Abstract
The ubiquitous presence of microplastics (MPs) in the environment is a major concern for the conservation of biodiversity. Numerous studies show the detrimental effects of MPs on marine species, especially because of their small size and their capacity to absorb organic contaminants from their surroundings. The bivalve mollusk Cerastoderma glaucum (Bruguière, 1789), because of its wide geographic distribution and immobile feeding habits, can be used as a sentinel and bioindicator species. By examining the presence, localization, and co-localization of Toll-like receptor 2 (TLR2) and inducible nitric oxide synthetase (iNOS), this study aims to evaluate the response of the internal defense system of C. glaucum to pristine MPs through the employment of confocal microscopy and bioinformatics techniques. The results show haemocytes immunoreactive to the antibodies tested; in particular, a higher number of TLR2-positive haemocytes can be observed in the group exposed to pristine MPs. These findings suggest that haemocytes can play a key biomarker role as sentinels to environmental pollutants. Furthermore, bioinformatics analyses on the antibodies tested confirmed an evolutionary conservation of these molecules. These data highlight the critical role of phagocytosis in identifying ecosystem damage and are helpful in developing biosensors with less negative effects on the environments in which they are applied.
1. Introduction
Anthropogenic activities have a significant impact on the biological conditions and ecosystems of the world, making them one of the most important threats to biodiversity. Changes in habitats to meet societal needs are causing serious effects (including global warming, environmental degradation, biodiversity loss, and mass extinction) extensively documented in various studies [1,2,3]. The warming, acidification, and deoxygenation of the ocean are already having a dramatic impact on its flora and fauna. These changes are resulting in significant shifts in population distribution and the decline of sensitive species [4]. Climate-related changes in the aquatic ecological conditions of species often occur alongside other anthropogenic stressors, such as pollution, eutrophication, or overfishing. The cumulative impact of these multiple anthropogenic stressors can have serious consequences on aquatic organisms [5,6,7,8].
Plastics are a highly versatile and cost-effective group of materials that are composed primarily of polymers. In recent decades, their use grew exponentially due to their strength, flexibility, and light weight. However, the issue of plastic pollution became a serious concern in recent years. It is estimated that the global annual plastic production is 270 million tons, with a corresponding waste generation of approximately 275 million tons [9]. Heavy plastic pollution results in approximately eight million tons of plastic entering the world’s water bodies each year. Unfortunately, this led to an estimated 10,000–100,000 tons of plastic in surface waters and the depths of the seabed. The presence of plastics in the marine environment is a clear indication of recent human impact [10].
Microplastics (MP) are plastic fragments smaller than 5 mm (mm), while nanoplastics (NP) are smaller plastic fragments (≤100 nm) [11]. The issue of MPs is critical for biodiversity conservation because they are one of the most long-lasting and ubiquitous anthropogenic pollutants [12]. These plastics are composed of various polymers, including polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), and polystyrene (PS), which are the most commonly used forms. Scientific research shows a significant increase in the negative impact of MPs on marine animals [13,14,15,16,17]. MPs are a significant environmental concern due to their small size, constituent monomers, and associated additives, readily available to marine animals at all trophic levels, with the potential for uptake by ingestion and transfer within the food web [18,19]. Moreover, it was scientifically proven that MPs can absorb organic pollutants, such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs), from their surrounding environment. These pollutants are potentially toxic, bioaccumulative, and persistent when ingested [20,21]. In recent years, there were numerous documented cases of MP ingestion in marine species, ranging from the smallest planktonic animals to top predators. These species include micro-zooplankton, fish, and sea turtles [22,23]. It is well established that organisms at all levels of the marine food chain ingest MPs. However, it was observed that those at lower trophic levels, such as zooplankton, invertebrates, and echinoderms, are more vulnerable to their harmful effects [24,25].
Cerastoderma glaucum (Bruguière, 1789) is a highly adaptable bivalve mollusk that belongs to the Mediterranean zoogeographic complex and the family Cardiidae. The species is widely distributed in the coastal zone, within 100–300 m from the shore, and can also be found in desalinized water bodies such as estuaries. It is known to settle on sandy and muddy substrates and is remarkably euryhaline and eurybiontic [26]. This species is an ideal candidate as a sentinel and bioindicator species due to its broad spatial distribution and stationary feeding habits on suspended and deposited matter. It was shown to effectively reflect environmental pollution levels and ecosystem impacts [27,28,29]. Biochemical alterations induced by contaminants in this species were limitedly studied in several aquatic systems, including those with higher levels of contamination, using a limited range of biomarkers, including acetylcholinesterase activity, lipid peroxidation, and metallothionein levels [30,31,32]. It is, therefore, necessary to evaluate the behavior of C. glaucum in contaminated environments given its ecological and economic significance and wide spatial distribution [33,34].
The impact of stressors on the internal defense system is a crucial factor in the relationship between environmental stress and the health of aquatic organisms. Alterations in immune performance may underlie emerging diseases, making immunity a point of convergence for many environmental stressors [35,36]. To better understand the relationship between environmental stress and health, it is essential to determine how the immune systems of aquatic organisms respond to stressors.
The innate immune system in invertebrates serves as the only defense mechanism against pathogens. It is composed of physical, chemical, and biological barriers [2]. Although invertebrates lack specific immunity, their defensive responses are highly efficient. These responses include phagocytosis, encapsulation, cytotoxicity, and synthesis and release of microbicidal agents by specialized cells such as haemocytes, coelomocytes, and amoebocytes [37,38,39]. While many studies investigated the toxicity of MPs, less attention was given to their impact on immune cells. Several authors commented on the lack of immunological knowledge regarding MP exposure [40,41,42,43], indicating a need for further investigation. Defense cells play a crucial role in eliminating pathogens and other external bodies, such as MPs, from marine animals [44]. Hence, the role of these cells can offer valuable insights into the fate of MPs and their potential toxicological effects.
It is well established that defense cells play a crucial role in aquatic animals as biomarkers for environmental monitoring. A growing body of evidence suggests that blood cells, in both invertebrates and vertebrates, can provide valuable insights into the effects of environmental stimuli [17,45,46,47,48,49]. However, there is still a paucity of data regarding the defense responses of certain metazoans, such as C. glaucum, to specific contaminants.
This study aims to provide for the first time an assessment of the response of the internal defense system of C. glaucum to pristine microplastics and evaluate the biomarker role of haemocytes as sentinels of harmful environmental alterations. Considering the previous reports, we hypothesize that the biological response of the haemocytes of C. glaucum specimens exposed to pristine MPs will be stronger than the control group, highlighting the role of these defense cells as environmental biomarkers.
Assessing the ecotoxicological risk of a substance and its impact on organisms is crucial in preventing the loss of target populations, estimating the potential impact of pollutants on the environment, and taking necessary measures to mitigate any negative effects. The population growth ratio is a widely recognized parameter for explaining the health status of the organism and serves as a biomarker for assessing the ecological risk of pollutants.
2. Materials and Methods
2.1. Contaminants
The MPs used in this study were 10 µm of Polybead® Blue Dyed Microspheres (Polystyrene microparticles #18138-2) purchased from Polysciences in a 2 mL package in a 2.5% aqueous suspension at 4.55 × 107 particles/mL.
2.2. Selection of Concentrations
According to the previous environmental studies [50,51,52] that reported microplastic concentrations for surface coastal waters, the concentration of 102 MPs/mL was selected.
2.3. Animal Preparation
In the present study, C. glaucum was used as a model organism. Clams were purchased from commercial vendors of Ganzirri Lake (Messina, Italy). A total of 100 clams (mean length = 6.51 ± 0.18 cm) were housed in a 40 L tank in the laboratory for acclimation for 10 days before starting the experiments. The water was not filtered before entering the cores, thus containing natural concentrations of phytoplankton and other organic suspensions. A subsample of the water was taken, filtered over 20 μm mesh, and analyzed to assess the potential contamination with microplastics from the environment. The same procedure was applied to the sediment of the microcosm. The overlying water in each tank (15 cm deep) was aerated by a pump (OxyMax 400, Oase, Germany).
2.4. Experimental Design
A total of 50 clams were exposed to pristine MPs for 7 days. The experiment was repeated for two times (25 clams per experiment). Both during the acclimation period and the exposure of bivalves to MPs the overlying water was constantly aerated, the temperature inside the tank was maintained around 15 °C, and each microcosm was controlled with a multiparameter sensor HI98194 (HANNA instrument, Villafranca Padovana (PD), Italy). The oxygen sensor was thoroughly rinsed with seawater after each measurement to avoid accidental removal of MPs from the tank. The fish food pellet Tetra Pleco Multi Wafers (Tetra, UK) was used to feed bivalves, ground to fine powder, and dissolved in water. About 0.1 g of powdered food was added per tank every two days. Approximately half of the overlying water amount was changed each week. The water was filtered through a 5 μm filter mesh to remove any possibility of microspheres being taken away from the microcosms. Any material that was retained on the filter was cleaned and then reintroduced back to the tank. Survival rate and water parameters were monitored daily. All dead bivalves found on the sediment surface were immediately removed from the tank and frozen in −80 °C.
2.5. Tissue Preparation
For 12–18 h, samples were fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4), dehydrated in graded ethanol, rinsed in xylene, and embedded in Paraplast® (McCormick Scientific LLC, St. Louis, MO, USA). Lastly, using a rotary microtome (LEICA 2065 Supercut), serial sections (3–5 µm thick) were obtained.
2.6. Histological Analysis
Serial slices, after being deparaffinized, were stained for light microscopic examination using the Alcian Blue pH 2.5-PAS (04-163802 Bio-Optica Milano S.p.A., Milan, Italy) and Masson Trichrome (04-010802 Bio-Optica Milano S.p.A., Milan, Italy) techniques.
2.7. Immunofluorescence
Following the deparaffinization and rehydration steps, slices were treated with a 2.5% bovine serum albumin (BSA) solution. Sections were then incubated in a humid chamber at 4 °C overnight with primary antibodies against TLR-2 and iNOS. Sections were then treated with Alexa Fluor 594 donkey anti-rabbit IgG TRITC conjugated and Alexa Fluor 488 donkey anti-mouse IgG FITC conjugated secondary antisera (Molecular Probes, Invitrogen, Eugene, OR, USA, 1:300) [53]. The slices were mounted using Vectashield (Vector Labs, Burlingame, CA, USA) to avoid photobleaching. As a negative control, the experiments were conducted without the primary antibodies. Rat skin tissues were used as a positive control to guarantee that the primary antibodies were immunopositive. Table 1 reports the data of antibodies used in this study.

Table 1.
Antibodies data.
2.8. Confocal Microscopy
Sections were examined and photographs were acquired using a Zeiss LSM DUO confocal laser scanning microscope equipped with a META module (Carl Zeiss MicroImaging GmbH, Jena, Germany). Two helium-neon (543 nm) and two argon (458 nm) lasers, included in this microscope, were used, with a scanning speed of 1 min and 2 s and up to eight averages, to obtain optical slices of fluorescence samples. The images were improved using Zen 2011 (LSM 700 Zeiss software, Oberkochen, Germany). Digital photo cropping was conducted in Adobe Photoshop CC (Adobe Systems, San Jose, CA, USA) to build the figure montage. The intensity curves are presented in graphs alongside the scanned images.
2.9. In Silico Prediction of TLR2 and iNOS Orthologs in the Genome of the Close Relative Cerastoderma edule (Linnaeus, 1758)
Due to the lack of available genomic data relative to the C. glaucum, we decided to look for the presence of the TLR2 and iNOS gene orthologs in Cerastoderma edule (Linnaeus, 1758), the taxonomically closest species with an available genome in GenBank (Accession: GCA_947846245.1). To achieve this, we first generated an ab initio annotation by running the BRAKER2 v2.1.6 pipeline with default parameters, providing as input the C. edule genome [54,55,56,57,58,59,60]. Secondly, the resulting annotation was used to extract the predicted proteins through the AGAT toolkit v0.8.0, which were queried along with the reference TLR2 and iNOS proteins (respectively, from Oryctolagus cuniculus and Mus musculus, accession: XP_051675243.1, NP_035057.1) to the Orthofinder v2.5.4 software, to identify the C. edule protein orthologs [61].
Finally, we performed a functional annotation of the protein domains present in both the reference and the newly found C. edule orthologs making use of the InterProScan v98.0 webserver [62].
2.10. Statistical Analysis
To gather data for the quantitative analysis, ten sections and twenty fields per specimen were examined (4 specimens came from the control group and 4 specimens were exposed to pristine MPs). To assess the positivity and number of the cells, ImageJ 1.53e was used. After the captured image was converted to 8-bit, the background was removed cells and were identified using a “Threshold” filter and a mask. The cells were then counted using the “Analyze Particles” plug-in. SigmaPlot version 14.0 (Systat Software, San Jose, CA, USA) was used to obtain the means and standard deviation of haemocytes positive for TLR2 and iNOS. Student’s t-test were used to evaluate the normally distributed data, assessed using the Shapiro–Wilk normality test. The mean values and standard deviations (SD) of haemocytes detected are presented as follows: ** p ≤ 0.01, * p ≤ 0.05.
3. Results
3.1. Mortality
During the entire exposure period, no statistically significant mortality was noticed in the group exposed to pristine MPs compared with the control group. The survival rate is described in Figure 1.
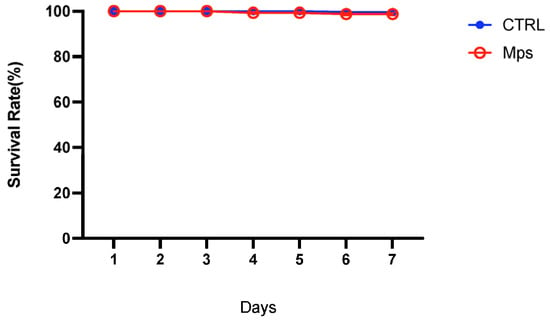
Figure 1.
The survival rate of C. glaucum during seven days of exposure to pristine MPs compared to the control group (CTRL).
3.2. Histology and Immunofluorescence
All clams share a distinctive feature: the two V-shaped demi-branches that form their gills. Each demi-branch is composed of an ascending and a descending lamella that border a water channel and are joined by inter-lamellar connections. The lamellae, which are constituted of filaments, are sequentially folded structures that form each lamella. Connective tissue connects each gill filament, which is composed of a layer of epithelial cells. The filaments display gill-ciliated cells of the frontal, lateral, laterofrontal, and paralaterofrontal types. The hemolymphatic sinuses are defined by connective tissue located at the base of the branchial filaments. Depressions are formed in the descending and ascending folds of the demi-branch to aid in the food channeling process. Near the tip of the filaments, low frontal columnar ciliated cells are visible. These cells are followed bilaterally and sequentially by anterior, laterofrontal, and paralaterofrontal ciliated cells. Additionally, there are mucous cells scattered among the ciliated cell clusters. The gills of control clams exhibit normal tissue organization with well-organized lamellae and no changes in epithelia. The gills of MP-exposed specimens show mild and insignificant alteration of the epithelium and occasionally minimal shedding of cells (Figure 2).
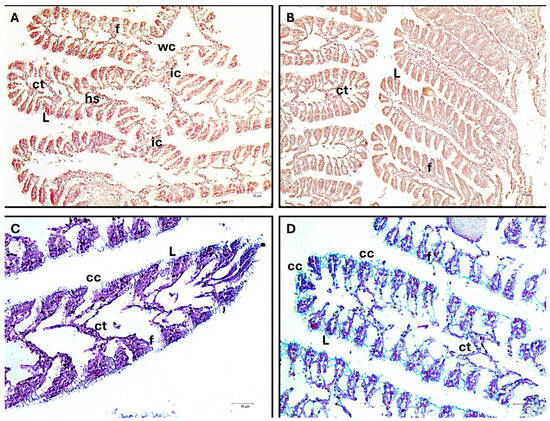
Figure 2.
Cross section of C. glaucum gills. (A,B) Masson trichrome, 10× (C,D) AB/PAS, 20×. Gills consist of two V-shaped demi-branches. An ascending and a descending lamella (L) that border a water channel (WC) and are connected by inter-lamellar connections (ic) make up each demi-branch. Each lamella is made up of successively folded structures called filaments (f). Every gill filament is connected by connective tissue (ct). In the filaments ciliated cells (cc) are present on the frontal, lateral, laterofrontal, and paralaterofrontal sides. The connective tissue at the base of the branchial filaments defines the hemolymphatic sinuses (hs). No significant histological changes are evident between the control group and the exposed group.
Confocal microscopy analysis revealed increased haemocytic infiltrates in the gills of the exposed group. The haemocytes were highlighted through immunohistochemistry using polyclonal (TLR2) and monoclonal (iNOS) antibodies. It is noteworthy that only a few cells exhibited colocalization for the tested antibodies, suggesting diversification of hemocyte functions. Additionally, there was a significant increase in TLR2+ haemocytes compared to both control and iNOS+ haemocytes (Figure 3 and Figure 4).
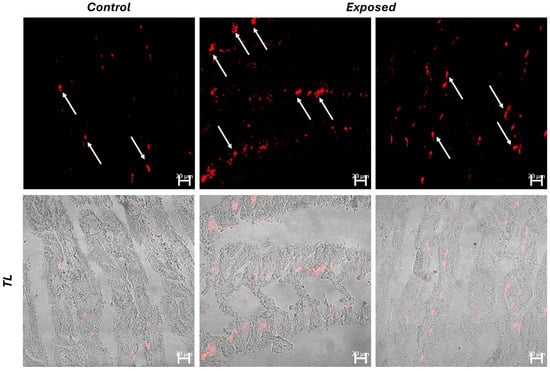
Figure 3.
Cross section of C. glaucum gills. An increase in haemocytes (arrow) immunoreactive to TLR2 (red) is evident in the exposed group. TL = Transmitted light.
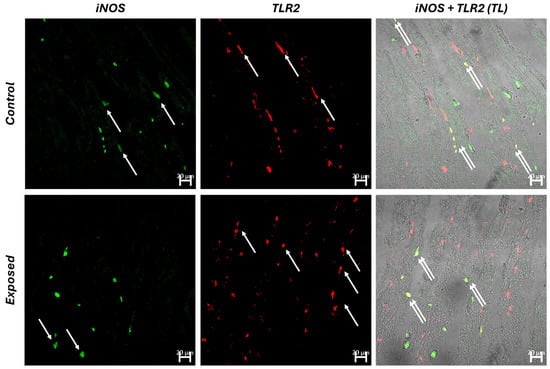
Figure 4.
Cross section of C. glaucum gills. Haemocytes (arrow) immunopositive for iNOS (green) and TLR2 (red) are well evident in both groups. TLR2-reactive haemocytes are increased in the exposed group. Interestingly, only some haemocytes (double arrow) colocalize (yellow) for the antibodies tested, suggesting a different function of the haemocytes.
This finding was confirmed by statistical analysis of the confocal microscopy results (Table 2).

Table 2.
Statistical analysis results (mean values ± standard deviations; N = 8).
3.3. TLR2 and iNOS Orthologs Are Found in the Cerastoderma edule Genome
Our analyses show the presence of a single TLR2 orthologous gene on chromosome 1 and a single iNOS orthologue on chromosome 8 in C. edule, respectively, coding for 903 aa and 1197 aa proteins. In particular, the annotation analysis shows that the TLR2 gene ortholog contains a total of 24 exons and 23 introns, whereas the iNOS gene ortholog is made up of a single coding sequence. The subsequent functional annotation analysis of both reference and newly predicted proteins finds all of the typical domains attributed to their corresponding protein families (Figure 5 and Figure 6).

Figure 5.
Structural and functional protein domains of C. edule TLR2 ortholog and Oryctolagus cuniculus TLR2 protein predicted by InterProScan.
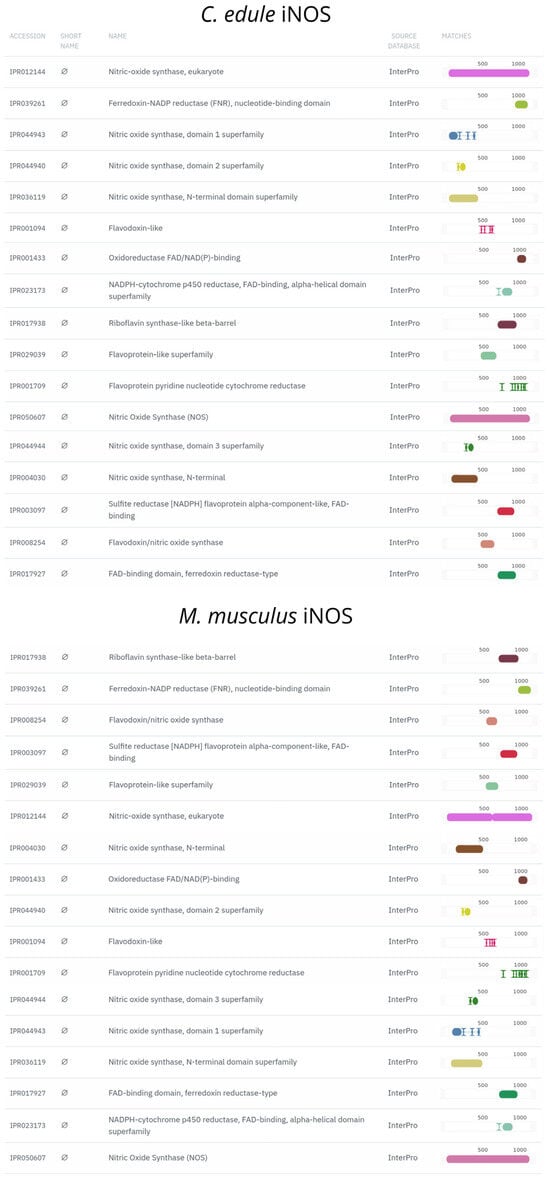
Figure 6.
Structural and functional protein domains of C. edule iNOS ortholog and Mus musculus iNOS protein predicted by InterProScan.
4. Discussion
This study analyzes the response of the internal defense system in C. glaucum gills, following exposure to pristine MPs. The results demonstrate an increase in the number of TLR2-immunoreactive haemocytes in response to exposure to MPs. This indicates an enhanced phagocytic activity of haemocytes in the gills, which are in close contact with the external environment.
Gills play a crucial role in respiration, gas and nutrient exchange, and the uptake and accumulation of pollutants [63]. Histologically, we observed mild and insignificant alterations in the gill lamellae, which is consistent with previous studies that found no significant organic tissue changes [64,65]. The histological structure of the gills analyzed reflects the normal histology of this organ [66,67,68,69]. Based on these results, the observed alterations are not indicative of significant tissue damage. This finding is supported by a study conducted by De Sales-Ribeiro et al. (2020), which argues that pristine MPs do not cause tissue pathological alterations in Danio rerio [70]. The suitability of C. glaucum as a model for exposure to pollutants was confirmed by several studies, making it an ideal candidate for experimental studies. However, the defense responses of this bivalve in relation to the pollutants tested were not addressed in these studies [71,72,73].
Cuvillier-Hot et al. (2014) demonstrated that coelomocyte cells in the polychaete Hediste diversicolor (O.F. Müller, 1776) respond to infection by releasing proteins and increasing phagocytosis, even in polluted environments over a long-term period [74]. According to Gomiero et al. (2018), cell viability, phagocytosis, acid phosphatase, and phenoloxidase activities are relevant biomarkers for assessing the immunotoxic effects of MPs in H. diversicolor [75]. The study demonstrated that PVC-MPs significantly increased the phagocytosis activity of immune cells. These findings suggest a potential impact of PVC-MPs on the immune system of H. diversicolor. As phagocytosis is commonly used as an indicator of immunocompetence in aquatic species [76], information on the phagocytic activity of coelomocytes could potentially serve as a valuable marker of MP exposure and its impact on the immune system. The research conducted by Marques-Santos et al. (2018) and Bergami et al. (2019) demonstrates that MPs have the potential to negatively impact the defense cells of sea urchin species, specifically Paracentrotus lividus (Lamarck, 1816) and Sterechinus neumayeri (Meissner, 1900) [42,77]. This can result in immunotoxicity, decreased lysosomal membrane stability, and apoptotic-like nuclear alterations in phagocytes. Based on this evidence, MPs pose a significant threat to the health of these sea organisms [41,43,77,78,79]. Therefore, haemocytes of Mytilus galloprovincialis (Lamarck, 1819) are potential biomarkers for hydrocarbon and pesticide pollution. This was observed through a significant increase in these cells when exposed to benzo[a]pyrene and thiacloprid [45].
TLR2 and iNOS were used for reliable labeling of C. glaucum haemocytes in this study. Toll-like receptors (TLRs) play a crucial role in regulating defense responses. TLRs were identified in the earliest multicellular invertebrates, including sponges, cnidarians [80], and oligochaetes [81]. In our previous studies, it the presence of TLR2 was evaluated in several metazoans, including Polititapes aureus (Gmelin, 1791), M. galloprovincialis [45], the ascidian Styela plicata (Lesueur, 1823) [82], Eptatretus cirrhatus (J. R. Forster, 1801) [83], the cartilaginous fish Scyliorhinus canicular (Linnaeus, 1758) [84], and several bony fishes [84,85,86,87]. The innate immune molecules of invertebrates enable them to recognize, bind, and kill pathogens through a rich variety of processes [88]. Additionally, the production of reactive O-(ROS) and N-(NOS) species is a key factor relied upon for oxidative killing. Similar mechanisms were extensively studied in fish. Inducible NO synthase is readily expressed following parasitic infection or environmental stimuli [89]. Furthermore, this molecule modulates innate immunity through the autocrine production of nitric oxide [90] and in the functioning of the invertebrate internal defense system [91]. The study conducted on the mollusk Hyriopsis cumingii (Lea, 1852) confirms that nitric oxide synthetase plays a crucial role in the immune response [92].
This study found a significant increase in the number of haemocytes that were immunoreactive to TLR2 and iNOS in the gills of clams that were exposed to pristine MP, as revealed by confocal microscopy. It is interesting to note that most of the cells did not colocalize for the antibodies tested, especially in the exposed group, partly due to the high increase in immunoreactive haemocytes at TLR2. This diversification of immunoreactive haemocytes may suggest different cellular competence. Based on the receptors on their surface, haemocytes were evaluated for their phagocytic and cytotoxic role in previous studies [38,39,93,94]. The increased number of TLR2-immunopositive haemocytes could indicate increased phagocytic activity of these cells, TLR2 being a crucial receptor involved in cell recognition and modulating phagocytosis [95,96,97]. This finding is consistent with previous studies on other animal species and suggests that phagocytic activity could serve as an excellent biomarker in this clam as well. Additionally, Ikuta et al. (2022) reported that microplastics are indeed phagocytosed at the gill level in Bathymodiolus japonicus (Hashimoto and Okutani, 1994) and Perna viridis (Linnaeus, 1758) [98]. Based on the results, Sun et al. (2020) identified distinct haemocyte populations in shrimp [99]. They classified granular haemocytes, which expressed genes associated with humoral immunity, and hyalinocytes, which expressed genes associated with phagocytosis. Flow cytometry analysis further revealed that hyalinocytes were the primary subpopulation of haemocytes responsible for the ingestion of foreign fluorescent microspheres [99]. De la Ballina et al. (2021) identified genes in Ostrea edulis (Linnaeus, 1758) that transcribe for granulocytes and hyalinocytes, indicating the latter as the haemocytes most involved in cell recognition and phagocytosis [100]. Moreover, our bioinformatics analysis and in silico predictions confirm the presence of TLR2 and iNOS orthologs in the genome of C. edule, suggesting their presence in C. glaucum as well. This corroborates the data obtained by confocal microscopy.
5. Conclusions
In conclusion, this study suggests that C. glaucum clam haemocytes play a sentinel role as biomarkers for monitoring the impact of pristine MPs on marine ecosystems. Furthermore, haemocytes and hemolymph can be easily collected without causing population impacts or invasive actions, making them a practical choice for biomonitoring purposes. Moreover, this research provides valuable insight into the internal defense system of bivalves. It strongly emphasizes the crucial role of phagocytosis in recognizing environmental damage. By expanding our understanding of the marine defense systems of the organisms, we can effectively aid in environmental protection and develop strategies to conserve biodiversity, which is a crucial component of ecosystem homeostasis. These data are useful for creating environmental biosensors based on the natural defense mechanisms of living organisms, using an approach that is less impactful on the environment in which they are applied.
Author Contributions
Conceptualization, A.A. and D.D.P.; formal analysis, A.A. and S.M. (Sebastian Marino); investigation, A.A., D.D.P., S.M. (Sebastian Marino), F.D.G., M.A., S.M. (Silvana Morgante), G.R., L.G., M.K., N.S. and E.R.L.; data curation, A.A., D.D.P., S.M. (Sebastian Marino), F.D.G., M.A., S.M. (Silvana Morgante), G.R., L.G., M.K., N.S. and E.R.L.; writing—original draft preparation, A.A. and D.D.P.; writing—review and editing, A.A., D.D.P., M.K., N.S. and E.R.L.; visualization, A.A.; supervision, N.S. and E.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been partially funded by the European Union (NextGeneration EU) through the MUR-PNRR project SAMOTHRACE (ECS00000022).
Institutional Review Board Statement
Ethical review and approval were waived for this study because the use of bivalves mollusks is not subjected to ethical restrictions.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bertocci, I.; Dell’Anno, A.; Musco, L.; Gambi, C.; Saggiomo, V.; Cannavacciuolo, M.; Lo Martire, M.; Passarelli, A.; Zazo, G.; Danovaro, R. Multiple Human Pressures in Coastal Habitats: Variation of Meiofaunal Assemblages Associated with Sewage Discharge in a Post-Industrial Area. Sci. Total Environ. 2019, 655, 1218–1231. [Google Scholar] [CrossRef]
- Sharifinia, M.; Bahmanbeigloo, Z.A.; Keshavarzifard, M.; Khanjani, M.H.; Lyons, B.P. Microplastic Pollution as a Grand Challenge in Marine Research: A Closer Look at Their Adverse Impacts on the Immune and Reproductive Systems. Ecotoxicol. Environ. Saf. 2020, 204, 111109. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.K.; Holen, E.; Segner, H. Editorial: Immunity and Disease of Aquatic Organisms under the Combined Impact of Anthropogenic Stressors: Mechanisms and Disease Outcomes. Front. Mar. Sci. 2023, 10, 1291639. [Google Scholar] [CrossRef]
- Bijma, J.; Pörtner, H.-O.; Yesson, C.; Rogers, A.D. Climate Change and the Oceans—What Does the Future Hold? Mar. Pollut. Bull. 2013, 74, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Crain, C.M.; Kroeker, K.; Halpern, B.S. Interactive and Cumulative Effects of Multiple Human Stressors in Marine Systems. Ecol. Lett. 2008, 11, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Schmitt-Jansen, M.; Sabater, S. Assessing the Impact of Multiple Stressors on Aquatic Biota: The Receptor’s Side Matters. Environ. Sci. Technol. 2014, 48, 7690–7696. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.E. Marine Parasites and Disease in the Era of Global Climate Change. Annu. Rev. Mar. Sci. 2021, 13, 397–420. [Google Scholar] [CrossRef] [PubMed]
- Hutson, K.S.; Davidson, I.C.; Bennett, J.; Poulin, R.; Cahill, P.L. Assigning Cause for Emerging Diseases of Aquatic Organisms. Trends Microbiol. 2023, 31, 681–691. [Google Scholar] [CrossRef] [PubMed]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an Emerging Threat to Terrestrial Ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Broad, S.; Caine, J.; Clout, M.; Dicks, L.V.; Doran, H.; Entwistle, A.C.; Fleishman, E.; Gibbons, D.W.; Keim, B.; et al. A Horizon Scan of Global Conservation Issues for 2016. Trends Ecol. Evol. 2016, 31, 44–53. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Xiong, F.; Xu, K.; Pu, Y.; Huang, J.; Zhang, J.; Pu, Y.; Sun, R.; Cheng, K. Research Advances of Microplastics and Potential Health Risks of Microplastics on Terrestrial Higher Mammals: A Bibliometric Analysis and Literature Review. Env. Geochem. Health 2023, 45, 2803–2838. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.P.; Cowie, W.J.; Maes, T.; Le Quesne, W.J.F. Marine Plastic Litter in the ROPME Sea Area: Current Knowledge and Recommendations. Ecotoxicol. Environ. Saf. 2020, 187, 109839. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of Microplastic Debris throughout the Marine Ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Lyons, B.P.; Galloway, T.S.; Lewis, C. Role of Marine Snows in Microplastic Fate and Bioavailability. Environ. Sci. Technol. 2018, 52, 7111–7119. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The Occurrence of Microplastic in Specific Organs in Commercially Caught Fishes from Coast and Estuary Area of East China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, C.; Kato, Y.; Ariyoshi, T.; Takasu, M.; Narazaki, T.; Nagasaka, S.; Tatsuta, H.; Kashiwada, S. Comparative Toxicities of Silver Nitrate, Silver Nanocolloids, and Silver Chloro-Complexes to Japanese Medaka Embryos, and Later Effects on Population Growth Rate. Environ. Pollut. 2018, 233, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, C.; Kashiwada, S. Ecological Risks Due to Immunotoxicological Effects on Aquatic Organisms. Int. J. Mol. Sci. 2021, 22, 8305. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Hazarika, R.P.; Kumar, V.; Roy, A.; Pandit, S.; Prasad, R. Microplastics in Marine and Aquatic Habitats: Sources, Impact, and Sustainable Remediation Approaches. Environ. Sustain. 2022, 5, 39–49. [Google Scholar] [CrossRef]
- Fauser, P.; Vorkamp, K.; Strand, J. Residual Additives in Marine Microplastics and Their Risk Assessment—A Critical Review. Mar. Pollut. Bull. 2022, 177, 113467. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in Freshwater Systems: A Review of the Emerging Threats, Identification of Knowledge Gaps and Prioritisation of Research Needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of Marine Mussels Mytilus Spp. to Polystyrene Microplastics: Toxicity and Influence on Fluoranthene Bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.G.; Omeyer, L.C.M.; et al. Microplastic Ingestion Ubiquitous in Marine Turtles. Glob. Change Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Raju, P.; Gunabal, S.; Santhanam, P. The Impact of Microplastics on Marine Copepods. In Basic and Applied Zooplankton Biology; Santhanam, P., Begum, A., Pachiappan, P., Eds.; Springer: Singapore, 2019; pp. 429–442. ISBN 978-981-10-7952-8. [Google Scholar]
- Ivar Do Sul, J.A.; Costa, M.F. The Present and Future of Microplastic Pollution in the Marine Environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in Aquatic Environments: Occurrence, Accumulation, and Biological Effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- Mirzoeva, A.T.; Demchenko, N.A. Morphological Response of Lagoon Cockle Cerastoderma glaucum (Poiret, 1789) to Eutrophication in the Sea of Azov. IOP Conf. Ser. Earth Environ. Sci. 2022, 1049, 012059. [Google Scholar] [CrossRef]
- Velez, C.; Pires, A.; Sampaio, L.; Cardoso, P.; Moreira, A.; Leandro, S.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. The Use of Cerastoderma glaucum as a Sentinel and Bioindicator Species: Take-Home Message. Ecol. Indic. 2016, 62, 228–241. [Google Scholar] [CrossRef]
- Karray, S.; Tastard, E.; Moreau, B.; Delahaut, L.; Geffard, A.; Guillon, E.; Denis, F.; Hamza-Chaffai, A.; Chénais, B.; Marchand, J. Transcriptional Response of Stress-Regulated Genes to Industrial Effluent Exposure in the Cockle Cerastoderma glaucum. Environ. Sci. Pollut. Res. 2015, 22, 17303–17316. [Google Scholar] [CrossRef]
- Machreki-Ajmi, M.; Hamza-Chaffai, A. Assessment of Sediment/Water Contamination by in Vivo Transplantation of the Cockles Cerastoderma glaucum from a Non Contaminated to a Contaminated Area by Cadmium. Ecotoxicology 2008, 17, 802–810. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Albentosa, M.; Campillo, J.A.; Viñas, L.; Fumega, J.; Franco, A.; Besada, V.; González-Quijano, A.; Bellas, J. Influence of Mussel Biological Variability on Pollution Biomarkers. Environ. Res. 2015, 137, 14–31. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Gonzalez, P.; Baudrimont, M.; Nili, H.; De Montaudouin, X. Short-Term Metallothionein Inductions in the Edible Cockle Cerastoderma Edule after Cadmium or Mercury Exposure: Discrepancy between mRNA and Protein Responses. Aquat. Toxicol. 2010, 97, 260–267. [Google Scholar] [CrossRef]
- Hamza-Chaffai, A. Usefulness of Bioindicators and Biomarkers in Pollution Biomonitoring. Int. J. Biotech. Well. Indus. 2014, 3, 19–26. [Google Scholar] [CrossRef]
- Kandeel, K.E.; Mohammed, S.Z.; Mostafa, A.M.; Abd-Alla, M.E. Reproductive Biology of the Cockle Cerastoderma glaucum (Bivalvia: Cardiidae) from Lake Qarun, Egypt. Egypt. J. Aquat. Res. 2013, 39, 249–260. [Google Scholar] [CrossRef]
- WoRMS—World Register of Marine Species—Cerastoderma Glaucum (Bruguière, 1789). Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=138999 (accessed on 15 March 2024).
- Rollins-Smith, L.A. Amphibian Immunity–Stress, Disease, and Climate Change. Dev. Comp. Immunol. 2017, 66, 111–119. [Google Scholar] [CrossRef]
- Palmer, C.V. Immunity and the Coral Crisis. Commun. Biol. 2018, 1, 91. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Balbi, T. Invertebrate Models for Investigating the Impact of Nanomaterials on Innate Immunity: The Example of the Marine Mussel Mytilus Spp. Curr. Bionanotechnol. 2017, 2, 77–83. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Fumia, A.; Albano, M.; Messina, E.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Coelomocytes of the Oligochaeta Earthworm Lumbricus terrestris (Linnaeus, 1758) as Evolutionary Key of Defense: A Morphological Study. Zool. Lett. 2023, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Fumia, A.; Albano, M.; Messina, E.; D’Angelo, R.; Mangano, A.; Miller, A.; Spanò, N.; Savoca, S.; Capillo, G. Investigating the Internal System of Defense of Gastropoda Aplysia depilans (Gmelin, 1791): Focus on Hemocytes. Fish Shellfish Immunol. 2023, 137, 108791. [Google Scholar] [CrossRef]
- Espinosa, C.; Cuesta, A.; Esteban, M.Á. Effects of Dietary Polyvinylchloride Microparticles on General Health, Immune Status and Expression of Several Genes Related to Stress in Gilthead Seabream (Sparus aurata L.). Fish. Shellfish. Immunol. 2017, 68, 251–259. [Google Scholar] [CrossRef]
- Espinosa, C.; García Beltrán, J.M.; Esteban, M.A.; Cuesta, A. In Vitro Effects of Virgin Microplastics on Fish Head-Kidney Leucocyte Activities. Environ. Pollut. 2018, 235, 30–38. [Google Scholar] [CrossRef]
- Marques-Santos, L.F.; Grassi, G.; Bergami, E.; Faleri, C.; Balbi, T.; Salis, A.; Damonte, G.; Canesi, L.; Corsi, I. Cationic Polystyrene Nanoparticle and the Sea Urchin Immune System: Biocorona Formation, Cell Toxicity, and Multixenobiotic Resistance Phenotype. Nanotoxicology 2018, 12, 847–867. [Google Scholar] [CrossRef]
- Tang, J.; Ni, X.; Zhou, Z.; Wang, L.; Lin, S. Acute Microplastic Exposure Raises Stress Response and Suppresses Detoxification and Immune Capacities in the Scleractinian Coral Pocillopora damicornis. Environ. Pollut. 2018, 243, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Di Paola, D.; Fumia, A.; Marino, S.; D’Iglio, C.; Famulari, S.; Albano, M.; Spanò, N.; Lauriano, E.R. Internal Defense System of Mytilus galloprovincialis (Lamarck, 1819): Ecological Role of Hemocytes as Biomarkers for Thiacloprid and Benzo[a]Pyrene Pollution. Toxics 2023, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Cicero, N.; Fumia, A.; Petrarca, C.; Mangifesta, R.; Nava, V.; Lo Cascio, P.; Gangemi, S.; Di Gioacchino, M.; Lauriano, E.R. Histological and Chemical Analysis of Heavy Metals in Kidney and Gills of Boops Boops: Melanomacrophages Centers and Rodlet Cells as Environmental Biomarkers. Toxics 2022, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Zelikoff, J.T.; Raymond, A.; Carlson, E.; Li, Y.; Beaman, J.R.; Anderson, M. Biomarkers of Immunotoxicity in Fish: From the Lab to the Ocean. Toxicol. Lett. 2000, 112–113, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, D.; More-Bayona, J.A.; Wakaruk, J.; Barreda, D.R. Innate Immunity Provides Biomarkers of Health for Teleosts Exposed to Nanoparticles. Front. Immunol. 2019, 9, 3074. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The Role of Biomarkers in the Assessment of Aquatic Ecosystem Health. Int. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Dubaish, F.; Liebezeit, G. Suspended Microplastics and Black Carbon Particles in the Jade System, Southern North Sea. Water Air Soil. Pollut. 2013, 224, 1352. [Google Scholar] [CrossRef]
- Albano, M.; Panarello, G.; Di Paola, D.; Capparucci, F.; Crupi, R.; Gugliandolo, E.; Spanò, N.; Capillo, G.; Savoca, S. The Influence of Polystyrene Microspheres Abundance on Development and Feeding Behavior of Artemia salina (Linnaeus, 1758). Appl. Sci. 2021, 11, 3352. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, V.; Chatterjee, S. Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies. Front. Mar. Sci. 2021, 8, 634934. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Żuwała, K.; Kuciel, M.; Budzik, K.A.; Capillo, G.; Alesci, A.; Pergolizzi, S.; Dugo, G.; Zaccone, G. Confocal Immunohistochemistry of the Dermal Glands and Evolutionary Considerations in the Caecilian, Typhlonectes natans (Amphibia: Gymnophiona). Acta Zool. 2016, 97, 154–164. [Google Scholar] [CrossRef]
- Hoff, K.J.; Lange, S.; Lomsadze, A.; Borodovsky, M.; Stanke, M. BRAKER1: Unsupervised RNA-Seq-Based Genome Annotation with GeneMark-ET and AUGUSTUS. Bioinformatics 2016, 32, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Hoff, K.J.; Lomsadze, A.; Stanke, M.; Borodovsky, M. BRAKER2: Automatic Eukaryotic Genome Annotation with GeneMark-EP+ and AUGUSTUS Supported by a Protein Database. NAR Genom. Bioinform. 2021, 3, lqaa108. [Google Scholar]
- Hoff, K.J.; Lomsadze, A.; Borodovsky, M.; Stanke, M. Whole-Genome Annotation with BRAKER. In Gene Prediction; Kollmar, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1962, pp. 65–95. ISBN 978-1-4939-9172-3. [Google Scholar]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Stanke, M.; Diekhans, M.; Baertsch, R.; Haussler, D. Using Native and Syntenically Mapped cDNA Alignments to Improve de Novo Gene Finding. Bioinformatics 2008, 24, 637–644. [Google Scholar] [CrossRef]
- Stanke, M.; Schöffmann, O.; Morgenstern, B.; Waack, S. Gene Prediction in Eukaryotes with a Generalized Hidden Markov Model That Uses Hints from External Sources. BMC Bioinform. 2006, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Dainat, J.; Hereñú, D.; Murray, K.D.; Davis, E.; Crouch, K.; LucileSol; Agostinho, N.; Pascal-Git; Zollman, Z. Tayyrov NBISweden/AGAT: AGAT-v1.2.0. 2023. Available online: https://zenodo.org/records/8178877 (accessed on 7 March 2024).
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Dobal, V.; Suárez, P.; Ruiz, Y.; García-Martín, O.; San Juan, F. Activity of Antioxidant Enzymes in Mytilus galloprovincialis Exposed to Tar: Integrated Response of Different Organs as Pollution Biomarker in Aquaculture Areas. Aquaculture 2022, 548, 737638. [Google Scholar] [CrossRef]
- Urban-Malinga, B.; Jakubowska, M.; Białowąs, M. Response of Sediment-Dwelling Bivalves to Microplastics and Its Potential Implications for Benthic Processes. Sci. Total Environ. 2021, 769, 144302. [Google Scholar] [CrossRef]
- Sıkdokur, E.; Belivermiş, M.; Sezer, N.; Pekmez, M.; Bulan, Ö.K.; Kılıç, Ö. Effects of Microplastics and Mercury on Manila Clam Ruditapes Philippinarum: Feeding Rate, Immunomodulation, Histopathology and Oxidative Stress. Environ. Pollut. 2020, 262, 114247. [Google Scholar] [CrossRef] [PubMed]
- Added, A.; Khalloufi, N.; Khazri, A.; Harrath, A.H.; Mansour, L.; Nahdi, S.; Boufahja, F.; Aldahmash, W.; Alrefaei, A.F.; Dellali, M. Effects of an Endocrine Disruptor Triclosan on Ruditapes Decussatus: Multimarker and Histological Approaches. Animals 2023, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Smolowitz, R. Mollusca: Bivalvia. In Invertebrate Histology; LaDouceur, E.E.B., Ed.; Wiley: New York, NY, USA, 2021; pp. 163–183. ISBN 978-1-119-50765-9. [Google Scholar]
- Liquin, F.; Hünicken, L.A.; Arrighetti, F.; Davies, D.; Paolucci, E.M.; Sylvester, F. Parasitism and Fitness of Invaders: Oligochaete Chaetogaster Limnaei Produces Gill Damage and Increased Respiration Rates in Freshwater Asian Clams. Hydrobiologia 2021, 848, 2213–2223. [Google Scholar] [CrossRef]
- Jemaà, M.; Morin, N.; Cavelier, P.; Cau, J.; Strub, J.M.; Delsert, C. Adult Somatic Progenitor Cells and Hematopoiesis in Oysters. J. Exp. Biol. 2014, 217, 3067–3077. [Google Scholar] [CrossRef] [PubMed]
- De Sales-Ribeiro, C.; Brito-Casillas, Y.; Fernandez, A.; Caballero, M.J. An End to the Controversy over the Microscopic Detection and Effects of Pristine Microplastics in Fish Organs. Sci. Rep. 2020, 10, 12434. [Google Scholar] [CrossRef] [PubMed]
- Karray, S.; Marchand, J.; Geffard, A.; Rebai, T.; Denis, F.; Chénais, B.; Hamza-Chaffai, A. Metal Contamination and Biomarkers in Cerastoderma glaucum: A Multi-Level Approach. Arch. Environ. Contam. Toxicol. 2023, 84, 484–503. [Google Scholar] [CrossRef] [PubMed]
- Mona, M.H.; El-Khodary, G.M.; Abdel-Halim, K.Y.; Omran, N.E.; Abd El-Aziz, K.K.; El-Saidy, S.A. Histopathological Alterations Induced by Marine Environmental Pollutants on the Bivalve Cerastoderma glaucum (Bruguière 1789) from Temsah Lake, Suez Canal, Egypt. Environ. Sci. Pollut. Res. 2022, 29, 9971–9989. [Google Scholar] [CrossRef] [PubMed]
- Ben Youssef-Dridi, S.; Magalhães, L.; Soares, A.M.V.M.; Pereira, E.; Freitas, R.; Gargouri, L. Trace Elements Assessment in Cerastoderma glaucum from Port Areas in the Tunisian Mediterranean Coast: The Influence of Parasites on Bioaccumulation. Mar. Pollut. Bull. 2024, 198, 115831. [Google Scholar] [CrossRef] [PubMed]
- Virginie Cuvillier-Hot; Céline Boidin-Wichlacz; Aurélie Tasiemski Polychaetes as Annelid Models to Study Ecoimmunology of Marine Organisms. J. Mar. Sci. Technol. 2014, 22, 2. [CrossRef]
- Gomiero, A.; Strafella, P.; Pellini, G.; Salvalaggio, V.; Fabi, G. Comparative Effects of Ingested PVC Micro Particles with and without Adsorbed Benzo (a) Pyrene vs. Spiked Sediments on the Cellular and Sub Cellular Processes of the Benthic Organism Hediste Diversicolor. Front. Mar. Sci. 2018, 5, 99. [Google Scholar] [CrossRef]
- Ellis, R.P.; Parry, H.; Spicer, J.I.; Hutchinson, T.H.; Pipe, R.K.; Widdicombe, S. Immunological Function in Marine Invertebrates: Responses to Environmental Perturbation. Fish. Shellfish. Immunol. 2011, 30, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Bergami, G.; Bertini, F.; Montesi, D. On Approximate Nesting of Multiple Social Network Graphs: A Preliminary Study. In Proceedings of the 23rd International Database Applications & Engineering Symposium—IDEAS’19, Athens, Greece, 10–12 June 2019; ACM Press: Athens, Greece, 2019; pp. 1–5. [Google Scholar]
- Chen, J.-C.; Chen, M.-Y.; Fang, C.; Zheng, R.-H.; Jiang, Y.-L.; Zhang, Y.-S.; Wang, K.-J.; Bailey, C.; Segner, H.; Bo, J. Microplastics Negatively Impact Embryogenesis and Modulate the Immune Response of the Marine Medaka Oryzias melastigma. Mar. Pollut. Bull. 2020, 158, 111349. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Yakovenko, N.; Caley, T.; Guillet, C.; Châtel, A.; Mouneyrac, C. Accumulation and Immunotoxicity of Microplastics in the Estuarine Worm Hediste Diversicolor in Environmentally Relevant Conditions of Exposure. Environ. Sci. Pollut. Res. 2020, 27, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Franzenburg, S.; Fraune, S.; Künzel, S.; Baines, J.F.; Domazet-Lošo, T.; Bosch, T.C.G. MyD88-Deficient Hydra reveal an Ancient Function of TLR Signaling in Sensing Bacterial Colonizers. Proc. Natl. Acad. Sci. USA 2012, 109, 19374–19379. [Google Scholar] [CrossRef] [PubMed]
- Škanta, F.; Roubalová, R.; Dvořák, J.; Procházková, P.; Bilej, M. Molecular Cloning and Expression of TLR in the Eisenia andrei Earthworm. Dev. Comp. Immunol. 2013, 41, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.R.; Aragona, M.; Alesci, A.; Lo Cascio, P.; Pergolizzi, S. Toll-like Receptor 2 and α-Smooth Muscle Actin Expressed in the Tunica of a Urochordate, Styela Plicata. Tissue Cell 2021, 71, 101584. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Savoca, S.; Fumia, A.; Mangano, A.; Albano, M.; Messina, E.; Aragona, M.; Lo Cascio, P.; Capillo, G.; et al. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology 2022, 11, 1366. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Fumia, A.; Messina, E.; Albano, M.; Aragona, M.; Lo Cascio, P.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Confocal Characterization of Intestinal Dendritic Cells from Myxines to Teleosts. Biology 2022, 11, 1045. [Google Scholar] [CrossRef]
- Alesci, A.; Albano, M.; Savoca, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lo Cascio, P.; Hussein, M.M.; Capillo, G.; Pergolizzi, S. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology 2022, 11, 1653. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Alesci, A.; Aragona, M.; Pergolizzi, S.; Miller, A.; Zuwala, K.; Kuciel, M.; Zaccone, G.; Germanà, A.; Guerrera, M.C. Immunohistochemistry of the Gut-Associated Lymphoid Tissue (GALT) in African Bonytongue (Heterotis niloticus, Cuvier 1829). Int. J. Mol. Sci. 2023, 24, 2316. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Fumia, A.; Calabrò, C.; Lo Cascio, P.; Lauriano, E.R. Mast Cells in Goldfish (Carassius auratus) Gut: Immunohistochemical Characterization. Acta Zool. 2023, 104, 366–379. [Google Scholar] [CrossRef]
- Ghosh, J.; Lun, C.M.; Majeske, A.J.; Sacchi, S.; Schrankel, C.S.; Smith, L.C. Invertebrate Immune Diversity. Dev. Comp. Immunol. 2011, 35, 959–974. [Google Scholar] [CrossRef]
- Sigh, J.; Lindenstrom, T.; Buchmann, K. The Parasitic Ciliate Ichthyophthirius multifiliis Induces Expression of Immune Relevant Genes in Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish. Dis. 2004, 27, 409–417. [Google Scholar] [CrossRef]
- Davies, S.-A.; Dow, J.A.T. Modulation of Epithelial Innate Immunity by Autocrine Production of Nitric Oxide. Gen. Comp. Endocrinol. 2009, 162, 113–121. [Google Scholar] [CrossRef]
- Rivero, A. Nitric Oxide: An Antiparasitic Molecule of Invertebrates. Trends Parasitol. 2006, 22, 219–225. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Zhao, X.; Chen, Y.; Liu, R.; Fang, Y.; Zhang, R. Molecular Characterization of the Nitric Oxide Synthase Gene and Its Immunomodulation of Nitric Oxide Production in the Triangle Shell Mussel (Hyriopsis cumingii). Dev. Comp. Immunol. 2021, 122, 104136. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Fumia, A.; Mastrantonio, L.; Marino, S.; Miller, A.; Albano, M. Functional Adaptations of Hemocytes of Aplysia depilans (Gmelin, 1791) and Their Putative Role in Neuronal Regeneration. Fishes 2024, 9, 32. [Google Scholar] [CrossRef]
- Alesci, A.; Albano, M.; Fumia, A.; Messina, E.; Miller, A.; Di Fresco, D.; De Oliveira Fernandes, J.M.; Spanò, N.; Savoca, S.; Capillo, G. Shell Formation in Two Species of Bivalves: The Role of Mantle Cells and Haemocytes. Zool. J. Linn. Soc. 2023, 200, 980–993. [Google Scholar] [CrossRef]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immun. 2012, 3, 79. [Google Scholar] [CrossRef]
- Taban, Q.; Mumtaz, P.T.; Masoodi, K.Z.; Haq, E.; Ahmad, S.M. Scavenger Receptors in Host Defense: From Functional Aspects to Mode of Action. Cell Commun. Signal 2022, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Gantner, B. Integration of Toll-like Receptor and Phagocytic Signaling for Tailored Immunity. Microbes Infect. 2004, 6, 1368–1373. [Google Scholar] [CrossRef]
- Ikuta, T.; Tame, A.; Takahashi, T.; Nomaki, H.; Nakajima, R. Microplastic Particles Are Phagocytosed in Gill Cells of Deep-Sea and Coastal Mussels. Front. Mar. Sci. 2022, 9, 1034950. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Zhang, X.; Xiang, J.; Li, F. Isolation and Transcriptome Analysis of Three Subpopulations of Shrimp Hemocytes Reveals the Underlying Mechanism of Their Immune Functions. Dev. Comp. Immunol. 2020, 108, 103689. [Google Scholar] [CrossRef]
- De La Ballina, N.R.; Villalba, A.; Cao, A. Shotgun Analysis to Identify Differences in Protein Expression between Granulocytes and Hyalinocytes of the European Flat Oyster Ostrea edulis. Fish. Shellfish. Immunol. 2021, 119, 678–691. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).