Abstract
The tropical gar (Atractosteus tropicus Gill, 1863) is a prehistoric fish of high nutritional value in southern Mexico and Central America. However, some aspects related to the effects caused by alternative protein sources, such as insect meal, as a substitute for fish meal on the growth and expression of digestive enzyme genes, are still unknown. A total of 225 juveniles of A. tropicus were used and fed five experimental diets, each in triplicate, with different levels of substitution of fishmeal (FM) protein with house cricket meal (HCM) protein. A control diet that contained no HCM (T1-0% HCM) was used, and substitutions ranged from 25 to 100% of FM protein by HCM (T2-25% HCM, T3-50% HCM, T4-75% HCM, and T5-100% HCM) for 45 days. The results of this study indicate that T4-75% HCM showed the best growth indices, such as feed efficiency (EF), feed conversion ratio (FCR), specific growth rate (SGR), as well as higher gene expression of pepsin and trypsin, while chymotrypsin showed higher expression in T3. The higher performance achieved in T4-75% HCM may be due to the fact that, in the early stages, insects are part of the natural diet of A. tropicus. The inclusion of cricket meal as a partial substitute for fish meal is not recommended in quantities greater than T4-75%.
Key Contribution:
House cricket meal increases gene expression in the digestive tract of tropical gar, increasing protein assimilation. House cricket meal can improve feed efficiency in tropical gar growth and production.
1. Introduction
In Mexico, the main objective of the 2020–2024 National Fisheries and Aquaculture Program was to increase aquaculture production by 20% through the farming of various species of commercial and cultural importance, including the tropical gar (Atractosteus tropicus Gill, 1863) [1]. This fish is prehistoric and is found from southern Mexico to Costa Rica in Central America. It has high nutritional value [2], is resistant to disease, tolerates low levels of dissolved oxygen in the water, and can be farmed at high densities and in polyculture. On the other hand, the high cost of fishmeal (FM) in recent years has led to the search for new nutritional alternatives for the preparation of diets for aquaculture. Diets formulated with plant and animal sources that deviate from the nutritional habits of fish tend to affect fish growth due to the presence of anti-nutrients [3,4].
Insect meal represents an alternative protein source that has recently been accepted as feed for aquaculture species [5] due to its high content of protein, fat, essential fatty acids, minerals, vitamins, caloric energy, fiber, and a good balance of essential amino acids, as well as its high feed conversion efficiency, which is very similar to FM, and in many cases, these insects are part of the main diet of freshwater fish [3,6,7]. The nutritional content varies between insect species and their diets [8], so substitution levels depend on the insect species and the fish species being farmed. For example, sea bass (Dicentrarchus labrax L.) tolerates 25% silkworm meal (Tenebrio molitor), Siberian sturgeon (Acipenser baerii) tolerates 30% soldier fly meal, African catfish hybrids (Clarias gariepinus × Heterobranchus longifilis) tolerate 30% soldier fly meal and Tenebrio molitor meal, Nile tilapia (Oreochromis niloticus) 40% cricket meal (A. domesticus), and guppy (Poecilia reticulata) 75% A. domesticus meal and 100% field cricket meal (Gryllus bimaculatus) [9,10,11,12,13,14]. This is because insect flours, compared to FM, tend to be deficient in growth-limiting amino acids such as methionine, arginine, lysine, and tryptophan [10,15,16,17], so an imbalance in these amino acids causes decreased growth, health, stress, disease resistance in fish, and metabolism [18,19]. This imbalance strongly influences the activity of digestive enzymes, determining the degree of nutrient utilization and the success of aquaculture production [20].
The three most studied enzymes are pepsin (EC: 3.4.23.1), an aspartic protease secreted in the form of a zymogen, such as pepsinogen, and is usually stable in a neutral environment and at a pH of 7 to 11. It is activated in gastric juice when the pH decreases due to the addition of hydrochloric acid. When pepsin is activated, it catalyzes the peptide bonds of aromatic amino acids where the second residue of the protein is tyrosyl, tryptophan, or phenylalanine in the stomach [21,22,23,24,25,26,27]. Trypsin (EC: 3.4.21.4.), on the other hand, is a serine protease secreted in the pancreas [21] that hydrolyzes protein residues and peptides to release small peptides and some amino acids for intestinal absorption. It predominantly cleaves on the carboxyl side where residue 1 is arginine or lysine, except when either of these is bound to a C-terminal proline [28]. This enzyme has been used as an indicator of digestive capacity, as well as the nutritional and physiological status of fish [29]. It also activates all pancreatic enzymes such as chymotrypsin, elastase, procarboxypeptidases A and B, and several hydrolases, including itself [28]. Finally, chymotrypsin (EC 3.4.21.1) can hydrolyze peptide bonds when residue 1 contains phenylalanine, tyrosine, or tryptophan [30] and usually exhibits greater catalytic activity in the proteins of plant origin [31]. Trypsin and chymotrypsin are intestinal proteases that function under alkaline conditions and are synthesized by the pancreas and secreted into the intestinal lumen in an inactive form [21]. Both enzymes are used as functional indicators of digestive enzyme activity [32]. Thus, the main objective of this research was to evaluate the effect of complete and partial replacement of fish meal protein with common house cricket meal protein on the growth and relative expression of digestive enzyme genes in juvenile A. tropicus.
2. Materials and Methods
2.1. Organisms and Experimental Design
A total of 225 juvenile A. tropicus were obtained from the Technological Institute of Centla Tabasco, Mexico (ITESC), with an average weight of 1.62 ± 0.04 g. The organisms were placed in 80 L semicircular tanks at the facilities of the Multidisciplinary Academic Division of Jalpa de Méndez (DAMJM-UJAT) of the Universidad Juárez Autónoma de Tabasco. Five treatments were carried out with different levels of fish meal (FM) replacement by cricket meal (HCM): T1-0%; T2-25%; T3-50%; T4-75%; and T5-100% HCM, respectively) (Table 1), with 5 daily feedings at 5% of their biomass at 8:00 a.m., 10:00 a.m., 12:00 p.m., 2:00 p.m., and 4:00 p.m. for 45 days. Cleaning was carried out using a siphon twice a day, with 50% water changes daily and constant aeration. Biometric measurements were taken every two weeks to collect the data. Mortality was recorded in a logbook; however, only one death occurred in repetition 2 of T1-25% on the first day of the trial, so it is assumed that this may have been due to the initial handling of the fish. All treatments were carried out in triplicate.

Table 1.
Experimental diet formulations with different levels of cricket flour (A. domesticus) inclusion. Values presented for 100 g preparation, and all ingredients were measured in grams.
At the end of the trial, the organisms were starved for 24 h, then three fish per culture unit were randomly sacrificed by hypothermia using ice water. Cold dissection was performed to extract the intestine and stomach, and the samples were placed in RNAlater at room temperature for 24 h and stored at −20 °C until subsequent genetic analysis.
2.2. Biometric Parameters
The biometric parameters were determined at the end of the experiment, as described by Concha-Frías et al. [33], using the following formulas.
Absolute weight gain (AWG):
Specific growth rate (SGR) in % day−1:
where Wi: initial weight; Wf: final weight; T: Number of days in the feeding period.
Feed conversion ratio (FCR):
Condition factor (K):
Protein efficiency rate (PER):
Daily weight gain (DWG) in g day−1:
Protein intake (PI) in g:
Daily growth coefficient (DGC):
And Feed Efficiency (FE):
2.3. Primers Design
For THE oligonucleotide design, the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/ (accessed on 11 August 2023)) was used to obtain FASTA sequences, and Primer3Plus (https://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi (accessed on 11 August 2023)) was used to design and create the primers (Table 2).

Table 2.
Oligonucleotides for quantitative polymerase chain reaction (qPCR) in the pejelagarto (A. tropicus).
The specific oligonucleotide design was obtained from the A. tropicus transcriptome (NCBI: PRJNA395289) National Center of Biotechnology Information (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ accessed on 12 may 2025). Melting curves were performed to evaluate the presence of dimers using Web-based LinRegPCR. To ensure that the regions where the oligonucleotides were designed were exons, the the reliable ExPASy translation software (https://web.expasy.org/translate/ (accessed on 23 May 2025)) was used to search for the open reading frame (ORF). Once the ORF was identified, it was translated to amino acid (AA) sequences using standard genetic codes.
A standard curve for each pair of primers was generated to estimate amplification efficiencies based on known amounts of cDNA (four serial dilutions corresponding to cDNA transcribed from 100 to 0.1 ng of total RNA). In the melting curve analysis, it was determined that the melting temperature peak varied between 81.5 and 83 °C, which corresponded to the product obtained by these primers. In addition, the absence of primer dimers and non-specificities was confirmed. Finally, in order to determine whether the oligonucleotide forms a single product, the melt curve was used, and no contamination or heterodimers were observed. Standard curves were also performed for each oligonucleotide. However, no product was sequenced, and we also ensured that the PCRs were efficient.
2.4. Quantitative Polymerase Chain Reaction (qPCR)
mRNA was extracted from the samples using the TRIzol reagent method (Invitrogen). A NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Mexico City, Mexico) was used to determine the concentration and purity in a 260/280 ratio. The product obtained from RNA extraction was rehydrated in ultrapure distilled water and was subsequently quantified at 260/280 to identify the purity and concentration of the samples. cDNA synthesis was performed using one microgram of RNA and random primer using the iScriptTM Select kit 170-8896 (BioRad, Hercules, CA, USA) following the manufacturer’s instructions.
The Cq value, the expression level of repeated fluorescence units, was captured from each run to detect each transcript. Each reaction was normalized by amplifying elongation factor 1alpha (ef-1) as a housekeeping gene to determine partial expression [34]. The control group used to perform the relative expression of pepsin was the stomach from treatment T0, which did not contain cricket meal as a dietary ingredient, while for the relative expression of trypsin and chymotrypsin, the intestine from the control group T0 was used. This research used a single reference gene, the elongation factor, since it is the most stable gene for the Atractosteus tropicus species, based mainly on the work of Jiménez-Martínez et al. [35], who evaluated various reference genes in this species and found the elongation factor to be the best.
The research was limited to the three enzymes involved in protein digestion within the digestive tract, because the emphasis of the evaluation of diets containing cricket meal (HCM) is on protein assimilation and the absence of negative gene expression during digestion.
2.5. Statistical Analysis
Biometric parameters and growth were evaluated with an ANOVA test for statistical differences (p < 0.05), and a Tukey test was performed for the distribution of means of the parameters (p < 0.05). To analyze the relative expressions of the gene in different organs, Kruskal–Wallis and a post hoc Nemenyi test were applied to observe significant differences between expressions according to diet, using a significant value of α = 0.05. All statistical analyses were performed using STATISTICA software (™ v.7.0, Statsoft, Tulsa, OK, USA), and Sigma Plot 12.0 (Grafiti LLC, Palo Alto, CA, USA) was used for the graphs.
3. Results
3.1. Growth and Biometric Parameters
Acheta domesticus meal (HCM) contains around 60% protein and has a nutritional content similar to FM. This study revealed a higher consumption of the control diet (0%) compared to the other treatments but was not statistically different compared to T3-50%, T4-75%, and T5-100%, with T2-25% being the diet with the lowest consumption and statistically different only to T1-0% (p < 0.05) (Figure 1a). In terms of feed efficiency, substitutions with T2-25% showed the highest value, being significantly different from T5-100% (p < 0.05), while T1-0%, T2-25%, T3-50%, and T4-75% did not show statistical differences between them (Figure 1b).

Figure 1.
(a) Feed consumption (FC), and (b) Feed efficiency (FE) in juvenile A. tropicus fed with different levels of A. domesticus meal (HCM). Different letters indicate statistical differences (p < 0.05), and bars represent the SD.
The effect of protein was evaluated, and we found that T1-0% and T5-100% were the treatments with the highest consumption, with a statistical difference compared to T3-50% and T4-75%, while T2-25% had the lowest consumption and a significant difference compared to some treatments (Figure 2a). The highest efficiency was observed in T2-25%, T3-50%, and T4-75%, which were statistically equal among each other but did not show statistical differences in relation to the fish fed with the T1-0% (Figure 2b). Likewise, the best feed conversion values were obtained in T2-25%, but was not statistically different compared to T1-0%, T3-50%, and T4-75%, which showed no significant differences between them. Meanwhile, T5-100% showed a significant difference compared T2-25%, but did not show statistical differences compared to the rest of the treatments (Figure 2c).
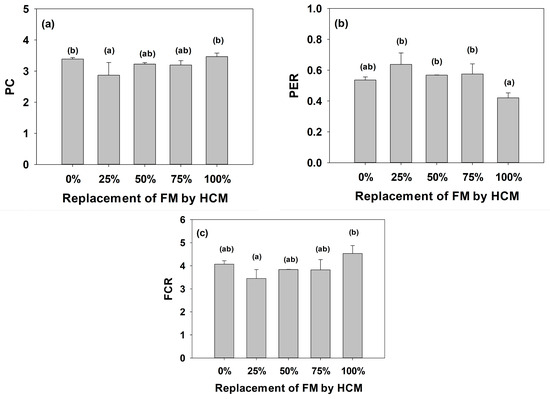
Figure 2.
(a) Protein consumption (PC), (b) Protein efficiency ratio (PER), and (c) Feed conversion ratio (FCR) in juvenile A. tropicus fed with different levels of A. domesticus meal (HCM). Different letters indicate statistical differences (p < 0.05), and bars represent the SD.
Absolute weight gain showed no significant difference between treatments T1-0%, T2-25%, T3-50%, and T4-75%, but did differ from treatment T5-100% (Figure 3a). The final results show that the highest growth was observed in T4-75%; meanwhile, T1-0%, T2-25%, T3-50%, and T4-75% did not show significant differences among themselves. A statistical difference (p < 0.05) between T4-75% and T5-100% was observed (Figure 3b).
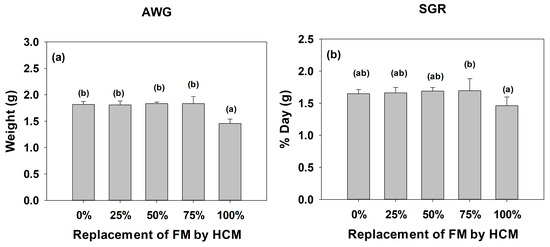
Figure 3.
(a) Absolute Weight Gain (AWG), and (b) Specific Growth Rate (SGR) in juveniles of A. tropicus fed with different levels of A. domesticus meal inclusion. Different letters indicate statistical differences (p < 0.05), and bars represent the SD.
3.2. Gene Expression
The highest relative expression values of the pepsin gene were observed in T1-0% and T4-75%, followed by T3-50% and T2-25% with no significant difference between them; however, values lower than the expression show in fish fed with the control diet and T4-75% (p < 0.05), with T5-100% being the treatment that showed the lowest relative expression values with significant differences compared to the other treatments (p < 0.05) (Figure 4a). Trypsin expression was similar to pepsin, with the highest relative expression values in T4-75%, followed by T1-0% which showed a statistical difference (p < 0.05). T2-25% and T3-50% showed no statistical difference (p > 0.05), while the lowest relative expression values were obtained in T5-100%, which was statistically different from the rest of the treatments (p < 0.05) (Figure 4b). Chymotrypsin expression showed no significant differences (p > 0.05) between treatments T1-0%, T2-25%, T3-50%, and T4-75%, but was statistically different (p < 0.05) from fish fed with treatment T5-100%, which had the lowest values (Figure 4c).
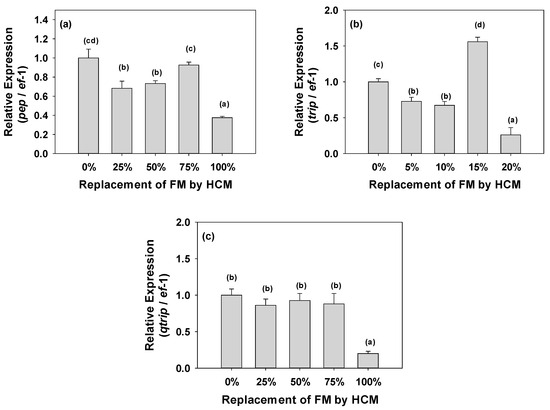
Figure 4.
(a) Gene expression of gastric pepsin in the stomach; (b) Gene expression of trypsin; and (c) Gene expression of chymotrypsin in the intestine of A. tropicus fed with different levels of A. domesticus. The values in the graph represent the means ± SD. Means with different superscripts are significantly different (p < 0.05).
4. Discussion
The digestive process in fish begins in the stomach, through pepsin-type enzymes, and continues in the intestine, where proteolysis continues and the majority of total free amino acids are absorbed, mainly through the action of trypsin and chymotrypsin. Protein is the most important nutrient in balanced fish diets, since, depending on the source and amino acid profile (animal, vegetable, microbial, among others), it will provide the proper conformation and higher filet yield [10,17]. In this regard, the proper selection of protein ingredients mainly promotes growth, feed efficiency, and protein efficiency in various fish species, so feeding habits must be considered for this selection. Another fundamental aspect related ts is amino acid profiles, which determine the correct conformation of muscle fibers (myofibrin and myelin), depending on the source of the protein included in the diet [10]. Therefore, including the right protein ingredients improves intake, feed efficiency, growth, and gene expression of digestive enzymes [36]. Based on the above, insect flours, depending on the species, stage of development, and production system, can be a rich source of protein (45–68% based on dry matter), because they have a good amino acid profile and are highly digestible by fish [37,38]. This is because, for several species, particularly omnivorous and carnivorous ones, insects are part of their prey, so the use of insect meal is in line with digestive capacity and, consequently, improves fish production by modulating blood biochemical parameters, gastrointestinal microbiota, oxidative stress, the immune system, and digestibility through a reduction in gastrointestinal transit [39,40,41,42,43,44].
In the present study, lower FC and PC, as well as better FE and PER values, were found in fish fed with the T2-25% HCM treatment, in addition to being similar to those obtained for fish fed with the T4-75% HCM treatment; however, in all cases, the values were not statistically different from the control treatment (p > 0.05). Fish fed with T5-100% HCM were negatively affected, which may be caused by the presence of anti-nutritional components in cricket meal, such as chitin or chitosan. In this regard, Mikołajczak et al. [45] indicate that PER and SGR values decreased for rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar) when 10% of fish meal was replaced with meal from silkworms (T. molitor) and mealworm (Zophobas morio) meal. Similarly, Tran et al. [15] indicate that some insect meals are deficient in tryptophan, methionine, and lysine, which can affect fish growth, although this depends on the level of substitution used [46].
In this study, it was found that FCR and AWG were statistically equal in fish fed with the control diet (T1-0% HCM) compared to those obtained with fish fed with treatments T2-25%, T3-50%, and T4-75% HCM, which were statistically higher than those obtained in fish fed with T5-100% HCM. These results are similar to those reported by Ahmed & Ahmad [46] in O. mikyss when including high concentrations of insect meal [11,47], which was related to a nutritional imbalance, as already documented by Susanto et al. [48], in juvenile Kelabau (Osteochilus melanopleurus); Ghosi-Mobaraki et al. [49], in pearl gourami (Trichogaster leeri); Odu-Onikosi et al. [50], in African catfish (Clarias gariepinus); Wang et al. [51], in spotted knifejaw (Oplegnathus punctatus); Hamdy et al. [52], in grass carp (Ctenopharyngodon idella); Baek and Cho [53], in red sea bream (Pagrus major), to name a few. On the other hand, a diet with the right ingredients and balanced nutrients can improve production parameters, as observed in sea bream (Megalobrama Pellegrini), where better FCR and growth are related to higher digestive enzyme activity [54], which is reflected in an appropriate balance of microbiota, serum biochemistry, and organosomatic indices, as tested by Mikołajczak et al. [37], in sea trout (Salmo trutta m. trutta) fry, when including 10% hydrolyzed meal from T. molitor and Z. morio as dietary ingredients. Similarly, Ndione et al. [55] report that the inclusion of 50% caterpillar meal (Cirina butyrospermi) did not have any negative effects on AWG and SGR in O. niloticus; however, at higher concentrations, the effects were lower. Other research indicates that insect meal is one of the most suitable alternative protein sources to fish meal in fish diets, as it has similar nutritional profiles to FM [10,39,55,56,57]. Promising results have been reported that allow FM to be partially replaced, although this depends on the fish and insect species [54]. Some authors indicate that replacing more than 30% of MF with insect meal can negatively affect fish growth [10,58,59,60]. However, the level of inclusion will depend on the type of insect from which the meal is derived, as demonstrated by Homska et al. [16], who reported greater growth in Ide (Leuciscus idus) with dietary inclusions of 20% meal from H. illucens and T. molitor larvae than with meal from Z. morio larvae and FM.
Although not statistically different (p > 0.05), fish fed T4-75% HCM had higher SGR compared to fish fed the control diet (T1-0% HCM), with no negative effect on the growth of juvenile A. tropicus. Similar results were reported by Ndione et al. [61] in O. niloticus when replacing 75% of MF with cricket meal (Ornithachris turbida Cavroisi). However, Lee et al. [62] report negative effects on growth and fat accumulation in the liver when more than 60% of MF is replaced in red hybrid tilapia (Oreochromis sp.). Perera and Bhujel [13] report for P. reticulata that replacing 75% of MF with A. domesticus and G. bimaculatus meal produced the best AWG and SGR values, while higher replacements negatively affecting these parameters. In addition, Irungu et al. [63] found that replacing 75% of FM with HCM increased phosphorus and potassium levels and reduced iron, magnesium, and sodium in fish feed, showing a low effect on feed leaching. Similarly, Perera et al. [64] indicate that replacing 100% of FM with HCM negatively affects the growth of O. niloticus. On the other hand, Tilami et al. [65] report an increase in linoleic acid when feeding with HCM and Z. morio meal, which has a positive effect as it helps regulate osmoregulation in freshwater fish when the inclusion of FM is reduced [66,67].
Digestive enzyme activity depends on feeding habits, gastrointestinal structure, and external factors, which affect the efficiency of nutrient hydrolysis in balanced feed, fish growth, and aquaculture profitability [68,69].
In fish with stomachs, such as A. tropicus, pepsin can maximize protein hydrolysis due to its high catalytic affinity with ingredients of animal origin, as has been observed in Antarctic rock cod (Trematomus bernacchii) [70]. Dietary components have been shown to regulate the expression of genes involved in nutrient digestion [71,72]. In this study, the expression of pepsin showed a negative effect on fish when FM was completely replaced by HCM. Similar data have been reported by Silva et al. [71] in diets with 30 and 42% HCM in O. niloticus fry, which was related to digestive capacity at this stage of development. Since they are omnivorous, they have feeding habits that allow them to hydrolyze insect larvae, as well as crustaceans, copepods, cladocerans, among other types of prey.
One aspect that should be highlighted is that each species has a different digestive capacity, so the use of insect meal depends on multiple factors such as the type of digestive tract and digestive enzymes. Particularly important is the presence of chitin in the exoskeleton of insects, and although some species can digest chitin, high concentrations of it in the diet reduce digestibility due to a greater amount of amino acids bound to chitin, as observed in O. niloticus and O. mykiss [57,73]. Thus, it has been reported that including adult insect meals increases the chitin content, so that digestion of the diet depends not only on proteases, but also on the synthesis and activity of the enzyme chitinase in the stomach and chitobiose in the intestine, which hydrolyzes chitin dimers into absorbable monomers of β N-acetyl-glucosamine [74]. On the other hand, trypsin and chymotrypsin carry out protein hydrolysis in the intestine, and their contributions to protein digestion vary among different fish species [69]. Both are synthesized in the exocrine pancreas in inactive forms as trypsinogen and chymotrypsinogen and are subsequently released into the intestine where enterokinase cleaves a short peptide from trypsinogen, converting it into active trypsin [28], which is more active when exposed to proteins of animal origin [75].
This study found that the highest expression was obtained in fish fed 75% HCM, although, it was not statistically different from the control treatment for the three enzymes analyzed. These results may be due to the fact that trypsin has highly conserved regions, which allow it to adapt to environmental stressors [76]; however, the effect of diet on relative expression may be due to the following: (1) the concentration of amino acids on which it exerts its action and their hydrolytic activity; (2) mRNA can be read repeatedly depending on the organism’s needs [77]; and (3) at insect meal concentrations higher than optimal, chitin decreases the palatability and digestibility of the diet [78], affecting in all cases the gene expression levels of certain digestive enzymes. The ability and plasticity of fish to catabolize different dietary nutrients as a biological evolution to meet their energy and nutrient requirements has been observed in most fish species studied [4,79,80].
Chymotrypsin is a protease that preferentially cleaves peptide amide bonds on the carboxyl side of aromatic amino acids such as tyrosine, tryptophan, and phenylalanine [28,30]. We observed that 100% HCM negatively affects its expression. Therefore, low expression could be due to amino acid deficiency; however, to counteract these deficiencies, these amino acids can be included in insect meal [7]. These amino acids play a very important role in fish physiology. For example, lysine participates in the acetylation of metabolic enzymes, modulating nutrient metabolism, and amino acid-induced lysine deacetylation in phosphoenolpyruvate carboxyquinase-1 improves enzyme stability and activity [81]. Sufficient amounts of amino acids such as leucine and arginine can activate the MTORC1 pathway (which is part of the mTOR protein kinase), which in turn increases protein synthesis and cell proliferation [82], while the absence of methionine induces the expression of C/EBP homology protein, a stress-induced transcription factor that regulates transcription, the cell cycle, and apoptosis. Likewise, tryptophan regulates the immune system and apoptosis together with methionine. Therefore, different mechanisms would be involved in the regulation of gene expression in the face of amino acid deficiency and stress [83,84], which is directly reflected in growth and could explain the low expression of chymotrypsin in our assay.
Unlike trypsin, which is usually sensitive when conditions favor growth but is genetically affected by dietary content, quimiotrypsin becomes vital when growth is limited or depressed [85]. This study showed that the highest expression of pepsin and trypsin in juvenile A. tropicus was achieved in fish fed with 75% HCM, while the highest expression of chymotrypsin was found in fish fed with the control diet, which was affected by 100% HCM.
Finally, using insect meal in the diets of native tropical fish appears to be an economically viable alternative to fishmeal-based feed production. However, due to the still-developing insect larvae meal industry, it would not be profitable for commercial-scale systems in the short term [86,87]. But the greater ecological sustainability of replacing other animal-based protein products means that there is potential for long-term reductions in production costs as the technology for industrializing insect meal advances [87].
5. Conclusions
In the present study, 75% inclusion of house cricket meal by fish meal showed the best growth rates and gene expression of pepsin and trypsin, while high levels of HCM inclusion by FM negatively modulated growth and gene expression in juvenile tropical gar, which may be the result of an amino acid deficiency. The high percentage of HCM acceptance in the diet may be due to A. tropicus in its early stages, since during this stage in their natural habitat, they commonly feed on insect larvae and crustaceans.
Author Contributions
Conceptualization, B.C.F., C.A.Á.-G. and G.G.-C.; methodology, F.J.D.l.C.-A. and B.C.F.; software, C.A.Á.-G.; validation, G.G.-C., D.J.P.-C. and S.H.-G.; formal analysis, F.J.D.l.C.-A. and S.H.-G.; investigation, B.C.F. and M.G.L.-C.; resources, J.L.A.-M., D.J.P.-C. and J.B.-O.; data curation, M.G.L.-C.; writing—original draft preparation, B.C.F.; writing—review and editing, C.A.Á.-G., J.B.-O. and D.J.P.-C.; visualization, J.L.A.-M. and J.B.-O.; supervision, C.A.Á.-G. and G.G.-C.; project administration, B.C.F. and D.J.P.-C.; funding acquisition, G.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Helsinki Declaration, the NOM-062-ZOO-2000 (Mexico), NOM-033-SAG/ZOO-2014 (Mexico), and the protocol and experiment were approved by the Institutional Commission of Ethics in Research of the Universidad Juárez Autónoma de Tabasco, Mexico (approval code: CIEI-2025-071 and approval date: 11 September 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request to the corresponding authors.
Acknowledgments
The authors would like to thank the Laboratory of Physiology in Aquatic Resources (LAFIRA) in the Academic Division of Biological Sciences of the Universidad Juárez Autónoma de Tabasco, Mexico. Likewise, we thank, César Antonio Sepúlveda Quiroz, and Luis Daniel Jiménez Martínez for their recommendations and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Official Journal of the Federation. National Aquaculture Charter 2021. National Fisheries Institute. Mexico, 15 April 2021. Available online: https://www.gob.mx/inapesca/documentos/carta-nacional-acuicola-2021 (accessed on 1 May 2024).
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J.; Contreras-Sánchez, W.M.; Álvarez-González, C.A. Sustainable Tropical Aquaculture: A Strategy for the Production and Conservation of Pejelagarto (Atractosteus tropicus) in Tabasco, Mexico, 2nd ed.; Juárez Autonomous University of Tabasco: Villahermosa, Mexico, 2015. [Google Scholar]
- Gougbedji, A.; Detilleux, J.; Lalèyè, P.A.; Francis, F.; Caparros Megido, R. Can Insect Meal Replace Fishmeal? A Meta-Analysis of the Effects of Black Soldier Fly on Fish Growth Performances and Nutritional Values. Animals 2022, 12, 1700. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Alvarado, F.J.; Álvarez-González, C.A.; Nolasco-Soria, H.; Martínez-García, R.; Piña-Gutierrez, J.M.; Concha-Frías, B.; Frás-Quintana, C.A.; Peña, E. Characterization and improvement of Cannavalia ensiformis flour as a balanced feed for Oreochromis niloticus. Hidrobiológica 2019, 29, 163–170. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processing. 2017. Available online: http://data.europa.eu/eli/reg/2017/893/oj (accessed on 18 September 2025).
- Ferrer-Llagostera, P.F.; Kallas, Z.; Reig, L.; De Gea, D.A. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Sawant, S.S.; Salin, K.R. Use of insect-based meals in aquaculture. J. Exp. Zool. India 2020, 23, 715–724. [Google Scholar]
- Siddiqui, S.A.; Zhao, T.; Fitriani, A.; Rahmadhia, S.N.; Alirezalu, K.; Ito, F. Acheta domesticus (house cricket) as human foods-An approval of the European Commission-A systematic review. Food Front. 2024, 5, 435–473. [Google Scholar] [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black soldier fly full-fat larvae meal is more profitable than fish meal and fish oil in Siberian sturgeon farming: The effects on aquaculture sustainability, economy and fish growth development. Animals 2021, 11, 604. [Google Scholar] [CrossRef]
- Sándor, Z.J.; Banjac, V.; Vidosavljević, S.; Káldy, J.; Egessa, R.; Lengyel-Kónya, É.; Tömösközi-Farkas, R.; Zalán, Z.; Adányi, N.; Libisch, B.; et al. Apparent Digestibility Coefficients of Black Soldier Fly (Hermetia illucens), Yellow Mealworm (Tenebrio molitor), and Blue Bottle Fly (Calliphora vicina) Insects for Juvenile African Catfish Hybrids (Clarias gariepinus × Heterobranchus longifilis). Aquac. Nutr. 2022, 2022, 4717014. [Google Scholar] [CrossRef] [PubMed]
- Perera, G.S.C.; Bhujel, R.C. Replacement of fishmeal by house cricket (Acheta domesticus) and field cricket (Gryllus bimaculatus) meals: Effect for growth, pigmentation, and breeding performances of guppy (Poecilia reticulata). Aquac. Rep. 2022, 25, 101260. [Google Scholar] [CrossRef]
- Cadena-Cadena, F.; Cuevas-Acuña, D.A.; Concha-Frias, B.; Hernández, R.C.; Nuñez, J.C.G.; Martinez, B.A.; Arias-Moscoso, J.L. Replacement of fishmeal by common cricket (Acheta domesticus) meal in diets for juvenile tilapia (Oreochromis niloticus). Isr. J. Aquac.-Bamidgeh 2023, 75, 1–12. [Google Scholar] [CrossRef]
- Tran, G.; Heuzé, V.; Makkar, H.P.S. Insects in fish diets. Anim. Front. 2015, 5, 37–44. [Google Scholar]
- Homska, N.; Kowalska, J.; Bogucka, J.; Ziółkowska, E.; Rawski, M.; Kierónczyk, B.; Mazurkiewicz, J. Dietary Fish Meal Replacement with Hermetia illucens and Tenebrio molitor Larval Meals Improves the Growth Performance and Nutriphysiological Status of Ide (Leuciscus idus) Juveniles. Animals 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hanboonsong, Y.; Totakul, P.; Matra, M.; Cherdthong, A.; Wanapat, M. Nutritional composition of various insects and potential uses as alternative protein sources in animal diets. Anim. Biosci. 2022, 35, 317–331. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, H.; Li, B.; Su, L.; Deng, J.; Cao, Z. Effects of dietary lysine level on the growth performance, protein metabolism, and antioxidant status in Hemibagrus wyckioides juveniles. J. World Aquac. Soc. 2023, 54, 1317–1336. [Google Scholar] [CrossRef]
- Liaqat, R.; Fatima, S.; Komal, W.; Minahal, Q.; Hussain, A.S. Dietary supplementation of methionine, lysine, and tryptophan as possible modulators of growth, immune response, and disease resistance in striped catfish (Pangasius hypophthalmus). PLoS ONE 2024, 19, e0301205. [Google Scholar] [CrossRef]
- Gutiérrez-Vela, M. Effect of Feeding Sea Bream (Sparus aurata L.) with Fishmeal-Free Feed on Digestive Enzyme Activity. Ph.D. Dissertation, Polytechnic University of Valencia, Valencia, Spain, 2017. [Google Scholar]
- Sunde, J. Digestive Protease Activities, Growth and Feed Utilisation in Atlantic Salmon (Salmo salar L.). Ph.D. Dissertation, University of Bergen, Bergen, Norway, 2006. [Google Scholar]
- De Melo-Oliveira, V.; de Souza Bezerra, R.; Assis, C.R.D. Fish pepsin: Basic characteristics, extraction, determination and biotechnological applications. Nat. Resour. 2014, 4, 6–14. [Google Scholar]
- Hassaan, M.S.; Mohammady, E.Y.; Adnan, A.M.; Abd Elnabi, H.E.; Ayman, M.F.; Soltan, M.A.; El-Haroun, E.R. Effect of dietary protease at different levels of malic acid on growth, digestive enzymes and haemato-immunological responses of Nile tilapia, fed fish meal free diets. Aquaculture 2020, 522, 735124. [Google Scholar] [CrossRef]
- Shah, S.Z.H.; Fatima, M.; Afzal, M.; Bilal, M. Interactive effect of citric acid, phytase and chelated mineral on growth performance, nutrient digestibility and whole-body composition of Labeo rohita fingerlings. Aquac. Res. 2021, 52, 842–858. [Google Scholar] [CrossRef]
- Brilianto-Muttaqin, R.A.; Surya, A.; Istiarini, I.; Duanassurya, M.; Samosir, W.; Makatita, S.; Nurhayati, T. Extraction and characterization of pepsin enzyme from the skipjack vicera (Katsuwonus pelamis). BIO Web Conf. 2024, 147, 01018. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurul-Izzati, S.A.; Nurhayati, T.; Jacoeb, A.M. Application of pepsin enzyme from yellowfin tuna gastrics on the physical and histological characteristics of beef. BIO Web Conf. 2024, 147, 01026. [Google Scholar] [CrossRef]
- Maryam; Shah, S.Z.H.; Fatima, M.; Nadeem, H.; Ashraf, S.; Hussain, M. Roles of dietary supplementation of exogenous protease in low fishmeal aquafeed—A mini review. Ann. Anim. Sci. 2024, 24, 27–39. [Google Scholar] [CrossRef]
- Solovyev, M.; Kashinskaya, E.; Gisbert, E. A meta-analysis for assessing the contributions of trypsin and chymotrypsin as the two major endoproteases in protein hydrolysis in fish intestine. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 278, 111372. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Khajavi, M.; Abedian Kenari, A.; Haghbin Nazarpak, M.; Solouk, A.; Esmaeili, M.; Gisbert, E. Physicochemical and Biochemical Properties of Trypsin-like Enzyme from Two Sturgeon Species. Animals 2023, 13, 853. [Google Scholar] [CrossRef]
- Whitaker, J.R. Principles of Enzymology for the Food Sciences, 2nd ed.; Dekker, M., Ed.; INC: New York, NY, USA, 1994. [Google Scholar]
- Hofer, R.; Schiemer, F. Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia 1981, 48, 342–345. [Google Scholar] [CrossRef]
- Moyano, F.J.; Diaz, M.; Alarcón, F.J.; Sarasquete, M.C. Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol. Biochem. 1996, 15, 121–130. [Google Scholar] [CrossRef]
- Concha-Frías, B.; González, C.A.A.; Gaxiola, G.; Chiappa, X.; Sánchez-Zamora, A.; Martínez-García, R.; Camarillo-Coop, S.; Peña, E.; Jiménez-Martínez, L.D.; De la Cruz-Alvarado, F.J. Dietary protein requirement in common snook (Centropomus undecimalis) juveniles reared in marine and brackish water. Ecosistemas Recur. Agropecu. 2018, 5, 45–54. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Jimenez-Martinez, L.D.; Morales-Garcia, V.; Frías-Quintana, C.A.; Castillo-Collado, A.C.; Asencio-Alcudia, G.G.; Álvarez-Villagomez, C.S.; Peña, E.; Concha-Frías, B.; Álvarez-González, C.A. Quality evaluation of reference gene expression on different tissues in adults of tropical gar Atractostreus tropicus. Pak. J. Zool. 2021, 54, 363–372. [Google Scholar] [CrossRef]
- Molinari, G.S.; Wojno, M.; Kwasek, K. Effects of dietary indispensable amino acid deficiencies on feed intake in stomachless fish. Comp. Biochem. Physiol. Part A 2024, 298, 111742. [Google Scholar] [CrossRef]
- Makinde, O.J. Maggot meal: A sustainable protein source for livestock production—A Review. Adv. Life Sci. Technol. 2015, 31, 35–42. [Google Scholar]
- Chaalala, S.; Leplat, A.; Makkar, H. Importance of insects for use as animal feed in low-income countries. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 303–319. [Google Scholar]
- Mikołajczak, Z.; Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. The effect of hydrolyzed insect meals in sea trout fingerling (Salmo trutta m. trutta) diets on growth performance, microbiota and biochemical blood parameters. Animals 2020, 10, 1031. [Google Scholar] [CrossRef]
- Bosi, A.; Bani, D.; Moroni, F.; Ceccotti, C.; Giron, M.C.; Antonini, M.; Giaroni, C.; Terova, G. Effect of partial substitution of ishmeal with insect meal (Hermetia illucens) on gut neuromuscular function in Gilthead sea bream (Sparus aurata). Sci. Rep. 2021, 11, 21788. [Google Scholar] [CrossRef]
- Peng, K.; Mo, W.; Xiao, H.; Hu, J.; Zhu, X.; Huang, Y.; Wang, G. Dietary black soldier fly pulp affects growth, antioxidant and immune capacity of Micropterus salmoides. J. Insects Food Feed 2021, 8, 1197–1203. [Google Scholar] [CrossRef]
- Terova, G.; Gini, E.; Gasco, L.; Moroni, F.; Antonini, M.; Rimoldi, S. Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 30. [Google Scholar] [CrossRef]
- Gasco, L.; Schiavone, A.; Caimi, C.; Trocino, A.; Lussiana, C.; Bellezza-Oddon, S.; Malfatto, V.; Anedda, R.; Serra, G.; Biasato, I.; et al. Digestibility of defatted insect meals for rainbow trout aquafeeds. J. Insects Food Feed 2022, 8, 1385–1399. [Google Scholar] [CrossRef]
- Sajid, Q.U.A.; Asghar, M.U.; Tariq, H.; Wilk, M.; Płatek, A. Insect Meal as an Alternative to Protein Concentrates in Poultry Nutrition with Future Perspectives (An Updated Review). Agriculture 2023, 13, 1239. [Google Scholar] [CrossRef]
- Mikołajczak, Z.; Mazurkiewicz, J.; Rawski, M.; Kierończyk, B.; Józefiak, A.; Świątkiewicz, S.; Józefiak, D. Black soldier fly full-fat meal in Atlantic salmon nutrition–Part A: Effects on growth performance, feed utilization, selected nutriphysiological traits and production sustainability in fries. Ann. Anim. Sci. 2022, 23, 225–238. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I. Effect of dietary protein levels on growth performance, hematological profile and biochemical composition of fingerlings rainbow trout, Oncorhynchus mykiss reared in Indian Himalayan region. Aquac. Rep. 2020, 16, 100268. [Google Scholar] [CrossRef]
- Adewumi, A.A. The impact of nutrition on fish development, growth and health. Int. J. Sci. Res. Publ. 2018, 8, 147–153. [Google Scholar]
- Susanto, A.; Hutabarat, J.; Anggoro, S.; Subandiyono. The effects of dietary carbohydrate level on the growth performance, body composition and feed utilization of juvenile Kelabau (Osteochilus melanopleurus). AACL Bioflux 2020, 13, 261–270. [Google Scholar]
- Ghosi-Mobaraki, M.R.; Abedian-Kenari, A.; Bahrami-Gorji, S.; Esmaeili, M. Effect of dietary fish and vegetable oil on the growth performance, body composition, fatty acids profile, reproductive performance and larval resistance in pearl gourami (Trichogaster leeri). Aquac. Nutr. 2020, 26, 894–907. [Google Scholar] [CrossRef]
- Odu-Onikosi, S.G.; Babalola, O.A.; Ogunbanwo, O. Effect of alternate feeding of normal and low protein diet on the growth performance and biochemical composition of Clarias gariepinus (Burchell, 1822) fingerlings. Int. J. Fish. Aquat. Stud. 2021, 9, 63–67. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Zheng, P.; Xu, H.; Su, H.; Han, T.; Yang, Y. Effect of dietary lipid levels on growth performance, body composition, and feed utilization of juvenile spotted knifejaw Oplegnathus punctatus. Aquac. Rep. 2021, 21, 100797. [Google Scholar] [CrossRef]
- Hamdy, A.M.S.; Emad, E.I.A.M.; Faiza, M.S.; Ahmed, E.A.B.; Alaa, G.M.O. Fish growth performance, body composition and water quality in integrated system producing Grass carp (Ctenopharyngodon idella) in the Eastern Desert. Sohag J. Sci. 2022, 7, 67–75. [Google Scholar] [CrossRef]
- Baek, S.I.; Cho, S.H. Dietary Replacement Effect of Fish Meal by Tuna By-Product Meal on Growth and Feed Availability of Red Sea Bream (Pagrus major). Animals 2024, 14, 688. [Google Scholar] [CrossRef]
- Qu, H.; Chen, L.; Yang, J.; Liao, J.; Wei, D.; Lu, X. Effects of feeding strategies on growth, body composition, intestine digestive enzymes activities and intestine histology of Megalobrama pellegrini (Tchang, 1930) early juveniles raised in flow-through system. Isr. J. Aquac.-Bamidgeh 2020, 72, 1–9. [Google Scholar] [CrossRef]
- Ndione, A.; Fall, J.; Mbaye, S.; Ndour, P.M.; Khady, L.F.S.; Jatta, S.; Loum, A.; Sagne, M.; Diouf, A.; Ndong, D.; et al. Effect of replacing fishmeal with caterpillar (Cirina butyrospermi) meal on the growth performance, feed efficiency, survival and body composition of Nile tilapia (Oreochromis niloticus) fry. J. Fish. Soc. Taiwan 2022, 49, 193–199. [Google Scholar] [CrossRef]
- Alfiko, Y.; Xie, D.; Tri Astuti, R.; Wong, J.; Wan, L. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Manan, M.C.A.; Naz, S.; Danabas, D. Effect of Insect Feed on Fish Growth: A Review. Asian Fish. Sci. 2024, 37, 52–68. [Google Scholar] [CrossRef]
- Reyes, M.; Rodríguez, M.; Montes, J.; Barroso, M.G.; Fabrikov, D.; Morote, E.; Sánchez-Muros, M.J. Nutritional and Growth Effect of Insect Meal Inclusion on Seabass (Dicentrarchuss labrax) Feeds. Fishes 2020, 5, 16. [Google Scholar] [CrossRef]
- Hua, K. A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture 2021, 530, 735732. [Google Scholar] [CrossRef]
- Liland, N.; Araujo, P.; Xu, X.; Lock, E.-J.; Radhakrishnan, G.; Prabhu, A.; Belghit, I. A meta-analysis on the nutritional value of insects in aquafeeds. J. Insects Food Feed 2021, 7, 743–760. [Google Scholar] [CrossRef]
- Ndione, A.; Fall, J.; Mbaye, S.; Ndour, P.M.; Khady, L.F.S.; Jatta, S.; Loum, A.; Sagne, M.; Diouf, A.; Ndong, D.; et al. Effects of replacement of fishmeal by cricket meal on the growth performance, feed efficiency, survival and body composition of Nile Tilapia (Oreochromis niloticus) fry. J. Fish. Soc. Taiwan 2022, 49, 183–191. [Google Scholar] [CrossRef]
- Lee, S.W.; Tey, H.C.; Wendy, W.; Wan Zahari, M. The effect of house cricket (Acheta domesticus) meal on growth performance of red hybrid tilapia (Oreochromis sp.). Int. J. Aquat. Sci. 2017, 8, 78–82. [Google Scholar]
- Irungu, F.G.; Mutungi, C.M.; Faraj, A.K.; Affognon, H.; Tanga, C.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Mineral content of extruded fish feeds containing cricket (Acheta domesticus) and black soldier fly (Hermetia illucens) fractions. Int. Aquat. Res. 2018, 10, 101–113. [Google Scholar] [CrossRef]
- Perera, G.S.C.; Perera, D.A.; Piyavorasakul, C.; Pumpuang, S. Fishmeal Replacement by House cricket (Acheta domesticus) and Field cricket (Gryllus bimaculatus) Meals in Nile Tilapia (Oreochromis niloticus) Fingerling Feed. Aquac. Stud. 2023, 23, AQUAST1187. [Google Scholar] [CrossRef]
- Tilami, S.K.; Turek, J.; Červený, D.; Lepič, P.; Kozák, P.; Burkina, V.; Sakalli, S.; Tomčala, A.; Sampels, S.; Mráz, J. Insect Meal as a Partial Replacement for Fish Meal in a Formulated Diet for Perch Perca fluviatilis. Turk. J. Fish. Aquat. Sci. 2020, 20, 867–878. [Google Scholar] [CrossRef]
- Silva, A. Marine Fish Farming; Catholic University of the North, Faculty of Marine Sciences: Santiago, Chile, 2005; p. 262. [Google Scholar]
- Baldisserotto, B.; Mancera, J.M.; Kapoor, B.G. Fish Osmoregulation; Science Publishers: Enfield, NH, USA, 2007; p. 527. [Google Scholar] [CrossRef]
- Navarro-Guillén, N.C.; Yúfera, M.; Perera, E. Biochemical features and modulation of digestive enzymes by environmental temperature in the greater amberjack, Seriola dumerili. Front. Mar. Sci. 2022, 9, 960746. [Google Scholar] [CrossRef]
- Lal, V.; Naeem, M.; Asad, M.; Tanveer, K.; Zulfiqar, A.; Kausar, S. Study of digestive enzymes in marine fish, Terapon jarbua, from Pakistan. Braz. J. Biol. 2024, 84, e267508. [Google Scholar] [CrossRef]
- Carginale, V.; Trinchella, F.; Capasso, C.; Scudiero, R.; Parisi, E. Gene amplification and cold adaptation of pepsin in Antarctic fish. A possible strategy for food digestion at low temperature. Gene 2004, 336, 195–205. [Google Scholar] [CrossRef]
- Silva, W.S.; Ribeiro, P.A.P.; Costa, L.S.; López-Olmeda, J.F.; Costa, N.C.S.; Santos, W.M.; Luz, R.K. Gene expression, enzyme activity and performance of Nile tilapia larvae fed with diets of different CP levels. Animal 2019, 13, 1376–1384. [Google Scholar] [CrossRef]
- Syed-Makhdoom, H.; Muniba, J.; Shafaqat, A.; Muhammad, M.S.; Majid, H.; Nisar, A.; Danish, R. Gene Expression, Growth Performance and Body Composition of Labeo rohita Fingerlings Fed on Polyphenols Supplement. Sains Malays. 2023, 52, 3357–3370. [Google Scholar] [CrossRef]
- Eggink, K.M.; Pedersen, P.B.; Lund, I.; Dalsgaard, J. Chitin digestibility and intestinal exochitinase activity in Nile tilapia and rainbow trout fed different black soldier fly larvae meal size fractions. Aquac. Res. 2022, 53, 5536–5546. [Google Scholar] [CrossRef]
- Gutowska, M.A.; Drazen, J.C.; Robison, B.H. Digestive chitinolytic activity in marine fishes of Monterey Bay, California. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 139, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Jonás, E.; Rágyanszki, M.; Oláh, J.; Boross, L. Proteolytic digestive enzymes of carnivorous (Silurus glanis L.), herbivorous (Hypophthalmichthys molitrix Val.) and omnivorous (Cyprinus carpio L.) fishes. Aquaculture 1983, 30, 145–154. [Google Scholar] [CrossRef]
- Kanno, G.; Klomklao, S.; Kumagai, Y.; Kishimura, H. A thermostable trypsin from freshwater fish Japanese dace (Tribolodon hakonensis): A comparison of the primary structures among fish trypsins. Fish Physiol. Biochem. 2018, 45, 561–571. [Google Scholar] [CrossRef]
- Krebs, J.E.; Goldstein, E.S.; Kilpatrick, S.T. Lewin’s Genes XII; Jones & Bartlett Learning, LLC.: Burlington, MA, USA, 2018; p. 3194. [Google Scholar]
- Kroeckel, S.; Harjes, A.G.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Steinberg, C.E. Diets and digestive tracts–‘Your food determines your intestine’. In Aquatic Animal Nutrition: A Mechanistic Perspective from Individuals to Generations; Springer: Cham, Switzerland, 2018; pp. 9–59. [Google Scholar]
- Méndez, E. Digestive and Metabolic Biochemical Physiology of Decapod Crustaceans and Teleost Fish of Regional, Ecological, and/or Economic Interest: Characterization and Modulation of Key Enzymes in the Digestive Tract. Ph.D. Thesis, National University of Mar del Plata, Mar del Plata, Argentina, 2020. [Google Scholar]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A.; Wu, G. Amino acids and conceptus development during the peri-implantation period of pregnancy. In Cell Signaling During Mammalian Bendhack Fabiano Early Embryo Development; Springer: New York, NY, USA, 2015; pp. 23–52. [Google Scholar]
- Morin, G.; Pinel, K.; Dias, K.; Seiliez, I.; Beaumatin, F. RTH-149 Cell Line, a Useful Tool to Decipher Molecular Mechanisms Related to Fish Nutrition. Cells 2020, 9, 1754. [Google Scholar] [CrossRef]
- Machado, M.; Serra, C.R.; Oliva-Teles, A.; Costas, B. Methionine and Tryptophan Play Different Modulatory Roles in the European Seabass (Dicentrarchus labrax) Innate Immune Response and Apoptosis Signaling—An In Vitro Study. Front. Immunol. 2021, 12, 660448. [Google Scholar] [CrossRef] [PubMed]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbø, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Biteau, C.; Bry-Chevalier, T.; Crummett, D.; Ryba, R.; St. Jules, M. Insect-based livestock feeds are unlikely to become economically viable in the near future. Food Humanit. 2024, 3, 100383. [Google Scholar] [CrossRef]
- Auzins, A.; Leimane, I.; Reissaar, R.; Brobakk, J.; Sakelaite, I.; Grivins, M.; Zihare, L. Assessing the socio-economic benefits and cost of insect meal as a fishmeal substitute in livestock and aquaculture. Animals 2024, 14, 1461. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).