Abstract
The Shortfin mako shark, Isurus oxyrinchus Rafinesque, 1810, is a globally distributed highly migratory pelagic shark species, occurring mostly in temperate and tropical regions, including the Mediterranean Sea where it is by-caught during fishing activities targeting other economically important fish species. The aim of this study is to investigate the genetic connectivity of the Shortfin mako from the central Mediterranean Sea to previously studied populations. The mtDNA control region (CR), 977 bp, of 37 I. oxyrinchus specimens collected between 2004 and 2012 from landings in Malta were analysed, and we identified nine haplotypes, including three newly discovered haplotypes that may be unique to the Mediterranean Sea and which represent 16.7% of the studied individuals. These haplotypes, together with variations in haplotype frequencies, led to significant FST and ϕST values between the Mediterranean population and other global populations, with the exception of that from the north Atlantic Ocean. This study provides the first insight of the mtDNA CR diversity of this critically endangered species in the Mediterranean Sea and highlights the importance of conserving this species in the region.
Keywords:
mtDNA control region; phylogeography; conservation genetics; population genetics; elasmobranches Key Contribution:
This work represents the first population genetics study of Isurus oxyrinchus from the Mediterranean Sea. Data from mitochondrial DNA control region show that there are unique haplotypes to the Mediterranean Sea and highlight the importance of conserving this species and its genetic diversity in the region.
1. Introduction
The Shortfin mako, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes, Lamniformes), is a cosmopolitan pelagic shark species that occurs mostly in temperate and tropical seas and is known for its highly migratory behaviour [1,2,3,4]. This species exhibits a slow growth rate, late maturity age, long reproductive cycle and produces small clutch sizes characterized by oophagy and uterine cannibalism [3,5,6,7,8,9]. The Shortfin mako is frequently by-caught during commercial and small-scale fishing activities that target other economically important pelagic species [3,8,10,11,12,13,14]. Steep population declines have been recorded in most oceans [13], with satellite tagging data indicating that, in some regions, the fishing mortality rate can be up to 10-fold higher than previous estimates from fisheries-dependent data [15]. Given its life history and susceptibility to fishing pressure, this species has been categorized as endangered at the global level [13], while at the Mediterranean level it has been classified as critically endangered [16], with an overall declining population trend [13,16]. Additionally, this species has been listed in the United Nations Convention on the Law of the Sea (UNCLOS—Annex I) [17], the Convention on Migratory Species (CMS—Appendix II) [18], the Bern Convention (Appendix III) [19], the Convention on International Trade in Endangered Species (CITES—Appendix II) [20] and the Protocol of the Barcelona Convention concerning Specially Protected Areas and Biological Diversity in the Mediterranean (SPA/BD—Annex II) [21]. Moreover, the International Commission for the Conservation of Atlantic Tunas (ICCAT) has recommended restrictions, while for the Mediterranean Sea the General Fisheries Commission for the Mediterranean (GFCM) has banned the capture and sale of Shortfin mako sharks [22,23,24,25].
In the Mediterranean, I. oxhyrhinus is by-caught on various types of gear [11,26,27] including pelagic long-lines targeting Swordfish, Xiphias gladius, or the Atlantic bluefin tuna, Thunnus thynnus [23,24]. This is because the bait used and the targeted species constitute part of the diet of Shortfin makos [28]. In Malta, to safeguard this species, I. oxyrinhus has been listed and protected under national law as a species in need of strict protection [29], and, through the adoption of the recommendation GFCM/36/2012/3 [22], the species has been banned from landings. Nonetheless, there are no species-specific fishing methods that discriminate pelagic sharks from other large target pelagic fish species and data on catch-and-release fishing indicate that this species exhibits a high post-release survival rate, which may be significantly influenced by the hook type used [30,31,32,33,34].
The Shortfin mako, being a highly migratory species, exhibits both large-scale and region-specific movements depending on prey availability and seasonal productivity [3,4]. While migration typically leads to genetic homogeneity across various geographical areas, mitochondrial DNA (mtDNA) studies nonetheless indicate the presence of a global population structure, which is mostly pronounced between hemispheres [35,36,37,38]. Matrilineal population substructuring has been noted in a number of other large circumglobal shark species [39,40], which in some studies has been associated with natal philopatry and long-term site fidelity for parturition [40,41,42]. The control region (CR) is the most commonly used mtDNA sequence in studies that look for phylogeographic patterns [35,36,39,40,43,44,45,46,47,48]. This region is the longest non-coding region of the Shortfin mako mitochondrial genome [49,50,51] and contains sequences related to mtDNA replication and transcription. With increasing DNA barcoding data, recent studies are also exploring the use of cytochrome oxidase subunit I (COI) to understand phylogenetic patterns [37]. However, while data on the CR usually originate from research dedicated to phylogeographical analyses, elasmobranch COI data tend to derive from varied sources and include imports of unknown origin [52], while fin product data may include individuals being represented by more than one fin, leading to a bias in haplotype frequencies.
There have been efforts to study the global population of I. oxhyrhinus using mtDNA CR data [35,36,37,38,53,54], however these works did not consider data of specimens caught from the Mediterranean Sea. This work sheds light on this species’ genetic diversity in the central Mediterranean while inferring phylogeographic connections through phylogeographic analyses with global mtDNA CR data.
2. Materials and Methods
2.1. Sampling
Specimens of I. oxyrinchus (n = 37) were by-caught by Maltese fishermen between 2005 and 2012 (Table 1) in the central Mediterranean Sea, namely the Strait of Sicily (Figure 1). All of these shark specimens were caught on pelagic longlines and landed at the only official fish market in Malta prior to the local protection of the species.

Table 1.
List of specimens analysed, including specimen code, date of collection, gender, total length, haplotype number and GenBank accession numbers.
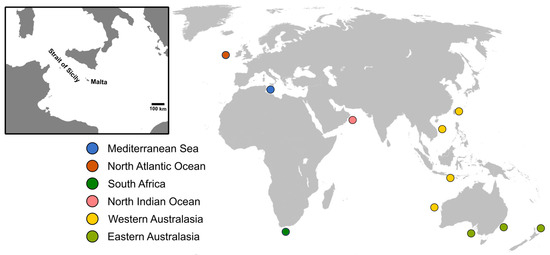
Figure 1.
A map showing the geographic area from which the Mediterranean specimens were collected and the geographical areas from which sequences of Shortfin mako (Isurus oxyrinchus) used for phylogeographic analyses were retrieved [36]. The insert indicates the position of Malta, which represents the landing site from which the sampled sharks were collected.
Whenever possible the total length of each specimen was measured to the nearest 1 cm and the gender was recorded, however there were instances where the specimens were sliced prior to sampling, thus having missing body parts for either gender determination or total length measurements. Chi-squared test was used to check for any significant bias in the sex ratio of the analysed specimens. Muscle tissue was collected from each encountered specimen and stored in 100% ethanol until further analysis.
2.2. DNA Extraction, PCR Amplification and Sequencing
The genomic DNA was extracted from 10 mg of tissue using GF-1 (Vivantis, Shah Alam, Malaysia), and the purified DNA was stored in Tris-HCl buffer at −20 °C until required. The mtDNA CR was amplified in a 25 μL volume using ~50 ng template DNA, 1× HOT FIREPol Blend Master Mix (Solis BioDyne, Tartu, Estonia) and 0.5 μM of the primers Shark tProF and Shark tPheR [36]. The PCR reactions were subject to an initial denaturation of 95 °C for 15 min, followed by 30 cycles of 95 °C for 30 s, 53 °C for 45 s, 72 °C for 1 min, and a final extension 72 °C for 10 min. Amplified PCR products were purified and sequenced using an ABI 3730XL Genetic Analyzer (Life Technologies, Carlsbad, CA, USA) with both forward and reverse primers, and two internal primers, mako405F and mako572R [36], to ensure good coverage.
2.3. Statistical Analyses
Sequences were trimmed, assembled, checked for consistencies and aligned through a Geneious R10 (Biomatters Ltd., Auckland, New Zealand) [55]. A 997 bp sequence of the CR, corresponding to the smallest homologous sequence was chosen for analysis. Sequences were deposited in GenBank under accession numbers OR497223–OR497259 (Table 1). Genetic variability was identified through Geneious R10 [55]. DnaSP v6 [56] was used to identify and group individuals on the basis of their respective haplotype. A haplotype rarefaction curve was constructed using Analytic Rarefaction v.1.3 (available at http://stratigrafia.org/software/index.html (accessed on 4 May 2023)) to depict the extent of saturation for the number of specimens sampled against the detected haplotypes. Curves showing rapid saturation and convergence of the confidence intervals indicate that the necessary sampling intensity has been conducted to document the current genetic variation present [57]. Haplotype diversity and nucleotide diversity were measured by Arlequin v3.5 [58]. A minimum spanning haplotype network showing the association between the various haplotypes identified in this study and from specimens of northeastern Atlantic origin [36] was constructed through TCS v1.21 [59] using POPart [60].
The currently generated data, representing 37 specimens of central Mediterranean Shortfin mako were compared with the genetic data presented in Corrigan et al. [36] (accession numbers MH759795–MH760159, excluding MH760060 as the sequence included degenerate bases). The latter study included circumglobal specimens that were collected from the northeastern Atlantic Ocean (n = 30), South Africa (n = 92), northern Indian Ocean (n = 77), Indo-Pacific Ocean (n = 22), southwestern Australia (n = 45), eastern Australia (n = 59) and New Zealand (n = 39) (Figure 1). Using the smallest homologous sequence, pairwise FST and ϕST values between the specimens of Mediterranean origin and those from other sampling locations were calculated using 1 × 105 permutations, via Arlequin v3.5 [58] and interpreted at p < 0.05 with Bonferroni correction.
3. Results
3.1. Specimens Sampled
The specimens analysed during this study consisted of 15 males, 13 females and 9 of undetermined gender, with a male:female ratio of 1:0.87, which is not significantly different from 1:1 (X2 = 0.143, p = 0.706). The total length (TL) of males ranged between 122 cm and 258 cm (mean 185 cm; SD ±43 cm), while that of females ranged between 81 cm and 350 cm (mean 226 cm; SD ±90 cm) (Table 1; Figure 2). The smallest two individuals were both females, with TLs of 81 cm and 84 cm, and were caught in May 2006 and April 2005, respectively. The largest male encountered during this study measured 258 cm and was caught in February 2008, while the largest female was 350 cm and was caught in May 2006. All collected specimens were by-caught on pelagic longlines, with 78% being landed between the end of April and the beginning of July. This coincides with the fishing season and the fishing gear that targets the Atlantic bluefin tuna [61].
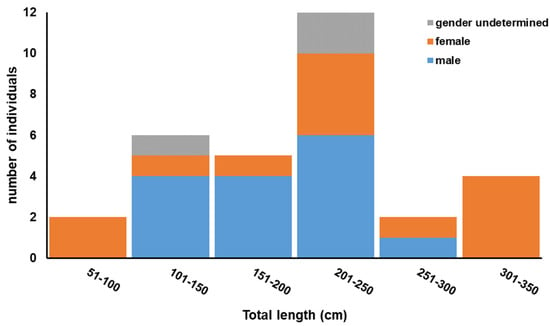
Figure 2.
Length frequency distribution of Shortfin mako (Isurus oxyrinchus) sampled from the central Mediterranean during this study.
3.2. Genetic Variation
A 977 bp sequence representing 94% of the mtDNA CR [50] was amplified and sequenced from each of the 37 specimens. An alignment of these data indicates the presence of 15 variable positions, 10 of which were transitions and 5 were transversions. Twelve of these sites were parsimony-informative sites, and no indels were detected during this study. This genetic variation led to the identification of nine haplotypes, with a haplotypic diversity of 0.784 (SD ± 0.048) and nucleotide diversity of 0.0042 (SD ± 0.0003). Although larger sample sizes may have led to the detection of other rare haplotypes, rarefaction analysis produced a curve that was almost levelling off with converging 95% confidence intervals, indicating that the sampling strategy of this study allowed for the representation of most haplotypes present in the region (Figure 3).
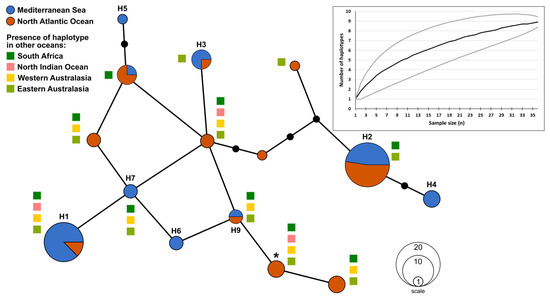
Figure 3.
A haplotype network including the nine haplotypes (H1 to H9) identified from the currently analysed Mediterranean specimens (n = 37; blue) and the haplotypes from the nearest population, i.e., from the northeastern Atlantic Ocean as identified in Corrigan et al. [36] (n = 30; orange). The boxes next to the haplotypes indicate other locations where the same haplotypes were found [36]. The haplotype frequencies are proportional with the area of the circle, while the black circles represent inferred putative haplotypes detected neither in the Mediterranean nor in the northeastern Atlantic Ocean. * Marks the most common global haplotype identified in Corrigan et al. [36]. Insert represents the haplotype rarefaction curve for the Mediterranean samples, including the 95% confidence intervals in grey.
The most common haplotypes identified here were H1 and H2 (Table 1; Figure 3) which belonged to 14 and 10 individuals, respectively. Three haplotypes (H4, H5 and H6; n = 3, 1 and 2, respectively), representing 16.2% of the specimens, were not detected in other studies on Shortfin mako [36,38,49,51] and did not match other sequences available on GenBank [62], thus, based on the currently available public data, these can be considered as unique haplotypes for the Mediterranean Sea.
3.3. Phylogeographic Analyses
For the phylogeographic analyses, data from the central Mediterranean were compared with the 791 bp mtDNA CR presented in Corrigan et al. [36]. For these analyses the sequences generated during this work were shortened to 791 bp, losing one of the variable positions on H9, reducing the distance to its closest haplotype, H6, from 2 bp to 1 bp (Figure 3).
The haplotype network used for this study included data of northeastern Atlantic origin [36], as that is physically the closest population to the Mediterranean Sea. No clear segregation of haplotypes was noted between these two geographic regions. From the nine haplotypes identified during this study, three were found to be unique to the Mediterranean Sea, five were shared with the Atlantic population and one rare haplotype was not detected in the Atlantic population but shared with other oceans (Figure 3). While the Mediterranean and northeastern Atlantic population shared almost half of their haplotypes, it must be noted that the haplotypic frequencies varied between regions, with H1, the most encountered haplotype for the Mediterranean, being recorded in 37.8% of the Mediterranean specimens, while being only found in 6.7% of the northeastern Atlantic specimens. Overall, H2 was the most common haplotype, and was recorded in 36.7% and 27.0% of the northeastern Atlantic and Mediterranean populations, respectively. Additionally, on a global scale, the most common haplotype identified in Corrigan et al. [36] (Figure 3) was shared amongst all locations and appeared in 30% of the analysed individuals. However, while common on a global scale, this haplotype only appeared in 10% of the northeastern Atlantic population sample [36] and was absent in the current work on the Mediterranean population sample.
A small significant genetic differentiation was initially detected between the Mediterranean population and the northeastern Atlantic Ocean through FST (0.063; p-value 0.0067), but this was not supported by ϕST (0.052; p-value 0.0532) and became non-significant after the Bonferroni correction for multiple tests. Therefore, based on the current dataset, these two sampling areas do not exhibit significant genetic divergence. The rest of the pairwise FST and ϕST values show significant genetic differentiation between the Mediterranean population and other populations from South Africa, the Indian Ocean and the Pacific Ocean (FST values 0.109–0.325; ϕST values 0.181–0.458; p-values < 0.0001) that remained significant after Bonferroni correction (Table 2).

Table 2.
The pairwise FST, ϕST and respective p-values values for the Mediterranean population against other populations as identified in Corrigan et al. [36]. Geographical locations: Mediterranean Sea (Med); north Atlantic Ocean (Natl); South Africa (SAfr); north Indian Ocean (NInd); Indo-Pacific Ocean (IndP); western Australia (WAus); eastern Australia (EAus); New Zealand (NZea).
4. Discussion
Shortfin mako records for the Mediterranean Sea indicate that juveniles may be as small as TL 65 cm [26] and adults may reach lengths of over 400 cm, with the largest female record being estimated at a total length of 585 cm [63]. While it is known that north Atlantic Shortfin makos mature at a TL larger than those of southern Atlantic origin [9], the Mediterranean hosts some of the largest known specimens for the species [63,64,65,66]. During the current study, at least 11 individuals were larger than the median TL at maturity, as it has been estimated that, in the north Atlantic Ocean, males mature at a median fork length (FL) of around 182 cm [7,67], while females mature at a median FL of 280 cm [7]. This corresponds to a TL of approximately 197 cm and 300 cm for males and females, respectively [68,69], with a recent study indicating that, along the Moroccan Atlantic coast, males mature at a TL of around 188 cm, corresponding to an estimated 6 years of age [70]. The difference in both the maximum total length and the length at maturity show that this species, as with several other shark species, exhibits sexual dimorphism [12,71], thus calling for detailed gender specific data collection.
During the current study we encountered four females larger than 300 cm; however, none of these mature individuals were pregnant. Nonetheless, given the size at birth and the growth rate for I. oxyrinchus [5,12], the smallest two individuals encountered during the current study represent young of the year, and are maybe suggestive of the presence of nursery grounds in the central Mediterranean. Moreover, the fact that one of these young of the year exhibited a haplotype (H4) that is not shared with any other geographic location further highlights the importance of the area under study. Likewise, the largest individual, which was a mature female, also had a newly identified haplotype (H6), unique to the Mediterranean Sea (Table 1; Figure 3).
The current work provides the first insights into the genetic diversity of Shortfin mako from the central Mediterranean Sea. The haplotype diversity index for this population was slightly lower (h = 0.784) than those observed in most other sampling locations [35,36,37,38]. In Corrigan et al. [36] this diversity index for the eight studied global populations ranged between 0.574 and 0.972 (global: h = 0.894), with only the northern Indian population showing a haplotype diversity index lower than the one found in the current study. Likewise, in Taguchi et al. [35] the haplotype diversity index for the six studied populations ranged between 0.82 and 0.96 (global: h = 0.92), while in studies on the Pacific Ocean populations this value ranged between 0.775 and 0.888 [37,38], with only the population from Chile [37] having a haplotype diversity index just below the one detected in this study. While further studies are required to correlate the slightly lower haplotype diversity index to the currently declining Mediterranean population [13,16], it is known that anthropogenic pressures negatively influence genetic diversity, impacting the long-term survival of species [72].
Though the Shortfin mako is a highly migratory shark, mtDNA data show a genetic structure with significant genetic differentiation between distant locations, especially across the northern and southern hemispheres [35,36,38]. Our results corroborate this observation, as the Mediterranean population is significantly different from the South African population and from those of the Indian and Pacific oceans. The current study also shows that any genetic differentiation between the Mediterranean and northeastern Atlantic population is weak and not significant (Table 2). This contrasts with some other shark genetic studies that have found significant genetic differentiation across the Strait of Gibraltar [39,47]. Therefore, while this strait may limit connectivity for some species [73], it may not be a strong barrier for certain large pelagic sharks, such as the Shortfin mako, that may migrate across the strait with migrating prey such as Xiphias gladius and Thunnus thynnus [74,75].
Though no significant genetic differentiation was noted between the Mediterranean population and the adjacent northeastern Atlantic population, it is interesting to note that the Mediterranean population was characterized by three unique haplotypes out of the nine detected haplotypes. This contrasts with the north Atlantic population, where the number of unique haplotypes identified in Corrigan et al. was of 1 unique haplotype out of the 11 detected, though pairwise FST and ϕST values showed that the latter was significantly different from all other studied populations [36]. This work reduces the gaps in knowledge on a critically endangered species from the Mediterranean Sea and encourages further research on its genetic diversity and conservation.
5. Conclusions
IUCN extinction risk assessments show that most of the Mediterranean Sea populations of elasmobranches are at a higher risk of extinction compared with their counterparts elsewhere, with most members of the family Lamniformes, including the Shortfin mako, being classified as critically endangered at the Mediterranean level, with the principal driver of decline being overfishing [76]. The rare and sporadic catches of Lamniformes [77] in the Mediterranean makes it difficult to carry out detailed studies on specimens of this family, leaving knowledge gaps that need to be filled to better understand and safeguard these species. Consequently, there has been an increasing need to improve data on the Shortfin mako to better assess its status in the region. The low genetic diversity, the identification of unique haplotypes, the significant genetic differences from other regions, the occurrence of young of the year and mature individuals in the central Mediterranean highlights the importance of the region for the effective conservation of this critically endangered species.
Author Contributions
Conceptualization, N.V.; methodology, N.V.; validation, N.V. and A.V.; formal analysis, N.V.; resources, N.V. and A.V.; data curation, N.V. and A.V.; writing—original draft preparation, N.V. and A.V.; writing—review and editing, N.V. and A.V.; visualization, N.V. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The research disclosed in this publication has been funded by the REACH HIGH Scholars Programme-Post Doctoral Grants awarded to NV. The latter was part-financed by the European Union, Operational Programme II—Cohesion Policy 2014–2020 “Investing in human capital to create more opportunities and promote the well-being of society”—European Social Fund.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as the specimens were caught by local fishermen during fishing activities, the specimens sampled were deceased and for sale at the official local fish market, prior to the species becoming locally protected.
Informed Consent Statement
Not applicable.
Data Availability Statement
Genetic sequences related to the analyses conducted during this study are available on GenBank under accession numbers OR497223–OR497259.
Acknowledgments
We would like to thank all of the Maltese fishermen for supporting sampling from their landings.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rogers, P.J.; Huveneers, C.; Page, B.; Goldsworthy, S.D.; Coyne, M.; Lowther, A.D.; Mitchell, J.G.; Seuront, L. Living on the continental shelf edge: Habitat use of juvenile shortfin makos Isurus oxyrinchus in the Great Australian Bight, southern Australia. Fish. Oceanogr. 2015, 24, 205–218. [Google Scholar] [CrossRef]
- Santos, C.; Domingo, A.; Carlson, J.; Natanson, L.; Cortés, E.; Miller, P.; Hazin, F.; Travassos, P.; Mas, F.; Coelho, R. Habitat use and migrations of shortfin mako in the Atlantic using satellite telemetry. Collect. Vol. Sci. Pap. ICCAT 2018, 75, 445–456. [Google Scholar]
- Nasby-Lucas, N.; Dewar, H.; Sosa-Nishizaki, O.; Wilson, C.; Hyde, J.R.; Vetter, R.D.; Wraith, J.; Block, B.A.; Kinney, M.J.; Sippel, T.; et al. Movements of electronically tagged shortfin mako sharks (Isurus oxyrinchus) in the eastern North Pacific Ocean. Anim. Biotelemetry 2019, 7, 12. [Google Scholar] [CrossRef]
- Vaudo, J.J.; Byrne, M.E.; Wetherbee, B.M.; Harvey, G.M.; Shivji, M.S. Long-term satellite tracking reveals region-specific movements of a large pelagic predator, the shortfin mako shark, in the western North Atlantic Ocean. J. Appl. Ecol. 2017, 54, 1765–1775. [Google Scholar] [CrossRef]
- Natanson, L.J.; Kohler, N.E.; Ardizzone, D.; Cailliet, G.M.; Wintner, S.P.; Mollet, H.F. Validated age and growth estimates for the shortfin mako, Isurus oxyrinchus, in the North Atlantic Ocean. Environ. Biol. Fishes 2006, 77, 367–383. [Google Scholar] [CrossRef]
- Francis, M.P.; Duffy, C. Length at maturity in three pelagic sharks (Lamna nasus, Isurus oxyrinchus, and Prionace glauca) from New Zealand. Fish. Bull. 2005, 103, 489–500. [Google Scholar]
- Natanson, L.J.; Winton, M.; Bowlby, H.; Joyce, W.; Deacy, B.; Coelho, R.; Rosa, D. Updated reproductive parameters for the shortfin mako (Isurus oxyrinchus) in the north atlantic ocean with inferences of distribution by sex and reproductive stage. Fish. Bull. 2020, 118, 21–36. [Google Scholar] [CrossRef]
- Joung, S.J.; Hsu, H.H. Reproduction and embryonic development of the shortfin mako, Isurus oxyrinchus Rafinesque, 1810, in the Northwestern Pacific. Zool. Stud. 2005, 44, 487–496. [Google Scholar]
- Mollet, H.F.; Cliff, G.; Pratt, H.L.; Stevens, J.D. Reproductive biology of the female shortfin mako, Isurus oxyrinchus Rafinesque, 1810, with comments on the embryonic development of lamnoids. Fish. Bull. 2000, 98, 299–318. [Google Scholar]
- O’Farrell, H.B.; Babcock, E.A. Shortfin mako hot sets–Defining high bycatch conditions as a basis for bycatch mitigation. Fish. Res. 2021, 244, 106123. [Google Scholar] [CrossRef]
- Erguden, D.; Ayas, D.; Kabasakal, H. Recent Occurrence of Shortfin Mako Shark, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamnidae), from the North-Eastern Mediterranean Coast of Turkey. COMU J. Mar. Sci. Fish. 2021, 4, 79–85. [Google Scholar] [CrossRef]
- Barreto, R.R.; De Farias, W.K.T.; Andrade, H.; Santana, F.M.; Lessa, R. Age, growth and spatial distribution of the life stages of the shortfin mako, Isurus oxyrinchus (Rafinesque, 1810) caught in the western and central Atlantic. PLoS ONE 2016, 11, e0153062. [Google Scholar] [CrossRef] [PubMed]
- Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Jabado, R.W.; Liu, K.M.; Marshall, A.; Pacoureau, N.; et al. Isurus oxyrinchus. The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/39341/2903170 (accessed on 8 August 2023).
- Vella, A.; Vella, N.; Schembri, S. A molecular approach towards taxonomic identification of elasmobranch species from Maltese fisheries landings. Mar. Genom. 2017, 36, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Cortés, E.; Vaudo, J.J.; Harvey, G.C.M.N.; Sampson, M.; Wetherbee, B.M.; Shivji, M. Satellite telemetry reveals higher fishing mortality rates than previously estimated, suggesting overfishing of an apex marine predator. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170658. [Google Scholar] [CrossRef] [PubMed]
- Walls, R.H.L.; Soldo, A. Isurus oxyrinchus (Mediterranean Assessment). The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/39341/16527941 (accessed on 8 August 2023).
- UNCLOS United Nations Convention on the Law of the Sea-Annex I. Available online: https://www.un.org/depts/los/convention_agreements/texts/unclos/annex1.htm (accessed on 8 August 2023).
- CMS Appendices I and II of the Convention on the Conservation of Migratory Species of Wild Animals. Available online: https://www.cms.int/sites/default/files/basic_page_documents/appendices_cop13_e_0.pdf (accessed on 8 August 2023).
- Convention on the Conservation of European Wildlife and Natural Habitats (Bern Convention) Annex III. Available online: https://rm.coe.int/168097eb57 (accessed on 8 August 2023).
- CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora. Appendices I, II and III. Available online: https://cites.org/sites/default/files/eng/app/2023/E-Appendices-2023-05-21.pdf (accessed on 8 August 2023).
- UNEP/MAP-SPA/RAC SPA-BD Protocol Annex II: List of Endangered or Threatened Species. Available online: https://www.rac-spa.org/sites/default/files/annex/annex_2_en_20182.pdf (accessed on 8 August 2023).
- GFCM Recommendation GFCM/36/2012/3 on Fisheries Management Measures for the Conservation of Sharks and Rays in the GFCM Area of Application; GFCM: Rome, Italy, 2012.
- ICCAT Recommendation by ICCAT on Shortfin Mako Caught in Association with ICCAT Fisheries (14-06 BYC); ICCAT: Madrid, Spain, 2014.
- ICCAT Recommendation by ICCAT on the Conservation of the North Atlantic Stock of Shortfin Mako Caught in Association with ICCAT Fisheries (17-08 BYC); ICCAT: Madrid, Spain, 2017.
- ICCAT Recommendation by ICCAT on the Conservation of the North Atlantic Stock of Shortfin Mako Caught in Association with ICCAT Fisheries (21-09 BYC); ICCAT: Madrid, Spain, 2021.
- Kabasakal, H. Occurrence of shortfin mako shark, Isurus oxyrinchus Rafinesque, 1810, off Turkey’s coast. Mar. Biodivers. Rec. 2015, 8, e134. [Google Scholar] [CrossRef]
- Bradai, M.N.; Saidi, B.; Enajjar, S. Elasmobranches of the Mediterranean and Black Sea: Status, ecology and biology. Bibliographic analysis. In Studies and Reviews. General Fisheries Commission for the Mediterranean; FAO: Rome, Italy, 2012; Volume 91, ISBN 9786021018187. [Google Scholar]
- Maia, A.; Queiroz, N.; Correia, J.P.; Cabral, H. Food habits of the shortfin mako, Isurus oxyrinchus, off the southwest coast of Portugal. Environ. Biol. Fishes 2006, 77, 157–167. [Google Scholar] [CrossRef]
- Legislation Malta Subsidiary Legislation 549.44: Flora, Fauna, and Natural Habitats Protection Regulations. Available online: https://legislation.mt/eli/sl/549.44/eng/pdf (accessed on 8 August 2023).
- French, R.P.; Lyle, J.; Tracey, S.; Currie, S.; Semmens, J.M. High survivorship after catch-and-release fishing suggests physiological resilience in the endothermic shortfin mako shark (Isurus oxyrinchus). Conserv. Physiol. 2015, 3, cov044. [Google Scholar] [CrossRef]
- Keller, B.; Swimmer, Y.; Brown, C. Review on the effect of hook type on the catchability, hooking location, and post-capture mortality of the shortfin mako, Isurus oxyrinchus. Collect. Vol. Sci. Pap. ICCAT 2020, 77, 240–251. [Google Scholar]
- Campana, S.E.; Joyce, W.; Fowler, M.; Showell, M. Discards, hooking, and post-release mortality of porbeagle (Lamna nasus), shortfin mako (Isurus oxyrinchus), and blue shark (Prionace glauca) in the Canadian pelagic longline fishery. ICES J. Mar. Sci. 2016, 72, 520–528. [Google Scholar] [CrossRef]
- Rogers, P.; Ward, T.; van Ruth, P.; Williams, A.; Bruce, B.; Connell, S.; Currie, D.; Davies, C.; Evans, K.; Gillanders, B.; et al. Physical Processes, Biodiversity and Ecology of the Great Australian Bight Region: A Literaure Review; CSIRO: Canberra, Australia, 2013. [Google Scholar]
- Musyl, M.K.; Brill, R.W.; Curran, D.S.; Fragoso, N.M.; McNaughton, L.M.; Nielsen, A.; Kikkawa, B.S.; Moyes, C.D. Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish. Bull. 2011, 109, 341–368. [Google Scholar]
- Taguchi, M.; Kitamura, T.; Yokawa, K. Genetic population structure of shortfin mako (Isurus oxyrinchus) inferred from mitochondrial DNA on inter-oceanic scale. Isc. Ac. Affrc. Go. Jp. 2011, 11, 19–21. [Google Scholar]
- Corrigan, S.; Lowther, A.D.; Beheregaray, L.B.; Bruce, B.D.; Cliff, G.; Duffy, D.J.; Foulis, A.; Francis, M.P.; Goldsworthy, S.D.; Hyde, J.R.; et al. Population Connectivity of the Highly Migratory Shortfin Mako (Isurus oxyrinchus Rafinesque 1810) and Implications for Management in the Southern Hemisphere. Front. Ecol. Evol. 2018, 6, 187. [Google Scholar] [CrossRef]
- González, M.T.; Leiva, N.V.; Zárate, P.M.; Baeza, J.A. Regional (south-eastern Pacific Ocean) population genetics and global phylogeography of two endangered highly migratory pelagic sharks, the blue shark Prionace glauca and shortfin mako Isurus oxyrinchus. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 1098–1115. [Google Scholar] [CrossRef]
- Kacev, D. Understanding Population Connectivity in Shortfin Mako Shark (Isurus oxyrinchus) at Multiple Spatial Scales. Ph.D. Thesis, University of California Davis and San Diego State University, San Diego, CA, USA, 2015. [Google Scholar]
- Vella, N.; Vella, A. Population genetics of the deep-sea bluntnose sixgill shark, Hexanchus griseus, revealing spatial genetic heterogeneity. Mar. Genom. 2017, 36, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, C.L.; Blower, D.C.; Broderick, D.; Giles, J.L.; Holmes, B.J.; Kashiwagi, T.; Krück, N.C.; Morgan, J.A.T.; Tillett, B.J.; Ovenden, J.R. A review of the application of molecular genetics for fisheries management and conservation of sharks and rays. J. Fish Biol. 2012, 80, 1789–1843. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.J.; Streich, M.K.; Topping, T.S.; Stunz, G.W. New insights into the seasonal movement patterns of Shortfin mako sharks in the Gulf of Mexico. Front. Mar. Sci. 2021, 8, 623104. [Google Scholar] [CrossRef]
- Feldheim, K.A.; Gruber, S.H.; Dibattista, J.D.; Babcock, E.A.; Kessel, S.T.; Hendry, A.P. Two decades of genetic profiling yields first evidence of natal philopatry and long-term fidelity to parturition sites in sharks. Mol. Ecol. 2014, 23, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gubili, C.; Bilgin, R.; Kalkan, E.; Karhan, S.Ü.; Jones, C.S.; Sims, D.W.; Kabasakal, H.; Martin, A.P.; Noble, L.R. Antipodean white sharks on a Mediterranean walkabout? historical dispersal leads to genetic discontinuity and an endangered anomalous population. Proc. R. Soc. B Biol. Sci. 2011, 278, 1679–1686. [Google Scholar] [CrossRef]
- Quattro, J.M.; Stoner, D.S.; Driggers, W.B.; Anderson, C.A.; Priede, K.A.; Hoppmann, E.C.; Campbell, N.H.; Duncan, K.M.; Grady, J.M. Genetic evidence of cryptic speciation within hammerhead sharks (Genus Sphyrna). Mar. Biol. 2006, 148, 1143–1155. [Google Scholar] [CrossRef]
- Gubili, C.; Sims, D.W.; Veríssimo, A.; Domenici, P.; Ellis, J.; Grigoriou, P.; Johnson, A.F.; McHugh, M.; Neat, F.; Satta, A.; et al. A tale of two seas: Contrasting patterns of population structure in the small-spotted catshark across Europe. R. Soc. Open Sci. 2014, 1, 140175. [Google Scholar] [CrossRef]
- Cardeñosa, D.; Hyde, J.; Caballero, S. Genetic Diversity and Population Structure of the Pelagic Thresher Shark (Alopias pelagicus) in the Pacific Ocean: Evidence for Two Evolutionarily Significant Units. PLoS ONE 2014, 9, e110193. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Urso, I.; Damalas, D.; Martinsohn, J.; Zanzi, A.; Mariani, S.; Sperone, E.; Micarelli, P.; Garibaldi, F.; Megalofonou, P.; et al. Genetic differentiation and phylogeography of Mediterranean-North Eastern Atlantic blue shark (Prionace glauca, L. 1758) using mitochondrial DNA: Panmixia or complex stock structure? PeerJ 2017, 5, e4112. [Google Scholar] [CrossRef] [PubMed]
- Keeney, D.B.; Heist, E.J. Worldwide phylogeography of the blacktip shark (Carcharhinus limbatus) inferred from mitochondrial DNA reveals isolation of western Atlantic populations coupled with recent Pacific dispersal. Mol. Ecol. 2006, 15, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Mehlrose, M.R.; Bernard, A.M.; Finnegan, K.A.; Krausfeldt, L.E.; Lopez, J.V.; Shivji, M.S. Three complete mitochondrial genomes of shortfin mako sharks, Isurus oxyrinchus, from the Atlantic and Pacific Oceans. Mitochondrial DNA Part B 2022, 7, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Shao, K.T.; Lin, Y.S.; Tsai, A.Y.; Su, P.X.; Ho, H.C. The complete mitochondrial genome of the shortfin mako, Isurus oxyrinchus (Chondrichthyes, Lamnidae). Mitochondrial DNA 2015, 26, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.; Marra, N.; Shivji, M.S.; Stanhope, M.J. The complete mitochondrial genome of an Atlantic Ocean Shortfin Mako Shark, Isurus oxyrinchus. Mitochondrial DNA Part B 2019, 4, 3642–3643. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.S.; Hung, T.C.; Chang, H.A.; Huang, C.K.; Shiao, J.C. The species and origin of shark fins in Taiwan’s fishing ports, markets, and customs detention: A DNA barcoding analysis. PLoS ONE 2016, 11, e0147290. [Google Scholar] [CrossRef] [PubMed]
- Schrey, A.W.; Heist, E.J. Microsatellite analysis of population structure in the shortfin mako (Isurus oxyrinchus). Can. J. Fish. Aquat. Sci. 2003, 60, 670–675. [Google Scholar] [CrossRef]
- Taguchi, M.; Kitamura, T.; Shigenobu, Y.; Ohkubo, M.; Yanagimoto, T.; Sugaya, T.; Nakamura, Y.; Saitoh, K.; Yokawa, K. Development of 15 polymorphic microsatellite markers for the shortfin mako, Isurus oxyrinchus, and cross-species amplification in lamniforme sharks. Conserv. Genet. Resour. 2013, 5, 675–678. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D.; Gwiazdowski, R.A.; Ashlock, D.; Hanner, R. An exploration of sufficient sampling effort to describe intraspecific DNA barcode haplotype diversity: Examples from the ray-finned fishes (Chordata: Actinopterygii). DNA Barcodes 2016, 3, 66–73. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Vella, A.; Vella, N.; Karakulak, F.S.; Oray, I.; Garcia-Tiscar, S.; de Stephanis, R. Population genetics of Atlantic bluefin tuna, Thunnus thynnus (Linnaeus, 1758), in the Mediterranean: Implications for its conservation management. J. Appl. Ichthyol. 2016, 32, 523–531. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kabasakal, H.; de Maddalena, A. A huge shortfin mako shark Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamnidae) from the waters of Marmaris, Turkey. Ann. Ser. Hist. Nat. 2011, 1, 5–8. [Google Scholar]
- Celona, A.; Piscitelli, L.; de Maddalena, A. Two large shortfin makos, Isurus oxyrinchus, Rafinesque, 1809, caught off Sicily, Western Ionian Sea. Ann. Ser. Hist. Nat. 2004, 14, 35–42. [Google Scholar]
- Lopez-Mirones, F.; de Maddalena, A.; Sagarminaga van Buiten, R. On a huge shortfin mako shark Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamnidae) observed at Cabrera Grande, Balearic Islands, Spain. Ann. Ser. Hist. Nat. 2020, 30, 25–30. [Google Scholar]
- Celona, A.; Donato, N.; de Maddalena, A. In relation to the captures of a great white shark, Carcharodon carcharias (Linnaeus, 1758), and a shortfin mako, Isurus oxyrinchus Rafinesque, 1809, in the Messina Strait. Ann. Ser. Hist. Nat. 2001, 11, 13–16. [Google Scholar]
- Maia, A.; Queiroz, N.; Cabral, H.N.; Santos, A.M.; Correia, J.P. Reproductive biology and population dynamics of the shortfin mako, Isurus oxyrinchus Rafinesque, 1810, off the southwest Portuguese coast eastern North Atlantic. J. Appl. Ichthyol. 2007, 23, 246–251. [Google Scholar] [CrossRef]
- Kohler, N.E.; Casey, J.G.; Turner, P.A. Lenght-weight relationships for 13 species of sharks from the western North Atlantic. Fish. Bull. 1995, 93, 412–418. [Google Scholar]
- Campana, S.E.; Marks, L.; Joyce, W. The biology and fishery of shortfin mako sharks (Isurus oxyrinchus) in Atlantic Canadian waters. Fish. Res. 2005, 73, 341–352. [Google Scholar] [CrossRef]
- Alahyene, J.; Chiahou, B.; El Habouz, H.; Ben-Bani, A. Sex ratio and male maturity for Shortfin mako shark in the Moroccan central Atlantic coast. Croat. J. Fish. 2022, 80, 67–75. [Google Scholar] [CrossRef]
- Vella, N.; Vella, A. A preliminary study of the Bluntnose sixgill shark, Hexanchus griseus, in the central Mediterranean region around the Maltese Islands. Rapp. Comm. Int. Mer Méditerranée 2010, 39, 695. [Google Scholar]
- Van Der Valk, T.; Sandoval-Castellanos, E.; Caillaud, D.; Ngobobo, U.; Binyinyi, E.; Nishuli, R.; Stoinski, T.; Gilissen, E.; Sonet, G.; Semal, P.; et al. Significant loss of mitochondrial diversity within the last century due to extinction of peripheral populations in eastern gorilla. Sci. Rep. 2018, 8, 6551. [Google Scholar] [CrossRef] [PubMed]
- Patarnello, T.; Volckaert, F.A.M.J.; Castilho, R. Pillars of Hercules: Is the Atlantic-Mediterranean transition a phylogeographical break? Mol. Ecol. 2007, 16, 4426–4444. [Google Scholar] [CrossRef]
- Carruthers, T.; Di Natale, A.; Lauretta, M.; Paga Garcia, A.; Tensek, S. Migratory behaviour of Atlantic Bluefin Tuna entering the Mediterranean. Collect. Vol. Sci. Pap. ICCAT 2018, 74, 3082–3099. [Google Scholar]
- Abid, N.; Bakkali, M.; Tserpes, G.; Idrissi, M. Swordfish growth pattern in the strait of Gibraltar; implications for Atlantic and Mediterranean stock mixing. Mediterr. Mar. Sci. 2014, 15, 135–144. [Google Scholar] [CrossRef][Green Version]
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H.L. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea; IUCN: Malaga, Spain, 2016. [Google Scholar]
- Vella, N.; Vella, A. The complete mitogenome of the Critically Endangered smalltooth sand tiger shark, Odontaspis ferox (Lamniformes: Odontaspididae). Mitochondrial DNA Part B 2020, 5, 3319–3322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).