Abstract
Global marine fish harvests have reached a plateau over the last decade, and efforts to increase aquaculture tend to face limitations in terms of water resources and contamination problems. Of the current fish harvest, at least 50% is discarded as waste. The current situation requires efforts to process, preserve, and utilize the fish capture to minimize waste. Chemical and microbiological contamination limit the utilization of harvested fish. There is a need to improve fish preservation to minimize spoilage and to process them into more appealing products. Instead of resorting to individual food-processing methods, the efficiency of processing could best be increased by a combination of conventional and modern processing methods or by combinations of modern processing methods. Fish waste is a rich source of oils containing essential fatty acids, polypeptides, and amino polysaccharides that could be utilized through the upscaling of current scientifically proven methods to new processing technologies. The separation of collagens, gelatins, bioactive peptides, edible fish oils, and chitosan form the primary stages in the utilization of fish waste. The products need purification to meet food quality and safety standards and to have desirable industrial characteristics. The diversity of information and products generated through new methods require advanced data handling and prediction systems, such as artificial intelligence, to address food safety and to derive the best out of fish processing and utilization.
Keywords:
fish spoilage; safety of food fish; fish processing; waste utilization; novel food additives and pharmaceuticals; artificial intelligence Key Contribution:
This article provides avenues for new ways of thinking to utilize the 50% waste occurring in the fish production–processing chain, namely through appropriate processing and by avoiding food safety hazards at production, harvesting, and handling, with the aim of attaining economic and nutritional benefits.
1. Introduction
Fish are a large group of vertebrates living in marine and freshwater environments. They consist of approximately 34,000 species. Fish and fish products form a valuable component of the human diet as sources of essential amino acids, highly unsaturated fatty acids, vitamin-A-rich oils, and micronutrients [1]. The presence of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) make fatty fish healthy foods, with the capacity to prevent cardiovascular diseases [2]. The above high-value proteins, omega-three fatty acids, and micronutrients make fish a nutritionally rich human food. Fish are harvested as a natural product or as a farmed product from marine and inland aquatic sources.
The total global marine fish capture has remained between 78 to 84 million tons of live weight from 1990 to 2020, with no signs of increased capture, suggesting the need for more efficient utilization of the marine fish harvest at the current level. The total capture of inland fisheries remained at 11–12 million tons between 2010 to 2020, indicating that limitations may arise in the availability of fish in the future [3]. Sustainability issues due to overfishing in natural fisheries are bound to occur unless fish production is increased to expand into more inland waters or the marine fish production is scientifically increased. China and India play a major role in increasing inland fisheries, with some African countries joining in the production. There are efforts to increase fish production through marine farming, using high-yielding and fast-growing species to meet the global demand, and through the genetic engineering of fish muscles [4,5]. The post-harvest loss of fish due to quality and safety problems is around 35%, with a range of 20–75% [6]. Reducing these losses is one approach to ensure that the global food-fish demand can be met. With 89% of the edible-quality food fish consumed by humans in 2020, only 11% is diverted, mainly for feed. Of the total harvest, about 40% is lost as non-edible components [3]. Amidst the efforts to increase the harvest and farming of fish, both marine and inland, there is room for a more effective utilization of fish and fish products through the improved processing, preservation, and conversion of hitherto unused fish components to foods. Effective utilization requires technologies to convert less-attractive fish components into new foods that carry appealing qualities for the consumer. Meeting future global food targets require a reduction in waste and the generation of new processed fish products. Current fish losses and available avenues for a more effective and profitable utilization of fish are shown in Figure 1.
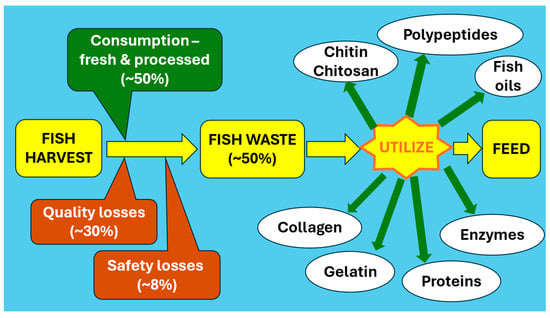
Figure 1.
Current fish losses and avenues for effective and profitable utilization.
In natural and farming systems, fish tend to become exposed to contaminants in the water, impairing product safety. The contaminants may be microorganisms proliferating in the environment, or chemicals entering the waters naturally or due to anthropogenic activities. Foodborne illnesses associated with fish between 2011 to 2018 were reported to be 6–8% against 1.6% for beef and 3.6% for chicken, as reported in 2018 by the Center for Disease Control and Prevention, USA [7]. Environmental stresses and contaminants reduce the sensory qualities of fish muscles, lowering their food value due to lipid oxidation, the breakdown of proteins, and other unexpected biochemical reactions [8]. Improved processing to ensure food-fish safety is continuously being addressed through research. Microbial contaminations increase the perishability of fish, further reducing the food value through biochemical changes in fish muscles, unless these are preserved or processed [7]. In domestic handling, only 50–60% of the fish components, consisting mainly of the edible muscles, are used, and the rest is discarded. The challenge is to preserve the captured fish, thus minimizing spoilage and extending their shelf-life. Fish waste, accounting for up to 50% of the losses, could be converted into novel products through value addition [9]. The processing of fish waste carries a high potential for meeting the increasing food demands and attaining profits in the fish industry. Utilizing the fish waste requires new management strategies that would contribute to effective environment mitigation too. Waste utilization is an important step in sustainable circular economies, a need identified in the current goals of the United Nations’ policies. There has been much research in the recent decades for the better utilization of fish muscles and currently unutilized components in fish by converting them into commercial products carrying sensory attributes preferred by consumers. This review examines the new avenues for making fish safe for human consumption, processing and preservation approaches to retain their quality, and the utilization of fish waste by converting them into rich nutrient sources.
The research publications for this review were selected initially through a Google search of a five-year period and then following relevant references in the publications to seek deep information on the specific areas discussed. The key words used were fish safety, fish processing, products from fish, fish technology, and fish availability. Articles of low depth i.e., with no new knowledge added to the subject, were not included in the review.
2. Safety of Fish and Fishery Products
Fish become unsafe for human consumption due to natural and man-made activities. The causes could mainly be of microbial or chemical origin. The changing microbial population in the environment affects the microbiota of fish prior to harvest. The high vulnerability of fish to microbial activity coupled with the natural degradation of muscles at post-harvest increase the food safety hazards from fish.
2.1. Autolysis of Fish Components
The perishability of fish begins with the natural degradation of their muscles after harvest due to autolysis by endogenous enzymes and the chemical oxidation of lipids [10]. The high-water activity of 0.98–0.99 makes fish muscles vulnerable to microbial growth and promotes the release of harmful microbial metabolites. During autolysis, the odor, flavor, and texture in the fish muscles change due to reactions triggered by endogenous enzymes. The dominance of spoilage bacteria in fish is reported to occur even before the muscles become unacceptable sensorily, with each species showing specific and predominant degradative biochemical reactions [11]. The outcome of autolysis and oxidation in producing unappealing constituents in fish is summarized in Table 1.

Table 1.
Unappealing constituents and properties caused during the autolysis of fish components [10,12,13].
Some of the metabolites produced during fish spoilage serve as substrates for the easy growth of pathogenic microorganisms or toxin producers, rendering food fish unsafe.
2.2. Microbial Food Safety Hazards
Pathogenic microorganisms are present in the marine environment to a lesser extent than in inland reservoirs and waterways due to the saline environment. However, pathogens tend to grow more commonly in coastal waters of the sea that are supported by the nutrients discharged by inland waterways. The common microorganisms associated with the pre-harvest and post-harvest environment of fish and the toxins produced by them, rendering the fish unsafe as a food, are presented in Figure 2.
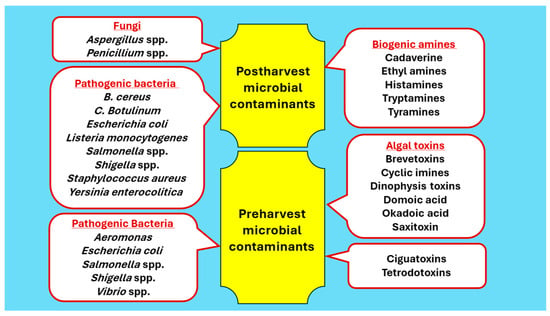
Figure 2.
Pre-harvest and post-harvest microorganisms and toxins render fish unsafe as food.
Symbiosis among the spoilage bacteria in fish appears to enhance spoilage and the spread of pathogenic bacteria. The spoilage bacteria appear to communicate with each other through signaling mechanisms during fish spoilage for mutual benefits [13]. Food safety hazards and quality changes in fish and fish products arising from biotoxins due to algal blooms in the oceans [14,15], diatoms releasing toxins [16], diarrhetic shellfish poisoning and azidinium poisoning [17], paralytic shellfish poisoning [14], ciguatoxin poisoning [18,19,20,21], and clupeotoxin [22] have been reported among the incidences of pre-harvest contamination of fish from different parts of the globe. The FAO [3] has observed the increasing appearance of hazardous biotoxins from tropical regions, predominantly from the upwelling regions of the Indian Ocean. These increased incidence of biotoxins are believed to be associated with increased contamination of coastal areas due to the discharge of nutrient-rich pollutants into the sea. The increase in food safety hazards through fish associated with harmful algal blooms (HAB) is beyond control. Avoiding fishing in the infected marine locations is currently practiced in temperate regions to prevent the exposure of humans to biotoxins. The spreading biotoxin production into new fishing zones is a challenge beyond control, especially considering seasonal changes, global warming, and oceanic currents. The movement of ships and the discharge of ballast water add to the spread of biotoxins [23]. Preventive control could be implemented based on digital satellite imaging of ocean surfaces to recognize the spread of algal blooms [16].
While the biotoxin contamination of fish is regional, the contamination by pathogenic microorganisms tends to be more local, triggered by coastal contaminations. Pre-harvest contamination by Vibrio parahaemolyticus, which produce virulent thermostable hemolysins, have been reported in shellfish from marine harvests in Italy [24], in mussels and oysters from commercial shellfish farms in New Zealand [25], in retail shrimp in Malaysia [26], and in shrimp and fish from retail markets in North China [27], among other reports. There is evidence of the increased spread of V. parahaemolyticus with summer temperatures. Vibrio cholerae, producing exotoxins and a blood coagulation factor, is detected in marine environments [28,29]. V. cholerae was reported to be initially of marine origin but later adapted to freshwater environments [30]. V. cholerae is, therefore, more associated with fish from rivers and reservoirs undergoing eutrophication. However, the ability of V. cholerae to contaminate fish in coastal areas cannot be ruled out. V. cholerae forms biofilms on fish skins pre-harvest, which are detected later as a post-harvest contaminant. In a rare situation, Campylobacter jejuni has been reported pre-harvest in marine fish from Kerala in India [31]. Aeromonas, producing enterotoxins and hemolysins, are associated more with locations of seawater mixing with river waters [32]. Aeromonas sp. are of a low food-safety risk.
Microbial pathogens of pre-harvest origin in fish may also appear as post-harvest pathogens, since they are carried with the harvest into the markets. Escherichia coli, Salmonella species, S. aureus, C. botulinum, L. monocytogenes, Shigella species, B. cereus, C. jejuni, Y. enterocolitica, and histamine producers have been identified as common post-harvest pathogens affecting fish [33,34]. E. coli, being a common bacterium of fecal origin, tends to be present more often with poorly handled fish. Salmonella species and Staphylococcus aureus, originating from human contact at handling, are found more frequently than other pathogens in market food fish. Spoilage of fish and food safety hazards originating from post-harvest microbiological contaminations has been reviewed by Sheng and Wang [7]. The authors have identified separately the contaminations originating during the handling, storage, and transport of harvested fish. The high genotypical and phenotypical diversity of Escherichia coli has made it possible for the bacteria to contaminate fish in marine and freshwater environments pre-harvest, and they appear in market products as post-harvest contaminants that release enterotoxins and Shiga toxins [35,36,37]. Although Salmonella is predominantly a freshwater microorganism, its presence in coastal waters and its appearance as a post-harvest contaminant after pre-harvest entry into fish has been noted. Salmonella could be present in high populations in fish, although the populations are low in seawater [38]. Clostridium is mostly a post-harvest contaminant that is capable of withstanding thermal processing below 100 °C, causing food safety hazards through canned fish [39].
Listeria monocytogenes is commonly present in food processing facilities, and it survives in a wide temperature range, high salt content, and wide pH range, posing problems for post-harvest fish handling and processing [40]. Listeria is reported in fish pastes and ready-to-consume smoked fish [41]. Food safety hazards from Listeria in fish and fish products are a considerable problem due to the ability of the microorganism to survive in a range of environments and to adhere to surfaces of food processing plants and food storage utensils.
During post-harvest handling, fish are exposed to pathogenic Staphylococcus aureus and its heat-stable enterotoxins. Enterotoxins withstand the processing of fish, leading to food safety hazards. Sivaraman et al. [42] has observed notable contaminations of S. aureus after primary processing and during storage, with some strains exhibiting resistance to methicillin. There is increasing evidence of the presence in fish of microorganisms resistant to a variety of antibiotics [43]. The consumption of fish carrying antibiotic residues tends to create antibiotic resistance in humans, leading to unforeseen health problems. Exposure of humans to post-harvest microbial contaminants is a continuing food safety hazard, which needs to be controlled through more effective and timely processing and preservation methods.
In view of the continuous food safety hazards associated with a variety of fish due to the formation of biogenic amines, especially histamines, more stringent guidelines and measures relating to histamine production associated with unhygienic fish handling and temperature control have been established by food authorities [3,33]. The guidelines help to minimize the consumption of food fish contaminated by a variety of microorganisms, thus ensuring food safety. The prevention of microbial contamination in food fish would save at least 25% of the harvested fish that are rejected.
2.3. Chemical Food Safety Hazards
Chemical pollutants tend to accumulate continuously in marine and inland waters and enter fish muscles, reaching concentrations hazardous to humans. Based on the facts and figures on marine pollution in a report by UNESCO, disposals from land contribute to 80% of sea pollutants, whereas disposals from ships and mineral explorations at the sea bottom account for the balance [44]. The accumulating pollutants create a negative impact on the marine and freshwater biota, threatening the existence and safety of food fish. The common chemical and physical contaminants affecting the safety of food fish are presented in Figure 3.
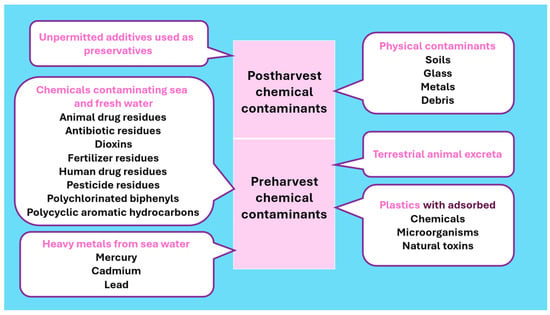
Figure 3.
Chemical and physical contaminants affecting the safety of food fish.
Among chemical contaminants, the residues of agrichemicals, animal drug residues from farms, and animal excreta containing toxic chemical residues end up in the muscles of food fish. With the rising sea levels, more contaminants from land tend to move into fish muscles through increased pollution in the seas [45].
Of the toxic residues, organochlorine (OC) pesticide residues in water are absorbed more through the gills than through stomach, being deposited in fish muscles. Following the ban on the use of OC pesticides in the developed countries, decreasing OC concentrations in Pacific salmon species were reported for the period from 2012 to 2018 [46]. However, OCs have continued to be used in some African and Asian countries, despite the knowledge about their potential hazards through food fish.
Among the organic compounds directly polluting the oceans and reservoirs, contamination by polycyclic aromatic hydrocarbons (PAHs) released from burned fuels into waterways is an increasing hazard. Of the PAHs released, low-molecular-weight compounds and the highly carcinogenic benzo[a]anthracene have been reported from Nigerian waters. The uncontrolled release of petroleum products from refineries and other sources containing PAHs is a continuing food safety concern as they enter food fish [47,48,49].
Toxic metal residues (arsenic mercury, cadmium, lead) enter the oceans due to the weathering of rocks and anthropogenic activities. The toxic metals enter the fish chain continuously, starting from sea plants through benthic fish to pelagic fish. Mercury is converted to the neurotoxic methyl mercury by marine flora, increasing the food safety hazards through food fish. The ability of sword fish and tuna fish to accumulate toxicologically high concentrations of mercury and cadmium are indicated by the high enrichment factors for them [50]. High cadmium content in sword fish is reported in all oceans due to its bottom-feeding nature. Cadmium is deposited in many tissues of fish, leading to toxicity, and this serves as a pathway to enter the human body [51]. Arsenic enters seawater from marine sediments. The concentration of arsenic in seawater is reported to be 100-fold higher than the US EPA human health criteria. However, the arsenates in seawater are converted through arsenites to the less toxic arsenobutane and other organic forms by marine algae, alleviating the dietary toxicity to humans through food fish significantly [52]. Open seas carry higher concentrations of lead than coastal areas, which are deposited into the water from the atmosphere [50]. Lead in fish livers and muscles are reported to exceed the EU tolerance limit of 0.1 mg/kg [53]. The atmospheric transfer of lead to seawater is a continuing threat beyond control, given the emission of lead from fuels in engines globally. It is not easy to handle the challenges associated with the natural contamination of oceans by toxic metals and with their accumulation in fish muscles and viscera.
Nurdles and plastics of differing origin are released continuously into waterways. They settle partly at ocean bottoms. Some plastics take about a thousand years to degrade, while others take a few hundred years [54]. Plastics account for 80% of marine litter [55]. Plastics themselves release carcinogens such as phthalates, bisphenol A, flame retardants, organophosphates, perfluorinated chemicals, and organo-tin compounds into the fish gut when swallowed. The nurdles provide a base for the algae to grow, opening a pathway to enter fish. The hazards arising from swallowing the nurdles by fish are great. Nurdles carry the potential to adsorb algal toxins, microorganisms, toxic metals, and organic compounds. The adsorbed constituents tend to become equilibrated with the environment and be released with changed osmolarity and acidities [56]. The digestive environment in the fish gut tends to release organic pollutants, transporting them into fatty tissues, thus exposing humans to a variety of toxicants transmitted through fish [57].
The bioaccumulation of toxins transferred through nurdles may create food safety hazards to humans through food fish. The plastics are also suggested to provide opportunities for horizontal gene transfer among the adhering microorganisms, creating new food safety hazards through food fish [58]. Microplastics have been reported in muscles of canned predator fish, suggesting a possible route for the entry of microplastics into human muscles [59]. Plastics bring in new and increasing hazards through food fish, which arise from a variety of toxicities that could be transferred to humans.
The use of unpermitted additives, such as formaldehyde (formalin), for the post-harvest preservation of food fish happens in some markets, introducing new food safety hazards [60,61]. At times, the inability to distinguish between added formaldehyde and formaldehyde naturally produced in fish in traces amounts makes regulatory decisions difficult. The use of unpermitted chemicals for fish preservation is a problem in countries where the food regulatory measures are not strong.
The food safety hazards arising from microorganisms and chemicals tend to increase with natural and anthropogenic activities in oceans and waterways [62]. The increasing challenges in handling food safety hazards through food fish are summarized in Figure 4, which identifies the origins of the hazards.
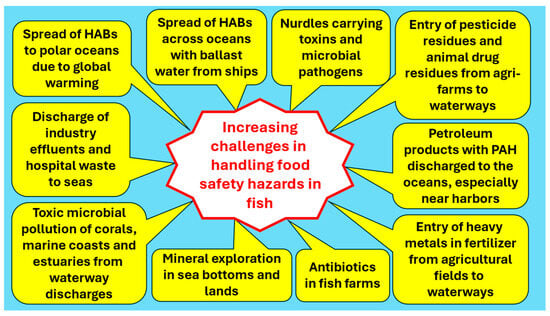
Figure 4.
Increasing challenges and origins of food safety hazards through food fish [62].
Public concern and responsible behavior by industries, with an understanding of the continuously aggravating potential hazards, are essential to protect the sources of food fish and their environment. The oceans, occupying 70% of the earth’s surface and holding 97% of the water, are unfortunately considered a sink to dispose of waste with low consideration for fish, a major source of food. The stagnant inland water reservoirs used for farming or harvesting fish tend to become polluted intensely due to eutrophication. The pollutants in the rivers are discharged into lagoons and coastal fishing areas. The above anthropogenic activities are a major challenge in maintaining a healthy food-fish supply in the human diet.
While it is possible to minimize the exposure of humans to pre-harvest microbial hazards in fish through appropriate handling and processing methods or by avoiding harvests from contaminated waters, the same does not work with chemical contaminants. Chemical contaminants tend to spread across oceans and waterways unnoticed unless regular chemical monitoring of water quality is performed. Being a difficult task, the examination of water quality is unlikely to happen, except for known contamination patterns or incidences. The bulk of fish harvest is processed or preserved. There is the need to eliminate hazardous materials in raw fish prior to processing or preservation, unless the treatment prevents the appearance of hazards in the market products.
3. Processing and Preservation of Fish and Fish Products
Raw fish need to be treated to make them appealing as food. Harvested fish are pre-processed, to get rid of adhering non-edible components and physical contaminants. Fish are processed to retain the organoleptic characteristics of muscles by freezing, heating, controlling the water activity by removing moisture, using additives, or by irradiation. Of the total capture of fish, 60% is processed into different forms in industrialized countries [63]. Processing aims to generate modified products from fresh fish that have consumer appeal. The value addition associated with processing brings increased profits and sustainability to the fish industry. From a consumer perspective, preserving fish to increase their shelf life and to prevent changes in their natural characteristics is not the top priority. Consumers mostly prefer fresh fish for their natural organoleptic characteristics. However, the need to make food fish continuously available as a rich nutrient source has compelled the industry to preserve food fish and, especially, to eliminate food safety hazards. Pre-process preservation of food fish by storing in ice (chilling) between harvest and processing helps in retaining fish quality. Processing food fish, which goes beyond preservation, carries the advantage of a more efficient utilization of edible muscles, developing products appealing to the consumers, and converting non-edible components into edible nutrient-rich forms.
3.1. Conventional Fish Processing and Preservation
The preservation of fish goes back to the early history of the food-gathering period for humans. Fish muscles can contain up to 90% water, which needs to be reduced to minimize microbial spoilage. Drying with salt, air drying, storing in ice, smoking, and fermentation continue to be practiced at the small and medium scale to preserve fish. The objectives of preservation are to prevent the autolysis of muscles and to eliminate the proliferation of pathogenic microorganisms due to the nutrients in fish. Two broad approaches to preserve fish are apparent among the conventional methods. The first is to reduce the water activity by drying with different techniques, with or without salt. The second is fermenting to competitively eliminate harmful microorganisms, allowing beneficial microorganisms and autolysis to bring about desirable organoleptic properties in the product.
In temperate countries, fish are suspended in air and exposed to low humidity at subzero ambient temperatures over weeks to reduce the water activity and to control microbial growth. Under tropical conditions, heat from solar radiation is applied to evaporate the moisture. Prolonged sun drying makes fish susceptible to microbial spoilage. Microbial activity is discouraged by reducing the duration of the sun drying of fish using dryers, platforms above the ground, and the addition of salt to reduce water activity in fish muscles. The combinations of techniques are diverse in conventional fish preservation methods. Some of the basic practices of fish preservation and their effects on the quality and safety of the products are summarized in Table 2.

Table 2.
Conventional fish preservation methods and their effects on quality and safety.
Conventional preservation methods reduce the nutritional quality of fish through changes in proteins, carbohydrates, and lipids due to heat and salt. Pathogenic microorganisms are controlled satisfactorily in the conventional methods, which either provide an environment unfavorable to growth or allow competition by non-pathogenic bacteria, reducing the food safety hazards. Reduced water activity due to salt, combined with drying, restricts microbial growth during preservation. However, halophilic bacteria and fungi may be active in salted dried fish unless optimal salt concentrations are maintained. In dried fish, growth of halophilic fungi has been observed at salt concentrations below 30% combined with more than 30% moisture [71]. The limitation arising from the improper combination of salt and moisture is visible in the poor quality of dried gray mullet carrying high moisture and low salt concentrations [72], by the presence of mycotoxins [73], and by reviewing fish drying methods [67]. Combining conventional fish drying methods with other controls, botanicals, and chemicals remains a possibility for ensuring product safety from microbial contaminants and mycotoxins. Spraying smoke liquid free of polycyclic aromatic hydrocarbons is practiced as a safe alternative to smoking, imparting appealing sensory characteristics to preserved food fish.
Conventional fish fermentation attracts low-value fish and undersized fish, as they do not find ready markets and advanced processing facilities. Fish fermentation is linked mostly with subsistent fishing. Fermentation carries the advantage of utilizing all the constituents of the fish, including micronutrients, essential fats, and vitamins. Microbial activity adds to the value of vitamins, particularly vitamin B12. Fermentation reduces waste and generates nutritious foods affordable for low-income populations in countries where sophisticated processing and preservation facilities are scarce.
Combining permitted food preservatives with drying remains a mechanism to ensure product safety in conventional drying. Acetic acids, ascorbates, benzoates, and potassium lactate are already permitted in certain fish products [74]. Additives are an important component in preserving fish in small- and medium-scale industries. Sodium chloride, essential oils (thyme, cinnamon, and clove extracts) have been used conventionally in reducing microbial activity in fish, aiming for food safety. Natural additives are comfortably accepted by consumers. With the identification of bio-preservatives as purified compounds, the use of nicin, pediocin, reutarin, bacteriocin, citric acid, and lactic acid have become accepted as safe for fish preservation. The chemicals BHA, BHT, EDTA, sodium nitrite, sulfites, and benzoates, permitted for use in foods, also may be good candidates to be used in the conventional drying of fish. The application of preservatives continues at home and in small-scale fish preservation in some countries. Adhering to minimum residue levels of the additives is expected when preserving fish combined with conventional methods.
Conventional processing and preservation methods affect the nutritional quality of the end products, change the texture of fish, and introduce chemicals in certain processes, raising questions on the safety of products. Thermal treatments tend to change the texture and generate new flavors, which are not preferred equally by consumers.
3.2. Emerging Food Processing and Preservation Technologies
The consumer demand is for fresh fish. Consumers seek fish which have undergone the fewest interactions and no thermal treatments. This has resulted in interest in non-thermal processing methods to preserve fish. More recent technologies aimed at using the irradiation and canning of fish to increase their shelf life. Canned fish is safe but is cooked, changing the sensory appeal, although fish canning is a major industry. The present consumer trend is to seek non-thermally processed and preserved fish that retain sensory properties as close as possible to those of fresh fish. Emerging fish processing and preservation methods along with their food quality and safety outcomes are summarized in Table 3.

Table 3.
Emerging processing and preservation methods and their effects on the quality and safety of fish.
The methods summarized above provide zero heat, low heat, or cooking conditions to preserve fish. Against the emerging methods in Table 3, quick freezing, in which the core temperature reaches −18 °C rapidly, continues to be the most widely used method to retain quality, control ice crystallization, and ensure the safety of the food-fish. Frozen fish may be stored for months at temperatures below −18 °C to prevent biochemical degradation and microbial spoilage. Fish stored above −10 °C tend to be vulnerable to slow enzymatic activity. In frozen fish, inoculated Salmonella has been observed to survive at −18 °C for up to 90 days, indicating that frozen fish may not remain safe if the initial microbial populations are high [105]. Freezing carries the advantage of eliminating the risk associated with the parasites A. simplex and P. decipiens in preparation for the Japanese cuisine of sushi and sashimi. Fish to be used for Japanese cuisine are flash frozen at −37 °C for 15 h or stored at −20 °C for 7 days to ensure food safety [106].
Non-thermal fish processing and preservation methods are gaining preference among consumers who expect fish to retain their freshness while ensuring quality and safety. Fish preserved by physical methods have an edge over conventionally preserved fish due to the retention of freshness to a greater extent. Although there is no direct transfer of heat to fish muscles in the non-thermal processes, there is internal heat generation, giving a cooked appearance and partial quality changes during microwave, high-pressure, and pulse treatments. However, consumers and sensory analyses have not detected changes in the odor and color of fish muscles preserved using some of the novel methods, although laboratory testing instruments detected discolorations, whitening, increased hardness, and the initiation of lipid oxidation [107]. Non-thermal processing possesses the advantage of retaining consumers’ preference for foods. However, emerging technologies possess limitations due to their differential effects on microorganisms and the capacity of microbial spores to survive the treatments. These limitations compel the processing industry to be aware of the types of microorganisms in fish in advance in order to manage processing conditions and hybrid processes.
With tuna fish, the HPP treatment is observed to be only bacteriostatic, leaving room for microbial proliferation during storage [108]. In view of the observed occasional limitations in single treatments for fish preservation, hybrid treatments combining non-thermal and mild thermal applications are being examined for efficient microbial destruction while retaining the freshness of fish muscles. The outcomes of modern methods are yet to be fully acceptable and their drawbacks need to be overcome. The synergistic effects of multi-technology may result in consumer acceptance, as the quality changes may be minimal compared with the application of drastic conditions in a single processing technique. Synergistic effects of combining freezing overnight and spraying liquid smoke, together with HPP treatment at 200 MPa, have been reported in the inactivation of Listeria monocytogenes on trout fillets [109]. The combination of low intense exposure by each of the methods in a combined treatment may accrue benefits of quality retention together with improved food safety. While the multiple hurdles in multi-technology preservation result in effective microbial control, the additive effects on quality changes need to be established scientifically for each combination.
Although the irradiation of fish as a non-thermal method has proven potential to eliminate pathogenic microorganisms, consumer acceptance is low due to several myths associated with irradiation, where knowledge about irradiation and radioactivity is mixed up [110]. The regulations requiring irradiated fish to be identified and labelled have added to consumer concerns.
The three major outcomes of applying new processing technologies to increase the shelf life of fish products are the retention of sensory and nutritional quality arising from reduced lipid oxidation and other reactions, the low cost, and the high consumer appeal. The cost continues to be a major deterrent in upscaling the new knowledge into industrial processes. Developing technologies to strike the optimum among the three concerns continues to be challenging. Combined treatments appear to meet the optimum processing requirements, but these need to be identified for each fish size and type, as well as by the microbial populations. Some of the fish products preserved using combinations involving chemical interaction by ozone or free radicals need examination for quality and safety. In selecting combined treatments, more refined operational standards and a deep scientific understanding of inhibitory mechanisms, unexpected biochemical reactions, and regulatory limitations need to be worked out for methods described in Table 3 for commercialization. The ultimate success of the new technologies will rest on the product cost and consumer acceptance as their ability to provide safe, sensory satisfaction to the consumers.
Fish processing has a vital role in improving the utilization of fish components. With inadequacies in the production to meet global demand, the need for better utilization of hitherto unused components in fish is clear. New processing technologies and fish-waste utilization should bring about cost benefits and increase the market for fish products.
4. Fish-Waste Utilization
The main edible component of fish is muscle. The muscles account for 30 to 56% of fish, of which the average consumption is about 49%. The remaining 51%, consisting of heads, viscera, skin, blood, belly flaps, trimmings, frame, etc., is discarded as waste. There are notable variations in the percentage proportions of the waste material among different species of fish and in the published analytical data. The ranges of constituents available from fish for direct consumption, processing, and valorization are summarized in Figure 5.
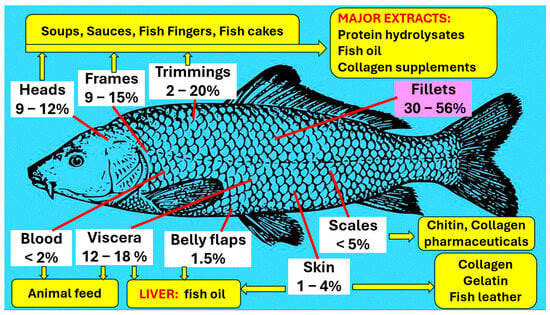
Figure 5.
Percent ranges of unutilized or underutilized constituents in fish [111].
The extractable unutilized nutritious fish constituents consist of proteins (collagen, gelatin, proteins, biopeptides, enzymes), fats (fish oils, squalene, vitamins), and amino polysaccharides (chitin and chitosan) in addition to bones (calcium, phosphates, carbonates). Most constituents can be processed into edible or medicinally valued products [112]. The current inability to meet the global demand for fish, even with increased aquaculture, compels the better utilization of hitherto unprocessed fish constituents into foods. Increasing the utility value of fish from the current 49% consumed directly to about 65% through the increased recovery of muscles, and value addition to components currently identified as waste, are priorities. In converting the unutilized components into novel products, the emphasis is expected to be on high nutritious values, economic benefits, value additions, and increased consumer acceptability while reducing environmental pollution. The byproducts of the fisheries industry could be purified to bio-functional ingredients, including omega-3-oils, polysaccharides, gelatin, chitin, enzymes, and bioactive peptides (BPs). Fish waste is a rich but unutilized source of nutrients and bioactive compounds exhibiting specific health benefits [113]. The major components to be separated from fish waste and the possible approaches for better utilization of fish waste are discussed below.
4.1. Collagen
Collagen is a structural protein in skin, bones, ligaments, cartilage, and tendons that performs structural functions in animals and humans. Collagen contains 30% protein. There are five main types of collagens, identified by their locations and functions in the animal and human body. The types extend to at least 28 based on molecular properties [114]. The degradation of collagens in human body tissues results in sagging skin, wrinkles, dry skin, and stiff joints. Chemically, collagen is a triple helix of three extended protein chains wrapped around each other. Collagen could be extracted from the skin, scales, cartilage, fins, bladder, and bones in fish, sustaining industrial production [115]. Fish bone comprises 30% collagen [112]. Fish collagen possesses the advantages of low molecular weight, easy absorption by the human body, and biocompatibility with human collagen. Fish collagen can be processed at low cost. Its low cost makes collagen an important material for biomedical applications [116]. Fish collagen carries no risk of transmitting zoonoses during healthcare applications compared with collagen from mammals. Collagens can be hydrolyzed to produce gelatin.
The collagens available in fish waste arise from skins (4.5%), scales (0.3%), and bones (0.64%) [114]. However, variations in the percentages of collagen are reported from different types and different organs of fish, even with the same extraction method. Collagens, being proteins of diverse characteristics arising from the arrangement of amino acid sequences and amino acid proportions in their structures, show diversity in their solubility and other properties. The applicable extraction methods may be specific to the source of collagens, using optimal pH, substrate–solvent ratios, the duration of heating, and temperature. Collagen-specific extraction processes using acid, pepsin–hydrolytic acid, eutectic solvents, or supercritical fluid extractions have been identified that suit the molecular characteristics of the triple helix and the end use [114]. The extractions cause changes in the molecular structure of collagens, altering their biochemical properties. The effects of different extraction methods on the functional properties limit the utilization of fish waste as a common source of collagen.
The diversity of the structural, chemical, and morphology of extracted collagens provides opportunities for pre-determined biomedical applications. These applications include tissue engineering (cartilage and tissue regeneration), drug delivery, wound dressing, skin regeneration, and vascular tissue regeneration among several other potential applications under investigation. The low denaturation of fish collagen at temperatures of 25–30 °C caused by low-molecular-weight amino acids compared with the denaturation of mammalian collagens at 39–40 °C limits some biomedical applications of fish collagen. The ability of hydrolyzed collagen along with vitamin C to accelerate wound healing through the proliferation and migration of fibroblast cells has been reported [117]. The combination generates a functional ingredient, exhibiting nutraceutical properties for skin nourishment. Skin nourishment is an aim in cosmetics. While the potential for the utilization of collagens in advanced biomedical applications is high, the sophistication needed to identify purpose-focused extraction and purification methods needs a deep scientific basis. However, each application requires optimization of the functional properties of the extracted collagen. The functional properties are linked with the chemical and molecular characteristics of the extracted fish collagens. Matching the molecular structures with the functionality of collagens, or even the modification of collagens for targeted application is a research need. Fish collagen extracted with sophisticated methods to retain their original properties would find more applications based on their compatibility with human health needs.
Low-cost extraction is needed to utilize fish collagen for a wide range of purposes. A simple method to recover collagens is by salt precipitation from salmon skin with low chemical interactions [118], and a method combining acid hydrolysis with ultrasonication has been described [119]. The extraction method would alter the molecular composition of the collagen extract. The correlation of fish species, tissues to be extracted, the extraction method, structural features of the extracted collagens, and their suitability for different food and biomedical applications require large databases for predictive scientific decisions. Artificial intelligence (AI) may provide solutions for handling data aimed at the better utilization of collagens.
4.2. Gelatin
The predicted global market for gelatin is USD 5 billion by 2025 [120]. The high market potential provides a great opportunity to increase fish gelatin production from the current utilization of 1.5%. Gelatins are multi-functional products characterized by moisture contents of slightly below 10%, protein contents of up to 85%, and ash content of around 5%. Fish gelatin derived by the partial hydrolysis of collagen proteins have a high concentration and diversity of amino acids, predominantly containing lysine, and methionine but low in tryptophan. The food industry targets gelatin as a constituent that provides multi-functional properties that appeal to consumers in processed foods. The structural and physical properties of gelatin, such as light penetration, mechanical strength, and networking depend on the molecular weight and the crosslinking patterns of amino acids [121]. Additionally, the balance of hydrophilic and hydrophobic interactions in the peptides decide their network characteristics on food surfaces.
In foods, gelatins provide structural features to meet rheological and organoleptic properties to suit each situation, generating creaminess, mouthfeel, chewiness, etc. Gelatin serves as an ingredient in many food products [122], providing gelling, stabilizing, emulsifying, dispersing, encapsulating, and thickening properties to the end products [115,123].
Comparing dry salting, wet salting, the use of proteases, and heat-based methods to extract fish gelatin, the enzymatic technique is reported to produce a more robust sponge-like gelatin, with a high water-absorption capacity of 10%, a fat-binding capacity of 10%, and better emulsifying properties [124], with protease-hydrolyzed gelatin exhibiting high utilization potential in the food industry [121]. The results from different studies indicate the relevance of the extraction method to the required properties of the gelatin for varying food uses. Fish gelatin, with a melting point about 10 °C below that of pork and beef gelatins, possesses advantages as a food ingredient with its easy melting in the mouth. The proteins in gelatin from the cannonball jellyfish exhibits antioxidative and antimutagenic properties. The antioxidative properties make gelatin a useful food preservative [125].
Fish gelatin is gaining recognition as a natural product against petroleum-based packaging for foods [126]. Fish gelatin is combined with plant materials to modify their properties. The incorporation of extracts from the mangrove plants Bruguiera gymnorhiza and Sonneratia alba has been reported to impart elongation, water vapor transmission, antioxidative properties, and antibacterial activities to fish-gelatin films used as active packaging materials [127]. The properties of gelatin are further modified by combining it with chitosan, xanthan gum, and antioxidants. Chemical modification with herbal extracts, essential oils, and the enzymatic action of transaminases modify the properties of gelatins for specific food uses [120,127]. Gelatin-based packages incorporating essential oils carrying antioxidative properties were found to be effective in quality retention in carp fish by slowing lipid oxidation during refrigerated storage [128]. However, the study reported setbacks due to color changes in the fillets. To suit the different food protection applications, gelatins are modified by enzymatic pretreatments during extraction, ultrasonication, and high-pressure processing [120]. Gelatin coatings have been improved, incorporating nano-capsulated essential oils and other additives to impart desirable properties as food coatings. Gelatin is used to coat nutrient food powders and pharmaceuticals as tablets and capsules. There is high potential for developing new processes and technologies for the product-specific modification of fish gelatins. The new processes need to work on the molecular rearrangement of amino acids and their crosslinks to generate gelatins with properties conducive to specific applications. The safety assessment of gelatins modified with additives needs research attention.
The main medical advantage of fish gelatin arises from the immunological safety of the proteins, allowing its wide use in healthcare products [129]. The low melting point of fish gelatin makes it suitable for the microencapsulation of oils and drug delivery. Gelatin from fish in cold climates has a low hydroxyproline content, which is more suitable for products requiring low gelling temperatures.
In the cosmetics industry, gelatin is used for the preparation of hair gels, creams, lotions, and shampoos as it plays an important role in protecting the human skin from UV irradiation, minimizing oxidative stress [130]. The food industry requires ingredients with standardized physical properties to maintain market characteristics of consumer preferences in foods.
4.3. Other Fish Proteins and Derivatives
Fish proteins generate a range of valuable products such as hydrolysates, active polypeptides, enzymes, and other derivatives for sustainable utilization in addition to collagen and gelatin [131]. The distribution of proteins in whole fish and in the components of Pacific Ocean perch are 17.9% in whole fish, 15.2% in the frames, 14.9% in the heads, and 11.3% in the viscera [132]. The authors indicate a high lysine content in fish heads and frames, suggesting the quantitative and qualitative importance of extracting protein derivatives from perch fish. A considerable proportion of the proteins available in fish is lost quantitatively with the fish waste.
The proteins in fish waste are separated mainly by chemical hydrolysis, enzymatic hydrolysis, or fermentation. Ultrasound technology is adding to the efficiency of chemical extraction of the fish proteins. Of the different hydrolytic methods, enzymatic hydrolysis is the best option, generating structurally and functionally uniform hydrolysates. The uniformity is linked to the molecular nature of the derived polypeptides. Through the selection of enzymes for proteolytic and other biochemical reactions, bioactive peptides (BPs) of specific characteristics can be generated. The extracted polypeptides are purified by membrane filtration and chromatographic methods to separate BPs of desirable and predictable properties having high economic values. Extraction conditions specific to fish types and the properties expected in hydrolysates have been established by different researchers.
BPs impart a positive impact on body functions and may influence human health positively or negatively, depending on the way the BP is used. There is potential to use BPs as food additives and as ingredients of pharmaceuticals designed to prevent lifestyle diseases such as obesity, hypertension, and type II diabetes [133]. However, retaining the activity of BPs in commercial production demands rigorous scientific scrutiny. Much research is needed to understand the interactive behavior of polypeptides in treating humans. With many hundreds of BPs generated from fish proteins, there is much room for future research to correlate the molecular properties with activities as pharmaceuticals [134]. The use of BPs in inhibiting angiotensin-converting enzymes (related to the treatment for hypertension), their antioxidative activity, antimicrobial activity, inhibition of dipeptidyl peptidase (in relation to diabetes control), and 49 possible other applications have already been recognized. This opens a vast field for the biomedical use of fish BPs. Biomedical applications need functionally specific BPs derived through well-defined extractions.
Biotechnological processes, using pure enzymes with known biochemical activities, provide opportunities to obtain BPs of predictable characteristics. The process for by-catch (low-value fish) using papain was found to extract BPs efficiently [135]. Cryoprotective effects of fish hydrolysates generated with alcalase® and protamex® enzymes appear to differ from each other in their performance in storage fish. The noted differences indicate diversities arising from the properties of generated polypeptides. The cryoprotective effects of fish-based gelatins appear to follow a mechanism different from that of the commonly used cryoprotective agent consisting of sucrose–sorbitol. The application of fish cryoprotectants in developing non-sweet surimi products is under investigation [136]. The diversity among BPs provides opportunities for a variety of applications. BPs consisting of 3–20 amino acids carry anti-inflammatory, antioxidative, anticancer, and antimicrobial activities that are linked to their structural features [137].
BPs from fish proteins carry the potential to generate novel compounds for the biomedical and food industries. Sanapala et al. [137] highlight the importance of improving and refining extraction methods to obtain BPs in their natural forms as far as possible to increase their utility value. Fish protein hydrolysates having known amino acid sequences, with the potential to generate high-value nutraceuticals and healthcare products industrially, are useful in gaining consumer acceptance and for treating several non-communicable diseases [138]. The potential to utilize hydrolysates for the benefit of human health justifies the development of processes to generate purpose-oriented products from low-cost raw materials from fish waste. Already, there are commercialized drugs extracted from fish, prescribed for noncommunicable diseases.
Fish protein hydrolysates have water-holding capacity, oil-absorption capacity, protein solubility, gelling activity, and foaming properties that are essential for application as food emulsifiers [139]. The properties of hydrolysates vary with the degree of hydrolysis and the distribution of peptides and amino acids in them. The product diversity in protein hydrolysates could be retained using specific enzymatic hydrolysis mechanisms to generate function-oriented BPs. The hydrolysis of fish proteins using purpose-oriented enzymes is an area to be explored.
A variety of proteolytic and other enzymes are used to extract protein-based hydrolyzed fractions from fish tissues. Hydrolysates prepared using the commercial enzymes alcalase® and flavourzyme® on minced amur sturgeon skin are reported to generate products having retarded lipid oxidation and protein oxidation when examined for their biochemical activities on fish mince. Controlled enzymatic hydrolysis appears to generate products with more specific properties, indicating the importance of refining the hydrolytic process [140]. Supplementation of poultry by-product meal with 10% fish protein hydrolysates is reported to reduce gut enteritis caused by the poultry by-product meal alone. Fish protein hydrolysates restore gut microbial composition in farmed juvenile seabass fish, indicating improvements on aquafeed formulations. The anti-inflammatory effect imparted by fish proteins is noteworthy [141]. The anti-inflammatory feature gives a competitive marketing edge to fish protein hydrolysates over poultry hydrolysates.
While fish protein hydrolysates can generate products carrying a variety of desirable properties, the processing technology need to be fine-tuned to handle different fish substrates aimed at envisaged properties in the hydrolysates, targeting novel foods and medicines.
4.4. Enzymes from Fish
Fish carry endogenous enzymes having diverse biochemical efficiencies, each with distinct characteristics. These could be used to target specific biotechnological reactions industrially. Of the enzymes available, proteases, lipases, and glutaminases are commonly utilized industrially. Borges et al. [142] describe the successful use of proteolytic enzymes extracted from the viscera of fish to hydrolyze fish proteins, generating comparable results with alcalase®, although the antihypertensive potential of the fish enzyme hydrolysate was lower. The viscera could be a useful source of proteolytic enzymes for the detergent industries, where high specific activity is a low requirement, and multiple effects of combined enzyme extracts may carry advantages.
The fish enzymes chitinases and collagenases are available from fish waste products. Enzymes obtained from fish living in a diverse range of temperature regimes may carry hitherto unexamined traits that can be harnessed beneficially for the industry [143]. Deep research into fish enzyme biochemistry may lead to new biotechnological openings to use fish waste.
4.5. Chitin and Chitosan
Chitins are linear amino polysaccharides, occurring in three forms as α-chitin, β-chitin, and γ-chitin. They are obtained predominantly from crustaceans. Living organisms in the ocean generate 102–104 tons of chitin annually [144]. The major marine waste products containing chitin are crustacean exoskeletons and finfish scales. Chitin is covalently bound to the proteins of shrimp [145]. Chitin is converted to chitosan, chito-oligosaccharides, and glucosamine for different applications [146].
Chitin is deacetylated to produce chitosan. Chitosan is extracted chemically (demineralization, deproteination, and deacetylation) [146], biologically (enzymatic deproteination, fermentation) [146,147] or by microwave irradiation combined with acid hydrolysis [145]. Chemical methods are the least preferred as the processed chitosans are non-uniform in size, charge, molecular weight, and the degree of deacetylation, limiting their applications [146]. However, chemical extraction of chitosan of industrial quality from shrimp has been described in Malaysia [148]. The viscosity of chemically generated chitosan is reported to be low [147], and the chemical processes generate hazardous chemical waste. Biological extraction of chitosan carries several advantages. Biological extraction provides the opportunity for the concurrent extraction of proteins, minerals, and pigments from fish waste with higher economic benefits. However, biological extraction requires well-controlled fermentation conditions, such as the pH, time, and temperature. Biological extraction needs to guard against possible microbial contamination during processing. It may be necessary to follow the biological hydrolysis with mild chemical methods to remove residual proteins from the chitosan hydrolysates. Ploydee and Chaiyanan [147] reported the production of high-viscosity chitosan by chemical treatment of biotechnologically extracted chitosan. Waste heads and exoskeletons of shrimp are biotechnologically extracted by fermentation with Lactobacillus pentosus to convert glucose into lactic acid together with decalcification and proteolysis by Bacillus thuringiensis to separate chitosan.
In the chemical extraction of chitosan, the ability of microwaves to generate heat inside the exposed fish waste carries an opportunity to increase the efficiency of thermal processes at low heat levels. Microwave heating coupled with chemical hydrolysis provide the advantages of rapid hydrolysis with low concentrations of alkali at low external heating [144]. In microwave heating, reducing the heating duration by one-third achieves the same degree of deacetylation as with chemicals, with no significant differences reported in the structures of chitosan compared to heating in a water bath [145]. Chitosan produced by microwave treatment coupled with alkali had higher crystallinity, lower molecular weight, and low zero-shear viscosity compared with extracts obtained by chemical treatment only. Laboratory experiments on different hydrolytic processes have generated a wealth of data that could be scaled up to industrial practices to generate purified chitosan from fish waste. The process design needs to work on the properties of chitosan required for specific uses, both in biomedical and food applications.
Chitosan is used in drug delivery, tissue engineering technology, wound healing in humans, and the production of sensors for electrical appliances [144]. Biodegradability, biocompatibility, antimicrobial, anti-tumor, and antioxidative activities make chitosan a valuable compound for use in food, pharmaceutical, agrichemical, and wastewater treatment processes [149]. Of the different potential markets for chitosan, nutraceutical and cosmeceutical appear to be the fastest growing, followed by the therapeutical and biomedical markets. The latter two need increased sophistication to lead to products with well-defined characteristics.
4.6. Fish Oil and Associated Products
Fish oil stands alongside fish muscles as a rich nutrient carrying health benefits. Crude fish oils and fish liver oils constitute the fish oil group. Tuna, salmon, sardine, and mackerel are rich sources of fish oils containing essential fatty acids, although all fish oils contain essential fatty acids. The association of fish oils with the prevention of cardiovascular diseases, diabetes, cancer, vision health, improvements in the immune systems, and development of nervous systems has been documented. The role of fish oils in managing health in the aging population has been identified by several international organizations. The fatty acids EPA and DHA are precursors for the production of mediators inhibiting the synthesis of proinflammatory cytokines in the human body [150].
With the depletion of fish harvests, capturing fish solely for oil is discouraged globally, compelling industries to extract fish oils from fish waste by novel technologies. Higher concentrations of fish oil appear to be in the discarded waste than in the muscles consumed. The lipid (oil) percentages in components of the Pacific Ocean perch were reported to be 7.8 in the whole fish, 10.5 in the frames, 9.3 in the heads, and 13.5 in the viscera, suggesting a high potential for oil recovery from fish waste [132]. The production of omega-3-polyunsaturated fatty acids by microalgae, though possible, is more expensive than its extraction from fish waste [150].
The technologies for the extraction of oils from fish waste have evolved, beginning from wet pressing (cooking, pressing, decanting, centrifugation, and chemical purification) through to solvent extractions using isopropyl alcohol to the more recent supercritical fluid extraction. The latter can be manipulated to separate dissolving-quality oil, which is rich in essential fatty acids [151]. Considering the health benefits associated with omega-3-fatty acids and the heavy global demand for them, utilizing discarded fish components, which amount to 50% of the whole fish weight, is an industry with massive potential. Microencapsulation, providing oxidative stability, appears to be the best option to market omega-3-fatty acids from fish. After extracting fish oil, the leftover meal is available either for the extraction of proteins or as a beneficial ingredient in animal feeds. The potential for a multi-product processing system should be targeted for the economic and nutritional benefits from fish waste [151].
Shark oil possesses outstanding moisturizing properties and the ability to penetrate human skin, strengthening its barrier properties. Consumption of shark oil for 6 weeks has been shown to result in a significant decrease in C-reactive proteins in human blood sera and in intracellular cholesterol levels in peripheral blood mononuclear cells. Shark oil also improves the human erythrocyte fatty acid composition and provides other health-promoting consequences [152]. Shark liver oil is a rich source of squalene, at concentrations higher than in vegetable sources. Squalene, a polyunsaturated hydrocarbon, carries health benefits, including imparting antioxidative properties to human skin. Squalene has high potential in pharmaceutical and nutraceutical preparations focusing on human health [153]. Demand for fish oils tends to increase with increasing population and with public interest in a healthy life. The increasing demand can be met only by using new sources of polyunsaturated fatty acids. Fish waste qualifies as the main inexpensive provider of heart healthy fish oils.
Annual global fish waste is around 75 million metric tons. It is recognized as a resource for producing components beneficial for health and for fuels. Fish oils possess potential for use as biodiesel. Distillation and supercritical fluid extraction can separate fish oils from fish waste material to be used as biodiesel [154]. Considering that antibiotics are used in fish farming, which could lead to residues in foods from fish, the conversion of fish waste from farmed fish to fuel is a safer option. However, the cost of supercritical fluid extraction may be a deterrent in producing fish-oil-based fuels economically. Distillation may be the best option currently. With impending fuel shortages globally, the increased production of biodiesel is a beneficial proposition.
4.7. Conventional Uses from Fish Waste
Calcium from fish bones; DHA extracted from tuna eyes, shark fins, and cartilages as a main ingredient in soup; fish albumins as a possible substitute for processes using egg albumen; fish meal as a source of B vitamins; and proteins as components in animal feeds are some of the end products that could be utilized with low-cost processing. However, benefits from the high-scale processing of fish waste bypass the benefits from low-cost processing.
5. Discussion
In a situation where expansion of the marine fish harvest is not possible and potential food safety hazards from contaminants in aquaculture are high, the efficient use of captured fish is essential. In the “blue growth” concept of the prevention of pollution and the circular economy concept of world-sustainable goals for utilizing waste for human benefit, the utilization of fish waste forms a significant component. There are many methods published on the efficient preservation and processing of fish products. The challenge lies in converting current scientific knowledge into cost-effective technologies. The conversion may be through combinations of preserving and processing methods to be energy efficient and cost effective.
Proteins are extracted from fish waste to generate collagen, gelatin, proteins, and biopeptides, listed in decreasing molecular size. The processes discussed in the above sections utilize chemical or enzymatic hydrolysis as major methods. Chemical hydrolysis produces a mixture of products listed above in different ratios. The mixture of hydrolyzed products can be fractionated by a single process to separate each of the products in the mixture for appropriate end uses. Enzymatic hydrolysis can generate products of more uniform quality and uniform properties beneficial to more refined purposes, such as biomedical production. The vision should be to establish chemical and biotechnological industries for fish waste, targeting the properties expected by the users of the end products.
The wealth of scientific data available on fish waste needs to be handled with a focus on selecting the best permutations and combinations of processes and treatments to arrive at the best end use. AI provides a platform for taking a better approach. The prospects of early warnings and risk identification tools to handle food safety through AI have been described [155]. The suitability of AI systems to predict algal blooms and mycotoxin production in foods is already under consideration. Hydrolysis of waste fish components to extract collagens, gelatins, proteins, enzymes, bioactive peptides, chitin, chitosan, and fatty acids are performed using several methods or combinations of them, each producing a different degree of hydrolysis, yielding diverse products. The products extracted are also dependent on the differences among fish species and the differences in fish habitats, e.g., temperate or tropical, etc. Given the diversity of the raw material quality and processed product characteristics, there is a need to select products that meet specific medical, food, and cosmetic applications. The future may lie in the application of AI to combine the knowledge on fish production, fish availability, fish spoilage, safety of food fish, and possible processing applications to predict the best means to utilize the fish capture. The beneficiaries will be the public, especially from economic benefits, an efficient food industry, and a sophisticated biomedical industry that minimizes environmental pollution. The amount of information appearing in the above areas demands high-scale data retention and analytical tools, where AI has a role to play.
6. Conclusions
More effective preservation and processing methods for food fish that ensure food safety and efficient waste utilization that applies the rapidly generated knowledge need to be converted into viable technologies for global benefit.
Funding
No external funding was received for the preparation of this article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Jayasekara, C.; Mendis, E.; Kim, S.-K. Seafood in the human diet for better nutrition and health. In Encyclopedia of Marine Biotechnology, 1st ed.; Kim, S.-K., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 2939–2956. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alge, H.M.; et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American heart association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Welch, A.W.; Knapp, A.N.; Tourky, S.E.; Daughtery, Z.; Hitchcock, G.; Benetti, D. The nutrient footprint of a submerged-cage offshore aquaculture facility located in the tropical Caribbean. J. World Aquac. Soc. 2019, 50, 299–316. [Google Scholar] [CrossRef]
- Rubio, N.; Datar, I.; Stachura, D.; Kaplan, D.; Krueger, K. Cell-Based Fish: A novel approach to seafood production and an opportunity for cellular agriculture. Front. Sustain. Food Syst. 2019, 3, 43. [Google Scholar] [CrossRef]
- Keerthana, P.S.; Gopan, S.; Rajabudeen, R.; Fathima, R. Postharvest losses in fisheries sector—Facts, figures, challenges and strategies. Int. J. Fish. Aquat. Stud. 2022, 10, 101–108. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L. The microbial safety of fish and fish products: Recent advances in understanding its significance, contamination sources, and control strategies. Comp. Rev. Food Sci. Food Saf. 2020, 20, 738–786. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Yin, T.; You, J.; Liu, R.; Huang, Q.; Shi, L.; Wang, L.; Liao, T.; Wang, W.; et al. Recent understanding of stress response on muscle quality of fish: From the perspective of industrial chain. Trends Food Sci. Technol. 2023, 140, 104145. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen derived from fish industry waste: Progresses and challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh fish degradation and advances in preservation using physical emerging technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Liu, Y.; Gao, S.; Tan, Y.; Hong, H.; Luo, Y. Mechanisms of fish protein degradation caused by grass carp spoilage bacteria: A bottom-up exploration from the molecular level, muscle microstructure level, to related quality changes. Food Chem. 2023, 403, 134309. [Google Scholar] [CrossRef]
- Duarte, A.M.; Silva, F.; Pinto, F.R.; Barroso, S.; Gil, M.M. Quality assessment of chilled and frozen fish-mini review. Foods 2020, 9, 1739. [Google Scholar] [CrossRef]
- Ying, X.; Li, T.; Deng, S.; Brennan, C.; Benjaku, S.I.; Liu, H.; Wang, F.; Xie, X.; Liu, D.; Li, J.; et al. Advancements in nonthermal physical field technologies for prefabricated aquatic food: A comprehensive review. Comp. Rev. Food Sci. Food Saf. 2024, 23, e13290. [Google Scholar] [CrossRef] [PubMed]
- Van Dolah, F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2002, 8 (Suppl. S1), 133–141. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Elorriaga-Verplancken, F.R.; Hernández-Camacho, C.J.; Álvarez-Santamaría, L.; Paniagua-Mendoza, A.; Robles-Hernández, R.; Rebolledo-Villa, F.; Rosales-Nanduca, H.; Ramos-Rodriguez, A.; Acevedo-Whitehouse, K. Largest mortality event to date of California sea lions in Mexico might be linked to a harmful algal bloom. Aquat. Mamm. 2022, 48, 59–67. [Google Scholar] [CrossRef]
- Hinder, S.L.; Hays, G.C.; Brooks, C.J.; Davies, A.P.; Edwards, M.; Walne, A.W.; Gravenor, M.B. Toxic marine microalgae and shellfish poisoning in the British Isles: History, review of epidemiology, and future implications. Environ. Health 2011, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Kibler, S.R.; Tester, P.A.; Kunkel, K.E.; Moore, S.K.; Litaker, R.W. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Model. 2015, 316, 194–210. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.I.; Ung, A.; Cruchet, P.; Revel, T.; Viallon, J.; Sibat, M.; Varney, P.; Laurent, V.; Hess, P.; et al. Evidence for the range expansion of ciguatera in French Polynesia: A revisit of the 2009 mass-poisoning outbreak in Rapa Island (Australes Archipelago). Toxins 2020, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Uddin, S.; Bottein, M.-Y.D.; Faizuddin, M. Ciguatera in the Indian Ocean with special insights on the Arabian Sea and adjacent Gulf and Seas: A Review. Toxins 2021, 13, 525. [Google Scholar] [CrossRef]
- Randell, J.E. Review of clupeotoxism, an often-fatal illness from the consumption of clupeoid fishes. Pac. Sci. 2005, 59, 73–77. Available online: http://hdl.handle.net/10125/24162 (accessed on 15 February 2024). [CrossRef]
- Zhang, F.Z.; Dickman, M.D. Mid-ocean exchange of container vessel ballast water. 1: Seasonal factors affecting the transport of harmful diatoms and dinoflagellates. Mar. Ecol. Prog. Ser. 1999, 176, 243–252. Available online: http://hdl.handle.net/10722/57256 (accessed on 15 February 2024). [CrossRef]
- Suffredini, E.; Mioni, R.; Mazzette, R.; Bordin, P.; Serratore, P.; Fois, F.; Piano, A.; Cozzi, L.; Croci, L. Detection and quantification of Vibrio parahaemolyticus in shellfish from Italian production areas. Int. J. Food Microbiol. 2014, 184, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Hedderley, D.; Fletcher, G.C. Long-term study of Vibrio parahaemolyticus prevalence and distribution in New Zealand shellfish. Appl. Environ. Microbiol. 2015, 81, 2320–2327. [Google Scholar] [CrossRef]
- Letchumanan, V.; Yin, W.-F.; Lee, L.-H.; Chan, K.-G. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cheng, J.; Wu, Q.; Zhang, J.; Xie, T. Prevalence, characterization, and antibiotic susceptibility of Vibrio parahaemolyticus isolated from retail aquatic products in North China. BMC Microbiol. 2016, 6, 32. [Google Scholar] [CrossRef]
- Escobar, L.E.; Ryan, S.J.; Stewart-Ibarra, A.M.; Finkelsteing, J.L.; Christine, A.; King, C.K.; Qiao, H.; Polhemas, M.E. A global map of suitability for coastal Vibrio cholerae under current and future climate conditions. Acta Trop. 2015, 149, 202–211. [Google Scholar] [CrossRef]
- Maheshwari, M.; Nelapati, K.; Kiranmayi, B. Vibrio cholerae—A review. Vet. World 2011, 4, 423–428. [Google Scholar] [CrossRef]
- Colwell, R.R. Infectious disease and environment: Cholera as a paradigm for waterborne. Int. J. Microbiol. 2004, 7, 285–289. Available online: http://scielo.isciii.es/scielo.php?pid=S1139-67092004000400008&script=sci_arttext (accessed on 26 December 2023).
- Deepa, J.; Sunil, B.; Latha, C.; Vrinda, K.M.; Mini, M.; Aravindakshan, T.V. Prevalence of Campylobacter spp. in marine fishes, crustaceans and molluscs in Kozhikode district, Kerala. J. Vet. Anim. Sci. 2022, 53, 32–38. [Google Scholar] [CrossRef]
- Aberoum, A.; Jooyandeh, H. A Review on occurrence and characterization of the Aeromonas species from marine fishes. World J. Fish Mar. Sci. 2010, 2, 519–523. Available online: https://www.researchgate.net/publication/312898449 (accessed on 15 February 2024).
- Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guidance; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition: Washington, DC, USA, 2022; p. 393. Available online: https://www.fda.gov/food/seafood-guidance-documents-regulatory-information/fish-and-fishery-products-hazards-and-controls (accessed on 15 January 2024).
- Ali, A.; Parisi, A.; Conversano, M.C.; Lannacci, A.; D’Emilio, F.; Mercurio, V.; Normanno, G. Food-borne bacteria associated with seafoods: A brief review. J. Food Qual. Hazards Control. 2020, 7, 4–10. [Google Scholar] [CrossRef]
- Rasko, D.A.; Rosovitz, M.J.; Myers, G.S.A.; Mongodin, E.F.; Fricke, W.F.; Gajer, P.; Crabtree, J.; Sebaihia, M.; Thomson, N.R.; Chaudhuri, R.; et al. The pangenome structure of Escherichia coli: Comparative genomic analysis of E. coli commensal and pathogenic isolates. J. Bacteriol. 2008, 190, 6881–6893. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.L.; Rabinowitz, P.; Weissman, S.J.; Roberts, M.C. Diversity of Escherichia coli found in the Salish Sea. Front. Mar. Sci. 2022, 9, 967435. [Google Scholar] [CrossRef]
- Prakasan, S.; Prabhakar, P.; Lekshmi, M.; Kumar, S.; Nayak, B.B. Isolation of Shiga- toxin producing Escherichia coli harboring variant Shiga-toxin genes from seafood. Vet. World 2018, 11, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, G.G.; Brandi, G.F.; Schiavano, G.F. Incidence and role of Salmonella in seafood safety. Food Res. Int. 2012, 45, 780–788. [Google Scholar] [CrossRef]
- Casalinuovo, F.; Gazzotti, T.; Rippa, P.; Ciambrone, L.; Musarella, R.; Pratticò, E. Microbiological stability of canned tuna produced in Italy and in non-European countries. Ital. J. Food Saf. 2015, 4, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How this pathogen survives in food-production environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, B.; Szymczak, M.; Trafiałek, J. Prevalence of Listeria species and L. monocytogenes in ready-to-eat foods in the West Pomeranian region of Poland: Correlations between the contamination level, serogroups, ingredients, and producers. Food Microbiol. 2020, 91, 103532. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, G.K.; Gupta, S.S.; Visnuvinayagam, S.; Muthulakshmi, T.; Elangovan, R.; Perumal, V.; Balasubramanium, G.; Lodha, T.; Yadav, A. Prevalence of S. aureus and/or MRSA from seafood products from Indian seafood products. BMC Microbiol. 2022, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Marijani, E. Prevalence and antimicrobial resistance of bacteria isolated from marine and freshwater fish in Tanzania. Int. J. Microbiol. 2022, 2022, 4652326. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Alleman, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human health and ocean pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef]
- Dvorak, A.C.; Solo-Gabriele, H.M.; Galletti, A.; Benzecry, B.; Malone, H.; Boguzewski, V.; Jason, B. Possible impacts of sea level rise on disease transmission and potential adaptation strategies, a review. J. Environ. Manag. 2018, 217, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Donets, M.M.; Tsygankov, V.Y.; Gumovskiy, A.N.; Gumovskaya, Y.P.; Boyarova, M.D.; Busarova, O.Y.; Livinenko, A.V.; Khristoforova, N.K. Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in Pacific salmon from the Kamchatka Peninsula and Sakhalin Island, Northwest Pacific. Mar. Pollut. Bull. 2021, 169, 112498. [Google Scholar] [CrossRef] [PubMed]
- Nasher, E.; Heng, L.Y.; Zakaria, Z.; Surif, S. Concentrations and sources of polycyclic aromatic hydrocarbons in the seawater around Langkawi Island. Malays. J. Chem. 2013, 975781. [Google Scholar] [CrossRef]
- Tongo, I.; Etor, E.E.; Ezemonye, L. Human health risk assessment of PAHs in fish and shellfish from Amariaria community, Bonny River, Nigeria. J. Appl. Sci. Environ. Manag. 2018, 22, 731–736. [Google Scholar] [CrossRef]
- Edward, H.S.; Rugebregt, M.J.; Opier, R.D.A. Polycyclic aromatic hydrocarbon (PAHs) compound in seawater of Cimandiri river estuary, Pelabuhan Ratu. IOP Conf. Ser. Earth Environ. Sci. 2021, 925, 012046. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Cai, W.; Xu, X.; Sun, M. The pollution status of heavy metals in the surface seawater and sediments of the Tianjin coastal area, North China. Int. J. Environ. Res. Public Health 2021, 18, 11243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic effects of cadmium on fish. Toxins 2022, 10, 622. [Google Scholar] [CrossRef]
- Neff, J.M. Ecotoxicology of arsenic in the marine environment. Environ. Toxicol. Chem. 2009, 16, 917–927. [Google Scholar] [CrossRef]
- Véron, A.; Bernier, I.; Hamelin, B. Lead abundances in bony fish from the world oceans: Significance for contaminated marine habitats and environmental policies. C. R. Géosci. Sci. Planète 2021, 353, 37–54. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Carney, A.B.; Eggert, H. Marine plastic pollution: Sources, impacts, and policy issues. Rev. Environ. Econ. Policy 2019, 13, 317–326. [Google Scholar] [CrossRef]
- Sewwandi, M.; Premarathne, K.S.D.; Wijesekara, H.; Rajapaksha, A.U.; Soysa, S.; Vithanage, M. Vector transport of microplastics bound potentially toxic elements (PTEs) in water systems. J. Natl. Sci. Found. Sri Lanka 2022, 50, 331–344. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent organic pollutants in food: Contamination sources, health effects and detection methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Siddiqui, J.A.; Sadaf, M.; Liu, S.; Wang, J. Enrichment and dissemination of bacterial pathogens by microplastics in the aquatic environment. Sci. Total Environ. 2022, 830, 154720. [Google Scholar] [CrossRef] [PubMed]
- Hussien, N.H.; Mohammadein, A.; Tantawy, E.M.; Khattab, Y.; Al Malki, J.S. Investigating microplastics and potentially toxic elements contamination in canned tuna, salmon, and sardine fishes from Taif markets, KSA. Open Life Sci. 2021, 16, 827–837. [Google Scholar] [CrossRef]
- Chandralekha, A.P.L.; Baranage, C.; Samarajeewa, U. Formaldehyde levels in fish from Kandy market. J. Natl. Sci. Coun. Sri Lanka 1992, 20, 115–121. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K.; Elliott, C.; Jayasinghe, G.D.T.M. A review of the presence of formaldehyde in fish and seafood. Food Control 2022, 136, 108882. [Google Scholar] [CrossRef]
- Samarajeewa, U. Emerging challenges in maintaining marine food-fish availability and food safety. Comp. Rev. Food Sci. Food Saf. 2023, 22, 4734–4757. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery wastes as a yet undiscovered treasure from the sea: Biomolecules sources, extraction methods and valorization. Mar. Drugs. 2020, 18, 622. [Google Scholar] [CrossRef]
- Bao, M.; Cipriani, P.; Giulietti, L.; Roiha, I.S.; Paoletti, M.; Palomba, M.; Levsen, A. Air-dried stockfish of Northeast Arctic cod do not carry viable anisakid nematodes. Food Control 2020, 116, 107322. [Google Scholar] [CrossRef]
- Patterson, J.; Ranjitha, G. Qualities of commercially and experimentally sundried fin fish Scomberoides tol. Afr. J. Food Sci. 2009, 3, 299–302. Available online: http://www.academicjournals.org/ajfs (accessed on 25 January 2024).
- Bantle, M.; Hanssler, J. Ultrasonic convective drying kinetics of clip fish during the initial drying period. Dry. Technol. 2013, 31, 1307–1316. [Google Scholar] [CrossRef]
- Al-Rubai, H.H.; Hassan, K.A.; Eskandder, M.Z. Drying and salting fish using different methods and their effect on the sensory, chemical and microbial indices. Multidiscip. Rev. 2020, 3, e2020003. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [PubMed]
- Narzary, Y.; Das, S.; Goyal, A.K.; Lam, S.S.; Sarma, H.; Sharma, D. Fermented fish products in South and Southeast Asian cuisine: Indigenous technology processes, nutrient composition, and cultural significance. J. Ethn. Food 2021, 8, 33. [Google Scholar] [CrossRef]
- Ly, D.; Mayrhofer, S.; Schmidt, J.-M.; Zitz, U.; Domig, K.J. Biogenic amine contents and microbial characteristics of Cambodian fermented foods. Foods 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Atapattu, R.; Samarajeewa, U. Fungi associated with dried fish in Sri Lanka. Mycopathologia 1990, 111, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Patir, B.; İnanlı, A.G.; Öksüztepe, G.; Ilhak, O.I. Microbiological and chemical qualities of salted grey mullet (Chalcalburnus tarichii PALLAS, 1811). Int. J. Food Sci. Technol. 2006, 1, 91–98. Available online: https://www.researchgate.net/publication/237505777 (accessed on 15 February 2024).
- Deng, Y.; Wang, Y.; Deng, Q.; Sun, L.; Wang, R.; Ye, L.; Tao, S.; Liao, J.; Gooneratne, R. Fungal diversity and mycotoxin contamination in dried fish products in Zhanjiang market, China. Food Control 2021, 121, 107614. [Google Scholar] [CrossRef]
- Health Canada. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-addtives/lists-permitted/11-preservatives.html (accessed on 11 February 2024).
- Ahmad, I.; Traynor, M.P. Impact of high-pressure processing and sous vide cooking on the physicochemical, sensorial, and textural properties of Fresh Whiteleg Shrimp (Litopenaeus setiferus). J. Aquat. Food Prod. Technol. 2022, 31, 508–524. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, D.; Liu, H.; Zhu, C.; Sun, Y.; Wu, J.; Zheng, M.; Zhang, D. Effects of water distribution and protein degradation on the texture of high pressure-treated shrimp (Penaeus monodon) during chilled storage. Food Control 2022, 132, 108555. [Google Scholar] [CrossRef]
- Roobab, U.; Fidalgo, L.G.; Arshad, R.N.; Khan, A.W.; Zeng, X.; Bhat, Z.F.; Bekhit, A.E.A.; Batool, Z.; Aadil, R.M. High-pressure processing of fish and shellfish products: Safety, quality, and research prospects. Comp. Rev. Food Sci. Food Saf. 2022, 21, 3297–3325. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Meng, L.; Wang, S.; Li, J.; Mao, X. Inactivation of Vibrio parahaemolyticus and retardation of quality loss in oyster (Crassostrea gigas) by ultrasound processing during storage. Food Res. Inter. 2023, 168, 112722. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, T.; Gowda, N.A.N.; Kambhampati, V. Ultrasonication in seafood processing and preservation: A comprehensive review. Appl. Food Res. 2022, 2, 100208. [Google Scholar] [CrossRef]
- Abel, N.; Rotabakk, B.T.; Lerfall, J. Mild processing of seafood—A review. Comp. Rev. Food Sci. Food Saf. 2022, 21, 340–370. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, K.A.; Benjakul, S.; Qi, H.; Zhang, B.; Deng, S. Combined hurdle effects of pulsed electric field and vacuum impregnation of Chamuang leaf extract on quality and shelf-life of Pacific white shrimp subjected to high voltage cold atmospheric plasma. Food Packag. Shelf Life 2021, 28, 100660. [Google Scholar] [CrossRef]
- Khouryieh, H.A. Novel and emerging technologies used by the U.S. food processing industry. Innov. Food Sci. Emerg. Technol. 2021, 67, 102559. [Google Scholar] [CrossRef]
- Shen, Z.; Luan, A.; Yi, S.; Wu, J.; Wang, F.; Liu, Y.; Li, X. Moderate protein degradation and lipid oxidation induced by cold plasma and its effect on the quality of dried fish products. J. Food Compos. Anal. 2023, 123, 105636. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Wu, Y.; Yi, C.; Lv, Y.; Luo, H.; Yang, T. The antibacterial mechanism of compound preservatives combined with low voltage electric fields on the preservation of steamed mussels (Mytilus edulis) stored at ice-temperature. Front. Nutr. 2023, 10, 1126456. [Google Scholar] [CrossRef]
- Mandal, R.; Mohammadi, X.; Wiktor, A.; Singh, A.; Pratap Singh, A. Applications of pulsed light decontamination technology in food processing: An overview. Appl. Sci. 2020, 10, 3606. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, G.; Ji, S.; Zou, L.; Liang, J.; Walayat, N.; Chen, J.; Lyu, F.; Ding, Y. Effect of pulse light on the quality of refrigerated (4 °C) large yellow croaker (Pseudosciaena crocea). LWT 2022, 167, 113855. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Dong, X.; Dong, S.; Xiang, Q.; Bai, Y. Effects of UVC light-emitting diodes on microbial safety and quality attributes of raw tuna fillets. LWT 2021, 139, 110553. [Google Scholar] [CrossRef]
- Sheng, L.A.; Li, X.R.; Wang, L.X. Photodynamic inactivation in food systems: A review of its application, mechanisms, and future perspective. Trends Food Sci. Technol. 2022, 124, 167–181. [Google Scholar] [CrossRef]
- Do Prado-Silva, L.; Brancini, G.T.P.; Braga, G.U.L.; Liao, X.Y.; Ding, T.; Sant’Ana, A.S. Antimicrobial photodynamic treatment (aPDT) as an innovative technology to control spoilage and pathogenic microorganisms in agri-food products: An updated review. Food Control 2022, 132, 108527. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lin, C.-S.; Zeng, W.-H.; Hwang, C.-C.; Chiu, K.; Ou, T.-Y.; Chang, T.-H.; Tsai, Y.-H. Effect of a novel microwave assisted induction heating (MAIH) technology on the quality of prepackaged Asian hard clam (Meretrix lusoria). Foods 2021, 10, 2299. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Hwang, C.-C.; Kao, J.-C.; Ou, T.-Y.; Chang, T.-H.; Lee, S.-H.; Lee, Y.-C. Cooking and pasteurizing evaluation of barramundi (Lates calcarifer) meats subjected to an emerging microwave-assisted induction heating (MAIH) technology. Innov. Food Sci. Emerg. Technol. 2022, 80, 103089. [Google Scholar] [CrossRef]
- Pankyamma, V.; Madhusudana Rao, B.; Debbarma, J.; Naga, P.P.V. Physicochemical, microstructural, and microbial qualities of dehydrated tuna chunks: Effects of microwave power and drying methods. J. Food Process. Preserv. 2021, 45, e15426. [Google Scholar] [CrossRef]
- Valø, T.; Jakobsen, A.N.; Lerfall, J. The use of atomized purified condensed smoke (PCS) in cold-smoke processing of Atlantic salmon—Effects on quality and microbiological stability of a lightly salted product. Food Control 2020, 112, 107155. [Google Scholar] [CrossRef]
- Waldenstrøm, L.; Gaarder, M.Ø.; Lerfall, J. Sensory methodology in product optimization of cold smoked Atlantic salmon (Salmo salar L.) processed with atomized purified condensed smoke. J. Food Sci. 2021, 86, 4650–4667. [Google Scholar] [CrossRef]
- Blogoslawski, W.; Stewart, M.E. Some ozone applications in seafood. Ozone Sci. Eng. 2011, 33, 368–373. [Google Scholar] [CrossRef]
- Goncalves, A.A. Ozone as a safe and environmentally friendly tool for seafood industry. J. Aquat. Food Prod. Technol. 2016, 25, 210–229. [Google Scholar] [CrossRef]
- Cosansu, S.; Mol, S.; Haskaraca, G. Sous-vide cooking: Effects on seafood quality and combination with other hurdles. Int. J. Gastron. Food Sci. 2022, 29, 100586. [Google Scholar] [CrossRef]
- Palamae, S.; Temdee, W.; Buatong, J.; Zhang, B.; Hong, H.; Benjakul, S. Enhancement of safety and quality of ready to-cook Asian green mussel using acidic electrolyzed water depuration in combination with sous vide cooking. Innov. Food Sci. Emerg. Technol. 2023, 87, 103391. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Paulino, B.N.; Silva, G.N.S.; Araújo, F.F.; Néri-Numa, I.A.; Pastore, G.M.; Bicas, J.L.; Molina, G. Beyond natural aromas: The bioactive and technological potential of monoterpenes. Trends Food Sci. Technol. 2022, 128, 188–201. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Torrieri, E.; Masi, P. Emerging technologies in seafood processing: An overview of innovations reshaping the aquatic food industry. Comp. Rev. Food Sci. Food Saf. 2024, 23, e13281. [Google Scholar] [CrossRef] [PubMed]
- Abelti, A.L.; Teka, T.A.; Forsido, S.F.; Tamiru, M.; Bultosa, G.; Alkhtib, A.; Burton, E. Bio-based smart materials for fish product packaging: A review. Int. J. Food Prop. 2022, 25, 857–871. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wei, A.; Li, H.; Zhang, H.; Zheng, L.; Xia, N.; Wang, J. Protein-based active films: Raw materials, functions, and food applications. Comp. Rev. Food Sci. Food Saf. 2024, 23, e13302. [Google Scholar] [CrossRef]
- Ibrahim, S.; Fahmy, H.; Salah, S. Application of interactive and intelligent packaging for fresh fish shelf-life monitoring. Front Nutr. 2021, 21, 677884. [Google Scholar] [CrossRef]
- Don, S.; Ammini, P.; Nayak, B.B.; Kumar, S.H. Survival behaviour of Salmonella enterica in fish and shrimp at different conditions of storage. LWT 2020, 132, 109795. [Google Scholar] [CrossRef]
- Podolska, M.; Pawlikowski, B.; Nadolna-Ałtyn, K.; Pawlak, J.; Szymczak, K.; Szostakowska, B. How effective is freezing at killing Anisakis simplex, Pseudoterranova krabbei, and P. decipiens larvae? An experimental evaluation of time-temperature conditions. Parasitol. Res. 2019, 118, 2139–2147. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhu, C.; Zhang, D.; Liu, H. Effects of high-pressure processing on aquatic products with an emphasis on sensory evaluation. Int. J. Food Sci. Technol. 2022, 57, 6980–6996. [Google Scholar] [CrossRef]
- Nema, P.K.; Sehrawat, R.; Ravichandran, C.; Kaur, B.P.; Kumar, A.; Tarafdar, A. Inactivating food microbes by high-pressure processing and combined nonthermal and thermal treatment: A review. J. Food Qual. 2022, 2022, 5797843. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Bulut, S.; Karatzas, K.A.G.; Boziaris, I.S. Inactivation of Listeria monocytogenes in raw and hot smoked trout fillets by high hydrostatic pressure processing combined with liquid smoke and freezing. Innov. Food Sci. Emerg. Technol. 2020, 64, 102427. [Google Scholar] [CrossRef]
- Castell-Perez, M.E.; Moreira, R.G. Irradiation and consumers acceptance. Innov. Food Process. Technol. 2021, 122–135. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Kim, S.-W.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Durazzo, A.; Di Lena, G.; Gabrielli, P.; Santini, A.; Lombardi-Boccia, G.; Lucarini, M. Nutrients and bioactive compounds in seafood: Quantitative literature research analysis. Fishes 2022, 7, 132. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.E.; Wassef, E.A.; Abdel-Mohson, H.H. Sustainable Fish and Seafood Production and Processing; Galankis, C.M., Ed.; Academic Press: London, UK, 2022; Available online: https://www.researchgate.net/publication/355081677 (accessed on 18 February 2024).
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Hutamekalin, P.; Nilsuwan, K.; Sukketsiri, W.; Aluko, R.E.; Abdul, N.R.; Benjakul, S. Combined effects of defatted hydrolyzed collagen from salmon skin and vitamin C on proliferation and migration of human fibroblast cell. Fishes 2022, 7, 265. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Patil, U.; Tu, C.; Zhang, B.; Benjakul, S. Salmon skin acid-soluble collagen produced by a simplified recovery process: Yield, compositions, and molecular characteristics. Fishes 2022, 7, 330. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Fusang, K.; Pripatnanont, P.; Benjakul, S. Properties and characteristics of acid-soluble collagen from salmon skin defatted with the aid of ultrasonication. Fishes 2022, 7, 51. [Google Scholar] [CrossRef]
- Rather, J.Z.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Francisco, J.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Fan, H.Y.; Dumont, M.-J.; Simpson, B.K. Preparation and physicochemical characterization of films prepared with salmon skin gelatin extracted by a trypsin-aided process. Curr. Res. Food Sci. 2020, 3, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166. [Google Scholar] [CrossRef] [PubMed]
- Aditi, M.; Varsha, L. Fish processing: Product and by-product, processing and marketing. Int. J. Fish. Aquat. Stud. 2020, 8, 74–77. [Google Scholar] [CrossRef]
- Yu, E.; Pan, C.; Chen, W.; Ruan, Q.; Luo, X.; Lv, M.; Fang, Y.; Jiang, L.; Ma, H. Gelatin from specific freshwater and saltwater fish extracted using six different methods: Component interactions, structural characteristics, and functional properties. LWT 2024, 191, 115656. [Google Scholar] [CrossRef]
- Esparza-Espinoza, D.M.; del Carmen Santacruz-Ortega, H.; Plascencia-Jatomea, M.; Aubourg, S.P.; Salazar-Leyva, J.A.; Rodríguez-Felix, F.; Ezquerra-Brauer, J.M. Chemical-structural identification of crude gelatin from jellyfish (Stomolophus meleagris) and evaluation of its potential biological activity. Fishes 2023, 8, 246. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and mechanical characteristics of gelatin-based films as a potential food packaging material: A review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Nurdiani, R.; Ma’rifah, R.D.A.; Busyro, I.K.; Jaziri, A.A.; Prihanto, A.A.; Firdaus, M.; Talib, R.A.; Huda, N. Physical and functional properties of fish gelatin-based film incorporated with mangrove extracts. PeerJ 2022, 10, e13062. [Google Scholar] [CrossRef]
- Derbew Gedif, H.; Tkaczewska, J.; Jamróz, E.; Zając, M.; Kasprzak, M.; Pajak, P.; Grzebieniarz, W.; Nowak, N. Developing technology for the production of innovative coatings with antioxidant properties for packaging fish products. Foods 2023, 12, 26. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Abu Dayah, A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Álvarez, G. Collagen hydrolysates for skin protection: Oral administration and topical formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ye, X.; Hou, Z.; Shiguo Chen, S. Sustainable utilization of proteins from fish processing by-products: Extraction, biological activities and applications. Trends Food Sci. Technol. 2024, 143, 104276. [Google Scholar] [CrossRef]
- Bechtel, P.J.; Morey, A.; Oliveira, A.C.M.; Wu, T.H.; Plante, S.; Bower, C.K. Chemical and nutritional properties of Pacific Ocean perch (Sebastes alutus) whole fish and by-products. J. Food Process. Preserv. 2010, 34, 55–72. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Du, J.; Li, Y. Review and perspective on bioactive peptides: A roadmap for research, development, and future opportunities. J. Agric. Food Res. 2022, 9, 100353. [Google Scholar] [CrossRef]
- Dinakarkumar, Y.; Krishnamoorthy, S.; Margavelu, G.; Ramakrishnan, G.; Chandran, M. Production and characterization of fish protein hydrolysate: Effective utilization of trawl by-catch. Food Chem. Adv. 2022, 1, 100138. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, F.-X.; Yu, J.; Li, X.-H.; Liu, Y.-L. Cryoprotective effects of protein hydrolysates prepared from by-products of silver carp (Hypophthalmuchthys molitrix) on freeze thawed surimi. Appl. Sci. 2019, 9, 563. [Google Scholar] [CrossRef]
- Sanapala, P.; Pola, S. Chapter 17—Bioactive Peptides as a Potential Antioxidants from Marine Byproducts. In Marine Antioxidants; Kim, S.-K., Shin, K.-H., Venkatesan, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 241–247. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef]
- Wangkheirakpam, M.R.; Mahanand, S.S.; Majumdar, R.K.; Sharma, S.; Hidangmayum, D.D.; Netam, S. Fish waste utilization with reference to fish protein hydrolysate—A Review. Fish. Technol. 2019, 56, 169–178. Available online: https://www.researchgate.net/publication/334823582 (accessed on 20 February 2024).
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.R.; Howieson, J.; Foysal, M.J.; Hanif, M.A.; Abdel-Latif, H.M.R.; Fotedar, R. Fish waste to sustainable additives: Fish protein hydrolysates alleviate intestinal dysbiosis and muscle atrophy induced by poultry by-product meal in Lates calcarifer juvenile. Front. Nutr. 2023, 10, 1145068. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Odila, J.; Voss, G.; Martins, R.; Rosa, A.; Couto, J.A.; Almeida, A.; Pintado, M. Fish by-products: A source of enzymes to generate circular bioactive hydrolysates. Molecules 2023, 28, 1155. [Google Scholar] [CrossRef] [PubMed]
- Klomklao, S.; Kuepethkaew, S.; Benjakul, S.; Zhang, Y.; Simpson, B.K. Enzymes from fish processing waste materials and their commercial applications. In Fish Waste to Valuable Products. Sustainable Materials and Technology; Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A., Eds.; Springer: Singapore, 2024; pp. 147–194. [Google Scholar] [CrossRef]
- Hisham, F.; Akmal, M.H.M.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer chitosan: Potential sources, extraction methods, and emerging applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, H.; Huang, J.; Zhao, J.; Yan, B.; Ma, S.; Zhang, H.; Fan, D. The physicochemical properties of chitosan prepared by microwave heating. Food Sci. Nutr. 2020, 8, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess 2019, 6, 8. [Google Scholar] [CrossRef]
- Ploydee, E.; Chaiyanan, S. Production of high viscosity chitosan from biologically purified chitin isolated by microbial fermentation and deproteinization. Int. J. Polym. Sci. 2014, 2014, 162173. [Google Scholar] [CrossRef]
- Ahing, F.A.; Wid, N. Extraction and characterization of chitosan from shrimp shell waste in Sabah. Trans. Sci. Technol. 2016, 3, 227–237. Available online: https://www.researchgate.net/publication/312498253 (accessed on 16 February 2024).
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. Sustainable Agriculture Reviews; Springer International Publishing AG: Cham, Switzerland, 2019; p. 338. ISBN 978-3-030-16538-3; 978-3-030-16537-6. [Google Scholar]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Varzakas, T.; Munekata, P.E.S.; Movilla Fierro, E.; Lorenzo, J.M. Omega-3-rich oils from marine side streams and their potential application in food. Mar. Drugs 2021, 19, 233. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Oszajca, K.; Zep, W.; Piekarska, A.; Sidorkiewicz, M. The impact of short-term shark liver oil supplementation on the fatty acid composition of erythrocyte membranes. Nutrients 2021, 13, 3329. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Karadeniz, F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Shalfoh, E.; Ahmad, M.I.; Binhweel, F.; Shaah, M.A.; Senusi, W.; Hossain, M.S.; Alsaadi, S. Fish waste oil extraction using supercritical CO2 extraction for biodiesel production: Mathematical, and kinetic modeling. Renew. Energy 2024, 220, 119659. [Google Scholar] [CrossRef]
- Mu, W.; Kleter, G.A.; Bouzembrak, Y.; Dupouy, E.; Frewer, L.F.; Natour, F.N.R.A.; Marvin, H.J.P. Making food systems more resilient to food safety risks by including artificial intelligence, big data and internet things into food safety, early warning and risk identification tools. Crit. Rev. Food Sci. Saf. 2024, 23, e13296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).