Abstract
Sinocyclocheilus grahami (Regan, 1904) is the only known cavefish used both in traditional medicine and aquaculture in the world, and it is also a national protected animal. However, its taxonomy and distribution remain ambiguous, hindering effective conservation and resource utilization. This study clarifies the taxonomy of S. grahami through field surveys, specimen examination, and literature reviews, combined with principal component analysis (PCA) for morphological comparison. The results indicate that S. grahami and S. guanduensis show no significant morphological differences, supporting the recognition of S. guanduensis as a junior synonym of S. grahami. Sinocyclocheilus huanglongdongensis, and S. hei, once synonyms of S. grahami, should be valid. The species faces a significant population decline, and its distribution is now restricted to a few unpolluted streams around Dianchi Lake. While artificial breeding has been successful, further studies are needed to determine the medicinal efficacy of these individuals. To address the conservation and sustainable use of this species, habitat restoration should be prioritized, particularly in protecting cave environments and maintaining groundwater connectivity. Additionally, artificial breeding and release in restored habitats are essential to establish a sustainable ecological governance system that supports biodiversity and regional development.
Key Contribution:
This study clarifies the taxonomy of Sinocyclocheilus grahami, confirming S. guanduensis as a junior synonym of the former. Sinocyclocheilus huanglongdongensis and S. hei, which were previously synonymized with S. grahami, may actually be valid species.
1. Introduction
Cavefish, whose life histories are adapted to caves and other subterranean environments, exhibit traits such as eye reduction, loss of pigmentation, and enhanced sensory abilities, which we call troglomorphism. Depending on whether they possess these traits and the degree of their adaptation to caves, cavefish can be further divided into stygobionts and stygophiles [1]. Cavefish are predominantly found in karst regions situated between the 40 degrees north latitude and the Tropic of Capricorn worldwide [2]. More than 75% of known cave fish populations are distributed in Southeast Asia and the Americas, where karst landscapes composed of limestone and other soluble rock types are prevalent [3,4]. China boasts the greatest species diversity of cavefish on the planet [5]. Presently, China is home to a minimum of 180 cavefish species, which constitute 11% of the country’s freshwater fish species. Over half of these species are considered stygobiont cavefish [6]. However, their population sizes are generally small and concentrated in only a few taxonomic groups. These species are found within a relatively narrow distribution range and exhibit distinct differentiation [1,7].
The Mexican blind cavefish, Astyanax mexicanus (De Filippi, 1853), has been widely used as a model organism [8] in research related to medicine and human health [9]. However, other cavefish species have hardly been utilized by humans. There are no known reports of cavefish being used as medicinal animals.
The fishes of the genus Sinocyclocheilus Fang, 1936, known as golden-line barbs, are endemic to China, have cave-dwelling behavior, and are the largest group of cavefishes globally [1]. Within this genus, Sinocyclocheilus grahami (Regan, 1904) holds the distinction of being the first cavefish (stygophile) ever documented worldwide [2]. Remarkably, the earliest record of S. grahami originates from a traditional Chinese medical medicine classic. In 1436, Mao Lan described a “golden lined fish” in his book, the Materia Medica of South Yunnan [10]. The fish was formally described as Barbus grahami by Regan [11]. It stands out as the sole cavefish species known to have been used as a medicinal resource in traditional medicine. However, due to habitat degradation, water pollution, and over-exploitation, populations have rapidly declined. Consequently, it was listed on the Chinese Key Protected Wildlife List in 1998 as a second-class protected species.
The taxonomy of Sinocyclocheilus grahami still remains ambiguous, and its distribution is unclear, posing challenges to the effective resource utilization and conservation. Based on our long-term field surveys, as well as thorough examinations of specimens, our objective is to clarify the taxonomy of S. grahami and to review its conservation status and utilization efforts.
2. Materials and Methods
2.1. Specimen Collection, Examination, and Preservation
Field collections adhered to the Guide to Collection, Preservation, Identification and Information Share of Animal Specimens [12], and the Implementation Rules of the Fisheries Law of the People’s Republic of China. All activities complied with the Laboratory Animal-Guideline for Ethical Review of Animal Welfare (GB/T 35892–2018) [13].
All examined specimens were collected using hand nets and mesh traps. Detailed specimen information is listed in the section Comparative Materials. Specimens used for morphological study were preserved in 10% formalin and then transferred to 70% ethanol for long-term storage. The specimens of Sinocyclocheilus grahami are deposited at the Institute of Zoology, Chinese Academy of Sciences, Beijing, China (ASIZB) and the personal collections of W. X. Li.
2.2. Morphological Study
Counts and measurements followed Zhao et al. [14]. All measurements were taken point-to-point on the left side of the specimens using digital calipers, accurate to 0.1 mm, with counts also made on the left side. Finer features (i.e., cephalic lateral line system, fins rays, nostrils distance) were examined under a microscope. The specimens (n = 10) used for vertebral counts were radiographed with an XDR-AZ1600 X-ray machine produced by Shanghai Anzhu Photoelectric Technology Co., LTD. (X-ray tube voltage 60 kV, tube current 1 mA, and exposure time of 2 s). To gain a general perception on external morphologic differences, 36 different measurable traits, log10-standardized to eliminate the allometries, were input into Past v. 4.03 [15] for principal component analysis (PCA) [16].
3. Results
3.1. Taxonomic Account
- Sinocyclocheilus grahami (Regan, 1904)
- Barbus grahami Regan, 1904: 190 (original description); Zhang (1959): 54.
- Percocypris grahami: Wu (1961): 89.
- Sinocyclocheilus grahami grahami: Wu et al. (1977): 263; Wu et al. (1979): 73; Chu and Cui (1989): 175.
- Sinocyclocheilus grahami: Li et al. (1994): 7; Li et al. (1996): 60; Romero et al. (2009): 226; Zhao and Zhang (2009): 162; Zhang and Zhao (2016): 100; Zhang et al.(2020): 148.
- Sinocyclocheilus (S.) grahami: Shan et al. (in Yue, 2000): 66.
- Sinocyclocheilus guanduensis Li et Xiao, 2004: Xiao et al. (2004): 522.
3.2. Materials Examined
3.2.1. Holotype
BMNH 1904.1.26.27 Barbus grahami. Collected in China. Data were provided by The Trustees of the Natural History Museum, London (Figure 1). Permanent Record URL: licensed under (http://creativecommons.org/licenses/by/4.0/ (accessed on 30 November 2024)).

Figure 1.
Sinocyclocheilus grahami, holotype, BMNH 1904.1.26.27.
3.2.2. Additional Materials Examined
ASIZB 03495–03499 (5), ASIZB 03501–03505 (5), ASIZB 03536–03539 (4), ASIZB 13202 (1), ASIZB 46922 (1), 94.1–121.6 mm SL, Dianchi Lake, Kunming, Jinshajiang River system; ASIZB 03534(1), 101.0 mm SL, Dianchi Lake, Yunnan, Jinshajiang River system, September 1947, ASIZB 3541–3542 (2), 107.6–117.1 mm SL, Dianchi Lake, Yunnan, Jinshajiang River system, ASIZB 56751–56759 (9), 99.8–121.1 mm SL, ASIZB 7260–7262 (3), 79.0–99.3 mm SL, Jinning, Yunnan, Jinshajiang River system; Li 9908006–7, 2 specimens, paratypes of Sinocyclocheilus guanduensis Li et Xiao, 2004, 99.9–106.7 mm SL; Jinxiandiaohulu, Kunming City, Yunnan Province, August 1999, collected by W.X. Li.
3.3. Diagnosis
Sinocyclocheilus grahami can be distinguished from all other congeners by the following combination of characteristics: (1) body shape normal, no prominent hump on the dorsal side of the head, abdomen profile slightly straight; (2) no horizontal black line along ventral side of body, several black spots scattered along lateral line; (3) the last unbranched dorsal-fin ray stiff, posterior margin serrated; (4) pectoral-fin not reaching pelvic-fin origin; (5) pelvic-fin origin anterior to dorsal-fin origin; (6) second pair of barbels not extending to posterior edge of operculum.
3.4. Description
Thirty-three specimens were measured. The standard body shape of the specimens is shown in Figure 1, with quantitative traits listed in Table 1.

Table 1.
Morphometric measurements of Sinocyclocheilus grahami, S. guanduensis, S. hei, and S. huanglongdongensis.
Body laterally compressed. Dorsal hump on junction between head and back insignificant, the highest point of body located slightly in front of dorsal-fin origin. Dorsal profile extends backward in an arc from snout to dorsal-fin origin, gradually sloping to caudal-fin base. Abdomen profile slightly straight.
Head compressed. Snout rounded, with a small protrusion at center of dorsal side. Nostrils situated on half of distance between tip of snout and anterior edge of eyes. Anterior nostrils rounded and in short tube shape. Posterior edge of tube wing shaped, covering the opening. Posterior nostrils open oblong. Mouth sub-inferior. Ventral side of oral cavity arc-shaped, upper jaw slightly longer than lower jaw. Lips simple and thin. Upper lip base covered by rostral cap. Upper and lower jaws connected at corners of mouth. Postlabial grooves extend forwards to chin; left and right grooves disconnected. Barbels two pairs: first pair located in front of anterior nostrils, medium length, just exceeding anterior edge of eyes; second pair medium length, exceeding posterior edge of eyes. Eye rounded, moderately in size. Gill opening large, dorsal corners below upper edge of eyes. Branchiostegal membrane connected at isthmus. Joint of dentary-angulars not adjacent at isthmus. Gill rakers triangular, well-developed and sparsely arranged, five to nine gill rakers on outer side of first gill arch. Epibranchial gill rakers one (16 specimens) or two (18), ceratobranchial gill rakers four (8), five (20), six (5) or seven (2). Total vertebrae 4 + 37 (9) or 38 (1) (Figure 2). Pharyngeal teeth “2,3,4–4,3,2” (in three rows).
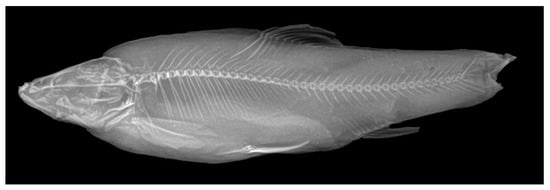
Figure 2.
Radiograph, showing the vertebrae of Sinocyclocheilus grahami, ASIZB 56753, 120.99 mm SL.
Dorsal fin with three unbranched and seven (31 specimens) branched rays, origin located approximately midway of tip of snout and caudal-fin base; pectoral fin with one unbranched and 15–18 (31) branched rays, inserted posterior to operculum, relatively short, not reaching pelvic-fin origin; pelvic fin with one unbranched and 7–9 (31) branched rays, origin anteroventral to dorsal fin, midway between pectoral and anal-fin origins, medium length, extending two-thirds of distance from pelvic-fin origin to anal-fin origin, not reaching anus; anal fin with three unbranched and five (31) branched rays; origin approximately half-way between pelvic-fin origin and base of caudal-fin base; caudal fin forked.
Lateral line complete, starting from upper corner of gill openings, curving above pectoral fin and extending to caudal-fin base. Body covered with scales, scales mostly buried below the skin. Lateral line scales 59–77 (72 in holotype), axillary scales present.
Coloration in life. Dorsal side of body golden, and ventral side grayish white. All fins relative golden, translucent. Body with scattered black spots along lateral line (Figure 3).

Figure 3.
Live specimen of Sinocyclocheilus grahami, uncatalogued.
Coloration in preservation. Color of aged specimen preservation in formalin brownish yellow.
3.5. Distribution
Sinocyclocheilus grahami was historically restricted to caves connected to Dianchi Lake in Yunnan Province and its nearby water bodies, part of the upper reaches of the Pudu River (Figure 4), a southern tributary of the Jinshajiang River. Habitat destruction has significantly contracted its range. A dragon pool is a unique geomorphic feature where a subterranean river emerges at the surface, sometimes flowing directly into surface rivers to form a small pond, or expanding into a larger surface lake.
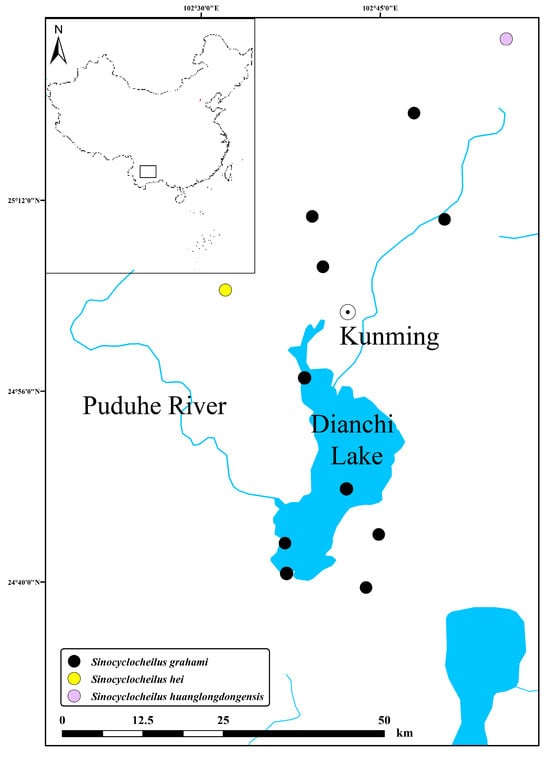
Figure 4.
Distribution of Sinocyclocheilus grahami, with the type localities of S. hei and S. huanglongdongensis.
3.6. Etymology
The species’ name, grahami, is derived from the surname of the American missionary John Graham, who provided the type specimens.
3.7. Protection Category
Listed in the “National Key Protected Wildlife List” (2021) as a national second-class protected animal and assessed as Critically Endangered (CR) in the “China’s Red List of Biodiversity: Vertebrates, Volume V, Freshwater Fishes (I)” [17]. Sinocyclocheilus grahami was also evaluated as Critically Endangered under criteria A2(c)(e) in the 2008 IUCN Red List of Threatened Species [18].
4. Discussion
Sinocyclocheilus grahami was described by a British ichthyologist, Charles Tate Regan, as Barbus grahami in 1904 [11], based on only one specimen collected from Dianchi Lake by John Graham. Until 1977, it was recognized as a member of the genus Sinocyclocheilus [19]. Historically, it was only known to be distributed in Dianchi Lake, and its cave-dwelling behavior was unknown. Therefore, the specific distribution of this species has been unclear for a long time. In 2004, Li and Xiao described S. hei, S. guanduensis, and S. huanglongdongensis in the same study [20]. Five years later, Zhao and Zhang concluded that these three species were synonymous with S. grahami, since no significant difference in morphology was detected after examining the type materials [1]. In 2013, Li recognized that these three species should be considered valid [21].
To further verify whether these three species are synonymous with Sinocyclocheilus grahami, we re-examined the holotype of this species, as well as type specimens of S. hei, S. guanduensis, and S. huanglongdongensis, and also compared these specimens with historical specimens deposited at ASIZB. The results indicate no significant differences in counts and measurable traits between S. guanduensis and S. grahami, whereas S. hei and S. huanglongdongensis show differences in barbel length with S. grahami (Table 1). The PCA on these morphological measurements were performed for detailed comparisons. The first three components contributed 75.9% of the variance. The PCA loadings (Table 2) indicate that the lengths of the first pair and second pair of barbels contributed most to PC 2 and PC 3, causing S. huanglongdongensis and S. hei to be separated from S. grahami (Figure 5).

Table 2.
Loadings on the first three principal components extracted from morphometric data of Sinocyclocheilus grahami, S. guanduensis, S. hei, and S. huanglongdongensis. Values in bold represent major contribution.
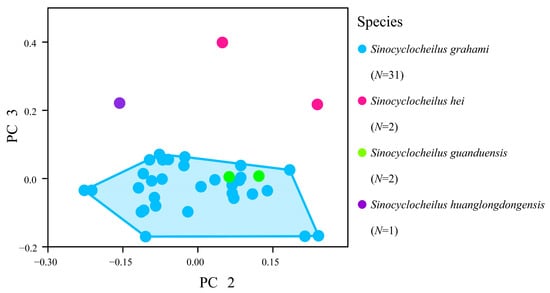
Figure 5.
Scatterplot of PC 2 against PC 3 derived from 36 morphometric data of Sinocyclocheilus grahami, S. guanduensis, S. hei, and S. huanglongdongensis.
Based on these findings, we agree with Zhao and Zhang (2009) that Sinocyclocheilus guanduensis is a synonym of the S. grahami [1]. However, the morphological differences observed in S. hei and S. huanglongdongensis in Principal Component 3 suggest that these two taxa may not be synonymous with S. grahami, and should be considered valid species. S. huanglongdongensis is found in Huanglongdong, east of Dianchi Lake, and does not extend into the lake, while S. hei is distributed in the northwestern areas of Dianchi Lake, i.e., Shalang and Fumin Dayingxiang [21]. Both species are located at a considerable distance from the recorded range of S. grahami (Figure 4). The genome and related morphological structures of Sinocyclocheilus grahami have been reported by Yang et al. [22]. Due to the limited number of type specimens, further collection and research on S. hei and S. huanglongdongensis are needed to confirm their validity.
Conservation stocking for fisheries of Sinocyclocheilus grahami is underway. However, unresolved taxonomy issues and unclear distribution information may affect the effectiveness of the stocking strategy [23]. Therefore, clarifying and revising the taxonomy of S. grahami is essential for the success of the program.
In traditional Chinese medicine, Sinocyclocheilus grahami is one of the six famous fish in Yunnan. With tender flesh and significant nourishing effects, it is regarded as a valuable medicinal material. Ming dynasty pharmacologist Mao Lan documented its therapeutic benefits in the Materia Medica of Southern Yunnan, noting that its flesh could be utilized to treat symptoms like chronic fatigue, weakness due to aging, and kidney deficiency with seminal leakage. It was believed to replenish yin, balance vital energy, and warm the kidneys, with regular use thought to enhance vitality and longevity. Modern research further supports its efficacy for treating childhood convulsions, fevers, and bacterial dysentery [24]. Additionally, genetic studies reveal that the purified cystatin gene from S. grahami exhibits potential applications in anti-tumor therapy [25]. These accounts not only highlight the medicinal significance of S. grahami in traditional medicine but also reflect China’s rational development and utilization of natural medicinal resources.
Under the combined effects of various factors such as lake enclosure for cultivation, water pollution, invasive species, and overfishing, the wild populations of Sinocyclocheilus grahami have declined sharply. For example, some caves along the lake have become isolated from Dianchi due to the diversion of water for industrial, agricultural, and domestic use, resulting in the destruction of breeding habitats and a reduction in distributional range [26]. Consequently, S. grahami has disappeared from the body of Dianchi Lake, and it is difficult to find them in the waters of Dianchi, with only a small number existing in the unpolluted streams, dragon pools, and reservoirs around Dianchi [27]. These threats have posed significant challenges to its survival, resulting in its designation as a national Class II protected species as early as 1988. However, with demand persisting, implementing more sustainable conservation strategies and alternative resources is crucial to alleviate the continued pressure of human activities on its population and habitat [28,29,30].
Artificial breeding has emerged as a crucial strategy for replenishing wild fish populations, particularly for rare and endangered species. This involves cultivating offspring and reintroducing them into natural habitats to gradually rebuild their numbers [23]. Sinocyclocheilus grahami has been the subject of such efforts since 2004. A research team from the Kunming Institute of Zoology, Chinese Academy of Sciences, embarked on extensive preliminary studies. They initiated artificial breeding research by sourcing the species from upstream tributaries of Dian Lake. The team delved into critical areas including the cultivation of broodstock, artificial spawning, the transfer and hatching of fertilized eggs, and fry cultivation. By 2008, they achieved a significant milestone by successfully breeding a second generation of fry exceeding 100,000 individuals [31,32]. Scientists also conducted detailed investigations into various aspects of S. grahami, including morphology [33,34], dietary changes from larval to juvenile stages [35], reproductive capacity of broodstock [36], nutrition [37], growth [38], and fish diseases [39].
The artificial propagation and release initiative for Sinocyclocheilus grahami commenced in 2009. By 2024, more than 3 million juveniles of S. grahami had been introduced into the Dianchi Lake basin, significantly contributing to the restoration of the species’ wild population. Long-term mark-recapture studies reveal that these juveniles have sustained a stable population size and experienced rapid growth over an extended period within Dianchi Lake and the Panlongjiang River. They have shown excellent adaptation to the prevailing water conditions in Dianchi Lake [40].
As previously stated, Sinocyclocheilus grahami has long been employed as a source of food and as a medicinal creature. In the ongoing efforts to rejuvenate and safeguard the wild populations of S. grahami, while also addressing the demand for this valuable fishery resource, a new breed has been developed that is well-suited for pond cultivation. This breed, known as S. grahami “Bayou No. 1”, is characterized by its fast growth and robust disease resistance. It successfully underwent evaluation by the National Aquatic Germplasm and Breeding Variety Evaluation Committee in 2018. Consequently, “Bayou No. 1” has been recognized as a new aquatic variety. This achievement represents the first instance worldwide of creating a new aquaculture variety derived from the genetic stock of a wild cavefish [41]. It not only offers a novel strategy for the conservation and resourceful exploitation of cavefish but also signifies a successful transition from the conservation to the sustainable utilization of S. grahami.
However, clinical research and application development remain insufficient, necessitating further comparative studies to determine whether the medicinal efficacy of artificially bred individuals has been retained [42,43]. Additionally, considering the advancements in modern medicine, the value of S. grahami as a medicinal resource is gradually declining; thus, it is recommended to completely cease its medicinal use.
5. Conclusions
This study revises the taxonomy of Sinocyclocheilus grahami, confirming S. guanduensis as a junior synonym of S. grahami, while S. huanglongdongensis and S. hei may be valid species. Furthermore, Sinocyclocheilus grahami is the first known cavefish to be successfully cultivated in aquaculture and used in traditional medicine. Its propagation and release efforts in China provide a valuable model for global conservation strategies. A clear taxonomic status is crucial for conservation efforts, while an accurate distribution delineation is essential for sustainable management.
6. Comparative Materials
- Sinocyclocheilus huanglongdongensis: Li 990807006, holotype, 106.2 mm SL; Huanglong Cave, Kunming City, Yunnan Province, 7th August 1999, collected by W.X. Li.
- Sinocyclocheilus hei: Li 020408004–5, 2 specimens, paratypes, 86.0–98.2 mm SL; Shalang, Kunming City, Yunnan Province, 9th April 2002, collected by W.X. Li.
Author Contributions
Conceptualization, Y.Z. and W.L.; validation, Y.Z. and J.H.; original draft, W.L; investigation, Y.Z. and J.H.; data curation, W.L.; writing—original draft preparation, W.L.; writing—review and editing, Y.Z., J.H. and W.L.; visualization, W.L.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC-31972868), Guangxi Natural Science Foundation (2021GXNSFBA220070) and Basic Ability Enhancement Program for Young and Middle-aged Teachers of Guangxi (2021KY0522).
Institutional Review Board Statement
Field collections observe the Guide to Collection, Preservation, Identification and Information Sharing of Animal Specimens and the Implementation Rules of the Fisheries Law of the People’s Republic of China. All activities complied with the Laboratory Animal-Guideline for Ethical Review of Animal Welfare (GB/T 35892–2018).
Data Availability Statement
The data presented in this study are available in this published paper.
Acknowledgments
We appreciate Yingnan Wang and Xiaowei Meng for assisting us in examining the fish specimens at the Institute of Zoology, Chinese Academy of Sciences (ASIZB), Beijing, China. We also appreciate two anonymous reviewers for their valuable comments on this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, Y.; Zhang, C. Endemic Fishes of Sinocyclocheilus (Cypriniformes: Cyprinidae) in China-Species Diversity, Cave Adaptation, Systematization and Zoogeography; Chinese Corporation for Promotion of Humanities: Beijing, China, 2009; pp. 1–271. ISBN 978-7-030-25813-7. (In Chinese) [Google Scholar]
- Zhao, Y.H.; Gozlan, R.; Zhang, C.G. Out of sight out of mind: Current knowledge of Chinese cave fishes. J. Fish Biol. 2011, 79, 1545–1562. [Google Scholar] [CrossRef]
- Torres-Dowdall, J.; Karagic, N.; Plath, M.; Riesch, R. Evolution in caves: Selection from darkness causes spinal deformities in teleost fishes. Biol. Lett. 2018, 14, 20180197. [Google Scholar] [CrossRef]
- White, W.B.; Culver, D.C.; Pipan, T. Encyclopedia of Caves; Elsevier Science: London, UK, 2019; pp. 1–768. ISBN 978-0-12-814124-3. [Google Scholar]
- Wynne, J.J. Cave Biodiversity: Speciation and Diversity of Subterranean Fauna; Johns Hopkins University Press: Baltimore, MD, USA, 2022; pp. 1–336. ISBN 978-1-4214-4457-4. [Google Scholar]
- Zhao, Y.; Zhang, C.; Proudlove, G. Fishes of the Genus Sinocyclocheilus (Cypriniformes: Cyprinidae) in China: Systematics, Biology, Biogeography and Cave Adaptation; Upfront publishing: London, UK, 2021; pp. 1–394. ISBN 978-1-784567-736. [Google Scholar]
- Romero, A.; Zhao, Y.; Chen, X. The Hypogean fishes of China. Environ. Biol. Fishes 2009, 86, 211–278. [Google Scholar] [CrossRef]
- Kowalko, J. Utilizing the blind cavefish Astyanax mexicanus to understand the genetic basis of behavioral evolution. J. Exp. Biol. 2020, 223 (Suppl. S1), jeb208835. [Google Scholar] [CrossRef] [PubMed]
- Rohner, N. The cavefish Astyanax mexicanus. Nat. Methods 2023, 20, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Lan, M. South Yunnan Materia Medica; Yunnan Science and Technology Press: Kunming, China, 2000; ISBN 978-7541643507. [Google Scholar]
- Regan, C.T. On a collection of fishes made by Mr. John Graham at Yunnan Fu. Ann. Mag. Nat. Hist. Zool. Bot. Geol. Artic. 1904, 13, 190–191. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Xue, D. Guide to Collection, Preservation, Identification and Information Share of Animal Specimens; Standard Press of China: Beijing, China, 2010; pp. 1–468. ISBN 978-7-5066-5983-3. (In Chinese) [Google Scholar]
- GB/T 35892-2018; Laboratory Animal—Guideline for Ethical Review of Animal Welfare. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Pepublic of China: Beijing, China, 2018.
- Zhao, Y.; Watanabe, K.; Zhang, C. Sinocyclocheilus donglanensis, a new cavefish (Teleostei: Cypriniformes) from Guangxi, China. Ichthyol. Res. 2006, 53, 121–128. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron 2001, 4, 1–9. [Google Scholar]
- Zhao, Y.; Kullander, F.; Kullander, S.O.; Zhang, C. A review of the genus Distoechodon (Teleostei: Cyprinidae), and description of a new species. Environ. Biol. Fish 2009, 86, 31–44. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, F.; Li, C.; Zhang, Y.; Wang, Z.; Zheng, G. China’s red list of biodiversity: Vertebrates. In Amphibians; Science Press: Beijing, China, 2021; Volume 5, pp. 1–375. ISBN 978-7030636560. (In Chinese) [Google Scholar]
- Chen, X.Y.; Yang, J.X. Sinocyclocheilus grahami. IUCN Red List of Threatened Species. 2008. Available online: https://doi.org/10.2305/IUCN.UK.2008.RLTS.T135152A4068202.en (accessed on 13 December 2024).
- Wu, X. Fauna of China: Fish Volume—Cyprinidae (Part II); Shanghai Scientific and Technological Publishers: Shanghai, China, 1964; pp. 1–601. (In Chinese) [Google Scholar]
- Xiao, H.; Li, W.; Zan, R. The three new species of Sinocyclocheilus from Kunming, Yunnan. Southwest China J. Agric. Sci. 2004, 17, 521–524, (In Chinese with English abstract). [Google Scholar]
- Li, W.X.; An, L. A new species of Sinocyclocheilus from Kunming, Yunnan—Sinocyclocheilus wui sp. nov. J. Jishou Univ. (Nat. Sci. Ed.) 2013, 34, 82–84, (In Chinese with English abstract). [Google Scholar]
- Yang, J.; Chen, X.; Bai, J.; Fang, D.; Qiu, Y.; Jiang, W.; Yuan, H.; Bian, C.; Lu, J.; He, S.; et al. The Sinocyclocheilus cavefish genome provides insights into cave adaptation. BMC Biology. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Pan, X.F.; Chen, X.Y.; Wang, X.A.; Zhao, Y.P.; Li, J.Y.; Li, Z.Y. Overview of the artificial enhancement and release of endemic freshwater fish in China. Zool. Res. 2013, 34, 267–280, (In Chinese with English abstract). [Google Scholar]
- Xu, J. The Survey and Research of Medicinal Fish Species in Shaoguan City. J. Shaoguan Univ. 1997, 18, 77–79, (In Chinese with English abstract). [Google Scholar]
- Liu, R. Transcriptome Sequencing in Sinocyclocheilus grahami and Characterization of A Cysteine Protease Inhibitor, Cystatin. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2015. (In Chinese with English abstract). [Google Scholar]
- Wang, S.; Zheng, G.; Wang, Q. China Red Data Book of Endangered Animals: Pisces; Science Press: Beijing, China, 1998; ISBN 7-03-006401-1. (In Chinese) [Google Scholar]
- Chen, Z.M.; Yang, J.X.; Su, R.F.; Chen, X.Y. Present Status of the Indigenous Fishes in Dianchi Lake, Yunnan. Biodivers. Sci. 2001, 9, 407–413, (In Chinese with English abstract). [Google Scholar]
- Nijman, V.; Nekaris, K. Provide context when reporting on the use of protected and endangered wildlife in ethnopharmacological surveys. J. Ethnopharmacol. 2016, 194, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Fernandes-Ferreira, H.; Leó Neto, N.A.; Brito, S.V.; Alves, R.R.N. The Trade of Medicinal Animals in Brazil: Current Status and Perspectives. Biodivers. Conserv. 2013, 22, 839–870. [Google Scholar] [CrossRef]
- Whiting, M.J.; Williams, V.L.; Hibbitts, T.J. Animals traded for traditional medicine at the Faraday market in South Africa: Species diversity and conservation implications. Anim. Tradit. Folk Med. Implic. Conserv. 2011, 284, 84–96. [Google Scholar] [CrossRef]
- Chu, Y. The Successful Artificial Breeding of the Golden-line Fish, a Specialty of Dianchi Lake. Pract. Rural. Technol. 2008, 11, 15. (In Chinese) [Google Scholar]
- Yang, J.X.; Pan, X.F.; Li, Z.Y. Preliminary report on the successful breeding of the endangered fish Sinocyclocheilus grahami endemic to Dianchi Lake. Zool. Res. 2007, 28, 329–331, (In Chinese with English abstract). [Google Scholar]
- Min, R.; Lian, Y.; Chen, X.Y.; Yang, J.X. Morphometrics Analysis of Sinocyclocheilus grahami (Cypriniformes: Cyprinidae). Zool. Res. 2010, 30, 707–712, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhao, T.Y.; Pan, X.F.; Chen, X.Y.; Yang, J.X. Resistibility of Sinocyclocheilus grahami, S. xichouensis and their hybrid F1 to Ichthyophthirius multifiliis and Trichodina spp. Freshw. Fish. 2016, 46, 33–39, (In Chinese with English abstract). [Google Scholar]
- Pan, X.F.; Yang, J.X.; Li, Z.Y.; Chen, X.Y. Feeding changes and growth performance of Sinocyclocheilus grahami (Pisces, Barbinae) larvae and juveniles in farm environment. Zool. Res. 2009, 30, 433–437, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Pan, X.F.; Yang, J.X.; Chen, X.Y.; Li, Z.Y. Broodstocks management, fecundity and the relationship between egg size and embryo survival ability of Sinocyclocheilus grahami. Zool. Res. 2011, 32, 195–203, (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.P.; Pan, X.F.; Yang, J.X.; Chen, X.Y.; Li, Z.Y.; Wang, X.A. Analysis of the nutritional components in muscle of Sinocyclocheilus grahami and S. tingi. Zool. Res. 2013, 34, 636–639, (In Chinese with English abstract). [Google Scholar]
- Zhang, X.; Zhou, L.; Gui, J. Biotechnological innovation in genetic breeding and sustainable green development in Chinese aquaculture. Sci. Sin. Vitae 2019, 49, 1409–1429, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhao, T.Y.; Pan, X.F.; Chen, X.Y.; Yang, J.X. Morphological analysis in Sinocyclocheilus grahami, S. xichouensis, and their hybrid F1 (S. grahami♀×S. xichouensis♂). Freshw. Fish. 2015, 45, 25–32, (In Chinese with English abstract). [Google Scholar]
- Zheng, J.R.; Peng, C.; Dang, H. Endangered Species Restored in Yunnan Province. Available online: https://www.chinadaily.com.cn/a/202408/25/WS66cb1999a31060630b924d1a.html (accessed on 25 August 2024).
- Zhang, Y.; Pan, X.; Yin, Y.; Yang, J. Fertilization and growth performance in reciprocal hybrids of Dianchi golden-line barbel (Sinocyclocheilus grahami) and domestic common carp (Cyprinus carpio) and crucian carp (Carassius auratus). Aquac. Rep. 2021, 21, 100893. [Google Scholar] [CrossRef]
- Wu, X.C.; Jia, X.B.; Ma, W.K.; Wu, Q.; Wang, L.; Yang, B.; Feng, L. Research on artificial substitutes of rare and endangered animal medicinal materials and industrialization. China J. Chin. Mater. Medica 2022, 47, 6278–6286, (In Chinese with English abstract). [Google Scholar]
- Ye, X.; Wu, X.; He, L.; Liu, Z.; Sun, X.; Wang, H.; Shen, C.; Wu, C. Substitutes for Endangered Animal Medicinal Materials: A Review. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 226–231, (In Chinese with English abstract). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).