Abstract
The morphological characteristics and surface roughness of substrata can significantly affect the settlement behaviour of planktonic larvae and the post-settlement survival of benthic organisms, such as mussels. Despite widespread recognition of these effects on ecological and aquaculture processes, species-specific complexities and limited research hinder a comprehensive understanding of the phenomenon and the potential to harness its application. In this study, the settlement of juvenile green-lipped mussels (Perna canaliculus; 0.32–3.59 mm shell length) on 42 different custom-designed artificial substrata with varied branch widths and surface microstructures were compared. Mussels smaller than 0.99 mm in shell length exhibited a clear preference for substrates with a thinner branch width (1.6 mm), wider roughness width (3.2 mm), and shorter roughness height (0.4 mm) on both V-shaped and squared-shaped surface microstructures. In contrast, for mussels larger than 1 mm, only the branch width of artificial substrata significantly influenced mussel attachment, while millimetre-scale surface features had no measurable effect. These findings indicate that, at the millimetre scale, the attachment of mussels > 1 mm does not conform to the surface contact theory, which proposes that settling organisms prefer substrates with microstructures that maximize their surface contact. Overall, a thinner branch width consistently yielded higher attachment densities, underscoring its dominant role. Our results reveal significant opportunities for optimizing the design of artificial substrata in mussel aquaculture, such as spat catching and nursery ropes, potentially improving seed collection efficiency and reducing the subsequent loss of seed mussels during their culture on mussel farms.
Keywords:
green-lipped mussel; juvenile mussel attachment; 3D printing; artificial substrata; aquaculture Key Contribution:
Mussels > 1 mm show no settling preference for specific millimetre-scale microstructures. Mussels < 0.99 mm prefer to settle on surfaces with wider (3.2 mm) and shorter (0.4 mm) substrate roughness.
1. Introduction
The majority of juvenile mussels used to initiate the aquaculture production of mussels are caught in the wild and transferred onto coastal farms for ongrowing. In many mussel farming locations, the supply of seed mussels is a constraint on production, with this problem being particularly acute for green-lipped mussels (Perna canaliculus) in New Zealand, where around 90% of the seed mussels are harvested from the wild [1,2,3,4]. Once the harvested wild juvenile mussels, commonly known as spat, are transferred onto mussel farms, only a very small proportion remain attached to the on-growing ropes to eventually become harvestable adult mussels [3,4]. The low retention of mussel spat on the culture ropes further aggravates the constrained mussel spat supply in New Zealand, limiting overall mussel aquaculture production. A predominant cause of the loss of mussel spat from on-growing ropes is thought to be their migration behaviour, which appears to be partly related to the suitability of the attachment substrata offered by the on-growing ropes used for seeding mussels [4,5,6]. Therefore, research aimed at determining the design of substrata that promotes the attachment behaviour of green-lipped mussel spat may greatly assist in addressing this problem by reducing the losses of the spat after they have been seeded onto mussel farms.
Close examination of natural material with attached green-lipped mussel spat has shown that the mussels have distinct preferences for attaching to specific natural substrata, particularly fine-branching algae and hydroids [7,8]. Collectively, these previous studies identified the macrostructure characteristics of substrata that were of potential importance in determining the numbers of attached early juvenile mussels, i.e., branch width, surface area-to-volume ratio, the presence of node areas, branching frequency and degree of branching. Although the effect of the surface microstructure of substrata on the attachment of juvenile green-lipped mussels has been tentatively explored together with branch width with varying results in one previous study [9], the role of variations in the surface microstructure of substrata in the attachment of juvenile green-lipped mussels remains to be examined in more detail. A further study identified differences in the attachment of mussel spat between the smooth and textured surfaces of plastic cable or zip ties [10]. The impacts of surface microstructure on the attachment behaviour of marine sessile organisms are not unidirectional; it can either enhance or reduce larval settlement and/or juvenile attachment [10,11]. Its effect varies with the scale, shape and other topographic features of the surface microstructure [12,13,14]. For example, the abundance of tropical epibenthic larvae was higher on panels with some specific combinations of surface grooves at scales of 0, 1 and 10 mm, with the larvae preferring to settle into the troughs of only some grooves, compared with the peaks of the grooves [13]. It has been suggested that the preferred surface structure of sessile marine invertebrate larvae may vary in relation to the relative scale of the settler and the surface microstructure [15]. It has been observed that settling larvae of marine organisms generally tend to settle on surfaces with a microstructure slightly larger than their body size, which can facilitate the physical protection of the settler from hydrodynamic influences [14]. In contrast, surfaces with a microstructure slightly smaller than that of settlers have been observed to be avoided by settlers [16].
If this principle applies to green-lipped mussels, then by changing the shape and scale of the surface microstructure on artificial substrata, it may be possible to produce a textured surface for promoting the increased attachment of the mussel spat, with the results of one preliminary study suggesting good potential for success. The textured side of plastic cable ties was found to increase the settlement of mussel larvae by up to 100% compared to the opposite, smooth side [10]. There are many other shapes of grooves, crevices or pits studied for the settlement behaviour of other marine organisms, but the general principles are summarized as the “attachment point theory”, which proposes that a microstructure of the substrate with more points of attachment that is slightly larger than the size of the settlers will promote the attachment of settlers [14,17]. Therefore, for this current research examining the importance of surface microstructure in influencing the attachment of juvenile green-lipped mussels, two variations of surface shape textures were used, a V-shape texture (Shape-V) and squared-shaped texture (Shape-Z). The Shape-V textured surface was chosen as a variation because the V-section of cable ties has previously been shown to promote the settlement of P. canaliculus larvae [10]. The Shape-Z textured surface was chosen as it has been used in similar settlement studies and found to be favourable to some marine invertebrate settlers [12,14]; therefore, it allows for the comparison of experiment results. The roughness height and roughness width were selected as two geometric variables of surface microstructure which can control the scale of the microstructure, with the extent of these two variables for experimentation being based on the size of mussel spat because previous studies have shown that a surface microstructure slightly larger than the spat size is more likely to promote attachment behaviour while surfaces with a microstructure slightly smaller than the spat size are more likely to decrease attachment [14]. To determine the presence of any interactive effect between the branching width of the filamentous material and surface microstructure, three variations in branch width were used in an experiment based on the results of one previous study as well as the measured widths of the thalli of macroalgae with attached juvenile mussels that were previously sampled from the wild [8,9].
The aim of this study was to experimentally determine how four geometric variables of the surface microstructure (i.e., shape, roughness, width, roughness height, and branch width) of artificial substrata affect the attachment of green-lipped mussel spat. The results of this study may ultimately assist in developing spat catching and seeding ropes with superior performance for retaining mussel spat.
2. Materials and Methods
2.1. Experimental Design
To determine the surface microstructure that promotes attachment behaviour in juvenile green-lipped mussels, artificial substrata were fabricated with designs based on two surface microstructure shape variations, i.e., Shape-V and Shape-Z surface microstructures (Figure 1 and Figure 2, respectively). Two corresponding sets of artificial substrata were fabricated, both with the same variables but one for Shape-V surface and the other for a Shape-Z surface microstructure (Table 1). Additionally, for both shapes of the surface microstructure, artificial substrata were fabricated for two surface roughness widths (r = 1.2, 3.2 mm); within each, there were three surface roughness heights (k = 0.4, 1.2, 3.2 mm), and within each of these, there were three branch widths (i.e., d = 1.6, 3.7, 5.6 mm), with six replicates of each (Table 1). For comparison, artificial substrata with no surface microstructure with three branch widths (i.e., d = 1.6, 3.7, 5.6 mm) were fabricated with six replicates of each (i.e., Shape-O, controls 1–3). Initial attempts to fabricate the designs using a proprietary blend of polypropylene, which includes amorphous carbon and calcium carbonate currently used for the commercial manufacture of green-lipped mussel spat catching and on-growing ropes, were unsuccessful (Quality Equipment Ltd., Auckland, New Zealand). Therefore, these 39 different artificial substrata (including controls 1–3) were made of resin (Photosensitive resin 9400 SLA) using ZHONGRUI SLA 600 3D printing machines operated by a specialist 3D printing company (Xiangxing Technology Ltd., Shenzhen, China). To provide some comparative benchmarking to previously tested polypropylene substrata, artificial substrata of three branch widths (i.e., d = 1.6, 3.7, 5.6 mm) were fabricated from a sheet (mean thickness of 0.15 ± 0.006 mm (S.E.)) of the proprietary blend of polypropylene (Quality Equipment Ltd.) using the Trotec Speedy 300 laser engraving and cutting machine (Trotec Laser GmbH, Marchtrenk, Austria) at the Bioengineering Institute at the University of Auckland. These polypropylene benchmarking substrata with three branch widths all had two sides with different textures (one side was smooth and the other side had evenly distributed ridge lines with a 1 mm interval) with six replicates of each substratum (i.e., Shape-T: controls 4–6). All 42 artificial substrata designs were 50 mm in length and were deployed in a mussel spat attachment experiment in a randomized arrangement to test the preference of attachment of juvenile green-lipped mussels. OpenSCAD software (version: 2015.03) (www.openscad.org (accessed on 18 January 2018)) was used to generate the designs for all artificial substrata.
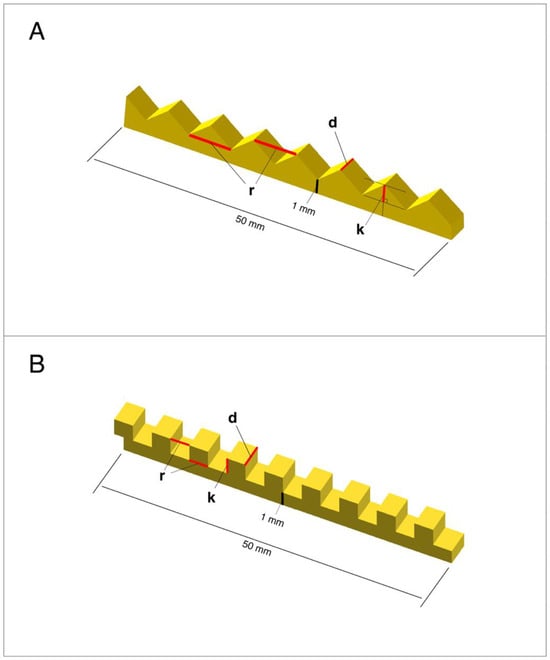
Figure 1.
The schematic diagrams of the design of an artificial substratum showing the morphological variables of the Shape-V (A) and Shape-Z (B) microstructures used for the 3D printing of artificial substrata from resin for the experiment (roughness height—k; roughness width—r; branch width—d).
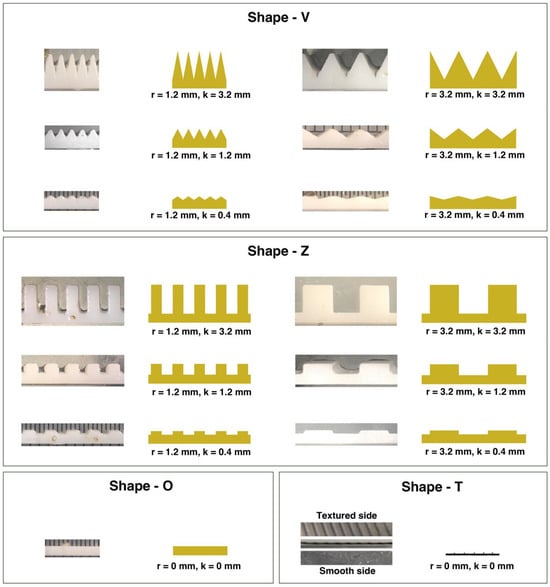
Figure 2.
Photographs and schematic diagrams of each artificial substratum showing the side view details, including the roughness width, r, and roughness height, k, of Shape-V, Shape-Z, and Shape-O (smooth controls 1–3) surface microstructures used for the fabrication of artificial substrata using 3D printing and photosensitive resin, and Shape-T polypropylene artificial substrata (controls 4–6) for the experiment.

Table 1.
The variables for 42 different designs of artificial substrata fabricated for this experiment to examine the importance of surface microstructure on the attachment of juvenile mussels. (Shape-V = V-shape surface microstructure on 3D-printed resin substrata; Shape-Z = squared-shaped surface microstructure on 3D-printed resin substrata; Shape-O = smooth surface microstructure on 3D-printed resin substrata; Shape-T = laser cut-polypropylene substrata with two sides with a textured and smooth microstructure). In total, 6 replicates were carried out for each of these 42 treatments.
2.2. Juvenile Mussels
Colonial hydroids (Amphisbetia bispinosa) and a wide range of species of seaweeds with attached juvenile green-lipped mussels were collected from the rocky intertidal of Maori Bay (36°50′11″ S, 174°25′36″ E) on 14 June 2018. The material was kept moist for 2 h while it was transported to the Leigh Marine Laboratory facilities. Upon arrival at the laboratory, the juvenile mussels and accompanying substrata were transferred to a 60 l conical plastic tank filled with filtered seawater (5 µm) at 26.5 ± S.E. 3.0 °C with vigorous aeration. The mussels were immediately fed with 150 mL of axenically cultured Tisochrysis lutea with a concentration of 4750 cells µL−1. The microalga Tisochrysis lutea was sourced from a stock culture (Strain CS-177) supplied by CSIRO—Australian National Algae Supply Service—and cultured in 10 l plastic bags in UV-sterilized and filtered seawater (0.45 μm) with standard F/2 enrichment media at 22–23 °C.
2.3. The Evaluation of Artificial Substrata in the Laboratory
All 252 replicates from the experimental artificial substrata of the 42 individual designs were attached at randomized positions on a plastic rack which was suspended in a 60 l plastic conical tank in the laboratory (Figure 3). The tank was filled with filtered seawater (5 µm) at 26.5 ± S.E. 3.0 °C and aerated vigorously through a concentric series of porous airlines at the base of the tank (Figure 3). Around 5000 juvenile mussels detached from their natural substrata (0.98 ± S.E. 0.04 mm) of the size range 0.32–3.59 mm were then added to the tank and given 5 days to select and attach to a substratum. During this time, the tank was supplied once a day with 150 mL of axenically cultured Tisochrysis lutea at a concentration of 4750 cells µL−1. After 5 days, the artificial substrata were removed from the plastic rack and high-resolution digital photographs were taken of the textured surface of each replicate artificial substratum. Image analysis software (ImageJ version 1.51n) was used to extract data from the digital images relating to the juvenile mussels attached to the textured side of the artificial substrata, i.e., the total number of mussels attached only to the textured surface of each replicate substratum, and the size of individual mussels as measured by their shell length (SL—maximum distance along the anterior/posterior axis of the mussel shell).
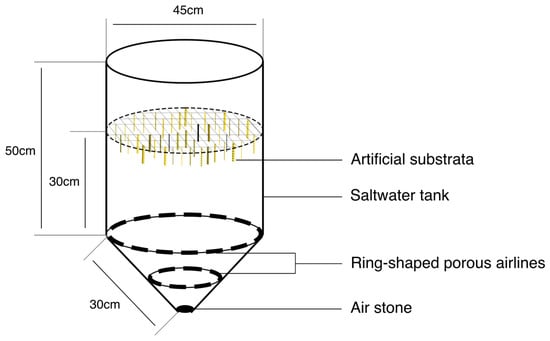
Figure 3.
The schematic diagram of the experimental set up used to determine patterns of attachment of juvenile mussels to artificial substrata.
From the perspective of catching wild spat for aquaculture, a higher density of attached mussels means that less substrata can be used to obtain more mussels. Such substrata are more economically efficient, and therefore the density of juvenile mussels was chosen as the indicator for comparing the influence of the microstructure of the substrate surface on the attachment of juvenile mussels. To focus on the effect of the surface microstructure, only the density of juvenile mussels attached on the textured surface (includes surface edges) of each replicate substratum was used as the experimental dependent variable and was compared among the experimental substrata. For the smooth resin substrata of three branch widths (i.e., Shape-O: controls 1–3), the density of attached juvenile mussels was only measured from one randomly selected side of the experimental substratum.
2.4. Statistical Analyses
To explore the effect of each of the morphometric dimensions (i.e., d—branch width; r—roughness width; k—roughness height) of two surface texture shapes (i.e., Shape-V, Shape-Z) on the density of attached juvenile mussels, rank-based regression was used because the parametric assumptions of homogeneity of variance and normality were not satisfied. Rank-based regression models were also used to further disaggregate the effect of each morphometric variable of the two surface texture shapes of substrata on the number and density of attached mussels across a range of different mussel size classes (i.e., <0.49 mm, 0.50–0.99 mm, 1.00–1.49 mm, 1.50–1.99 mm, >0.20 mm). As the resin substrata with a smooth surface microstructure (Shape-O) and laser-cut polypropylene substrata (Shape-T) have no roughness width and roughness height, only the effect of the branch width of these two substrata on the density of attached mussels were evaluated. To compare the overall performance of each of the substrata that incorporated these four different design variables (i.e., d—branch width; r—roughness width; k—roughness height; and shape—surface texture shape) on mussel attachment, the mean mussel density (MMD) and the mean percentage of small-size mussels (average percentage of mussels < 0.99 mm shell length) attached to the textured surface of 42 different designs/treatments of artificial substrata were calculated and then compared, respectively, by individual Kruskal–Wallis rank sum tests together with further pairwise comparisons using Wilcoxon rank sum tests.
The rank-based models were constructed by the “rfit” function of package “Rfit” in the statistical software R (Version 4.3.1 through integrated development environment (IDE): RStudio Version 2023.06.1+524). The Kruskal–Wallis rank sum tests and post hoc comparisons deployed the “kruskal.test” function and “pairwise.wilcox.test” functions from the package “stats”, respectively, in R.
3. Results
3.1. Mussel Settlement Preference to Isolated Different Morphometric Variables
Although the shape of the surface microstructure had a significant overall influence on the mean density of mussels attached to the artificial substrata (Kruskal–Wallis tests, χ2 = 9.02, p < 0.05), there were no significant pairwise differences found in the mean mussel density among the four shapes of the artificial substrata. However, the mean density of mussels attached to each of the four shapes of the artificial substrata was significantly affected by branch width, with narrower substrata for each of the four shapes consistently having significantly higher densities of attached mussels (i.e., Shape-V: p < 0.01; Shape-Z: p < 0.01; Shape-O: p < 0.01; Shape-T: p < 0.01) (Table 1 and Figure 4). This result was also consistent between the two different size classes of mussels for three shapes of artificial substrata, with the density of mussels of less than 0.99 mm SL and >1.00 mm SL both being significantly positively influenced by the decreasing branch width of the artificial substrate for shapes V, Z and O (Table 1). For Shape-T, the attachment response of the mussels to branch width of the artificial substrata varied with mussel size, with an overall positive effect on the density of attached mussels with decreasing branch width regardless of mussel size and for <0.99 SL mussels only, but for mussels of >1.00 mm SL, there was no effect of branch width. Both roughness width and roughness height were also found to have an influence on mussel attachment which varied with mussel size. For Shape-V greater roughness width (from 1.2 to 3.2 mm) resulted in a significant increase in mussel density for regardless of mussel size and for <0.99 SL mussels only, but for >1.00 mm SL mussels, there was no effect of branch width (Table 1 and Figure 4). In contrast, for Shape-Z substrata, only <0.99 SL mussels showed an increase in density in response to greater roughness width, whereas all sizes of mussel combined and mussels > 1.0 mm were not affected by roughness width. Greater roughness height (0.4 to 3.2 mm) decreased the mean density of <0.99 SL small mussels in Shape-V substrata, but not for 1.0 mm SL mussels or all sizes combined (Table 1 and Figure 4). In Shape-Z, greater roughness decreased mussel density for <0.99 SL small mussels and all sizes combined, but not for the larger sized mussels, i.e., 1.0 mm SL ones.
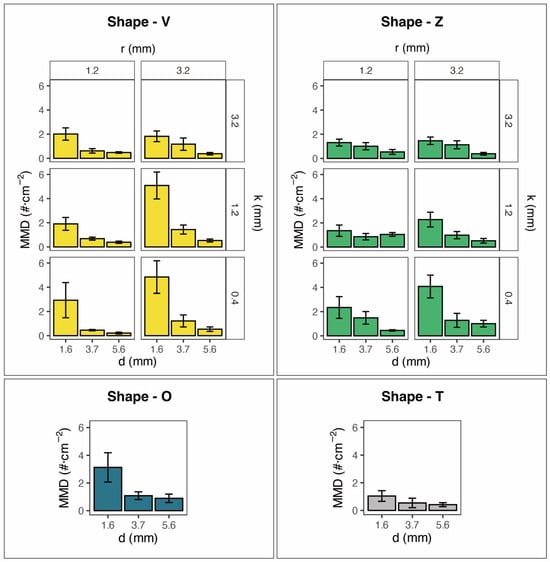
Figure 4.
Mean mussel density (MMD) (±S.E.) attached to experimental substrata with four types of surface microstructures of substrata (i.e., Shape-V = Shape-V surface microstructure on 3D-printed resin substrata; Shape-Z = Shape-Z surface microstructure on 3D-printed resin substrata; Shape-O = smooth surface microstructure on 3D-printed resin substrata; Shape-T = laser-cut polypropylene substrata), all with three different branch widths, d, two different roughness widths, r, and three different roughness heights, k (n = 6 for each experimental substratum).
3.2. Mussel Settlement Preferences for Combinations or Morphological Variables
Although there was a significant overall difference in the density of mussels attached on the 42 different designs of artificial substrata (Kruskal–Wallis tests, χ2 = 108.85, p < 0.01) (Table 2), there were no significant pairwise differences between any of them. This includes the absence of any differences in the density of attached mussels between the two types of smooth control substrata (i.e., Shape-O and Shape-T) and the two types of substrata with surface microstructures (i.e., Shape-V and Shape-Z). Among the 42 designs of artificial substrata, those that had higher densities of attached juvenile mussels all had a 1.6 mm branch width, including Treatment-13 (i.e., Shape-V, 3.2 mm roughness width, 1.2 mm roughness height and 1.6 mm branch width), Treatment-16 (i.e., Shape-V, 3.2 mm roughness width, 0.4 mm roughness height and 1.6 mm branch width), and Treatment-34 (i.e., Shape-Z, 3.2 mm roughness width, 0.4 mm roughness height and 1.6 mm branch width) (Figure 5). The morphological characteristics of these three substrata with the highest density of attached mussels were consistent with the general trends identified previously for the effects of individual morphological features of substrates on mussel attachment density, with increasing branch width having a negative effect, increasing roughness width tending to have a positive effect, and increasing roughness height tending to have a negative effect. These designs with higher densities of attached juvenile mussels all utilized a combination of the trends that promote a higher density of mussel attachment, demonstrating the potential for cumulative effects when the structural features promoting mussel settlement identified in this study were combined.

Table 2.
The overall effect of the morphological variations in experimental substrata (i.e., branch width, roughness width and roughness height) with four types of surface microstructure on the density of attached mussels within each of the two size classes (i.e., <0.99 and >1.00 mm) of mussel spat (bolded—significant correlation). V = Shape-V surface microstructure on 3D-printed resin substrata; Z = Shape-Z surface microstructure on 3D-printed resin substrata; T = Shape-T laser-cut polypropylene substrata; O = smooth surface microstructure on 3D-printed resin substrata.
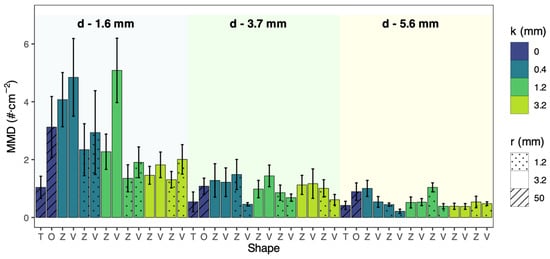
Figure 5.
Mean density (MMD) (±S.E.) of juvenile mussels attached to the surface of 42 different designs of artificial substrata. V = Shape-V surface microstructure on 3D-printed resin substrata; Z = Shape-Z surface microstructure on 3D-printed resin substrata; T = Shape-T laser-cut polypropylene substrata (i.e., r = 50 mm, k = 0 mm); O = smooth surface microstructure on 3D-printed resin substrata (i.e., r = 50 mm, k = 0 mm), d = branch widths, r = roughness widths, and k = roughness heights.
4. Discussion
The limited supply of wild mussel spat and its poor retention after being seeded onto mussel farms constrains the production in the green-lipped mussel aquaculture industry in New Zealand [3,4,18], and similar phenomena occur in mussel aquaculture in other parts of the world [19,20,21]. Therefore, improving the larval settlement and attachment of juvenile mussels on artificial substrates used in aquaculture has the potential to result in increases in the overall efficiency of mussel aquaculture and concomitantly reduce the harvesting pressure on wild mussel spat. The surface microstructure of substrata has been recognized as an important factor in the active selection behaviour of green-lipped mussel larvae, with the V-shaped grooves on plastic cable ties being previously found to promote the settlement of P. canaliculus larvae [10]. Similarly, a higher mean density of juvenile mussels was found to attach to the textured side (i.e., 1 mm spaced surface ridges) versus the smooth side of artificial substrata regardless of the branch width and degree of branching of the substrata [9]. However, the results of the current study showed that the branch width, roughness width and roughness height of artificial substrata all play a role in influencing the attachment of mussel spat to substrata, especially for mussels of a smaller size, i.e., <0.99 mm SL. Furthermore, although pairwise comparisons could not isolate significant differences for the individual morphometric variations in substrata, the overall significance of mussel settlement preferences for the combination of morphological variations indicates that they can influence each other, with the potential to produce a synergistic effect on juvenile mussel attachment.
Previous studies have postulated the attachment point theory, which posits that settling benthic invertebrates prefer substrates that maximize the points of contact between the settler and the substratum, and that providing a surface microstructure that is slightly larger than the size of settler will promote attachment behaviour in settlers [14,17]. However, the attachment point theory is inconsistent with the results of this current study, where juvenile mussels did not show a clear preference to attach to experimental substrata that maximized their points of contact with the substratum, especially for mussels > 1 mm SL; none of the surface microstructure variables had any significant effects on mussel attachment. The attachment point theory focuses more on larvae expressing settlement behaviour at the micrometre scale (i.e., typically less than 1 mm) of surface structures, especially in laboratory settings where water velocities are typically low and there is no interference from other naturally occurring species that are competing for settlement substrate [12,14,22,23,24,25]. In the present study, the juvenile mussels of different size classes did not show strong preferences for attaching to artificial substrata of dimensions slightly larger than their size, indicating that this theory may not apply at this larger scale for juvenile mussels. For example, mussels in the size range < 0.99 mm SL did not show preferences for attaching to surface microstructure designs with a 1.2 mm roughness width, which should have facilitated greater points of contact between the attaching juvenile mussel and the substrate; instead, they preferred to attach to surface microstructures with a much wider roughness width, namely 3.2 mm.
Recent studies that have simulated the turbulent boundary layer flow over rectangular prism roughness found that the ratio of the roughness width to the roughness height at the millimetre scale can lead to varied boundary layer flows that have distinct promoting or inhibiting effects on mussel settlement [26,27]. When the surface roughness width-to-height ratio is less than 1, the structure creates a skimming flow regime where the water flows over the ridges, making it difficult to carry the larvae onto the substrate, whereas when the ratio is larger than 1, the flow regime is near or beyond the transition to the reattached flow regime and consequently promotes the settlement of larvae. This phenomenon could provide a possible explanation for why the larger roughness width (3.2 versus 1.2 mm) in this current study resulted in an increase in the density of mussels attached, while the higher roughness height (0.4 versus 3.2 mm) significantly decreased the density of mussels attached on both V-shaped and squared-shaped (i.e., Z-shape) substrata. It might also explain the reason for different mussels showing particular preferences for certain substrate morphological variables in this study and that these structural effects interacted with each other to produce complex synergistic or counteracting effects. Furthermore, in this study, the attachment preferences for both of the two morphometric variables of surface microstructure were not significant for mussels larger than 0.99 mm SL, which points toward a possible change in the behaviour of these larger-sized mussels.
Controlling the branch width of substrata could be useful for increasing the settled mussel density in substrates used to collect settling wild larvae for aquaculture because branch widths were found to have a significant overall negative effect on mussel density. Consequently, the results from this current study suggest that a substratum with a branch width of 1.6 mm, a 3.2 mm roughness width, and a 1.2 mm roughness height might be the best for facilitating greater densities of mussel spat attachment based on the tendency of these surface microstructures toward higher attachment of mussel spat. However, there were no statistical differences found in the mussel attachment densities between the smooth control substrata (i.e., Shape-O and Shape-T) and the other experimental substrata (i.e., Shape-V and Shape-Z) with a textured surface, so a smooth substrate with a branching width of 1.6 mm should also be able to achieve good mussel attachment densities compared to any textured counterpart.
Overall, the results of this study indicate that the surface microstructure variables examined in this experiment are not major determinants of attachment behaviour in the mussel spat of this species and are therefore not critical for inclusion in the design of higher-performing spat catching and seeding ropes, while utilizing a branching width of 1.6 mm to achieve a higher density of juvenile mussel attachment to such products could be expected to result in superior performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10030135/s1, Table S1: Details of mussel size and attachment.
Author Contributions
Conceptualization, W.W. and A.G.J.; methodology, W.W. and A.G.J.; software, W.W.; validation, W.W. and A.G.J.; formal analysis, W.W.; investigation, W.W.; resources, A.G.J.; data curation, W.W.; writing—original draft preparation, W.W.; writing—review and editing, A.G.J.; visualization, W.W.; supervision, A.G.J.; project administration, A.G.J.; funding acquisition, A.G.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Marine Farming Association in New Zealand and the Ministry for Primary Industries, grant number #S3F-23044.
Institutional Review Board Statement
This research was fully compliant with Animal Welfare Act 1999, which controls the use of animals in research within New Zealand.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
Thank you to Dyahruri Sanjayasari and Supono Supono for their help in the field sampling and microalgal culture. Thank you to Errol Murray for helping to build the aeration system and thanks to both Errol and Peter Browne for providing essential tools and practical suggestions in the maintenance of experimental facilities. Thanks to Quality Equipment Ltd. for providing the polypropylene plastic sheet. Thank you to Iain Anderson and his students Katie Wilson and Max Pitoo from the Bioengineering Institute at the University of Auckland for assistance with laser cutting the artificial substrata. Thank you to Boyd Taylor, Jaime Rowntree, Moyang Li, Harry Allard, and Michael Kelly for providing advice and assistance in the field sampling and work in Leigh Laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Shape-V | V-shape texture |
| Shape-Z | squared-shaped texture |
| Shape-O | smooth surface microstructure |
| Shape-T | polypropylene artificial substrata |
| SL | shell length |
| MMD | mean mussel density |
| IDE | integrated development environment |
References
- Alfaro, A.C.; Jeffs, A.G. Variability in mussel settlement on suspended ropes placed at Ahipara Bay, Northland, New Zealand. Aquaculture 2003, 216, 115–126. [Google Scholar] [CrossRef]
- Jeffs, A.G.; Holland, R.C.; Hooker, S.H.; Hayden, B.J. Overview and bibliography of research on the greenshell mussel, Perna canaliculus, from New Zealand waters. J. Shellfish Res. 1999, 18, 347–360. [Google Scholar]
- Skelton, B.M.; South, P.M.; Jeffs, A.G. Inefficiency of conversion of seed into market-ready mussels in New Zealand’s Greenshell™ mussel (Perna canaliculus) industry. Aquaculture 2022, 560, 738584. [Google Scholar] [CrossRef]
- South, P.M.; Delorme, N.J.; Skelton, B.M.; Floerl, O.; Jeffs, A.G. The loss of seed mussels in longline aquaculture. Rev. Aquac. 2021, 14, 440–455. [Google Scholar] [CrossRef]
- Skelton, B.M.; Jeffs, A.G. The loss of spat following seeding onto coastal GreenshellTM mussel (Perna canaliculus) farms. Aquaculture 2021, 544, 737115. [Google Scholar] [CrossRef]
- Skelton, B.M.; Jeffs, A.G. The importance of physical characteristics of settlement substrate to the retention and fine-scale movements of Perna canaliculus spat in suspended longline aquaculture. Aquaculture 2020, 521, 735054. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Jeffs, A.G. Small-scale mussel settlement patterns within morphologically distinct substrata at Ninety Mile Beach, northern New Zealand. Malacologia 2002, 44, 1–15. [Google Scholar]
- Kelly, S. Improving the Supply of Mussel Spat; Technology New Zealand—Final Report: Contract Number: KPR90; Kinnears Ropes Ltd. & Kaitaia Spat Ltd.: Auckland, New Zealand, 2001. [Google Scholar]
- Wu, W.; Anderson, I.; Jeffs, A.G. The role of substrata width and millimeter scale surface micro-structure in the attachment of juvenile mussels. Aquaculture 2025, 595, 741593. [Google Scholar] [CrossRef]
- Gribben, P.E.; Jeffs, A.G.; de Nys, R.; Steinberg, P.D. Relative importance of natural cues and substrate morphology for settlement of the New Zealand GreenshellTM mussel, Perna canaliculus. Aquaculture 2011, 319, 240–246. [Google Scholar] [CrossRef]
- Berntsson, K.M.; Jonsson, P.R.; Lejhall, M.; Gatenholm, P. Analysis of behavioural rejection of micro-textured surfaces and implications for recruitment by the barnacle Balanus improvisus. J. Exp. Mar. Biol. Ecol. 2000, 251, 59–83. [Google Scholar] [CrossRef]
- Carl, C.; Poole, A.J.; Williams, M.R.; de Nys, R. Where to settle-settlement preferences of Mytilus galloprovincialis and choice of habitat at a micro spatial scale. PLoS ONE 2012, 7, e52358. [Google Scholar] [CrossRef] [PubMed]
- Pech, D.; Ardisson, P.L.; Bourget, E. Settlement of a tropical marine epibenthic assemblage on artificial panels: Influence of substratum heterogeneity and complexity scales. Estuar. Coast. Shelf Sci. 2002, 55, 743–775. [Google Scholar] [CrossRef]
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Walters, L.; Wethey, D. Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: The importance of refuge dimensions and adult morphology. Mar. Ecol. Prog. Ser. 1996, 137, 161–171. [Google Scholar] [CrossRef]
- Brenner, M.; Buck, B.H. Attachment properties of blue mussel (Mytilus edulis L.) byssus threads on culture-based artificial collector substrates. Aquac. Eng. 2010, 42, 128–139. [Google Scholar] [CrossRef]
- Callow, M.E.; Jennings, A.R.; Brennan, A.B.; Seegert, C.E.; Gibson, A.; Wilson, L.; Feinberg, A.; Baney, R.; Callow, J.A. Microtopographic cues for settlement of zoospores of the green fouling alga Enteromorpha. Biofouling 2002, 18, 229–236. [Google Scholar] [CrossRef]
- Jeffs, A.G.; Delorme, N.J.; Stanley, J.; Zamora, L.N.; Sim-Smith, C. Composition of beachcast material containing green-lipped mussel (Perna canaliculus) seed harvested for aquaculture in New Zealand. Aquaculture 2018, 488, 30–38. [Google Scholar] [CrossRef]
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernández Polanco, J.M.; et al. The decline of mussel aquaculture in the European Union: Causes, economic impacts and opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Gentry, R.R.; Froehlich, H.E.; Grimm, D.; Kareiva, P.; Parke, M.; Rust, M.; Gaines, S.D.; Halpern, B.S. Mapping the global potential for marine aquaculture. Nat. Ecol. Evol. 2017, 1, 1317–1324. [Google Scholar] [CrossRef]
- Kamermans, P.; Capelle, J.J. Provisioning of mussel seed and its efficient use in culture. In Goods and Services of Marine Bivalves; Smaal, A.C., Ferreira, J.G., Grant, J., Petersen, J.K., Strand, Ø., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 27–49. [Google Scholar]
- Carl, C.; Poole, A.J.; Sexton, B.A.; Glenn, F.L.; Vucko, M.J.; Williams, M.R.; Whalan, S.; de Nys, R. Enhancing the settlement and attachment strength of pediveligers of Mytilus galloprovincialis by changing surface wettability and microtopography. Biofouling 2012, 28, 175–186. [Google Scholar] [CrossRef]
- Scardino, A.J.; Zhang, H.; Cookson, D.J.; Lamb, R.N.; de Nys, R. The role of nano-roughness in antifouling. Biofouling 2009, 25, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Scardino, A.J.; de Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Vucko, M.J.; Poole, A.J.; Carl, C.; Sexton, B.A.; Glenn, F.L.; Whalan, S.; de Nys, R. Using textured PDMS to prevent settlement and enhance release of marine fouling organisms. Biofouling 2014, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gysbers, D.; Levenstein, M.A.; Juarez, G. The effect of surface topography on benthic boundary layer flow: Implications for marine larval transport and settlement. arXiv 2024, arXiv:2401.17216. [Google Scholar]
- Levenstein, M.A.; Gysbers, D.J.; Marhaver, K.L.; Kattom, S.; Tichy, L.; Quinlan, Z.; Tholen, H.M.; Kelly, L.W.; Vermeij, M.J.A.; Johnson, A.J.W.; et al. Millimeter-scale topography facilitates coral larval settlement in wave-driven oscillatory flow. PLoS ONE 2022, 17, e0274088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).