Abstract
In order to study the muscle quality of different crustaceans, we aim to provide a comprehensive assessment of their muscle quality characteristics as a theoretical reference. In this work, seven major species of farmed and consumer crustaceans were selected, including crayfish (Procambarus clarkii), freshwater prawns (Macrobrachium rosenbergii), pacific white shrimp (Litopenaeus vannamei), black tiger shrimp (Penaeus monodon), kuruma prawns (Penaeus japonicus), river prawns (Macrobranchium nipponense), and Chinese shrimp (Penaeus chinensis). Their morphometric parameters, nutritional composition, textural properties, and physical and chemical indexes were comparatively analyzed. The results showed that the meat content (MC, about 14.78%) of crayfish was significantly lower than that of the other six species. By contrast, pacific white shrimp had the highest MC, although the MCs of black tiger shrimp, kuruma prawns, and Chinese shrimp are greater than 40%. All seven crustacean species were high in protein and low in fat, while pacific white shrimp had the highest crude protein, crude lipid, and crude ash content compared to the other crustaceans. The content of threonine (Thr) was the highest in crayfish. The content of methionine (Met) and lysine (Lys) was the highest in freshwater prawns. The content of isoleucine (Ile), leucine (Leu), and non–essential amino acid (NEAA) was the highest in pacific white shrimp. C18:2n-6 (linoleic acid, LA) was the highest in freshwater prawns and pacific white shrimp; C18:3n-3 (linolenic acid, LNA), C20:4n-6 (arachidonic acid, AA), unsaturated fatty acids (UFA), and monounsaturated fatty acids (MUFA) were all the highest in crayfish; and polyunsaturated fatty acids (PUFA) was the highest in freshwater prawns, but the content was not significantly different from crayfish, pacific white shrimp, black tiger shrimp, and Chinese shrimp. Pacific white shrimp had the highest values for hardness, cohesiveness, gumminess, and chewiness. The redness values of crayfish, black tiger shrimp, and Chinese shrimp were significantly higher than those of the other three species except kuruma prawns. Compared to other crustaceans, river prawns had the highest drip loss and cooking loss. Black tiger shrimp and Chinese shrimp had the lowest cooking loss rates. The research shows that the tail muscle of the seven species of crustaceans is rich in protein, essential amino acids, unsaturated fatty acids and low in fat, representing a high-quality protein. Among these crustaceans, the main essential amino acids and essential fatty acids in the tail muscle of pacific white shrimp, freshwater prawns, and crayfish are higher in content and better in nutritional value.
Keywords:
muscle quality; amino acid composition; fatty acid composition; textural properties; crustaceans Key Contribution:
A comprehensive evaluation system for the muscle quality of crustaceans is initially established. These seven crustaceans species are high-protein and low-lipid quality aquatic products. Pacific white shrimp have better nutritional value and taste compared with other crustaceans.
1. Introduction
With the improvement of consumption levels, more consumers are not satisfied with only demanding nutritional quality, but also have higher demands on the taste, toughness and freshness of food. In recent years, various aquatic products have gained great popularity among consumers and have become a unique culture on the table, characterized by their crispness and tenderness, texture and chroma, and delicious taste. More consumers are sparing no expense in pursuing their tastes. Muscle quality is a comprehensive evaluation index, which is mainly evaluated based on the two aspects of muscle nutrition and muscle flavor [1]. It is important to analyze muscle quality indexes and explore ways to improve muscle quality to meet consumers’ demand for food production.
Current domestic and international studies on muscle quality have focused on the meat content, nutrient content, amino acid, fatty acid content, and textural properties of aquatic animals [2,3,4]. The physicochemical properties of muscles, such as textural characteristics, meat color, water holding capacity (WHC), and pH are also important indicators for assessing muscle quality [5,6]. It has been found that the addition of various nutrients [7,8,9,10] and additives [11,12,13] to feed can affect the nutrient, amino acid, and fatty acid content of the muscles of aquatic animals. Xu et al. [14] showed that optimal amounts of dietary protein improved the physical (or chemical) flavor quality of grass carp fillets by increasing the shear force, pH, hydroxyproline, protein, lipid, free amino acid, and nucleotide content (5’ inosinic acid), and decreased cooking loss, cathepsin B and L activities, and lactate content. Myofiber content was approximately 15% higher in Atlantic salmon (Salmo salar L.) fed high-protein diets than those fed low-protein diets [15]. In a study on rainbow trout (Oncorhynchus mykiss), the muscles of rainbow trout fed a high-protein diet had lower brightness levels and higher redness levels [16]. Under normal conditions, most of the muscle coloration of aquatic animals is derived from carotenoids in feedstuffs, so the flesh color of aquatic animals can be influenced by feeding feedstuffs containing pigments [5]. Astaxanthin is a non-vitamin-A-derived carotenoid with strong pigment deposition ability. Its use as a dye in aquatic animals improves the value of (a*) in the shell and muscle of black tiger shrimp [17]. Furthermore, muscle quality varies significantly between species; for example, silver carp (Hypophthalmichthys molitrix) has significantly lower meat quality than that of mandarin fish (Siniperca chuatsi) [18]. However, the assessment of muscle quality of aquatic animals is not unified. There are studies on nutrient composition and there are studies on textural properties, but there is not yet a unified standard. In addition, there are few comparisons of the muscle quality of different aquatic economic animals, and the reasons for the differences between them are not yet known.
Crayfish (Procambarus clarkii), freshwater prawns (Macrobrachium rosenbergii), pacific white shrimp (Litopenaeus vannamei), black tiger shrimp (Penaeus monodon), kuruma prawns (Penaeus japonicus), river prawns (Macrobranchium nipponense), and Chinese shrimp (Penaeus chinensis) are the most consumed crustaceans in China. They are loved by the majority of consumers and have significant economic importance. Crayfish are widely distributed in various aquatic ecosystems due to their remarkable environmental adaptability and robust viability. Crayfish have rapidly developed in the industry in China in recent years due to their bright color, delicious taste, and rich nutritional value and the high opinion of consumers [19]. China’s farmed production of crayfish reached 2.891 million tons in 2022. Freshwater prawns, with their rapid growth, extensive feeding habits, and short production cycles achieved a high production of 177,836 tons in 2022 [20]. Pacific white shrimp are one of the most important commercial aquaculture species due to their rapid growth rate and resistance to adversity, accounting for about 80% of global shrimp production [21], and they are farmed in China. Their production reached 1.340 million tons in 2022. Black tiger shrimp are one of the largest and fastest-growing commercial shrimps; they live mainly in the Indo-West Pacific region, the east coast of Hainan Island and the southeast coast of China, and are widely farmed in China [22]. China’s farmed production reached 114.36 thousand tons in 2022. Kuruma prawns are a commercially important species in several countries, including China, Japan, and the Mediterranean regions around Egypt, Israel, and Turkey. Kuruma prawns are one of the largest penaeid prawns. Due to their high desiccation resistance, good taste, and rich nutritional content, kuruma prawns are one of the most economically important members of the Penaeidae family [23]. China’s farmed production reached 46,199 tons in 2022. River prawns occur over a wide geographical range in East Asia. They are of significant importance as a commercial aquaculture species in China; China’s farm production reached 226,312 tons in 2022 [24]. Chinese shrimp are one of the most economically important commodities in the global fishing industry. They are rich in nutrients needed by the human body and are preferred by consumers owing to their taste. China’s farmed production reached 30,929 tons in 2022 [25]. However, the aquaculture industry faces issues such as intensive farming, high culturing density, and water quality that affect muscle quality; therefore, these issues, among others, need to be addressed.
Consequently, in this study, the muscle quality of these seven crustaceans species was more comprehensively evaluated and compared, and the differences between their muscle qualities were more comprehensively described to provide a reference for the study of muscle quality in aquatic animals.
2. Materials and Methods
2.1. Animals and Sample Collection
A total of 2 kg of crayfish, freshwater prawns, pacific white shrimp, black tiger shrimp, kuruma prawns, river prawns, and Chinese shrimp were all purchased from the Xiaolinwei fair trade market in Nanjing (all met the adult crustacean market specifications). All animals were treated in accordance with the following license established by the Animal Experiment Ethics Committee (Approval Code: SHOU-DW-2022-024). Whole and homogeneous individuals were selected and then washed under running water, surface moisture was wiped off with gauze, the head and shell were removed, and the specimens were stored at −20 °C under preservation conditions until the proximate composition, amino acids, and fatty acids of the muscle were to be measured.
2.2. Experimental Parameters Measured
2.2.1. Morphometric Parameters
Thirty intact and homogeneous individuals of each crustaceans species were randomly selected from the purchased crustaceans. The crustaceans were dried, and their body length and weight were measured. The head, chest plate, and shell were removed, and then the muscles and hepatopancreas were weighed.
Meat content (MC, %) = muscle weight/body weight × 100%
Hepatosomatic index (HSI, %) = hepatopancreas weight/body weight × 100%
Condition factor (CF) = body weight/body length3 × 100%
2.2.2. Proximate Composition Analysis
Muscles were measured using protocols based on the AOAC [26]. Moisture, crude protein, crude lipid, and crude ash were measuredusing the direct drying method, semi-micro Kjeldahl nitrogen determination method, Soxhlet extraction method, and high-temperature ashing method, respectively.
2.2.3. Amino Acids
Amino acids were determined according to the method of Thiago et al. [27] using a high-speed amino acid analyzer (L8800, Hitachi, Tokyo, Japan). A sample of approximately 0.2 g was weighed and 20 mL of 1 + 1 HCl were added. The sample was hydrolyzed in an electric air-drying oven at 110 °C for 22 h. Then, exactly 100 μL of the sample was dried at 60 °C for 2 h (all solvent was dried), mixed with distilled water to 0.5 mL, and then pressed through the 0.45 μm organic membrane on the machine. Five replicates of each crustacean species were used.
2.2.4. Fatty Acids
The fatty acid content was analyzed by gas chromatography (GC-2010, Shimadzu, Kyoto, Japan) according to the method of Thiago et al. [27]. A sample of approximately 0.2 g was taken randomly and added to 2 mL of a petroleum ether–ether mixture (v/v, 1:1) overnight. Then, 1.5 mL of a KOH–methanol solution was added and mixed, and the sample was allowed to stand for 1 h. After centrifugation at 10,000 rpm for 10 min, the supernatant was removed and 1.5 mL of BF3-CH3OH at a mass fraction of 14% was added. The fatty acids were placed in a 55 °C water bath for 30 min. After cooling, 1.5 mL each of hexane and saturated NaCl solution were added and left to stratify at 4 °C, then the supernatant was removed completely, and the solution was filtered through an organic phase filter membrane with a pore size of 0.22 μm and then analyzed by gas chromatography. Five replicates of each crustaceans species were used.
2.2.5. Textural Properties
The textural properties (hardness, cohesiveness, elasticity, adhesiveness, and chewability) of crustaceans muscles were analyzed using a texture analyzer (TMS-Touch, FTC, Washington, DC, USA) equipped with an 8 mm flat-end cylinder probe. A double compression method was used to construct the parameters. The test conditions were two consecutive rounds of compression at a speed of 30 mm/min, with a deformation of 60% of the original length and an initial force of 0.1 N [28].
2.2.6. Physical and Chemical Properties
Each sample was placed under the lens of a colorimeter (CR-410, Konica Minolta, Tokyo, Japan) to measure the chroma of the muscle samples. Five crustaceans were measured in each group, and three replicates were measured of each crustacean. According to the recommendation of the International Commission on Illumination [29], the skin color parameters were represented by L*, a*, and b*. After correction by a whiteboard, samples’ L*, a*, and b* were measured. L* stands for brightness, where a positive value is brighter, and a negative value is darker; a* is red–green value, where positive values are red, and negative values are green; and b* is yellow–blue value, where positive values are yellow, and negative values are blue.
Drip loss and cooking loss were measured using the methods described in previous studies [30,31] with minor modifications. To determine drip loss, five crustaceans of each species were randomly sampled, and the weight of each crustacean muscle was measured as W0. Five crustaceans’ muscles were tied with thin threads and then hung in the refrigerator at 4 °C for 24 h. The body surfaces were wiped dry with filter paper, and the weight of each crustacean muscle was measured again as W1. For cooking loss, the meat of the remaining five crustaceans was placed in a zip-lock bag labeled with the group and then placed in a water bath heated to 70 °C for 10 min. The weight of each crustacean muscle was measured again as W2. The calculations are as follows:
Drip loss (%) = (W0 − W1)/W0 × 100%
Cooking loss (%) = (W0 − W2)/W0 × 100%
Muscle pH was measured referring to the method of Fuentes et al. [32]. An amount of 1 g of muscle was added to 10 mL of distilled water, homogenized, and left to stand for 30 min, and then the pH of the meat samples was measured with a pH meter (PB-10, Sartorius, Göttingen, Germany).
2.3. Statistical Analyses
The experimental data were analyzed using SPSS 25.0 for biological statistics. The assumptions of normality and mean square deviation were confirmed before any statistical analysis. A one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test, was used to determine whether different levels of substitution had a significant effect on the measurements. The level of significance was set as p < 0.05. The statistics were expressed as mean ± SEM (standard error of the mean) and differences were considered significant at p < 0.05.
3. Results
3.1. Morphometric Parameters of Seven Crustaceans Species
Table 1 shows the morphometric parameters of crayfish, freshwater prawns, pacific white shrimp, black tiger shrimp, kuruma prawns, river prawns, and Chinese shrimp. In the present study, river prawns had the lowest body weight compared to other species, while crayfish had the highest body weight (p < 0.05). Pacific white shrimp and black tiger shrimp had the longest body lengths and were significantly longer than those of the other groups, while river prawns had the shortest body lengths. The hepatosomatic index (HSI) of crayfish and freshwater prawns was significantly higher than that of the other five species (p < 0.05), while the difference in HSI among the other five crustaceans species was not significant (p > 0.05). The meat content (MC) of crayfish (14.74%) was the lowest value and significantly lower than that of the other six crustaceans species (p < 0.05), whereas pacific white shrimp had the highest MC, followed by black tiger shrimp and kuruma prawns, with 48.04%, 46.61%, and 44.60%, respectively, and there were no significant differences among these three crustaceans species (p > 0.05). The condition factor (CF) of crayfish was significantly higher than that of the other six crustaceans species (p < 0.05).

Table 1.
Morphometric parameters of seven crustaceans species.
3.2. Proximate Composition of Muscle
As shown in Table 2, pacific white shrimp had the lowest moisture, and crayfish and river prawns had the highest moisture values, with no significant difference (p > 0.05) between these two species. Pacific white shrimp, black tiger shrimp, and Chinese shrimp had the highest crude protein contents, which were significantly higher than those of crayfish and river prawns (p < 0.05). The crude lipid content of freshwater prawns and pacific white shrimp was significantly higher than the other five crustaceans species, while kuruma prawns had the lowest value (p < 0.05). The crude ash content of pacific white shrimp was significantly higher than that of crayfish and river prawns and reached the highest value (p < 0.05), while river prawns had the lowest crude ash content.

Table 2.
Muscle composition of seven crustaceans species.
3.3. Amino Acid Composition of Muscle
As shown in Table 3, 16 amino acids could be detected in all seven crustaceans species, including nine essential amino acids (EAAs) and seven non-essential amino acids (NEAAs), while cysteine (Cys) could only be detected in crayfish, freshwater prawns, and pacific white shrimp. The content of threnonine (Thr) was the highest in crayfish. The contents of methionine (Met) and lysine (Lys) were the highest in freshwater prawns. The contents of isoleucine (Ile) and leucine (Leu) were the highest in pacific white shrimp. The content of histidine (His) was the highest in crayfish and freshwater prawns. The contents of valnine (Val) and phenylalanine (Phe) were the highest in freshwater prawns and pacific white shrimp. Arg was the most abundant essential amino acid and the most abundant of all the amino acids, with no significant differences among the seven crustaceans species. Pacific white shrimp had the highest NEAA and umami amino acids (UAA) values, but there was no significant difference in EAAs (p > 0.05). Pacific white shrimp and black tiger shrimp had the highest NEAA content, while crayfish, freshwater prawns, and river prawns had the lowest NEAA contents and were significantly lower than those of the other four crustaceans species (p < 0.05). The UAA contents of pacific white shrimp, black tiger shrimp, and Chinese shrimp were the highest and significantly higher than those of the other four crustaceans species (p < 0.05). However, there was no significant difference in EAA content among the seven crustaceans species (p > 0.05).

Table 3.
Amino acid composition of muscles of seven crustaceans species.
3.4. Fatty Acid Composition of Muscle
As shown in Table 4, crayfish, freshwater prawns, river prawns, and Chinese shrimp had the most abundant fatty acids with 18, followed by black tiger shrimp with 17, while pacific white shrimp and kuruma prawns had 16. C18:2n-6 (LA) and C18:3n-3 (LNA) are essential fatty acids, and there were significant differences in their content among the seven crustaceans species (p < 0.05). The C18:2n-6 content of freshwater prawns and pacific white shrimp, which had the highest values, was significantly higher than that of the other five crustaceans species (p < 0.05). The C18:3n-3 content of crayfish was significantly higher than that of the other six species (p < 0.05) and was the highest value. The C20:4n-6 (AA) content of crayfish was significantly higher than that of the other six species (p < 0.05) and was the highest value. The content of saturated fatty acids (SFAs) in black tiger shrimp and kuruma prawns was the highest value; the SFA content in crayfish, which was the lowest value, was significantly lower than that of the other six crustaceans (p < 0.05). Crayfish had the highest unsaturated fatty acid (UFA) and monounsaturated fatty acid (MUFA) values. Freshwater prawns had the highest polyunsaturated fatty acid (PUFA) content, but the value was not significantly different (p < 0.05) from crayfish, pacific white shrimp, black tiger shrimp, and Chinese shrimp (p > 0.05).

Table 4.
Fatty acid composition of muscles of seven crustaceans species.
3.5. Textural Properties
As shown in Table 5, the hardness of freshwater prawns and pacific white shrimp was significantly higher than that of the other five crustaceans species (p < 0.05) and had the highest value, while the hardness of river prawns had the lowest value. The cohesiveness values of pacific white shrimp, black tiger shrimp, and river prawns were significantly higher than those of crayfish and freshwater prawns (p < 0.05). There was no significant difference from the other two crustaceans species (p > 0.05). The springiness of black tiger shrimp had the highest value, and the springiness of river prawns, which was the lowest value, was significantly lower than that of the other six species (p < 0.05). The gumminess and chewiness of pacific white shrimp were the highest values and were significantly higher than those of the other six crustaceans species (p < 0.05). The gumminess and the chewiness of crayfish and river prawns, which were the lowest values, were significantly lower than those of the other five crustaceans species (p < 0.05).

Table 5.
Textural properties of muscles of seven crustaceans species.
A radar map of the textural properties of seven crustaceans species is shown in Figure 1. It shows that the best textural properties (hardness, gumminess, and chewiness) were found in the pacific white shrimp, followed by freshwater prawns; the worst textural properties were found in river prawns.
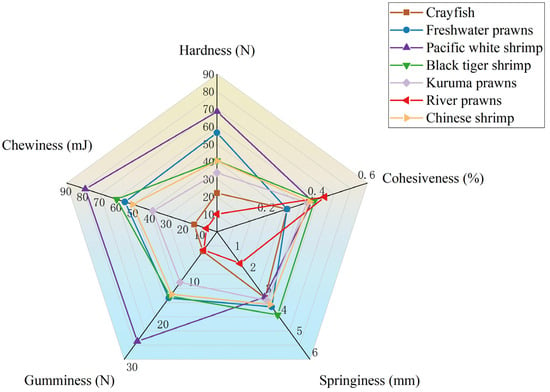
Figure 1.
Radar map of textural properties of seven crustaceans species.
3.6. Physical and Chemical Index
As shown in Table 6, the brightness (L*) of river prawns was significantly higher than that of the other six species (p < 0.05), while the L* of black tiger shrimp was significantly lower than that of the other five crustaceans species except for pacific white shrimp (p < 0.05). The redness (a*) of crayfish, black tiger shrimp, and Chinese shrimp was significantly higher than that of freshwater prawns, pacific white shrimp, and Chinese shrimp (p < 0.05). The yellowness (b*) values of freshwater prawns, pacific white shrimp, black tiger shrimp, and Chinese shrimp were negative and tended towards blue. The b* values of crayfish, kuruma prawns, and river prawns were positive and tended towards yellow. The b* of river prawns was significantly higher than that of the other six species (p < 0.05), and the b* of freshwater prawns was significantly lower than that of the other species except for black tiger shrimp (p < 0.05).

Table 6.
Physical and chemical indexes of muscles of seven crustaceans species.
As shown in Table 6, the drip loss of river prawns was the highest value. There was no significant difference among the other six crustaceans species (p > 0.05). The cooking loss rates of black tiger shrimp and Chinese shrimp were significantly lower than those of freshwater prawns, pacific white shrimp, and river prawns (p < 0.05). The pH value of kuruma prawns, which was the highest value, was significantly higher than that of crayfish, pacific white shrimp, and black tiger shrimp (p < 0.05); the pH of crayfish, which was the lowest value, was significantly lower than that of the other six crustaceans (p < 0.05).
4. Discussion
With the improvement of people’s living standards, more and more attention has been paid to the quality of crustaceans’ meat. This article selects seven main types of commercial crustaceans in China and makes a comprehensive assessment of their meat quality.
MC is one of the indexes for evaluating the quality, parental germplasm, and economic characteristics of aquatic products. It varies depending on the species, growth stage, living environment, and diet. In the present study, the body weight of crayfish was significantly higher than that of the other six crustaceans species. Nevertheless, the MC of crayfish was the lowest. This may be related to the thickening of the crayfish carapace, strong chelate foot, developed appendage foot, gonad development, and other factors. This indicates that the MC of crayfish is lower than that of other commercial crustaceans and depends on the growth stage, living environment, and origin [33]. Tian et al. [34] concluded that the MC of crustaceans is influenced by the environment in which they live, and the results of this work are similar; for example, the MC of crayfish in the Dongting Lake area was 20.21% [34] and that of crayfish in Nanwan reservoir area was 19.96% [35]. In the present study, pacific white shrimp had the highest MC, indicating that they contained more edible parts. Whether the higher MC is due to higher muscle quality needs to be further investigated. The nutritional value and flavor of these seven crustaceans species were also examined to determine differences in muscle quality.
The main nutritional component of crustaceans is muscle, and the nutrients in muscles are mainly protein and lipid, which are elementary indicators for evaluating muscle quality [36]. In the present study, the crude protein content of the seven crustaceans species was significantly higher than the crude lipid and ash content, indicating that crustaceans are a high-protein and low-lipid food [37]. It is known that protein is an essential part of the diet of aquatic animals and is required for the growth, development, reproduction, and survival of fish. The amount of protein content could affect the growth and nutritional values in the muscle of crustaceans [13,38]. Pacific white shrimp, black tiger shrimp, and Chinese shrimp had the highest crude protein content, but no significant difference from freshwater prawns and kuruma prawns; crude lipid content was also the highest value for freshwater prawns and pacific white shrimp. Therefore, freshwater prawns and pacific white shrimp are high-quality edible crustaceans with high protein and lipid contents.
Protein consists of amino acids. EAA content is an important nutritional value index for muscle production [39]. In the present study, the content of Thr was the highest in crayfish; Met and Lys were the highest in freshwater prawns; Ile and Leu were the highest in pacific white shrimp; His was the highest in crayfish and freshwater prawns; and Val and Phe were the highest in freshwater prawns and pacific white shrimp. In addition, umami amino acids (Phe, Ala, Gly, Asp, Glu, and Tyr) reflect the flavor of the muscle and are important indicators of muscle quality [38]. In the present study, the UAA contents of pacific white shrimp, black tiger shrimp, and Chinese shrimp were the highest and significantly higher than those of the other four crustaceans species, indicating that these three crustaceans species have better flavors. Overall, crayfish, freshwater prawns, and pacific white shrimp have high nutritional values of amino acids; however, pacific white shrimp, black tiger shrimp, and Chinese shrimp have better flavor.
More and more attention has been paid to the crucial role of fatty acids and predominantly unsaturated fatty acids in the human body so as to reduce blood lipids, prevent cardiovascular diseases, and promote growth and development. The World Health Organization (WHO) reported that reducing the saturated fatty acid (SFA) content and increasing the UFA content in the human diet is part of effective nutrition and prevention of chronic diseases [40]. In addition, PUFAs can significantly enhance the scent and reflect the juiciness of the muscle to a certain extent [41]. In terms of fatty acid composition, the fatty acid types of crayfish, freshwater prawns, river prawns, and Chinese shrimp were higher than those of the other three crustaceans species. The C18:3n-3, C20:4n-6, UFA, and PUFA contents had their highest values in crayfish, PUFA was highest in freshwater prawns, and C18:2n-6 had its highest values in freshwater prawns and pacific white shrimp. This shows that crayfish have the highest fatty acid value and strongest flavor, followed by freshwater prawns and pacific white shrimp. The highest content of SFA was palmitic acid (16:0), and seven of these were consistent. Palmitic acid can increase blood lipids and may cause the accumulation of blood cholesterol [42]. The crude lipid content of the seven crustaceans species was low, so palmitic acid would not cause nutritional problems. Recent studies have shown that MUFAs also play a role in regulating lipid metabolism, reducing the oxidation sensitivity of LDL cholesterol, protecting vascular endothelium, and reducing blood hypercoagulability [43]. Oleic acid (18:1) was the most abundant MUFA among the seven crustaceans species. Oleic acid is a benign fatty acid that can lower cholesterol and LDL [44]. In this paper, crayfish, freshwater prawns, and river prawns had high levels of oleic acid. Therefore, among the seven crustaceans species, crayfish have the highest nutritionally valuable fatty acids.
In addition to nutrients, the textural properties of muscle are important factors for their performance and represent the most important sensory and physicochemical index of muscle quality [45]. Hardness is represented by the human body’s sense of touch—softness or hardness, the force required to deform a food, the internal bond that maintains its shape. The greater the hardness, the greater the deformation force required for fracture and the greater the springiness [46]. Springiness can reflect the binding condition of prawn muscle tissue; the better the crucial ability, the higher the springiness. Both springiness and chewiness reflect the edible taste of prawn muscle [47]. In this study, the hardness, cohesiveness, gumminess, and chewiness of pacific white shrimp were the highest. On the contrary, the hardness, springiness, gumminess, and chewiness of river prawns were all minimal. Overall, the pacific white shrimp had the best textural properties, while the river prawns had the worst textural properties.
One of the key characteristics of meat quality is muscle chroma, which can be identified through visual perception. Muscle chroma itself does not contribute much to muscle flavor but is considered as a fleshy trait primarily because it represents the external appearance of the physiological, biochemical, and microbial changes of the muscle itself. Ferrous myoglobin (Mb) and hemoglobin (Hb) play an important role in muscle color. Therefore, muscle chroma is still commonly used to indicate meat quality. Muscle chroma is typically represented by a brightness value (L*), redness value (a*), and yellowness value (b*), with the change in a* having the best correlation with muscle chroma sensation [48]. In this study, the a* of crayfish, black tiger shrimp, and Chinese shrimp were significantly higher than those of the other species, except for kuruma prawns, suggesting that crayfish, black tiger shrimp, and Chinese shrimp have better visual indication of quality.
The amount of WHC is directly related to muscle texture and nutritional composition. The loss of water deprives the muscle not only of moisture but also nutrients and heme, affecting the sensory morphology and flesh color of the muscle [14]. Therefore, cooking loss rate and drip loss rate are essential parameters that reflect muscle water retention and show a negative correlation to quality [49]. In this study, the cooking loss of pacific white shrimp and river prawns was significantly higher than that of the other four crustaceans species except freshwater prawns, which is consistent with the study by Cui et al. [50], which showed that pacific white shrimp and river prawns lose more nutrients after cooking. The drip loss of river prawns was significantly higher than that of the other six crustaceans species, and the structural parameters of the hardness, springiness, and chewiness properties of river prawns were the lowest among the seven groups. Relevant studies also showed that drip loss is negatively correlated with the textural characteristics of muscle hardness, springiness, and chewiness [51], which was consistent with the results of this study. This also explains the high drip loss of river prawns. The pH value of muscle has a significant influence on muscle quality. When the pH of muscle decreases to the electrical value of the muscle protein or protein denaturation occurs, the mechanical properties of muscle are directly affected, especially retention, cooking loss, and dry processing capacity [52]. In the present study, the pH value of kuruma prawns was the highest. Still, it was not significantly different from that of freshwater prawns, river prawns, and Chinese shrimp. In contrast, the pH of crayfish was significantly lower than that of the other six species, suggesting that kuruma prawns, freshwater prawns, river prawns, and Chinese shrimp would be better preserved, boiled, and processed, while the opposite was true for crayfish.
5. Conclusions
In conclusion, our results suggest that each of these seven crustaceans species has its own advantages and that they are all high-quality sources of protein and rich in amino acids and fatty acids, capable of meeting the daily nutritional needs of the human body. Among them, the main essential amino acids and essential fatty acids in the tail muscles of pacific white shrimp, freshwater prawns, and crayfish are higher in content and have better nutritional value. In addition, the textural properties of pacific white shrimp are relatively better.
Author Contributions
Conceptualization, Z.Y.; methodology, S.X. and Y.W.; validation, Z.Y. and W.Z.; formal analysis, H.T.; investigation, Y.Y. and W.Y.; resources, F.L.; data curation, Q.J.; writing—original draft preparation, Q.J.; writing—review and editing, W.Z.; visualization, Y.Z.; supervision, Z.X. and Z.G.; project administration, A.C.; funding acquisition, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 32273154), the Natural Science Foundation of Shanghai (22ZR1427300), the earmarked fund for CARS-48, the earmarked fund for the Jiangsu Agricultural Industry Technology System (JATS [2023] 471), the key research projects for seed industry revitalization with the principle of “the open competition mechanism to select the best candidates” (JBGS❲2021❳032), and the National Key R&D Program of China (2022YFD2400700). This work is also affiliated with the National Natural Science Foundation of China (No. 32102767).
Institutional Review Board Statement
All animals were treated following the license established by the Animal Experiment Ethics Committee. On 19 January 2022, the animal study was reviewed and approved by the Animal Experimental Ethical Inspection of Shanghai Ocean University. The study was conducted in accordance with the local legislation and institutional requirements (Approval Code: SHOU-DW-2022-024.).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Lie, Ø. Flesh quality–the role of nutrition. Aquac. Res. 2001, 32, 341–348. [Google Scholar] [CrossRef]
- Wei, Q.H.; Sun, Q.X.; Dong, X.P.; Kong, B.H.; Ji, H.W.; Liu, S.C. Effect of static magnetic field-assisted freezing at different temperatures on muscle quality of pacific white shrimp (Litopenaeus vannamei). Food Chem. 2024, 438, 138041. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Guan, W.L.; Lyu, X.M.; Chen, R.C.; Wu, Y.Y.; Mao, L.C. Impacts of acute ammonia-N exposure on the muscle quality of whiteleg shrimp (Penaeus vannamei): Novel insights into lipid and protein oxidation. Food Chem. 2024, 437, 137701. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.X.; Fan, J.J.; Su, H.H.; Zhu, H.P.; Ma, D.M. Proximate compositions evaluation, histology and transcriptome analysis revealed the effects of formulated diets on muscle quality in Micropterus salmoides. Reprod. Breed. 2023, 3, 50–58. [Google Scholar] [CrossRef]
- Menasveta, P.; Worawattanamateekul, W.; Latscha, T.; Clark, J.S. Correction of black tiger prawn (Penaeus monodon Fabrieius) coloration by astaxanthin. Aquacult. Eng. 1993, 12, 203–213. [Google Scholar] [CrossRef]
- Anders, N.; Breen, M.; Skara, T.; Roth, B.; Sone, I. Effects of capture-related stress and pre-freezing holding in refrigerated sea water (RSW) on the muscle quality and storage stability of Atlantic mackerel (Scomber scombrus) during subsequent frozen storage. Food Chem. 2023, 405, 134819. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wu, L.Y.; Wang, Y.; Han, D.; Liu, H.K.; Zhu, X.M.; Yang, Y.X.; Xie, S.Q.; Liu, Z.; Jin, J.Y. Glutamate improves flesh quality and muscle growth of triploid crucian carp. Aquacult. Rep. 2023, 33, 101832. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, M.Q.; Jiang, J.L.; Yu, A.G.; Yu, D.H.; Huang, F. Effects of dietary lipid levels on fiber quality, lipidomic profiles, antioxidant and inflammation responses in muscle of yellow catfish Pelteobagrus fulvidraco. Aquacult. Rep. 2023, 33, 101855. [Google Scholar] [CrossRef]
- Jing, J.Z.; Wang, J.Y.; Xiang, X.Y.; Yin, S.G.; Tang, J.Y.; Wang, L.Q.; Jia, J.; Liu, G.M.; Chen, X.L.; Tang, G.; et al. Selenomethionine alleviates chronic heat stress-induced breast muscle injury and poor meat quality in broilers via relieving mitochondrial dysfunction and endoplasmic reticulum stress. Anim. Nutr. 2024, 16, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tian, S.J.; Yuan, J.; Zhang, Z.Y.; Zhou, H.H.; Gao, W.H.; Zhang, W.B.; Mai, K.S. Effects of Clostridium autoethanogenum protein as substitute for dietary fishmeal on the growth, feed utilization, intestinal health and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 2022, 561, 738591. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Li, C.Y.; Xu, G.; Zhao, Q.; Wei, Z.H.; Liu, S.J. Effects of dietary glutathione on growth performance, muscle quality and lipid metabolism of hybrid crucian carp (Carassius auratus cuvieri ♀ × Carassius auratus red var. ♂) fed a high-fat die. Reprod. Breed. 2023, 3, 89–98. [Google Scholar] [CrossRef]
- Yang, H.C.; Li, Y.C.; Wang, G.J.; Xie, J.; Kaneko, G.; Yu, E.M. Dietary grape seed proanthocyanidin extract improved the chemical composition, antioxidant capacity, myofiber growth and flesh quality of Nile tilapia muscle. Aquacult. Rep. 2023, 33, 101878. [Google Scholar] [CrossRef]
- Meng, X.L.; You, F.; Cao, H.; Cai, H.M.; Li, Y.; Yang, G.K.; Zhang, Y.M.; Chang, X.L.; Zhang, X.D.; Tian, X. Effects of dietary licorice (Glycyrrhiza uralensis) supplementation on growth performance, muscle quality, and immunity in the common carp (Cyprinus carpio haematopterus). Aquacult. Rep. 2022, 27, 101331. [Google Scholar]
- Xu, J.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Different dietary protein levels affect flesh quality, fatty acids and alter gene expression of Nrf2-mediated antioxidant enzymes in the muscle of grass carp (Ctenopharyngodon idella). Aquaculture 2018, 493, 272–282. [Google Scholar] [CrossRef]
- Johnston, I.A.; Manthri, S.; Alderson, R.; Campbell, P.; Mitchell, D.; Whyte, D.; Dingwall, A.; Nickell, D.; Selkirk, C.; Robertson, B. Effects of dietary protein level on muscle cellularity and flesh quality in Atlantic salmon with particular reference to gaping. Aquaculture 2002, 210, 259–283. [Google Scholar] [CrossRef]
- Ma, H.G.; Xiong, K.D.; Wu, J.W.; Ji, X.R.; Yang, S.H. Noncontact photoacoustic angiography with an air-coupled ultrasonic transducer for evaluation of burn injury. Appl. Phys. Lett. 2019, 114, 133701. [Google Scholar] [CrossRef]
- Huang, S.T.; Chen, Q.; Zhang, M.M.; Chen, S.M.; Dai, J.Y.; Qian, Y.X.; Gong, Y.Y.; Han, T. Synthetic astaxanthin has better effects than natural astaxanthins on growth performance, body color and n-3 PUFA deposition in black tiger prawn (Penaeus monodon). Aquacult. Rep. 2023, 33, 101816. [Google Scholar] [CrossRef]
- Tang, J.Z.; Zhang, D.Y.; Cheng, J.; Liu, F.; Fu, G.H.; Zhang, J.S. Comparative analysis of the amino acid composition and proteomic patterns of the muscle proteins from two teleosts, Siniperca chuatsi L. and Hypophthalmichthys molitrix L. J. Fish. China 2007, 31, 361–368. [Google Scholar]
- Zhang, Y.; Wang, L.; Ma, X.; Guan, T.Y.; Shi, W.J.; Zhu, C.K.; Wang, H.; Li, J.L. Response of antioxidation and immunity to combined influences of ammonia and temperature in red swamp crayfish (Procambarus clarkii). Aquaculture 2023, 563, 738906. [Google Scholar] [CrossRef]
- Sun, C.; Tadese, D.A.; Wangari, M.R.; Zhou, Q.; Zheng, X.; Liu, B.; Tamiru, M.; Dagne, A.; Janssens, G.P.J.; Zhao, Y. Amelioration of ammonia-induced intestinal oxidative stress by dietary Clostridium butyricum in giant freshwater prawn (Macrobrachium rosenbergii). Fish Shellfish Immunol. 2022, 131, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.L.; Shen, X.J.; Wang, Y.; Hu, J.J.; Bao, Z.M.; Wang, M.Q. Comparative study of five anti-lipopolysaccharide factor genes in Litopenaeus vannamei. Dev. Comp. Immunol. 2022, 139, 104557. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.B.; Li, Y.D.; Jiang, S.G.; Yang, Q.B.; Jiang, S.; Huang, J.H.; Yang, L.S.; Chen, X.; Zhou, F.L. Transcriptome analysis of hepatopancreas in penaeus monodon under acute low pH stress. Fish Shellfish Immunol. 2022, 131, 1166–1172. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Yao, N.; Zhang, C.T.; Sun, X.S.; Huang, J.X.; Zhao, B.R.; Li, H.D. Genetic analysis of survival in Penaeus japonicus exposed to white spot syndrome virus. Aquaculture 2022, 559, 738424. [Google Scholar] [CrossRef]
- Sun, S.M.; Wu, Y.; Jakovlic, I.; Fu, H.T.; Ge, X.P.; Qiao, H.; Zhang, W.Y.; Jin, S.B. Identification of neuropeptides from eyestalk transcriptome profiling analysis of female oriental river prawn (Macrobrachium nipponense) under hypoxia and reoxygenation conditions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 241, 110392. [Google Scholar]
- Xu, W.Y.; Ma, Q.Y.; Sun, J.F.; Li, Y.L.; Wang, J.; Tang, Y.W.; Liu, Y.Q.; Mu, J.L.; Wang, W.X. Changes in quality characteristics of shrimp (Penaeus chinensis) during refrigerated storage and their correlation with protein degradation. J. Food Compos. Anal. 2022, 114, 104773. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of the Association of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Thiago, M.T.D.N.; Cleber, F.M.M.; Helena, P.; Fábio, H.F.R.; Kifayat, U.K.; Rafael, S.R.; Nilva, K.S.; João, B.K.F. Determination of the optimum dietary essential amino acid profile for growing phase of Nile tilapia by deletion method. Aquaculture 2020, 523, 135204. [Google Scholar]
- Ginés, R.; Valdimarsdottir, T.; Sveinsdottir, K.; Thorarensen, H. Effects of rearing temperature and strain on sensory characteristics, texture, colour and fat of Arctic charr (Salvelinus alpinus). Food Qual. Prefer. 2004, 15, 177–185. [Google Scholar] [CrossRef]
- CIE. Official Recommendations on Uniform Colour Space, Colour Difference Equations and Metric Colour Terms. Color. Publ. CIE No 1976, 15, 9–12. [Google Scholar]
- Cao, L.P.; Rasco, B.A.; Tang, J.M.; Niu, L.H.; Lai, K.Q.; Fan, Y.X.; Huang, Y.Q. Effects of freshness on the cook loss and shrinkage of grass carp (Ctenopharyngodon idellus) fillets following pasteurization. Int. J. Food Prop. 2016, 19, 2297–2306. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Haji-Maleki, R.; Borderias, A.J. Wheat fiber as a functional ingredient in restructured fish products. Food Chem. 2007, 100, 1037–1043. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernandez-Segovia, I.; Serra, J.; Barat, J. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- Yu, Z.L.; Li, D.Y.; Yin, F.W.; Zhao, Q.; Liu, Z.Y.; Song, L.; Zhou, D.Y.; Wang, T. Lipid profiles in by-products and muscles of three shrimp species (Penaeus monodon, Penaeus vannamei, and Penaeus chinensis). Eur. J. Lipid Sci. Tech. 2020, 122, 1900309. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Q.Q.; Tian, L.; Hu, W.; Yang, C.G.; Gao, W.H. The Muscle Composition Analysis and flesh quality of Procambarus clarlia in the dongting lake. Acta Hydrobiol. Sin. 2017, 41, 870–877. (In Chinese) [Google Scholar]
- Li, L.C. Analysis of muscle nutrient composition of Macrobrachium nipponense and Procambarus clarkii in Nanwan Reservoir. J. Hydroecology 2005, 03, 28–29. (In Chinese) [Google Scholar]
- Cai, L.; Tong, F.L.; Tang, T.; Ao, Z.P.; Wei, Z.H.; Yang, F.Z.; Shu, Y.Q.; Liu, S.J.; Mai, K.S. Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 2021, 541, 736780. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.C.; Li, J.L. Comparison of biochemical composition and nutritional value of antarctic krill (Euphausia Superb) with several species of shrimps. Adv. Mater. Res. 2011, 361–363, 799–803. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Sentandreu, M.Á.; Lorenzo, J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014, 56, 226–235. [Google Scholar] [CrossRef]
- Zhao, F.; Zhuang, P.; Song, C.; Shi, Z.H.; Zhang, L.Z. Amino acid and fatty acid compositions and nutritional quality of muscle in the pomfret, Pampus punctatissimus. Food Chem. 2010, 118, 224–227. [Google Scholar] [CrossRef]
- Who, J.; Consultation, F.E. Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- Zhu, T.Y.; Yang, R.; Xiao, R.G.; Ni, W.Q.; Liu, L.W.; Zhao, J.; Ye, Z.Y. Effect of swimming training on the flesh quality in Chinese Perch (Siniperca chuatsi) and its relationship with muscle metabolism. Aquaculture 2023, 577, 739926. [Google Scholar] [CrossRef]
- Xu, C.; Song, D.; Holck, A.L.; Zhou, Y.Y.; Liu, R. Identifying Lipid Letabolites Influenced by oleic acid administration using high-performance liquid chromatography–mass spectrometry-based lipidomics. ACS Omega 2020, 5, 11314–11323. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wu, P.; Tang, R.J.; Liu, Y.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; Tejeda, J.F.; Noguera, J.L.; Ibanez-Escriche, N.; Gonzalez, E. Effect of the genetic line and oleic acid-enriched mixed diets on the subcutaneous fatty acid composition and sensory characteristics of dry-cured shoulders from Iberian pig. Meat Sci. 2020, 159, 107933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.W.; Tang, R.; He, X.G.; Li, L.; Takagi, Y.; Li, D.P. Improvement of muscle quality of grass carp (Ctenopharyngodon idellus) with a bio-floating bed in culture ponds. Front. Physiol. 2019, 10, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Larsson, T.; Koppang, E.O.; Espe, M.; Terjesen, B.F.; Krasnov, A.; Moreno, H.M.; Rørvik, K.A.; Thomassen, M.; Mørkøre, T. Fillet quality and health of Atlantic salmon (Salmo salar L.) fed a diet supplemented with glutamate. Aquaculture 2014, 426–427, 288–295. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Fernandez-Jover, D.; Black, K.D.; Ladoukakis, E.; Bayle-Sempere, J.T.; Sanchez-Jerez, P.; Dempster, T. Differentiating the wild or farmed origin of Mediterranean fish: A review of tools for sea bream and sea bass. Rev. Aquaculture. 2013, 5, 137–157. [Google Scholar] [CrossRef]
- Jeremiah, L.E.; Carpenter, Z.L.; Smith, G.C. Beef color as related to consumer acceptance and palatability. Food Sci. 1972, 31, 476–479. [Google Scholar] [CrossRef]
- Han, M.Y.; Wang, P.; Xu, X.L.; Zhou, G.H. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, L.; Shang, H.H.; Xuan, X.T.; Yu, J.F.; Lin, X.D.; Kang, M.L.; Lin, J.G. Effects of low temperature combined with high-humidity thawing on water holding capacity and biochemical properties of myofiberillar protein of Penaeus vannamei. J. Food Sci. Techn. 2020, 38, 81–89. [Google Scholar]
- El-Dengawy, R.; Sharaf, A.; El-Kadi, S.; Mahmoud, E.; Baidoon, E. Effect of frozen storage on the chemical, physical and microbiological quality of imported mackerel (Scomber scombrus). J. Food Dairy Sci. 2017, 8, 287–293. [Google Scholar] [CrossRef]
- Chen, S.M.; Chen, J.C. Effects of pH on survival, growth, molting and feeding of giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 2003, 218, 613–623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).