O2Bi3Ag0.6Sn0.4S5.7Se0.3. (b) The (T) for La2O2Bi3Ag0.6Sn0.4S5.7Se0.3, the inset low-temperature (T) under magnetic fields up to 9 T. Reproduced from Sci. Rep. 9, 13346 (2019) [73], copyrighted by the Springer Nature Limited.
Figure 11b shows the temperature dependence of resistivity. Superconductivity at Tc (zero) = 3.0 K in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3 has been confirmed by ρ(T) measurements, while the Tc (onset) was 3.5 K. From resistivity measurements under magnetic fields up to 9 T, the upper critical field Bc2 and the irreversible field Birr were estimated as 2.15 T and 1.0 T, respectively.
3.3.4. Eu-Substitution Effect on La2-xEuxO2Bi3Ag0.6Sn0.4S6
Since Se substitution was effective to induce bulk superconductivity in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3, we have tested another way to increase in-plane chemical pressure by La-site substitution by RE elements smaller than La. The first investigation was examined with RE = Eu [74]. The Sn amount was fixed as La2O2Bi3Ag0.6Sn0.4S6. With increasing Eu concentration, Tc increased, and the highest Tc (zero) of 4.0 K was observed for the x = 0.4 (Eu), which has been confirmed by the χ(T) and ρ(T) measurements (see Figure 12a,b). Figure 12c represents the phase diagram on Tc vs. x in La2-xEuxO2Bi3Ag0.6Sn0.4S6. Tc (zero) extracted by ρ(T) data and Tc from χ(T) curve has been plotted with Eu concentration x in Figure 12c. Tc gradually increases with increasing Eu concentration up to x = 0.4 in La2-xEuxO2Bi3Ag0.6Sn0.4S6. Furthermore, for a higher Eu contents, x = 0.5 and 0.6, Tc slightly decreased in the ρ(T) data, while Tc slightly increased for the same in χ(T) data. The small shielding volume fraction for x = 0.5 and 0.6 suggests that bulk nature of superconductivity was suppressed for x = 0.5 and 0.6. The upper critical field was estimated from ρ(T, B) data, which was above the 3.0 T for the La2-xEuxO2Bi3Ag0.6Sn0.4S6 (x = 0.4) confirmed the robustness of the superconductivity against the magnetic field.
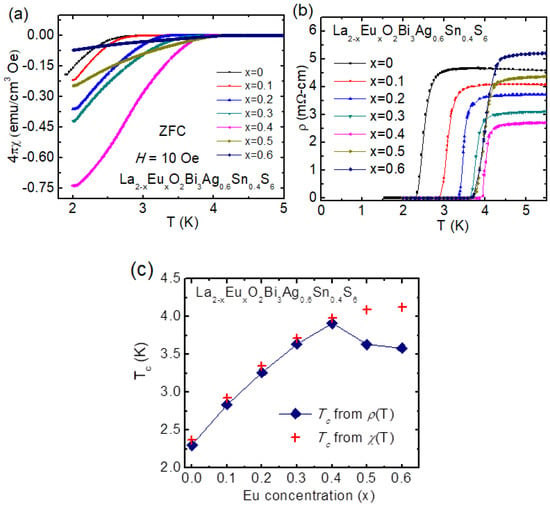
Figure 12.
(a) The χ(T) for La2-xEuxO2Bi3Ag0.6Sn0.4S6 (x = 0–0.6) for an applied magnetic field of 1 mT, compounds measured in the ZFC. (b) The ρ(T) curve is near to the Tc; the temperature range from 5.5 K to 1.5 K for the La2-xEuxO2Bi3Ag0.6Sn0.4S6 (x = 0–0.6) compounds. (c) The phase diagram, x dependence of Tc for La2-xEuxO2Bi3Ag0.6Sn0.4S6 compounds and Tc obtained from χ(T) data, Tc obtained from ρ(T) data [74].
The increase in Tc by Eu substitution can be basically understood by the in-plane chemical pressure effect. The valence state of Eu in La2-xEuxO2Bi3Ag0.6Sn0.4S6 is close to +3, which was analyzed from the temperature dependence of magnetic susceptibility. Since Eu3+ is smaller than La3+, lattice should be compressed with increasing Eu concentration, As a fact, lattice parameter a decreased by Eu substitution. According to the a-axis shrinkage, in-plane chemical pressure should be enhanced in the Bi-S plane.
3.3.5. Rare Earth (RE) Substitution Effects in La1.6RE0.4O2Bi3Ag0.6Sn0.4S6
In the Eu-doped La2-xEuxO2Bi3Ag0.6Sn0.4S6, the superconducting properties were optimized for x = 0.4. Therefore, to investigate the effects of substitution of other RE for the La site, we have synthesized samples of La2-xRExO2Bi3Ag0.6Sn0.4S6 with RE = Y, Pr, Nd, Sm, and Gd. The XRD patterns for La1.6RE0.4O2Bi3Ag0.6Sn0.4S6 (RE = Y, Pr, Nd, Sm, Eu, and Gd) are shown in Figure 13. All the XRD patterns were indexed using a tetragonal model with the space group of P4/nmm. The lattice parameter a decreases by RE doping, according to the difference in ionic radii of doped RE.
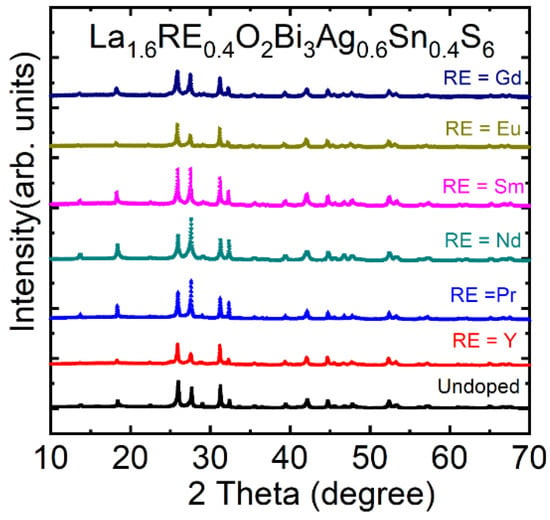
Figure 13.
(color online) The room temperature XRD pattern of the La1.6RE0.4O2Bi3Ag0.6Sn0.4S6 (RE = Y, Pr, Nd, Sm, Eu, and Gd) compounds [74].
The temperature dependence of electrical resistivity data for La1.6RE0.4O2Bi3Ag0.6Sn0.4S6 (RE = Y, Pr, Nd, Sm, Eu, and Gd) has been shown in Figure 14a. The normal state resistivity shows similar behavior for all the samples, as discussed above for the Eu-doped samples. A clear difference in Tc was observed for those samples, as shown in Figure 14b. Our structural analyses for the RE-doped sample suggest that the lattice parameter a is shrinking for all the RE-doped samples. The lattice parameter a is the smallest for the Sm-doped sample, which shows the highest Tc = 4.1 K.
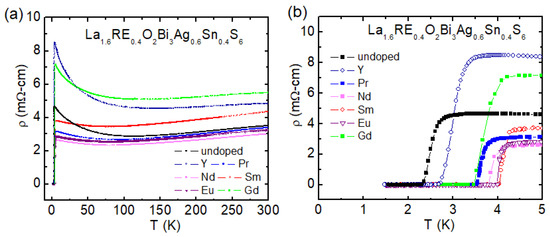
Figure 14.
(color online) (a) The ρ(T) from 300–1.5 K for the La1.6RE0.4O2Bi3Ag0.6Sn0.4S6 (RE = Y, Pr, Nd, Sm, Eu, and Gd) compounds. (b) The ρ(T) from 5.0–1.5 K for the La1.6RE0.4O2Bi3Ag0.6Sn0.4S6 compounds [74].
The Tc is higher for the samples having the smallest lattice parameter a, which suggests in-plane chemical pressure is essential for Tc in La1.6RE0.4O2Bi3Ag0.6Sn0.4S6. As shown in Figure 15, Tc shows a correlation with lattice parameter. Tc increases with decreasing lattice parameter a, which is suggesting in-plane chemical pressure can improve superconducting properties of La1.6RE0.4O2Bi3Ag0.6Sn0.4S6, which is a common feature with two-layer-type REO0.5F0.5BiCh2 superconductor [22,69]. Recently, enhanced superconductivity in the Nd- and Pr-doped La2-xRExO2Bi3Ag0.6Sn0.4S6 system has been reported in Reference 75. In the work, Tc increased for the 50%-Nd and 50%-Pr doping for the La site. The lattice parameter decreased by the Nd and Pr doping [75], which is consistent with our results.
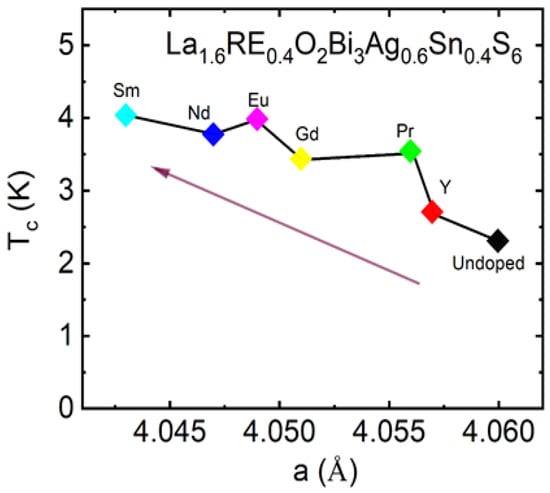
Figure 15.
The lattice parameter a (Å) dependence of Tc for La1.6RE0.4O2Bi3Ag0.6Sn0.4S6 (RE = Y, Pr, Nd, Eu, Sm, and Gd) obtained from ρ(T) data [74].
There are limited studies on the theoretical investigation of La2O2M4S6-type compounds; only a few have been executed for La2O2(M2Ch2)(Pn2Ch4) where M = Sn, Pb, Pn = Sb, Bi, and Ch = S, Se [51,84]. They suggested that a band structure could be changed by substitutions at the M, Pn, and Ch sites. No one has reported about the change in electronic states by the RE-site substitution. Based on previous theoretical studies for two-layer-type LnO1-xFxBiS2 systems, we can assume that the RE substitution does not largely influence the band structure for La2-xRExO2Bi3Ag0.6Sn0.4S6 systems. As a result that theoretical studies for LnO1-xFxBiS2 suggested that, the carrier density at the Fermi level could change slightly by changing Ln = RE in the block layers [85].
Our recent research on the four-layer-type compounds was focused to enhance the Tc at least above the 10 K, which has not been achievements yet. The maximum Tc is 4.1 K for the four-layer-type compounds. The applications of these compounds are not identified yet, as we have seen for hybrid superconducting-ferromagnetic thin films [86,87,88]. The study of superconducting and ferromagnetic states is important to understand the interacting mechanism of Cooper pairs and ferromagnetism [86,87,88], also hybrid superconducting-ferromagnetic systems are useful for the applications like; split-ring resonators, superconducting-spintronic devices, topological quantum computing, and magnetic-field-sensors as discussed in the reference [88]. There are many challenges to developing devices for the application purpose from the four-layer-type compounds.
4. Summary
In this review article, we have summarized the crystal structure and physical properties of the four-layer-type La2O2M4S6 system. The La2O2M4S6 phase was synthesized in 2014 with M = Bi2Pb2 by Sun et al. In 2018, superconductivity was observed in La2O2Bi3AgS6, where M = Bi3Ag, and Tc increased up to 4.1 K by element substitution in the La2O2Bi3AgS6-based systems. The in-plane chemical pressure effects seem to be essential for the emergence of bulk superconductivity and improvement of superconducting properties in the La2O2M4S6 system, which is common feature with the two-layer-type BiCh2-based systems.
La2O2Bi2Pb2S6 (M = Bi2Pb2) is the firstly-synthesized La2O2M4S6-type compounds and has been reported as a thermoelectric material. The crystal structure of La2O2Bi2Pb2S6 is composed of the stacks of a La2O2Bi2S4 block and a rock-salt-type Pb2S2 layer. Thus, we can design new four-layer-type La2O2M4S6 compounds by tuning the composition of the M2S2 layer. Although La2O2Bi2Pb2S6 shows insulating behavior at low temperatures, the electrical conductivity (metallicity) has been improved by Se substitution for the La2O2Bi2Pb2S6 compound. Superconductivity has been observed for Se-doped La2O2Bi2Pb2S6-xSex. The highest Tc was 1.9 K for La2O2Bi2Pb2S6-xSex (x = 1.0).
La2O2Bi3AgS6 (M = Bi3Ag) was synthesized according to the material-design strategy of La2O2M4S6. La2O2Bi3AgS6 shows metallic conductivity, which is in contrast to the case of La2O2Bi2Pb2S6. La2O2Bi3AgS6 shows superconductivity at Tc (zero) = 0.5 K. Tc slightly decreased by In doping at the Ag site for La2O2Bi3Ag1-xInxS6. In contrast, Tc increased by Sn doping at the Ag site; the maximum Tc was 2.5 K for La2O2Bi3Ag0.6Sn0.4S6. Since the in-plane chemical pressure effect worked positively for the two-layer-type BiCh2-based REO0.5F0.5BiS2 compounds, a similar approach was applied for the four-layer-type La2O2M4S6 systems. Bulk superconductivity with an enhanced Tc has been obtained for the Se-doped La2O2Bi3Ag0.6Sn0.4S5.7Se0.3. By Se doping at the S site in La2O2Bi3Ag0.6Sn0.4S5.7Se0.3, in-plane chemical pressure was generated, which enhanced the superconducting properties of the La2O2Bi3Ag0.6Sn0.4S5.7Se0.3 system. In addition, Tc increased by RE-site substitution in La2-xRExO2Bi3Ag0.6Sn0.4S6. The effects of the RE-site substitution can be regarded as in-plane chemical pressure effects as well as the case of Se substitution. The highest Tc of 4.1 K was observed for x = 0.4 in La2-xSmxO2Bi3Ag0.6Sn0.4S6. Bulk nature of superconductivity has been confirmed by magnetic susceptibility measurements, and the corresponding shielding volume fraction exceeded 75% for x = 0.4 for the Eu- and Sm-doped systems. The structural analysis suggested the solubility limit of RE in the La2-xRExO2Bi3Ag0.6Sn0.4S6 system is x ~ 0.4, at which the high Tc was observed. In La2-xRExO2Bi3Ag0.6Sn0.4S6, the lattice parameter a and Tc showed good correlation, which is suggesting the importance of in-plane chemical pressure. Our review article may enhance the visibility of four-layer-type La2O2M4S6 systems, which should be necessary for further development of Bi-based layered superconductors.
5. Experimental Details
We are showing new data for the La2O2Bi2Pb2-xCdxS6, La2O2Bi2Pb2-xSnxS6, and La2O2Bi2Pb2-xSbxS6 samples. The polycrystalline samples of La2O2Bi2Pb2-xCdxS6, La2O2Bi2Pb2-xSnxS6, and La2O2Bi2Pb2-xSbxS6 were synthesized by a solid-state reaction method. Powders of La2S3 (99.99%), Bi2O3 (99.9%), Pb (99.99%), Cd (99.99%), Sn (99.99%), Sb (99.99%), and grains of Bi (99.999%), S (99.99%) with a nominal composition were mixed in a pestle and mortar, pelletized, sealed in an evacuated quartz tube, and heated at 720 °C for 15 h. For the homogeneity the samples were reground, pelletized, and heated at 720 °C for 15 h. The phase purity has been checked by X-ray diffraction (XRD) with Cu-Kα radiation. The crystal structure parameters were refined by using the Rietveld method with RIETAN-FP [89]. The schematic images of the crystal structure were drawn using VESTA [90]. The actual compositions of the synthesized samples were investigated by using energy-dispersive X-ray spectroscopy (EDX) with TM-3030 (Hitachi). The electrical resistivity down to T = 2.0 K was measured by the four-probe technique. The temperature dependence of magnetic susceptibility χ (T) was measured by using a superconducting quantum interference device (SQUID) magnetometer (MPMS-3, Quantum Design).
Author Contributions
R.J. and Y.M.; writing—original draft preparation, R.J. and Y.M.; writing—review and editing, R.J. and Y.M.; visualization, Y.M.; supervision, Y.M.; project administration, Y.M.; funding acquisition, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Grants in Aid for Scientific Research (KAKENHI) (Grant No. 15H05886) and the Advanced Research Program under the Human Resources Funds of Tokyo (Grant Number: H31-1).
Acknowledgments
We gratefully appreciate O. Miura, Y. Goto, R. Higashinaka, T. D. Matsuda, and Y. Aoki of Tokyo Metropolitan University for fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bednorz, J.G.; Muller, K.A. Possible high Tc superconductivity in the Ba−La−Cu−O system. Z. Phys. B 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Kamihara, Y.; Watanabe, T.; Hirano, M.; Hosono, H. Iron-Based Layered Superconductor La[O1-xFx]FeAs (x = 0.05−0.12) with Tc = 26 K. J. Am. Chem. Soc. 2008, 130, 3296–3297. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, Y.; Fujihisa, H.; Gotoh, Y.; Suzuki, K.; Usui, H.; Kuroki, K.; Demura, S.; Takano, Y.; Izawa, H.; Miura, O. BiS2-based layered superconductor Bi4O4S3. Phys. Rev. B 2012, 86, 220510. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Demura, S.; Deguchi, K.; Takano, Y.; Fujihisa, H.; Gotoh, Y.; Izawa, H.; Miura, O. Superconductivity in Novel BiS2-Based Layered Superconductor LaO1-xFxBiS2. J. Phys. Soc. Jpn. 2012, 81, 114725. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, A.; Gahtori, B.; Kirtan, S.; Sharma, G.; Patnaik, S.; Awana, V.P.S. Bulk Superconductivity in Bismuth Oxysulfide Bi4O4S3. J. Am. Chem. Soc. 2012, 134, 16504–16507. [Google Scholar] [CrossRef] [PubMed]
- Demura, S.; Mizuguchi, Y.; Deguchi, K.; Okazaki, H.; Hara, H.; Watanabe, T.; Denholme, S.J.; Fujioka, M.; Ozaki, T.; Fujihisa, H.; et al. New Member of BiS2-Based Superconductor NdO1-xFxBiS2. J. Phys. Soc. Jpn. 2013, 82, 033708. [Google Scholar] [CrossRef]
- Jha, R.; Kumar, A.; Singh, S.K.; Awana, V.P.S. Superconductivity at 5 K in NdO0.5F0.5BiS2. J. Appl. Phys. 2013, 113, 056102. [Google Scholar] [CrossRef]
- Jha, R.; Kumar, A.; Singh, S.K.; Awana, V.P.S. Synthesis and Superconductivity of New BiS2-Based Superconductor PrO0.5F0.5BiS2. J. Supercond. Nov. Magn. 2013, 26, 499–502. [Google Scholar] [CrossRef]
- Xing, J.; Li, S.; Ding, X.; Yang, H.; Wen, H.H. Superconductivity appears in the vicinity of semiconducting-like behavior in CeO1−xFxBiS2. Phys. Rev. B 2012, 86, 214518. [Google Scholar] [CrossRef]
- Yazici, D.; Huang, K.; White, B.D.; Chang, A.H.; Friedman, A.J.; Maple, M.B. Superconductivity of F-substituted LnOBiS2 (Ln=La, Ce, Pr, Nd, Yb) compounds. Philos. Mag. 2012, 93, 673–680. [Google Scholar] [CrossRef]
- Yazici, D.; Huang, K.; White, B.D.; Jeon, I.; Burnett, V.W.; Friedman, A.J.; Lum, I.K.; Nallaiyan, M.; Spagna, S.; Maple, M.B. Superconductivity induced by electron doping in La1−xMxOBiS2(M= Ti, Zr, Hf, Th). Phys. Rev. B 2013, 87, 174512. [Google Scholar] [CrossRef]
- Krzton-Maziopa, A.; Guguchia, Z.; Pomjakushina, E.; Pomjakushin, V.; Khasanov, R.; Luetkens, H.; Biswas, P.; Amato, A.; Keller, H.; Conder, K. Superconductivity in a new layered bismuth oxyselenide: LaO0.5F0.5BiSe2. J. Phys.-Condens. Matter 2014, 26, 215702. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, Y.; Omachi, A.; Goto, Y.; Kamihara, Y.; Matoba, M.; Hiroi, T.; Kajitani, J.; Miura, O. Enhancement of thermoelectric properties by Se substitution in layered bismuth-chalcogenide LaOBiS2-xSex. J. Appl. Phys. 2014, 116, 163915. [Google Scholar] [CrossRef]
- Lin, X.; Ni, X.; Chen, B.; Xu, X.; Yang, X.; Dai, J.; Li, Y.; Yang, X.; Luo, Y.; Tao, Q.; et al. Superconductivity induced by La doping in Sr1−xLaxFBiS2. Phys. Rev. B 2013, 87, 020504. [Google Scholar] [CrossRef]
- Zhai, H.F.; Tang, Z.T.; Jiang, H.; Xu, K.; Zhang, K.; Zhang, P.; Bao, J.K.; Sun, Y.L.; Jiao, W.H.; Nowik, I.; et al. Possible charge-density wave, superconductivity, and f-electron valence instability in EuBiS2F. Phys. Rev. B 2014, 90, 064518. [Google Scholar] [CrossRef]
- Kotegawa, H.; Tomita, Y.; Tou, H.; Izawa, H.; Mizuguchi, Y.; Miura, O.; Demura, S.; Deguchi, K.; Takano, Y. Pressure Study of BiS2-Based Superconductors Bi4O4S3 and La(O,F)BiS2. J. Phys. Soc. Jpn. 2012, 81, 103702. [Google Scholar] [CrossRef]
- Wolowiec, C.T.; Yazici, D.; White, B.D.; Huang, K.; Maple, M.B. Pressure-induced enhancement of superconductivity and suppression of semiconducting behavior in LnO0.5F0.5BiS2 (Ln=La,Ce) compounds. Phys. Rev. B 2013, 88, 064503. [Google Scholar] [CrossRef]
- Wolowiec, C.T.; White, B.D.; Jeon, I.; Yazici, D.; Huang, K.; Maple, M.B. Enhancement of superconductivity near the pressure-induced semiconductor–metal transition in the BiS2-based superconductors LnO0.5F0.5BiS2 (Ln = La, Ce, Pr, Nd). J. Phys. Condens. Matter 2013, 25, 422201. [Google Scholar] [CrossRef]
- Jha, R.; Tiwari, B.; Awana, V.P.S. Impact of Hydrostatic Pressure on Superconductivity of Sr0.5La0.5FBiS2. J. Phys. Soc. Jpn. 2014, 83, 063707. [Google Scholar] [CrossRef]
- Jha, R.; Tiwari, B.; Awana, V.P.S. Appearance of bulk superconductivity under hydrostatic pressure in Sr0.5RE0.5FBiS2 (RE = Ce, Nd, Pr, and Sm) compounds. J. Appl. Phys. 2015, 117, 013901. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Miura, A.; Kajitani, J.; Hiroi, T.; Miura, O.; Tadanaga, K.; Kumada, N.; Magome, E.; Moriyoshi, C.; Kuroiwa, Y. In-plane chemical pressure essential for superconductivity in BiCh2-based (Ch: S, Se) layered structure. Sci. Rep. 2015, 5, 14968. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, J.; Hiroi, T.; Omachi, A.; Miura, O.; Mizuguchi, Y. Chemical Pressure Effect on Superconductivity of BiS2-Based Ce1−xNdxO1−yFyBiS2 and Nd1−zSmzO1−yFyBiS2. J. Phys. Soc. Jpn. 2015, 84, 044712. [Google Scholar] [CrossRef]
- Paris, E.; Joseph, B.; Iadecola, A.; Sugimoto, T.; Olivi, L.; Demura, S.; Mizuguchi, Y.; Takano, Y.; Mizokawa, T.; Saini, N.L. Determination of local atomic displacements in CeO1−xFxBiS2 system. J. Phys. Condens. Matter 2014, 26, 435701. [Google Scholar]
- Mizuguchi, Y.; Paris, E.; Sugimoto, T.; Iadecola, A.; Kajitani, J.; Miura, O.; Mizokawa, T.; Saini, N.L. The effect of RE substitution in layered REO0.5F0.5BiS2: Chemical pressure, local disorder and superconductivity. Phys. Chem. Chem. Phys. 2015, 17, 22090–22096. [Google Scholar] [CrossRef] [PubMed]
- Athauda, A.; Yang, J.; Lee, S.; Mizuguchi, Y.; Deguchi, K.; Takano, Y.; Miura, O.; Louca, D. In-plane charge fluctuations in bismuth-sulfide superconductors. Phys. Rev. B 2015, 91, 144112. [Google Scholar] [CrossRef]
- Nagasaka, K.; Nishida, A.; Jha, R.; Kajitani, J.; Miura, O.; Higashinaka, R.; Matsuda, T.D.; Aoki, Y.; Miura, A.; Moriyoshi, C.; et al. Intrinsic Phase Diagram of Superconductivity in the BiCh2-Based System Without In-Plane Disorder. J. Phys. Soc. Jpn. 2017, 86, 074701. [Google Scholar] [CrossRef]
- Mizuguchi, Y. Material Development and Physical Properties of BiS2-Based Layered Compounds. J. Phys. Soc. Jpn. 2019, 88, 041001. [Google Scholar] [CrossRef]
- Jinno, G.; Jha, R.; Yamada, A.; Higashinaka, R.; Matsuda, T.D.; Aoki, Y.; Nagao, M.; Miura, O.; Mizuguchi, Y. Bulk Superconductivity Induced by In-Plane Chemical Pressure Effect in Eu0.5La0.5FBiS2−xSex. J. Phys. Soc. Jpn. 2016, 85, 124708. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamaki, T.; Matsushita, Y.; Fujioka, M.; Denholme, S.J.; Yamaguchi, T.; Takeya, H.; Takano, Y. Site selectivity on chalcogen atoms in superconducting La(O,F)BiSSe. Appl. Phys. Lett. 2015, 106, 112601. [Google Scholar] [CrossRef]
- Hiroi, T.; Kajitani, J.; Omachi, A.; Miura, O.; Mizuguchi, Y. Evolution of Superconductivity in BiS2-Based Superconductor LaO0.5F0.5Bi(S1−xSex)2. J. Phys. Soc. Jpn. 2015, 84, 024723. [Google Scholar] [CrossRef]
- Tanaka, M.; Nagao, M.; Matsushita, Y.; Fujioka, M.; Denholme, S.J.; Yamaguchi, T.; Takeya, H.; Takano, Y. First single crystal growth and structural analysis of superconducting layered bismuth oxyselenide; La(O,F)BiSe2. J. Solid State Chem. 2014, 219, 168. [Google Scholar] [CrossRef]
- Nagao, M.; Tanaka, M.; Watauchi, S.; Tanaka, I.; Takano, Y. Superconducting Anisotropies of F-Substituted LaOBiSe2 Single Crystals. J. Phys. Soc. Jpn. 2014, 83, 114709. [Google Scholar] [CrossRef]
- Feng, Y.; Ding, H.C.; Du, Y.; Wan, X.; Wang, B.; Savrasov, S.Y.; Duan, C.G. Electron-phonon superconductivity in LaO0.5F0.5BiSe2. J. Appl. Phys. 2014, 115, 233901. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Shi, X.; Peng, Y. First-principles study of the electronic structure and thermoelectric properties of LaOBiCh2 (Ch = S, Se). Mod. Phys. Lett. B 2017, 31, 1750265. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Li, Y.; Zhu, X.; Wen, H.H. Pressure-tuned enhancement of superconductivity and change of ground state properties in LaO0.5F0.5BiSe2 single crystals. Phys. Rev. B 2014, 90, 094507. [Google Scholar] [CrossRef]
- Shao, J.; Liu, Z.; Yao, X.; Zhang, L.; Pi, L.; Tan, S.; Zhang, C.; Zhang, Y. Superconducting properties of BiSe2-based LaO1−xFxBiSe2 single crystals. EPL 2014, 107, 37006. [Google Scholar] [CrossRef][Green Version]
- Fujioka, M.; Tanaka, M.; Denholme, S.J.; Yamaki, T.; Takeya, H.; Yamaguchi, T.; Takano, Y. Pressure-induced phase transition for single-crystalline LaO0.5F0.5BiSe2. EPL 2014, 108, 47007. [Google Scholar] [CrossRef]
- Hoshi, K.; Goto, Y.; Mizuguchi, Y. Selenium isotope effect in the layered bismuth chalcogenide superconductor LaO0.6F0.4Bi(S,Se)2. Phys. Rev. B 2018, 97, 094509. [Google Scholar] [CrossRef]
- Jha, R.; Awana, V.P.S. Anomalous Impact of Hydrostatic Pressure on Superconductivity of Polycrystalline LaO0.5F0.5BiSe2. J. Supercond. Nov. Magn. 2015, 28, 2229–2233. [Google Scholar] [CrossRef][Green Version]
- Nishida, A.; Miura, O.; Lee, C.H.; Mizuguchi, Y. High thermoelectric performance and low thermal conductivity of densified LaOBiSSe. Appl. Phys. Express 2015, 8, 111801. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Hiroi, T.; Miura, O. Superconductivity phase diagram of Se-substituted CeO0.5F0.5Bi(S1-xSex)2. J. Phys. Conf. Ser. 2016, 683, 012001. [Google Scholar] [CrossRef]
- Hoshi, K.; Kimata, M.; Goto, Y.; Matsuda, T.D.; Mizuguchi, Y. Two-Fold-Symmetric Magnetoresistance in Single Crystals of Tetragonal BiCh2-Based Superconductor LaO0.5F0.5BiSSe. J. Phys. Soc. Jpn. 2019, 88, 033704. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Miura, A.; Nishida, A.; Miura, O.; Tadanaga, K.; Kumada, N.; Lee, C.H.; Magome, E.; Moriyoshi, C.; Kuroiwa, Y. Compositional and temperature evolution of crystal structure of new thermoelectric compound LaOBiS2−xSex. J. Appl. Phys. 2016, 119, 155103. [Google Scholar] [CrossRef]
- Lee, C.H.; Nishida, A.; Hasegawa, T.; Nishiate, H.; Kunioka, H.; Ohira-Kawamura, S.; Nakamura, M.; Nakajima, K.; Mizuguchi, Y. Effect of rattling motion without cage structure on lattice thermal conductivity in LaOBiS2−xSex. Appl. Phys. Lett. 2018, 112, 023903. [Google Scholar] [CrossRef]
- Kase, N.; Terui, Y.; Nakano, T.; Takeda, N. Superconducting gap symmetry of the BiS2-based superconductor LaO0.5F0.5BiSSe elucidated through specific heat measurements. Phys. Rev. B 2017, 96, 214506. [Google Scholar] [CrossRef]
- Nagao, M.; Demura, S.; Deguchi, K.; Miura, A.; Watauchi, S.; Takei, T.; Takano, Y.; Kumada, N.; Tanaka, I. Structural Analysis and Superconducting Properties of F-Substituted NdOBiS2 Single Crystals. J. Phys. Soc. Jpn. 2013, 82, 113701. [Google Scholar] [CrossRef]
- Liu, J.; Fang, D.; Wang, Z.; Xing, J.; Du, Z.; Zhu, X.; Yang, H.; Wen, H.H. Giant superconducting fluctuation and anomalous semiconducting normal state in NdO1−xFxBi1−yS2 single crystals. EPL 2014, 106, 67002. [Google Scholar] [CrossRef]
- Nagao, M. Growth and characterization of R(O,F)BiS2 (R = La, Ce, Pr, Nd) superconducting single crystals. Nov. Supercond. Mater. 2015, 1, 64–74. [Google Scholar] [CrossRef]
- Sun, Y.L.; Ablimit, A.; Zhai, H.F.; Bao, J.K.; Tang, Z.T.; Wang, X.B.; Wang, N.L.; Feng, C.M.; Cao, G.H. Design and Synthesis of a New Layered Thermoelectric Material LaPbBiS3O. Inorg. Chem. 2014, 53, 11125–11129. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Hijikata, Y.; Abe, T.; Moriyoshi, C.; Kuroiwa, Y.; Goto, Y.; Miura, A.; Lee, S.; Torii, S.; Kamiyama, T.; et al. Crystal structure, site selectivity, and electronic structure of layered chalcogenide LaOBiPbS3. EPL 2017, 119, 26002. [Google Scholar] [CrossRef][Green Version]
- Hijikata, Y.; Abe, T.; Moriyoshi, C.; Kuroiwa, Y.; Goto, Y.; Miura, A.; Tadanaga, K.; Wang, Y.; Miura, O.; Mizuguchi, Y. Synthesis, Crystal Structure, and Physical Properties of New Layered Oxychalcogenide La2O2Bi3AgS6. J. Phys. Soc. Jpn. 2017, 86, 124802. [Google Scholar] [CrossRef]
- Jha, R.; Goto, Y.; Higashinaka, R.; Matsuda, T.D.; Aoki, Y.; Mizuguchi, Y. Superconductivity in Layered Oxychalcogenide La2O2Bi3AgS6. J. Phys. Soc. Jpn. 2018, 87, 083704. [Google Scholar] [CrossRef]
- Wu, M.K.; Ashburn, J.R.; Torng, C.J.; Hor, P.H.; Meng, R.L.; Gao, L.; Huang, Z.J.; Wang, Y.Q.; Chu, C.W. Superconductivity at 93 K in a new mixed-phase Y-Ba-Cu-O compound system at ambient pressure. Phys. Rev. Lett. 1987, 58, 908. [Google Scholar] [CrossRef] [PubMed]
- Cava, R.J.; Van Dover, R.B.; Batlogg, B.; Rietman, E.A. Bulk superconductivity at 36 K in La1.8Sr0.2CuO4. Phys. Rev. Lett. 1987, 58, 408. [Google Scholar] [CrossRef]
- PELIKÁN, P. The relation between structure and superconductive properties of high-temperature superconductors. Chem. Papers 1990, 44, 721–736. [Google Scholar]
- Hazen, R.M.; Prewitt, C.T.; Angel, R.J.; Ross, N.L.; Finger, L.W.; Hadidiacos, C.G.; Veblen, D.R.; Heaney, P.J.; Hor, P.H.; Meng, R.L.; et al. Superconductivity in the high-Tc Bi-Ca-Sr-Cu-O system: Phase identification. Phys. Rev. Lett. 1988, 60, 1174. [Google Scholar] [CrossRef]
- Maeda, H.; Tanaka, Y.; Fukitoki, M.; Asano, T. A New High-Tc Oxide Superconductor without a Rare Earth Element. Jpn. J. Appl. Phys. 1988, 27, L209. [Google Scholar] [CrossRef]
- Eisaki, H.; Kaneko, N.; Feng, D.L.; Damascelli, A.; Mang, P.K.; Shen, K.M.; Shen, Z.X.; Greven, M. Effect of chemical inhomogeneity in bismuth-based copper oxide superconductors. Phys. Rev. B 2004, 69, 064512. [Google Scholar] [CrossRef]
- Brandt, B.; Ginzburg, N.I. Critical Fields of the Crystalline Modifications Bi II and Bi III. SoV. Phys. JETP 1963, 17, 326. [Google Scholar]
- Stromberg, H.D.; Stephens, D.R. Effects of pressure on the electrical resistance of certain metals. J. Phys. Chem. Solids 1964, 25, 1015. [Google Scholar] [CrossRef]
- Brandt, N.B.; Ginzburg, N.I. Superconductivity at high pressures. Comtemp. Phys. 1969, 10, 355. [Google Scholar] [CrossRef]
- Weitzel, B.; Micklitz, H. Superconductivity in granular systems built from well-defined rhombohedral Bi-clusters: Evidence for Bi-surface superconductivity. Phys. ReV. Lett. 1991, 66, 385. [Google Scholar] [CrossRef]
- Liu, L.Y.; Xing, Y.T.; Merino, I.L.C.; Micklitz, H.; Franceschini, D.F.; Baggio-Saitovitch, E.; Bell, D.C.; Solorzano, I.G. Superconductivity in Bi/Ni bilayer system: Clear role of superconducting phases found at Bi/Ni interface. Phys. Rev. Mater. 2018, 2, 014601. [Google Scholar] [CrossRef]
- Chao, S.P. Superconductivity in a Bi/Ni bilayer. Phys. Rev. B 2019, 99, 064504. [Google Scholar] [CrossRef]
- Tian, M.; Wang, J.; Kumar, N.; Han, T.; Kobayashi, Y.; Liu, Y.; Mallouk, T.E.; Chan, M.H.W. Observation of Superconductivity in Granular Bi Nanowires Fabricated by Electrodeposition. Nano Lett. 2006, 6, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, A.; Vajpayee, A.; Gahtori, B.; Sharma, D.; Ahluwalia, P.K.; Auluck, S.; Awana, V.P.S. Physical property and electronic structure characterization of bulk superconducting Bi3Ni. Supercond. Sci. Technol. 2011, 24, 085002. [Google Scholar] [CrossRef]
- Jha, R.; Avila, M.A.; Ribeiro, R.A. Hydrostatic pressure effect on the superconducting properties of BaBi3 and SrBi3 single crystals. Supercond. Sci. Technol. 2017, 30, 025015. [Google Scholar] [CrossRef]
- Fang, Y.; Yazici, D.; White, B.D.; Maple, M.B. Enhancement of superconductivity in La1−xSmxO0.5F0.5BiS2. Phys. Rev. B 2015, 91, 064510. [Google Scholar] [CrossRef]
- Jeon, I.; Yazici, D.; White, B.D.; Friedman, A.J.; Maple, M.B. Effect of yttrium substitution on the superconducting properties of La1−xYxO0.5F0.5BiS2. Phys. Rev. B 2014, 90, 054510. [Google Scholar] [CrossRef]
- Thakur, G.S.; Selvan, G.K.; Haque, Z.; Gupta, L.C.; Samal, S.L.; Arumugam, S.; Ganguli, A.K. Synthesis and Properties of SmO0.5F0.5BiS2 and Enhancement in Tc in La1–ySmyO0.5F0.5BiS2. Inorg.Chem. 2015, 54, 1076–1081. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Hoshi, K.; Goto, Y.; Miura, A.; Tadanaga, K.; Moriyoshi, C.; Kuroiwa, Y. Evolution of Anisotropic Displacement Parameters and Superconductivity with Chemical Pressure in BiS2-Based REO0.5F0.5BiS2 (RE = La, Ce, Pr, and Nd). J. Phys. Soc. Jpn. 2018, 87, 023704. [Google Scholar] [CrossRef]
- Paris, E.; Mizuguchi, Y.; Hacisalihoglu, M.Y.; Hiroi, T.; Joseph, B.; Aquilanti, G.; Miura, O.; Mizokawa, T.; Saini, N.L. Role of the local structure in superconductivity of LaO0.5F0.5BiS2-x Sex system. J. Phys.-Condes. Matter 2017, 29, 145603. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Goto, Y.; Matsuda, T.D.; Aoki, Y.; Nagao, M.; Tanaka, I.; Mizuguchi, Y. Bulk superconductivity in a four-layer-type Bi-based compound La2O2Bi3Ag0.6Sn0.4S5.7Se0.3. Sci. Rep. 2019, 9, 13346. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Goto, Y.; Higashinaka, R.; Miura, A.; Moriyoshi, C.; Kuroiwa, Y.; Mizuguchi, Y. Improvement of superconducting properties by chemical pressure effect in Eu-doped La2-xEuxO2Bi3Ag0.6Sn0.4S6. arXiv 2019, arXiv:1908.09311. [Google Scholar]
- Kim, G.C.; Cheon, M.; Choi, W.; Ahmad, D.; Kwon, Y.S.; Ko, R.; Kim, Y.C. Superconductivity in Oxychalcogenide LaREO2Bi3Ag0.6Sn0.4S6 (RE = Pr and Nd). J. Supercond. Nov. Magn. 2019, 33, 625. [Google Scholar] [CrossRef]
- Ruan, B.B.; Zhao, K.; Mu, Q.G.; Pan, B.J.; Liu, T.; Yang, H.X.; Li, J.Q.; Chen, G.F.; Ren, Z.A. Superconductivity in Bi3O2S2Cl with Bi–Cl Planar Layers. J. Am. Chem. Soc. 2019, 141, 3404. [Google Scholar] [CrossRef]
- Jha, R.; Goto, Y.; Matsuda, T.D.; Aoki, Y.; Mizuguchi, Y. Superconductivity in Se-doped La2O2Bi2Pb2S6-xSex with a Bi2Pb2Ch4-type thick conducting layer. arXiv 2019, arXiv:1912.11981. [Google Scholar]
- Jha, R.; Goto, Y.; Matsuda, T.D.; Aoki, Y.; Mizuguchi, Y. Effect of Indium doping on the superconductivity of layered oxychalcogenide La2O2Bi3Ag1-xInxS6. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1293, p. 012001. [Google Scholar]
- Jerome, D.; Berthier, C.; Molinie, P.; Rouxel, J. Layer compounds. Charge density waves in transitions metal compounds. Electronic properties of transition metal dichalcogenides: Connection between structural instabilities and superconductivity. J. Physique Colloq. 1976, 37, C4-125–C4-135. [Google Scholar]
- Li, L.; Deng, X.; Wang, Z.; Liu, Y.; Abeykoon, M.; Dooryhee, E.; Tomic, A.; Huang, Y.; Warren, J.B.; Bozin, E.S.; et al. Superconducting order from disorder in 2H-TaSe2−xSx. npj Quantum Mater. 2017, 2, 11. [Google Scholar] [CrossRef]
- Goto, Y.; Sogabe, R.; Mizuguchi, Y. Bulk Superconductivity Induced by Se Substitution in BiCh2-Based Layered Compounds Eu0.5Ce0.5FBiS2−xSex. J. Phys. Soc. Jpn. 2017, 86, 104712. [Google Scholar] [CrossRef]
- Pallecchi, I.; Lamura, G.; Putti, M.; Kajitani, J.; Mizuguchi, Y.; Miura, O.; Demura, S.; Deguchi, K.; Takano, Y. Effect of high-pressure annealing on the normal-state transport of LaO0.5F0.5BiS2. Phys. Rev. B 2014, 89, 214513. [Google Scholar] [CrossRef]
- Nishida, A.; Nishiate, H.; Lee, C.H.; Miura, O.; Mizuguchi, Y. Electronic Origins of Large Thermoelectric Power Factor of LaOBiS2−xSex. J. Phys. Soc. Jpn. 2016, 85, 074702. [Google Scholar] [CrossRef]
- Kurematsu, K.; Ochi, M.; Usui, H.; Kurok, K. First-principles Study of LaOPbBiS3 and Its Analogous Compounds as Thermoelectric Materials. J. Phys. Soc. Jpn. 2020, 89, 024702. [Google Scholar] [CrossRef]
- Morice, C.; Artacho, E.; Dutton, S.E.; Kim, H.J.; Saxena, S.S. Electronic and magnetic properties of superconducting LnO1−xFxBiS2 (Ln = La, Ce, Pr, and Nd) from first principles. J. Phys.-Condes. Matter 2016, 28, 345504. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Perea, J.D.; Castillo, R.; Diosa J., E.; Baca, E. Hybrid Superconducting-Ferromagnetic [Bi2Sr2(Ca,Y)2Cu3O10]0.99(La2/3Ba1/3MnO3)0.01 Composite Thick Films. Materials 2019, 12, 861. [Google Scholar] [CrossRef]
- Rouco, V.; Córdoba, R.; De Teresa, J.M.; Rodríguez, L.A.; Navau, C.; Del-Valle, N.; Via, G.; Sánchez, A.; Monton, C.; Kronast, F.; et al. Competition between Superconductor-Ferromagnetic stray magnetic fields in YBa2Cu3O7−x films pierced with Co nano-rods. Sci. Rep. 2017, 7, 5663. [Google Scholar] [CrossRef]
- Zhang, G.; Samuely, T.; Xu, Z.; Jochum, J.K.; Volodin, A.; Zhou, S.; May, P.W.; Onufriienko, O.; Kacmarcík, J.; Steele, J.A.; et al. Superconducting Ferromagnetic Nanodiamond. ACS Nano 2017, 11, 5358–5366. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-Dimensional Visualization in Powder Diffraction. Solid State Phenom. 2007, 130, 15. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).