Abstract
Collichthys lucidus is an important small-scale economic fish species in the Yangtze River Estuary. To improve the accuracy of acoustic stock assessments for C. lucidus, it is necessary to accurately measure its target strength (TS). This study obtained precise morphological parameters of C. lucidus through X-ray scanning and established a Kirchhoff ray mode (KRM) model to simulate the changes in TS of the fish body and swimbladder at different acoustic frequencies and pitch angles. At the same time, the TS was measured using the tethered method to analyze and compare the broadband scattering characteristics obtained from both methods. An empirical formula of C. lucidus relating TS to body length at two conventional frequencies was established using the least squares method. The results show that the C. lucidus TS changes, with body length ranging from 10.91 to 16.61 cm, are significantly influenced by the pitch angle at 70 kHz and 200 kHz frequencies, and the fluctuation of TS for both the fish body and swimbladder increases with the rise in frequency. The broadband TS values estimated by the KRM model and measured by the tethered method fluctuate within in the ranges from −45 dB to −55 dB and −40 dB to −55 dB, respectively. The TS of C. lucidus tends to increase with the increase in swimbladder length. When the probability density function of the pitch angle is N(−5°, 15°), the measured by the KRM and the tethered method at 70 kHz are −71.94 dB and −69.21 dB, respectively, while at 200 kHz they are −72.58 dB and −70.55 dB. This study provides a scientific basis for future acoustic target discrimination and stock assessment of C. lucidus in the Yangtze River Estuary.
Key Contribution:
This study marks the inaugural exploration of the broadband acoustic signature of Collichthys lucidus, a keystone species in the Yangtze River Estuary’s ecosystem, by fusing X-ray morphometry-informed acoustic modeling with ex situ experimental data. This pioneering work established an empirical formula that correlates target strength with body length of C. lucidus, contributing significantly to the understanding and conservation of this ecologically and economically vital species in one of China’s most important aquatic habitats.
1. Introduction
The Yangtze River Estuary, the largest estuary in the western Pacific Ocean, is located at the confluence of saline water from the East China Sea and freshwater from the Yangtze River, featuring a unique ecosystem and geographical characteristics [1]. The estuary is rich in nutrients and abundant in prey organisms, offering significant biodiversity and potential for fishery production [2]. It serves as a breeding, foraging, and fattening habitat for various marine, freshwater, estuarine, and migratory fish species and biological communities [3,4]. In recent years, pressures from prolonged overfishing and water pollution have gradually degraded the aquatic habitats in the Yangtze River Estuary, leading to a rapid decline in numerous fish stocks and bringing some unique and rare aquatic species to the brink of extinction [5]. To mitigate these issues, a ten-year fishing ban was implemented across the entire Yangtze River Basin in 2020, aimed at restoring the aquatic ecological environment and protecting biodiversity. This measure also supports the sustainable economic development of the region [6].
Collichthys lucidus, belonging to the order Perciformes and family Sciaenidae, is a warm-temperate demersal fish species. It is characterized by rapid growth, wide distribution, and high yield, mainly found in the waters of the East China Sea and the Yellow Sea. C. lucidus is an important small-scale economic fish species in the Yangtze River Estuary [7]. In the context of a ten-year fishing ban, long-term monitoring of its resource distribution and understanding its current status and dynamic changes during the ban in the Yangtze River Estuary are crucial. Current research on C. lucidus mostly focuses on resource distribution [8], biological characteristics [9], growth and development [10], and genetic traits [11], with fewer studies on stock assessment, which mainly relies on traditional trawling methods [12]. Considering the impact of the fishing ban, traditional net surveys are restricted because they may cause some damage to the fish resources and environment in the area. Therefore, more suitable methods are needed to conduct resource surveys.
Fishery acoustic assessment is an important method for surveying fishery resources, known for its efficiency, high resolution, wide coverage, and non-destructive nature to the environment [13]. Among its key parameters, target strength (TS) is crucial for acoustic stock assessment, as it reflects the individual’s ability to reflect incident sound waves and directly affects the accuracy of acoustic stock assessment [14]. The determination and estimation of fish TS can be divided into in situ methods, ex situ methods, and modeling approaches [15]. Ex situ methods include the tethered method and the cage method, while modeling methods comprise the Kirchhoff ray mode (KRM) model [16], prolate spheroidal model [17], the distorted wave Born approximation [18], and others. Unlike in situ methods that require the target fish to be discretely distributed, ex situ methods allow for free measurement of any fish species in the laboratory [19]. The tethered method, in particular, allows for better control of target posture and is suitable for studying the relationship between TS and pitch angle, body length, body weight, and sound frequency. KRM can simulate the process of sound waves entering the fish body and swimbladder [20], providing a reasonable balance between model complexity and accuracy of results [21], making it widely used.
Under the framework of major conservation efforts in the Yangtze River, acoustic surveys are increasingly valued in the fisheries resource investigations of the Yangtze River Basin. However, there is currently a lack of research on the TS of fish species in the Yangtze River Basin. TS is influenced by both physical and biological factors, making it challenging to accurately measure TS values under every variation [22]. Combining the tethered method with the KRM can relatively accurately determine the TS of target fish under various conditions. To enhance the accuracy of acoustic fishery methods in assessing C. lucidus resources, this study obtained the precise morphological parameters of C. lucidus from the Yangtze River Estuary using X-ray scanning and estimated its TS and broadband scattering characteristics through experiments using the KRM and the tethered method. This study therefore aims to aid the acoustic resource survey of C. lucidus in the Yangtze River Estuary, provide a scientific basis for long-term monitoring of fish resources in the estuary, and promote the sustainable development, utilization, and management of economic fish species in these waters.
2. Materials and Methods
2.1. Sample Collection
The C. lucidus samples used in this study were collected from bottom trawl surveys conducted in November 2022 at the Yangtze River Estuary and its adjacent waters. With 14 sampling stations set up, the survey area extends from 121°12.54′ to 122°11′ E and from 31°18.09′ to 31°44.17′ N. The survey vessel was a single-boat bottom trawler with a net mouth measuring 6 m long and 2 m high, a net rope length of 6 m, and a cod-end mesh size of 20 mm. The trawl depth was about 10 m. All C. lucidus samples collected by trawling were immediately preserved in seawater and then frozen at low temperatures to ensure maximum freshness and the integrity of the swimbladder.
2.2. Measurement of Biological Parameters
The obtained samples were thawed in the laboratory and X-ray images were taken. Before imaging, the equipment was calibrated, preheated, and adjusted according to the guidelines in the operation manual. During the imaging process, the fish were placed horizontally to obtain lateral images; then, supported by foam boards, the fish were positioned vertically to capture dorsal images. Based on the integrity of the swimbladders in the X-ray images, this study selected 10 samples for biological measurements and TS measurements, and they were sequentially numbered for recording. The morphological parameters of each C. lucidus body and swimbladder were measured using Photoshop 2024 software, including body length (BL), body width (BW), body height (BH), swimbladder length (SL), swimbladder width (SW), swimbladder height (SH), and the angle between body and swimbladder (Figure 1). According to the requirements of the KRM, the fish were approximated into equidistant segments, and the position coordinates of each segment were measured.
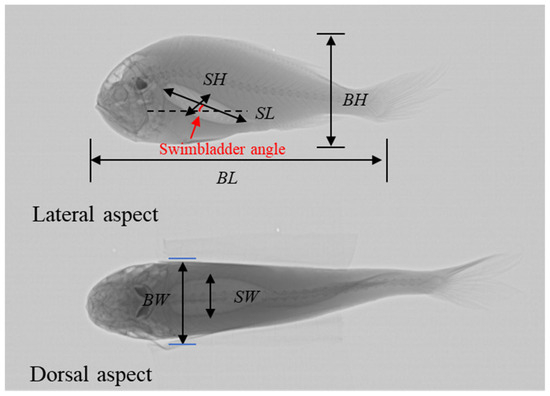
Figure 1.
Dorsal and lateral X-ray images of C. lucidus, along with morphological parameters. The brighter ellipsoid part within the fish body is the swimbladder.
2.3. Construction of the Tethered Experiment System
The tethered experiment was conducted in an anechoic pool, with dimensions of 15 m × 7 m × 6 m (Figure 2). The broadband split-beam Simrad EK80 scientific echosounder was used. Transducers at frequencies of 70 kHz and 200 kHz were used for measurements with their basic settings and parameters, as shown in Table 1. Before the experiment began, the two transducers were calibrated using the standard sphere method [23].
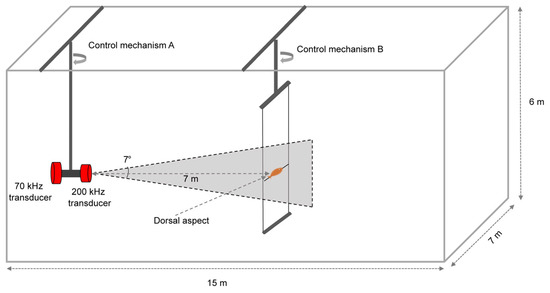
Figure 2.
Schematic diagram of TS measurement using the tethered method for C. lucidus.

Table 1.
Basic settings and main parameters of the Simrad EK80 Scientific echosounder.
All signal transmission pulses were set to a length of 1.024 ms, utilizing the FAST ramp mode, which applies minimal tapering to the transmitted signals. Due to anomalously high values at the frequency band edges of the 200 kHz transducer, only the frequency ranges of 45~90 kHz and 169~250 kHz were selected for subsequent analysis [24]. In the anechoic pool, one transducer was mounted on control mechanism A and positioned at a depth of 2 m. The selected samples of C. lucidus were individually fixed at the same depth on control mechanism B. The horizontal distance between the samples and the transducer was maintained at 7 m, with the fish’s posture kept horizontal and capable of rotation along the horizontal axis. The samples were suspended below a metal rod using fishing lines, with weights hung underneath to prevent floating. The fish were centered on the acoustic axis and oriented horizontally with their backs facing the transducer. TS measurements were conducted by rotating the fish bodies in 5° increments clockwise.
2.4. KRM Model
The KRM approximates the fusiform body and swimbladder of a fish as a series of continuous cylinders with varying radii along the head–tail direction. The model accounts for changes in acoustic impedance of the external medium, fish body, and swimbladder (related to the ratios of sound speed and density) as well as the distances between different media. Based on these parameters, it calculates the reflection and transmission coefficients for each cylindrical element, then computes the scattering strength of each element in both the fish body and swimbladder and performs coherent superposition to obtain the scattering strengths of the body and swimbladder separately. The scattering strengths of the fish body and swimbladder are coherently superimposed to yield the backscatter cross-section of an individual fish, which is then converted into target strength. The environmental and acoustic parameters used for KRM modeling in this study are the typical parameters (Table 2) [16]. The model computations are performed in MATLAB R2021b.

Table 2.
Acoustic parameters used to calculate the TS of C. lucidus using the KRM.
2.5. The TS of C. lucidus
In this study, the pitch angles of C. lucidus were selected within the range of −50° to 50°, reflecting the normal swimming activities of the fish (when the incident sound wave is perpendicular to the fish body, the angle is 0°; the angle is positive when the fish head faces the transducer and negative otherwise) [25]. The probability density function for the distribution of pitch angles was chosen to be the normal distribution N(−5°, 15°), which is commonly used in TS studies [20].
2.6. Data Analysis
The acoustic data collected from the tethered experiment were analyzed using the Echoview 13.0 post-processing software. Based on the distance between the fish target signals and the transducer, the single target detection method was used to extract the TS values of individual fish at different angles. During the detection process, the TS threshold was set to −80 dB. The upper and lower distance boundaries for analyzing the individual TS were defined based on the distance between the fish sample and the transducer and the trend in the echogram. The determined individual TS echograms were selected and defined as analyzable area data for analysis. The frequency distribution of TS values from multiple measurements was extracted, and the average TS value was calculated using the following formula:
where represents the average TS measured, P represents the frequency probability, and is the frequency of the distribution.
The least squares method was used to fit the relationship between the TS of C. lucidus and body length at 70 kHz and 200 kHz for the two methods, establishing empirical formulas for TS–body length at different frequencies:
where represents the body length of the fish. represents the slope, and represents the intercept. The formula is then standardized as follows:
where represents the intercept after standardization.
3. Results
3.1. Morphological Measurements of C. lucidus
This study measured the morphological parameters of 10 C. lucidus. Details such as body length, swimbladder length, and body weight are shown in Table 3. The range of body lengths was from 10.91 to 16.61 cm, with an average body length of 13.49 cm. The swimbladder length ranged from 2.30 to 8.76 cm, with an average of 4.18 cm. The body weight ranged from 19.01 to 57.75 g, with an average weight of 38.88 g.

Table 3.
Basic biological information of C. lucidus samples.
3.2. TS of C. lucidus at Different Pitch Angles
The variations in the TS of the body, swimbladder, and overall fish of C. lucidus with pitch angle are shown in Figure 3. The TS changes with pitch angle vary among individuals of different body lengths, but the overall trend is consistent. For example, for a sample with a body length of 16.61 cm, the maximum TS values estimated by KRM at 70 kHz and 200 kHz occurred around −20°. Moreover, the overall TS trend of C. lucidus aligns closely with that of the swimbladder TS but differs significantly from the body TS. This indicates that the swimbladder is the main source of sound scattering in C. lucidus, playing a decisive role in its TS. At the same time, the changes in TS of C. lucidus at 70 kHz and 200 kHz showed a multi-peaked distribution and were significantly influenced by posture pitch angle. As the frequency increased, the scattering curves became more fluctuating with more side lobes, indicating that the impact of posture pitch angle on scattering strength was increasingly significant.
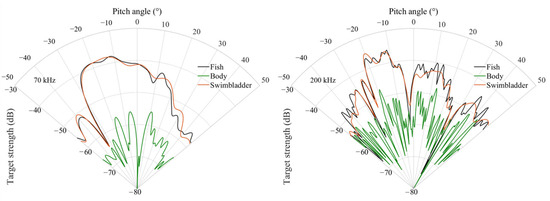
Figure 3.
TS changes with posture pitch angles for C. lucidus No. 1, estimated by KRM at different frequencies.
3.3. Broadband Scattering Characteristics of C. lucidus
Figure 4 shows the broadband scattering characteristics of TS with changes in pitch angle, measured using both the KRM model and the tethered method. Both methods clearly observe the changes in fish TS relative to angle and frequency. When the frequency was below 50 kHz, the TS at different angles was similar due to the influence of side lobes. However, as the sound frequency increased, the directionality of individual TS became more pronounced, and the differences in TS at the same frequency became more evident. In the KRM model, the maximum TS for C. lucidus with a body length of 16.61 cm occurred around −20° at all frequencies. Under the tethered method, there was some variance between the maximum TS angles at high frequencies (169 kHz to 250 kHz) and low frequencies (45 kHz to 90 kHz) compared to the KRM results. At low frequencies, the maximum TS was around −5°, while at high frequencies, it was around −15°.
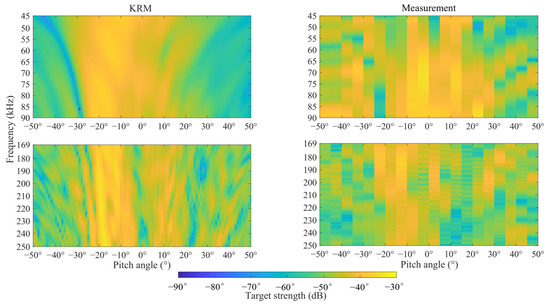
Figure 4.
Broadband scattering characteristics of C. lucidus no. 1 with pitch angle variation, as measured by the KRM model and the tethered method.
Figure 5 shows the broadband TS scattering characteristics of C. lucidus. Figure 5a displays the curves representing the broadband TS estimated for all fish samples by the KRM model, while Figure 5b shows the curves measured directly in the tethered method experiments. The broadband TS estimated by the KRM model fluctuated within a range from −45 dB to −55 dB, and the TS measured by the tethered method fluctuated within a range from −40 dB to −55 dB, showing a difference in the trend of changes between the measured values and the KRM estimates. The KRM-measured TS of C. lucidus was higher at lower frequencies, showing slight vibrations as the frequency increases, and then tended to stabilize. The TS measured by the tethered method showed an increasing trend at lower frequencies and a decreasing trend at higher frequencies. Regardless of the method used, the overall trend in broadband TS of C. lucidus generally increased with the swimbladder length.
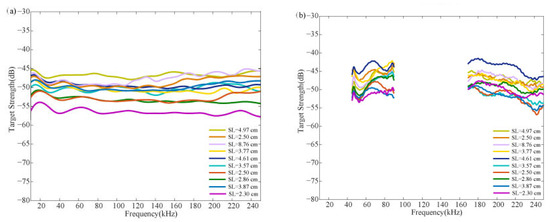
Figure 5.
Broadband scattering spectra of C. lucidus with different swimbladder lengths, as obtained from the (a) KRM model and (b) tethered method measurements.
3.4. TS-BL Empirical Formula
Using the KRM model and tethered method, the average TS of C. lucidus at two different frequencies was calculated for various body lengths under a fish body posture pitch angle density function of N(−5°, 15°) (Table 4). The relationship between average TS and body length was analyzed (Figure 6).

Table 4.
Average target strength results for 10 samples of C. lucidus under two frequencies and methods.
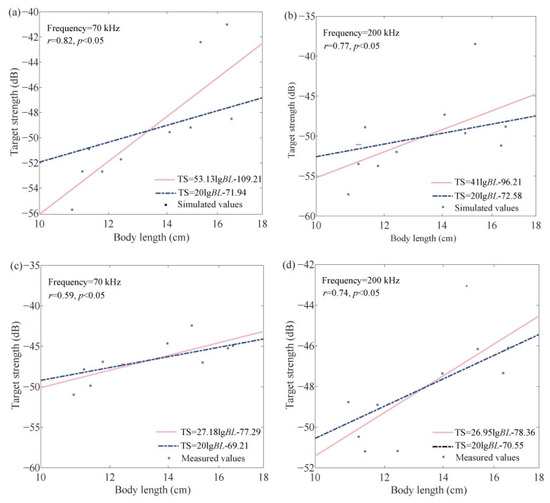
Figure 6.
Relationship between body length and TS of C. lucidus: (a) 70 kHz KRM; (b) 200 kHz KRM; (c) 70 kHz tethered method; (d) 200 kHz tethered method.
The TS-BL formula in Figure 6 are as follows:
70 kHz:
200 kHz
4. Discussion
Accurate measurement of the morphological parameters of the fish body and swimbladder is crucial for simulating fish TS using the KRM model. Therefore, we used an X-ray imaging system to obtain X-ray images of the sample fish, which reliably reflected the size of the swimbladder and its relative position within the body. This is one of the commonly used methods in fish TS-modeling studies [26,27]. Meanwhile, the freshness of the samples can also significantly affect the imaging quality, potentially leading to unclear swimbladder images or unusable data. The samples used in this study were all freshly collected, injected with seawater, and frozen to ensure the stability of the internal structure of the fish. However, swimbladder morphology changes may inevitably occur after freezing. And due to prolonged transportation, maintaining a constant temperature was not possible, which may cause sample degradation. Therefore, we selected only 10 specimens of C. lucidus with clear X-ray images of both the swimbladder and body for the final modeling and tethered experiments.
The research indicates that there is a relationship between the TS of fish and their body pitch angles [28,29]; hence, it is necessary to consider changes in these angles when studying TS. This study used the KRM and the tethered method to analyze the relationship between TS and pitch angles of C. lucidus at 70 kHz and 200 kHz. The results show that at 70 kHz, the TS of the subjects remained relatively stable, with fewer side lobes as the angle changed. At higher frequencies, TS oscillated more significantly with changes in angle, exhibiting more pronounced side lobes. At high frequencies, the TS of C. lucidus was particularly sensitive to changes in pitch angle, with minor variations causing significant increases or decreases in TS. Simultaneously, the maximum TS values at both frequencies corresponded to pitch angles of about −20°. Studies have shown that the swimbladder, as the primary organ for sound scattering, accounts for over 90% of a fish’s scattering capabilities [30]. The pitch angle of the swimbladder for fish no. 1 was 18.4°, which aligns with the angle corresponding to the maximum TS values obtained from the model (Figure 3). According to the broadband scattering spectra of TS variations with pitch angles measured using the tethered method (Figure 4), there is a significant difference in the angles corresponding to the maximum TS at high and low frequencies and also some discrepancies with the KRM results. This is due to the experimental setup where TS measurements were taken at intervals of 5°, and such intervals may skip the actual angle corresponding to the maximum TS, causing the maximum TS to appear at other angles. Consequently, it is necessary to use smaller angle intervals for more precise measurements in future studies.
In recent years, the application of broadband acoustic technology in fishery resource acoustic surveys, especially in species identification and classification, has become a hot topic [31]. Integrating the broadband TS of fish with dB difference and other methods can aid in the acoustic target identification of C. lucidus. Therefore, this study summarizes the broadband TS characteristics of C. lucidus measured using the KRM and tethered method (Figure 5). The KRM results indicate that the TS of C. lucidus is higher at lower frequencies and exhibits slight vibrations as frequency increases, stabilizing eventually. Simultaneously, there is a trend of increasing TS with increasing swimbladder length, which aligns with the broadband scattering spectrum characteristics of the chub mackerel (Scomber japonicus) reported by Tong et al. [32] and conforms to the general characteristics of swimbladdered fish, with TS varying with frequency. However, the results obtained from the tethered method were different, especially at higher frequencies. On one hand, discrepancies between model predictions and actual measurements are inevitable; on the other hand, from the time of X-ray imaging to the start of actual measurements, the fish undergo physiological changes due to being out of water, leading to changes in scattering characteristics. Later, when the fish samples are placed underwater for tethered experiments, the swimbladder volume changes again under pressure, leading to deviations in TS measurements [33]. Future research should strive to minimize the impacts of these factors and could attempt to use adhesive to seal the mouth and anus of the samples for more accurate measurements. In addition, the discrepancy at high frequencies between the two methods is because the directivity of the target strength becomes more and more obvious with the increase of frequency. And the small angle difference brings greater changes in TS. However, the high TS values may not be detected because of the large interval of pitch angle, resulting in the low average TS of single fish. Therefore, the difference between the two methods becomes more pronounced as the frequency increases.
Fish body length is a critical parameter characterizing individual fish size and has a significant correlation with TS. Therefore, this study established empirical relationships for TS-BL of C. lucidus, with body lengths ranging from 10.91 to 16.61 cm at different frequencies, using both the KRM and tethered method. The results show that at 70 kHz, both the KRM and tethered method produced well-fitting TS-BL equations. The values measured by the two methods were −71.94 dB and −69.21 dB, respectively, a difference of 2.73 dB. At higher frequencies, the TS-BL fitting equations from both KRM and tethered methods also fit well, with values measured at −72.58 dB and −70.55 dB, respectively, a difference of 2.03 dB. Since the tethered method requires specialized acoustic equipment and the construction of an experimental platform, it is more costly and time-consuming. In contrast, KRM is flexible, simple, and not easily restricted by external environmental factors [32], and the differences between the two methods are also small. Therefore, in acoustic resource surveys, the TS-BL formulas based on KRM can be used to calculate the TS of C. lucidus and be applied in acoustic stock assessment. Additionally, acoustic surveys are well adapted to the ten-year fishing ban policy in the Yangtze River, aiding in the formulation of scientific and sustainable management strategies to promote the rational use and protection of economically important fish species. Therefore, as TS serves as a crucial parameter for acoustic surveys and resource assessments, future studies on fish TS should be expanded to better meet the needs of acoustic stock assessment in the Yangtze River Estuary.
5. Conclusions
This study enriches the understanding of the acoustic properties of C. lucidus, a key species in the Yangtze River Estuary ecosystem. The TS of C. lucidus shows a multi-peak distribution with changes in pitch angle and is more sensitive at high frequencies. The comparative analysis between the KRM and the tethered method provides insightful observations regarding the broadband scattering characteristics. Both methods demonstrate a general trend of increasing TS with swimbladder length, but discrepancies at higher frequencies highlight the need for further refinement of ex situ measurement techniques. The TS–body length formula established in this study offers a simple and flexible tool for the acoustic survey and identification of C. lucidus and paves the way for more environmentally friendly fishery resource surveys.
Author Contributions
Conceptualization, S.L.; funding acquisition, J.T.; methodology, S.L., J.T., C.Q., Z.Z. and M.X.; project administration, J.T.; software, S.L., M.X., C.Q., Z.Z. and Y.Q.; supervision, J.T.; writing—original draft, S.L.; writing—review and editing, J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Technology Innovation Project of Shanghai Municipal Commission of Agriculture (Shanghai Agricultural Science and Technology Innovation No. 2-1, 2022).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of scientific research office, shanghai ocean university (SHOU-DW-2023-097 and 2022-11-30).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors of this research would like to thank all our colleagues from the Research Laboratory Quantitative Fisheries Stock & Ecosystem Assessment and Management for their work in sample analyses. Special thanks to Fishery Machinery and Instrument Research Institute, Chinese Academy of Fishery Sciences, for providing experimental site support. Thanks to Shanghai Aquatic Wildlife Conservation and Research Center for help. The authors would also like to thank Ruitong Yang of Hokkaido University for help.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, J.; Tian, S.; Gao, C.; Kindong, R.; Zhao, J. Evaluation of sampling designs for different fishery groups in the Yangtze River estuary, China. Reg. Stud. Mar. Sci. 2020, 38, 101373. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, F.; Feng, S.; Dai, F.; Wang, Y. Biological community of fishery resources in the Yangtze River Estuary and adjacent sea areas in the summer of 2015. Mar. Sci. 2017, 39, 490–499. [Google Scholar]
- Wang, R.; Yang, G.; Geng, Z.; Zhao, F.; Feng, X.; Zhang, T. Application of environmental DNA technology in fish diversity analysis in the Yangtze river estuary. Acta Hydrobiol. Sin. 2023, 47, 365–375. [Google Scholar]
- Chen, J.; Wang, X.; Tian, S.; Wu, J.; Dai, L.; Gao, C.; Tong, J.; Zhao, J. Community structure of fishery biology in the Yangtze River estuary and its adjacent waters. Resour. Environ. Yangtze Basin 2021, 30, 122–136. [Google Scholar]
- Ma, J.; Huang, J.; Chen, J.; Li, B.; Zhao, J.; Gao, C.; Wang, X.; Tian, S. Analysis of spatiotemporal fish density distribution and its influential factorsbased on generalized additive model (GAM) in the Yangtze River estuary. J. Fish. China 2020, 44, 936–946. [Google Scholar]
- Zhao, J.; Yang, K.; Ma, J. Optimization of sampling effort for different fishery groups in the Yangtze River Estuary, China. Mar. Coast. Fish. 2022, 14, e10214. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F.; Song, C.; Yang, G.; Hou, J.; Zhuang, P. Diet composition and seasonal variation in feeding habits of Collichthy lucidus in Yangtze Estuary, China. Chin. J. Appl. Ecol. 2016, 27, 291–298. [Google Scholar]
- Xiong, P.; Xu, S.; Chen, Z.; Zhang, S.; Jiang, P.; Fan, J. Spatiotemporal distribution of Collichthy lucidus in the Pearl River Estuary and its relationship with environmental factors. Mar. Sci. 2022, 46, 79–87. [Google Scholar]
- Xiong, P.; Chen, Z.; Hou, G.; Zhang, S.; Qiu, Y.; Fan, J.; Xu, S. Decadal change in biological traits of Collichthys lucidus in Pearl River Estuary. South China Fish. Sci. 2021, 17, 31–38. [Google Scholar]
- Liu, J.; Song, W.; Jiang, K.; Liang, S.; Zhang, F.; Chen, W.; Ma, L. Observation of embryonic development and larval morpholoy of Collichthys lucidus. Mar. Fish. 2018, 40, 691–702. [Google Scholar]
- Zhao, L.; Qu, F.; Song, N.; Han, Z.; Gao, T.; Zhang, Z. Population genomics provides insights into the population structure and temperature-driven adaptation of Collichthys lucidus. BMC Genom. 2021, 22, 729. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhang, K.; Cai, Y.; Xu, Y.; Sun, M.; Xu, S.; Zhu, J.; Chen, Z. Stock assessment of Collichthys lucidus in the Pearl River Estuary in data-limited conditions. Mar. Fish. 2022, 44, 435–445. [Google Scholar]
- Zhu, Z.; Tong, J.; Xue, M.; Sarr, O.; Gao, T. Assessing the influence of abiotic factors on small pelagic fish distribution across diverse water layers in the Northwest Pacific Ocean through acoustic methods. Ecol. Indic. 2024, 158, 111563. [Google Scholar] [CrossRef]
- Wan, S.; Chen, X.; Tong, J. Review on acoustic scattering models and its applications used in fish body target strength and fish species classification. J. Shanghai Ocean Univ. 2023, 32, 171–180. [Google Scholar]
- Henderson, M.J.; Horne, J.K. Comparison of in situ, ex situ, and backscatter model estimates of Pacific hake (Merluccius productus) target strength. Can. J. Fish. Aquat. Sci. 2007, 64, 1781–1794. [Google Scholar] [CrossRef]
- Clay, C.S. Composite ray-mode approximations for backscattered sound from gas-filled cylinders and swimbladders. J. Acoust. Soc. Am. 1992, 92, 2173–2180. [Google Scholar] [CrossRef]
- Madirolas, A.; Membiela, F.A.; Gonzalez, J.D.; Cabreira, A.G.; dell’Erba, M.; Prario, I.S.; Blanc, S. Acoustic target strength (TS) of argentine anchovy (Engraulis anchoita): The nighttime scattering layer. ICES J. Mar. Sci. 2017, 74, 1408–1420. [Google Scholar] [CrossRef]
- Smith, J.N.; Ressler, P.H.; Warren, J.D. A distorted wave Born approximation target strength model for Bering Sea euphausiids. ICES J. Mar. Sci. 2013, 70, 204–214. [Google Scholar] [CrossRef][Green Version]
- Sobradillo, B.; Boyra, G.; Pérez-Arjona, I.; Martinez, U.; Espinosa, V. Ex situ and in situ target strength measurements of European anchovy in the Bay of Biscay. ICES J. Mar. Sci. 2021, 78, 782–796. [Google Scholar] [CrossRef]
- Clay, C.S.; Horne, J.K. Acoustic models of fish: The Atlantic cod (Gadus morhua). J. Acoust. Soc. Am. 1994, 96, 1661–1668. [Google Scholar] [CrossRef]
- Li, C.; Chu, D.; Horne, J.; Li, H. Comparison of coherent to incoherent kirchhoff-ray-mode (KRM) models in predicting backscatter by swim-bladder-bearing fish. J. Mar. Sci. Eng. 2023, 11, 473. [Google Scholar] [CrossRef]
- Yoon, E.; Oh, W.-S.; Lee, H.; Hwang, K.; Kim, D.-N.; Lee, K. Comparison of target strength of Pacific Herring (Clupea pallasii Valenciennes, 1847) from Ex-Situ measurements and a theoretical model. Water 2021, 13, 3009. [Google Scholar] [CrossRef]
- Demer, D.A.; Berger, L.; Bernasconi, M.; Bethke, E.; Boswell, K.; Chu, D.; Domokos, R.; Dunford, A.; Fassler, S.; Gauthier, S. Calibration of Acoustic Instruments, 1st ed.; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2015; pp. 34–39. [Google Scholar]
- Loranger, S.; Jech, M.J.; Lavery, A.C. Broadband acoustic quantification of mixed biological aggregations at the New England shelf break. J. Acoust. Soc. Am. 2022, 152, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.D.; Stanton, T.K.; Wiebe, P.H.; Seim, H.E. Inference of biological and physical parameters in an internal wave using multiple-frequency, acoustic-scattering data. ICES J. Mar. Sci. 2003, 60, 1033–1046. [Google Scholar] [CrossRef]
- Sawada, K.; Ye, Z.; Kieser, R.; McFarlane, G.A.; Miyanohana, Y.; Furusawa, M. Target strength measurements and modeling of walleye pollock and Pacific hake. Fish. Sci. 1999, 65, 193–205. [Google Scholar] [CrossRef][Green Version]
- Yasuma, H.; Sawada, K.; Ohshima, T.; Miyashita, K.; Aoki, I. Target strength of mesopelagic lanternfishes (family Myctophidae) based on swimbladder morphology. ICES J. Mar. Sci. 2003, 60, 584–591. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Liu, J.; Gao, X.; Huang, H.; Wang, F.; Huang, Z. Acoustic target strength of Thornfish (Terapon jarbua) based on the Kirchhoff-Ray Mode model. Electronics 2024, 13, 1279. [Google Scholar] [CrossRef]
- Yan, N.; Mukai, T.; Hasegawa, K.; Yamamoto, J.; Fukuda, Y. Broadband target strength of arabesque greenling, Pacific sand lance, and pointhead flounder. ICES J. Mar. Sci. 2024, 81, 195–203. [Google Scholar] [CrossRef]
- Foote, K.G. Importance of the swimbladder in acoustic scattering by fish: A comparison of gadoid and mackerel target strengths. J. Acoust. Soc. Am. 1980, 67, 2084–2089. [Google Scholar] [CrossRef]
- Kang, M.; Adrianus, A.; Cho, K.-H.; Kim, J.-H.; Son, W.; Yoo, J.; Yang, E.J.; La, H.S. Characterization of pelagic communities in the Pacific sector of the Arctic Ocean using a broadband acoustic system, net samplers, and optical instruments. J. Mar. Syst. 2024, 244, 103976. [Google Scholar] [CrossRef]
- Tong, J.; Xue, M.; Zhu, Z.; Wang, W.; Tian, S. Impacts of morphological characteristics on target strength of chub mackerel (Scomber japonicus) in the Northwest Pacific Ocean. Front. Mar. Sci. 2022, 9, 856483. [Google Scholar] [CrossRef]
- Yang, Y.; Gastauer, S.; Proud, R.; Mangeni-Sande, R.; Everson, I.; Kayanda, R.J.; Brierley, A.S. Modelling and in situ observation of broadband acoustic scattering from the Silver cyprinid (Rastrineobola argentea) in Lake Victoria, East Africa. ICES J. Mar. Sci. 2023, fsad137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).