Abstract
The parasitic disease scuticociliatosis poses significant economic losses to shrimp culture. In this study, an unidentified species of ciliates was discovered in Litopenaeus vannamei. Morphological and molecular analyses were conducted to identify the parasite species, while a reinfection experiment was performed to assess its virulence towards L. vannamei. Simultaneously, Chinese herbal medicine was assessed for potential prevention and control of this pathogen. The results revealed that the SSU rDNA (93.87%), LSU rDNA (98.20%), and ITS1-5.8S-ITS2 rDNA (87.10%) genes exhibited high homology with Metanophrys sinensis, suggesting its classification within the genus Metanophry sp. The reinfection experiment demonstrated median lethal dosages of 6638 individuals/mL at 24 h and 4658 individuals/mL at 48 h for this ciliate on the host L.vannamei. In terms of drug control, we conducted a screening of 23 Chinese herbal extracts through in vivo injection trials and observed that Mume fructus extract exhibits potent biocidal activity. Specifically, the M. fructus extract solution with a final concentration of 5 mg/mL (0.5% v/v) eliminated all ciliates within 3 h. In conclusion, this study identified the ciliate parasite as Metanophrys sinensis and demonstrated its virulence to Litopenaeus vannamei. Mume fructus extract was found to effectively control the parasite, offering potential for managing scuticociliatosis in shrimp culture.
Key Contribution:
1. A novel pathogenic ciliate, Metanophrys sinensis, was identified in Litopenaeus vannamei. 2. This ciliate caused significant pathogenicity in Litopenaeus vannamei. 3. Mume fructus extract exhibited efficacy against Metanophrys sinensis.
1. Introduction
Litopenaeus vannamei is a globally significant shrimp species, providing consumers with high-quality protein and generating substantial economic benefits for the aquaculture industry []. Currently, intensive high-density culture systems are predominantly employed for L. vannamei production. However, as shrimp production increases, disease issues have become increasingly prominent [,,]. Among numerous pathogens, there has been a gradual rise in the incidence and scale of ciliate-induced parasitic diseases in shrimp, attracting widespread attention.
Scuticociliatid ciliates are facultative parasites that feed on bacteria and algae in natural seawater, but are also parasitic to fish, shrimp, crabs, and other hosts []. For instance, Metanophrys sinensis has been documented infecting larvae of the giant river prawn (Macrobrachium rosenbergii), causing large-scale mortalities of up to 80–90% in Indian hatcheries [,]. Especially in the case of aquaculture animals injured and environmental deterioration, scuticociliatid ciliates can cause infections in aquaculture animals, leading to large numbers of deaths [,]. Scuticociliatid ciliates not only infect the body surface and gills of farmed aquatic animals, but also parasitize internal organs, such as the brain, kidney, spleen, and even the blood []. Early diagnosis and treatment are essential to prevent large outbreaks of scuticociliatosis.
In the existing methods for preventing and controlling ciliate diseases, three approaches are commonly employed: 1. preventing the entry of pathogens into the aquaculture environment; 2. promptly interrupting transmission routes of local infections; and 3. enhancing host immunity to protect susceptible hosts. The control methods for ciliates typically involve using chemical drugs such as copper sulfate, formalin, and hydrogen peroxide to eliminate their presence [,]. However, these methods have notable limitations as they only target surface ciliates and may cause severe irritation to hosts. Moreover, the overuse of chemical drugs increases the accumulation of toxic substances in the ecological environment and threatens food safety [,,]. Therefore, the development of new drugs to replace chemical drugs is urgently needed for the prevention and control of scuticociliatid ciliates.
The use of traditional Chinese herbal medicine has attracted much attention for the treatment of pathogenic diseases of aquaculture species due to limited toxicity, relatively few side effects, and various biological activities, such as enhanced immune function and promotion of growth []. For example, Aegle marmelos not only promotes Nile tilapia growth but also enhances the immune system and antioxidant levels []. Additionally, Phyllanthus amarus extraction can improve the immune system of shrimp, promote growth, and increase resistance to pathogens []. Traditional Chinese herbs play a significant role in controlling parasitic diseases. While chemical agents like formalin and CuSO4 exhibit potent insecticidal effects, they carry risks such as disrupting aquatic microbial communities and inducing drug. Studies have found that the therapeutic doses of formaldehyde and oxytetracycline administered to sea bass (Dicentrarchus labrax L.) result in liver damage []. Formaldehyde could induce oxidative stress in rainbow trout (Oncorhynchus mykiss) hepatocytes by suppressing antioxidant defense mechanisms, thereby producing cytotoxicity []. Moreover, Zhou et al. found that trichloroisocyanuric acid and methanol extract of M. cordata are both viable alternatives for formaldehyde in aquaculture [].
Currently, Chinese herbal medicine has found two applications in aquaculture. Firstly, unilateral or compound Chinese herbal medicine can effectively eliminate pathogenic microorganisms present in aquaculture water. Generally, compound Chinese herbal medicine preparations exhibit superior efficacy compared to unilateral ones []. Secondly, the addition of feed containing Chinese herbal extracts enhances the immune response of farmed animals against pathogenic microorganisms. Chemical chemotaxis plays a crucial role in determining parasite virulence []. By incorporating Chinese herbal medicine extracts into animal feed, the concentration of specific active compounds within animals increases, thereby inhibiting ciliate penetration and parasitization. Studies have demonstrated that when hosts remain unparasitized for more than 8 h, ciliate infectivity is significantly reduced [].
During an investigation of aquatic diseases, our team discovered a significant mortality event of L. vannamei. Samples of diseased and dying shrimps were collected, and a large number of ciliates were identified through microscopic examination of the gills. In this study, the collected ciliates were purified and identified while establishing a passage system for further experimentation. Pathogenicity assessment was conducted using reinfection experiments, and herb screening was performed to identify effective preventive and therapeutic measures against these pathogens. We anticipate that the findings from this research will provide crucial foundational knowledge for managing outbreaks caused by the ciliates in aquaculture.
2. Materials and Methods
2.1. Isolation and In Vitro Culture
Six diseased shrimps were dissected, and the gill filaments were placed in Petri dishes with sterilized seawater for 3 h to release the scuticociliatid ciliates from the body. Then, a fixed amount of liquid was aspirated onto a slide, and the ciliates were observed under a microscope (Nikon Eclipse Ni-U, Osaka, Japan). The water drop separation method was used to isolate the ciliates. A single ciliate was isolated and transferred into conical flasks containing a mixture of sterilized seawater and L-15 cell nutrient solution (5:1) for monoclonal culture at 25 ± 1 °C. The nutrient solution was changed weekly to ensure an adequate supply of nutrients.
2.2. Morphological and Growth Characteristics
After isolation, purification, and stable growth, the morphological characteristics of the ciliates were assessed by fluorescent staining. In brief, 1 mL ciliate solution cultured for 24 h was transferred into polyethylene tubes, fixed with formaldehyde for 15 min, and centrifuged. The individual ciliates were washed with 1 × phosphate-buffered saline (Solarbio, Beijing, China) and stained with 20 µL of MitoTracker™ Green dye solution (Thermo Fisher Scientific, Waltham, MA, USA) for 15 min in the dark, followed by propidium iodide for 15 min, then centrifuged, resuspended in 1 × phosphate-buffered saline, added to glass slides, and observed under a differential interference contrast microscope (Nikon Corporation, Tokyo, Japan).
To determine the growth characteristic of ciliates, 18 mL of nutrient solution and 1 mL of ciliates (1000 individuals) were combined in a large Petri dish. Each day, 2 µL of the mixture was collected from each of nine spots on the Petri dish (Figure 1) and the ciliates were counted under a microscope. The growth rate was calculated as the formula G = (lnNt − lnN0)/t, where Nt and N0 are the final and initial population densities, respectively, and t is time.
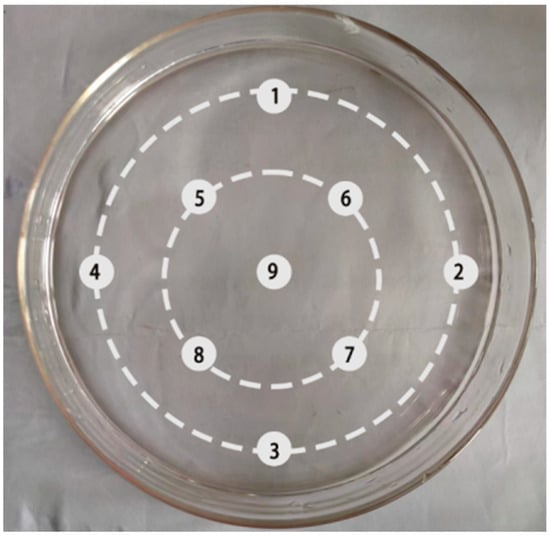
Figure 1.
Sampling protocol for ciliate growth assessment. The culture was initiated with 1000 ciliates in a Petri dish. Microscopic counts were performed daily on 2 µL samples taken from each of the nine marked locations.
2.3. DNA Extraction, Amplification and Phylogenetic Analysis
To extract DNA from scuticociliatid ciliates, 2 mL of purified ciliates was removed from the culture medium. Then, 200 µL formaldehyde solution was added to fix the ciliates. Subsequently, scuticociliatid ciliates were collected through centrifugation at 4000 r/min for 10 min. The Magen DNA Universal extraction kit was utilized to extract DNA. The DNA was amplified for the ITS1 region, small subunit (SSU) rDNA, and large subunit (LSU) rDNA using primers (Table 1). The PCR reaction system consisted of a 50 µL volume containing 1 × PCR buffer, 1.5 mmol/L MgCl2, 200 µmol/L dNTP mixture, 1 µmol/L primer, and 1 µL DNA template. PCR products were purified and retrieved using the AGAR gel DNA recovery kit. The sequencing results were spliced and blasted on the NCBI. The Neighbor-joining method was utilized to construct the phylogenetic relationships of scuticociliatid ciliates using MEGA 11.0 software, and the confidence was checked by bootstrap analysis with 1000 bootstrapping datasets. Some species of scuticociliatid ciliates were used to construct the phylogenetic tree based on SSU rDNA, LSU rDNA and ITS1 region.

Table 1.
Oligonucleotide primers for PCR amplification.
2.4. Virulence and Reinfection Experiments In Vivo
In reinfection experiments, 120 healthy L. vannamei (6.0 ± 0.5 cm body length) were randomly selected and raised in indoor barrels containing filtered seawater at a temperature of 18 ± 1 °C. Approximately one-third of the seawater was changed daily. After acclimation, the shrimp were randomly allocated to six groups (one control group and five experimental groups), which consisted of 20 shrimps in each plastic bucket. Shrimps in the five experimental groups were subject to intramuscular injections of 50 μL ciliates (proportionally diluted ciliate fluid with the sterile seawater at the ratios of 1:1, 1:2, 1:4, 1:8, and 1:16), while those in the control group were injected with an equal volume of culture medium. The specific number of ciliates used in each injection was provided in Table 2. After injection, the shrimp in both groups were temporarily kept in plastic buckets containing filtered seawater, with approximately one-third of the seawater changed daily. The behavior, feed intake, and mortality of the shrimp were monitored every 8 h during the experimental period. Finally, the ciliates were isolated from dying L. vannamei for identification and calculation of the median lethal dose (LD50). The LD50 values were calculated using the Reed-Muench method, with the formula LD50 = log^ [X_m − I (Σp − 0.5)], where X_m is the logarithm of the maximum dose, I is the logarithm of the adjacent dose ratio (higher/lower), p is the group mortality rate, and Σp is the sum of mortality rates.

Table 2.
Pathogenicity of Metanophrys sinensis to Litopenaeus vannamei, including mortality and LD50 values.
To investigate the effect of ciliates on the hemolymph of L. vannamei, 1 mL aliquots of hemolymph were collected from L. vannamei into EP tubes. The experimental group was supplemented with 100 μL of ciliate solution, while the control group was treated using culture medium only, without any ciliate solution. The control group was used to compare the effects of ciliate treatment on the hemolymph. After 24 h, ciliate growth in the hemolymph samples was evaluated both visually and microscopically.
2.5. Chinese Herbal Medicines Solution Extract
By consulting relevant journals, a total of 23 effective Chinese herbal medicines suitable for parasitic diseases in aquatic animals (such as Cryptocaryon irritans and scuticociliatosis) were selected (Supplementary Table S1). The herbs are dried and sealed in bags. The traditional decoction method is employed to prepare the herbal extract. Specifically, 30 g of dried herbs are finely chopped and wrapped in double-layered gauze to ensure complete immersion in 300 mL of distilled water without excessive compression that may impede boiling of the enclosed medicine. After boiling the mixture for 30 min, the filtrate is collected, and another 300 mL of water is added for an additional round of boiling and filtration lasting 30 min each time. Following these steps, the filtrates are combined and heated until evaporated to a volume of 30 mL, resulting in a medicine concentration of 1 g/mL. A portion of 15 mL liquid is stored at 4 °C for subsequent experiments, while the remaining liquid is preserved long-term at −20 °C.
2.6. Screening of Herbs In Vitro
After 24 h incubation, 1970 μL ciliate culture solution was added to 24-well plates with 30 μL of the corresponding herb solution, and the final volume of each well was 2 mL. The wells contained sterilized seawater as a control. All assays were performed in triplicate, with each replicate consisting of independent ciliate cultures exposed to the extract under identical conditions for 24 h or shorter intervals. Antiparasitic effects of the herbs were observed under a microscope. Then, 50 μL of herb liquid with the obvious antiparasitic effects was diluted in a Petri dish with 1950 μL of the new nutrient solution. After 24 h, the herb was considered to have a good antiparasitic effect if no living ciliates were found.
The experiment was conducted in a 24-well plate with a total volume of 2 mL. Each herbal medicine was tested in five groups, where the experimental group consisted of both ciliate liquid and herbal liquid. The volumes of herbal liquid and ciliate liquid were as follows:
Group 1: 1 μL of herbal liquid and 1999 μL of ciliate liquid, with herbal liquid accounting for 0.05% of the total volume;
Group 2: 2 μL of herbal liquid and 1998 μL of ciliate liquid, with herbal liquid accounting for 0.10% of the total volume;
Group 3: 10 μL of herbal liquid and 1990 μL of ciliate fluid, with herbal liquid accounting for 0.50% of the total volume;
Group 4: 20 μL of herbal liquid and 1980 μL of ciliate liquid, with herbal liquid accounting for 1.00% of the total volume;
Group 5: 50 μL of herbal liquid and 1950 μL of ciliate liquid, with herbal liquid accounting for 2.50% of the total volume.
The ciliate culture solution added to each well contained approximately 4775–4900 ciliates, as determined by the cultivation rate and density, with the range accounting for minor volume variations across groups.
The control group included only ciliate liquid and culture medium. The vitality of the ciliates in all groups was monitored at 3 h, 6 h, 12 h, 24 h, and 48 h. Given the high ciliate density, microscopic observation of ciliate mortality was conducted by aspirating a portion of the sample. Records were kept to determine which herb had the obvious insecticidal effect and its optimal concentration.
3. Results
3.1. Phenotypic Characteristics
Under a microscope, scuticociliatid ciliates in the diseased shrimps are long, oval, and appear slightly flattened. Live ciliates measured a mean length of 21.39 ± 4.71 μm and a mean width of 9.21 ± 2.14 μm, with a length-to-width ratio of 3:1 to 2:1. The morphology is similar to a sunflower seed, as the anterior end is slender, while the posterior end is blunt and rounded. The whole body is covered by cilia for locomotion, including a terminal ciliary tail (Figure 2A). Ciliates prefer to gather and move freely in nutrition-rich substrates via rotation and bending of the soft and flexible body. This ciliate reproduces by binary fission (Figure 2B). The vegetative nucleus is located in the middle to posterior part of the body, while the reproductive nucleus is relatively smaller and located next to the vegetative nucleus (Figure 2C).
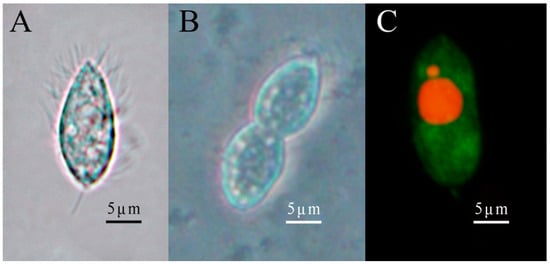
Figure 2.
Morphological characteristics of ciliates. (A) depicts the fission morphology of ciliates observed using a light microscope. (B) represents the binary ciliates. (C) is the fluorescence staining of the ciliates. The vegetative nucleus (big) and reproductive nucleus (small) are stained red, while the cytoplasm is stained green.
3.2. Molecular Identification
The rDNA sequences of the SSU, LSU, and ITS1 regions for this scuticociliatid ciliate were 1702 bp, 1772 bp, and 542 bp, respectively. Based on the blast results, the SSU rDNA sequences of this scuticociliatid ciliate exhibited a remarkable resemblance to M. sinensis HM236336.1 (93.87%). The LSU rDNA sequences displayed close similarity with M. sinensis JN885114.1 (98.20%). Moreover, ITS1 rDNA sequences of scuticociliatid ciliates were found to be significantly associated with Metanophrys sp. OR282527.1 (95.96%) and M. sinensis JN885092.1 (86.36%). Based on the phylogenetic tree depicted in Figure 3, it can be inferred that this species should be taxonomically classified within Metanophrys sinensis.
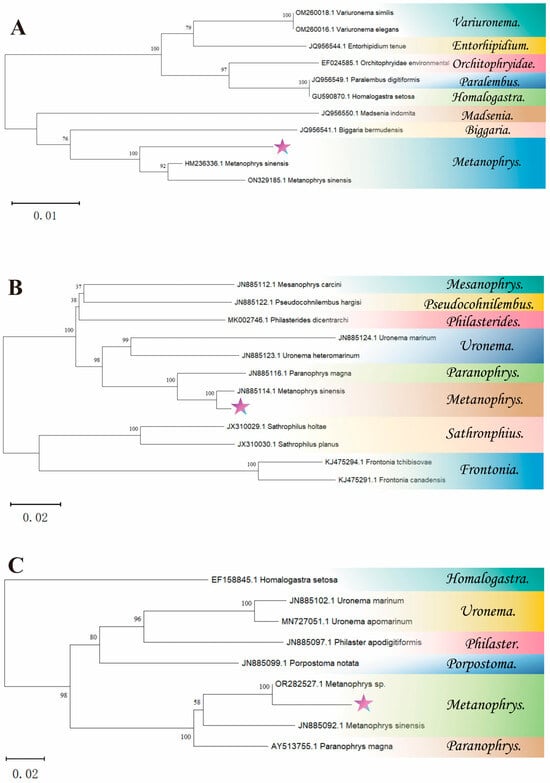
Figure 3.
The phylogenetic tree is based on SSU rDNA, LSU rDNA and ITS1 rDNA. (A) presents the SSU rDNA phylogenetic tree, (B) illustrates the LSU rDNA phylogenetic tree, and (C) displays the ITS1 rDNA phylogenetic tree. The neighbor-joining phylogenetic trees were constructed using MEGA 11.0. The symbol star indicated the scuticociliatid ciliate.
3.3. Life Characteristics
Ciliates were cultured in a 19 mL medium at 25 ± 1 °C and exhibited a growth phase on day 3, reaching a maximum on day 4. Then, the number of ciliates began to decrease and returned to the initial level on day 8. The average natural growth rate was 0.577 from day 1 to 4 (Figure 4).
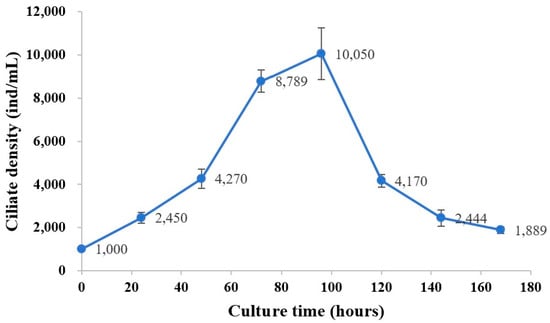
Figure 4.
Population growth dynamics of the scuticociliate ciliates isolated from Litopenaeus vannamei. Ciliates were cultured in L-15 medium mixed with sterilized seawater at 25 ± 1 °C. Ciliate density was enumerated daily with a hemocytometer. Values and deviation bars represent the means and standard errors (n = 3), respectively.
3.4. Virulence and LD50
In the reinfection experiment, the dying L. vannamei exhibited no obvious changes on the body surface, but the gill filaments were red in color (Figure 5). Meanwhile, ciliates in gill filaments were observed under the microscope (Figure 6). Furthermore, the infection experiment results are presented in Table 2 and Figure 7. The LD50 values of this ciliate at 24 and 48 h were 6638 and 4258 individuals/mL, respectively. The hemolymph was examined at 24 h, and there was no obvious difference by direct visual observation. However, a large number of rapidly swimming ciliates were observed in the experimental group under the microscope (Figure 8). The ciliates in the experimental group exhibited excellent vitality, with elongated movement trails observed in the photographs.

Figure 5.
Clinical symptoms of diseased shrimp. The red arrow shows the blue-green color hepatopancreas in the diseased shrimp, and the white arrow indicates red gill filaments.
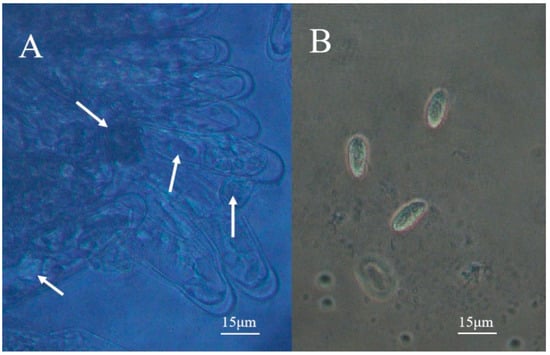
Figure 6.
Ciliates in the gill of a diseased shrimp. (A) is the gill filaments of the affected prawn under the microscope, and the white arrow indicates the ciliates in the gill filaments; (B) is the ciliate released from the gill filament.
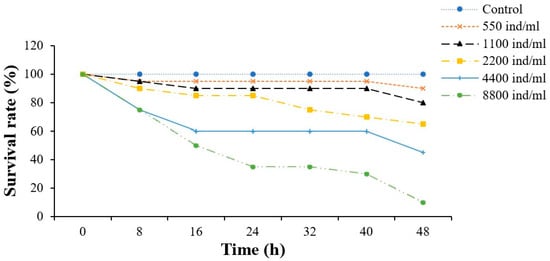
Figure 7.
Survival rate of L. vannamei in the reinfection experiment. The experimental group received intramuscular injections of 50 μL of ciliates, while the control group received injections of an equal volume of culture medium. Mortality rates were monitored every 8 h throughout the experiment.
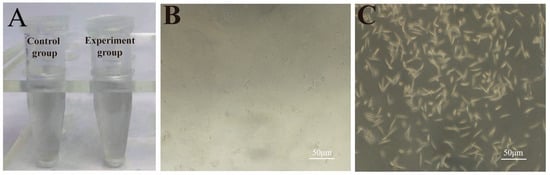
Figure 8.
Activity of ciliates in hemolymph after 24 h. (A) displays the hemolymph that was subjected to 24 h of treatment. The left side represented the control group with culture medium only, while the right side depicted the experimental group with ciliates. (B) presents the light microscope images of the control group, while (C) shows the observed fast-moving ciliate in the experimental group.
3.5. Sensitivity of Ciliates to Herbs
Three herbs (Scutellaria baicalensis, Mume fructus, Astragalus membranaceus) exhibited antiparasitic activities. Drug screening (Figure 9) revealed that Scutellaria baicalensis, Mume fructus, and Astragalus membranaceus exhibit anti-M. sinensis effects. Notably, when the volume ratio was set at 0.05% and 0.1%, a concentration of 1 g/mL of M. fructus extract demonstrated significant inhibitory effects on ciliates after 24 h; however, the most pronounced effect was observed with a volume ratio of M. fructus extract at 0.5%, resulting in lysis and eradication of ciliates within just three hours. Furthermore, the concentration of S. baicalensis and A. membranaceus extract at 1 g/mL also exhibited substantial inhibitory effects on ciliates when the volume ratio exceeded 1.00%. Consequently, based on our experimental findings, M. fructus emerges as the most effective drug against ciliates.
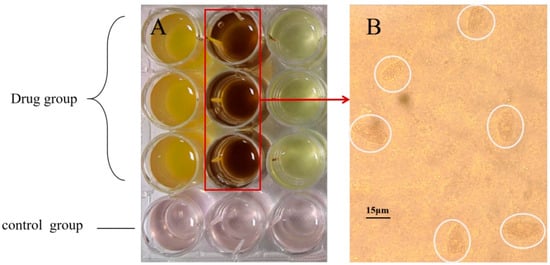
Figure 9.
Herb screening and morphology of ciliates after herb treatment. Notes: (A) indicates the drug group and the control group, in which the drug group from left to right is Scutellaria baicalensis, Mume fructus, and Astragalus membranaceus; (B) indicates the ciliate lysed and died under the action of plum extract, and the part circled in white represents the ciliate lysed and died. Each treatment had three replicates.
4. Discussion
The term “scuticociliatid ciliates” refers to the ciliates of the phylum Ciliophora, class Oligohymenophorea, and family Scuticociliatidae. The study of scuticociliatid ciliates originated from Müller’s description of Cyclidium glaucoma in 1773 [], and over a hundred species have been discovered thus far, with continuous reports on potential new species []. M. sinensis belongs to the genus Metanophrys within the group of shield ciliates. It is a facultative parasite that can survive in the environment by feeding on organic debris. When wounds occur on aquatic animals’ surfaces, they can invade and infect them, leading to mass mortality in aquaculture populations []. Morphological analysis reveals that M. sinensis has an average length and width of 21 μm and 9 μm, respectively, which is similar in size to M. sinensis found in Korea (20 × 8.5 μm), but slightly smaller than populations found in Qingdao (25–50 × 10–20 µm) and Zhanjiang (25–30 × 10–15 µm) in China []. Due to their microscopic size (ranging from 20~45 μm) [] and morphological similarities among populations (ciliary pattern, caudal cilium, contractile vacuole), confusion may arise when distinguishing between different scuticociliatid ciliate species. Additionally, the size of ciliates also varies depending on their food source. Research has shown that M. sinensis parasitizing Scophthalmus maximus tend to be larger while those living in artificial saltwater are thinner. Furthermore, there are differences in size among different growth stages of ciliates as well []. Therefore, accurate molecular classification is preferred to avoid morphological variations caused by nutritional sources.
After amplification and analysis of the SSU, ITS1-5.8S-ITS2, and LSU rDNA sequences of the ciliate, this study revealed that its closest phylogenetic relationship was with Metanophrys sinensis. Specifically, based on sequence alignment and molecular phylogenetic tree results, the SSU and LSU sequences indicate that this ciliate first clusters with Metanophrys sinensis before clustering with other species in the genus Metanophrys, suggesting its classification as Metanophrys sinensis. However, concerning ITS1-5.8S-ITS2 rDNA sequence alignment, the highest similarity is limited to species within the genus Metanophrys. The genus Metanophrys was established by de Puytorac et al. (1974) with Metanophrys durchoni as the type species and subsequently included four additional species: Metanophrys selongata, Metanophrys echini, Metanophrys sinensis, and Metanophrys similis []. Nevertheless, M. sinensis is currently the only reported species with the ability to infect and parasitize cultivated animals, other species are only free-living. Therefore, based on consideration of the ciliates’ infectivity and sequence alignment results, it was determined that this ciliate should be classified as Metanophrys sinensis.
The study used L-15 medium and sterilized seawater (1:5) as the nutritional source for this ciliate. L-15 medium contains the necessary inorganic salts, amino acids, and vitamins for the growth and reproduction of the ciliate [,]. Under the influence of L-15 medium, detached monoclonal cultures of M. sinensi exhibited a growth curve similar to that of a logistic function, with a natural growth rate reaching 0.577 within 96 h, indicating a relatively fast reproductive speed. It is worth noting that even under conditions of reduced nutrition, the ciliates were still able to survive. Ciliates were observed to persist for up to four weeks in a 2 mL culture solution containing 20% L-15 and a small number of ciliates, although they appeared slightly emaciated in morphology. To elucidate the virulence of M. sinensis on South American white shrimp, acute toxicity experiments were conducted using the muscle injection method. The results demonstrated LD50 values of 6638 ind/mL at 24 h and 4258 ind/mL at 48 h (Table 2), indicating that M. sinensis exhibits low virulence as a ciliate. However, shrimps exhibit cannibalistic behavior, with a particular preference for cephalothorax and pleopods. Multiple instances of predation were observed every 8 h during the recording period, potentially leading to repeated infections in shrimps and causing more severe harm. Furthermore, when a small quantity of ciliates was introduced into shrimp hemolymph, their rapid proliferation could be observed within 24 h. Previous studies on Paranophrys marina have revealed that hemolymph serves as an ideal growth medium for ciliates []. Concurrently, the ciliates in this study originated from affected aquaculture farms where all farmed shrimp were infected. The initial site of pathogen infection occurred in the gills, as gill tissue was in direct contact with the water. The pathogen could survive in water, and exposure to the water resulted in a 100% infection rate among the shrimp. These findings indicate that M. sinensis possesses the ability to parasitize and rapidly reproduce in multiple tissues of shrimp, such as gills and hemolymph, and it can survive in nutritionally deficient environments. These biological characteristics collectively suggest that this parasite may pose a potentially serious threat to the white shrimp aquaculture industry.
In recent years, there has been limited research on M. sinensis. The most recent specialized report was published in 2018, documenting a large-scale infection caused by M. sinensis in Macrobrachium rosenbergii []. However, due to significant variations in the immune systems of crustaceans and other aquatic animals, it remains uncertain whether M. sinensi can infect other cultured species. South Korea is recognized as a high-incidence region for ciliate diseases, with frequent outbreaks observed in farmed fish; at least four scuticociliatid ciliates have been identified as infecting flounder (Uronema marinum, Miamiensis avidus, Pseudocohnilembus persalinus, and Pseudocohnilembs hargisi) []. Furthermore, Uronema marinum has been confirmed to infect American oysters, European oysters, and Philippine clams [,]. Henceforth, it can be speculated that M. sinensis may possess the potential to infect other cultured animals; however, further experiments are required to obtain definitive results.
In this study, we observed that when the concentration of M. fructus was set at 1 g/mL, and its water extract proportion reached 0.5%, it exhibited significant efficacy in eradicating M. sinensis, thereby demonstrating a robust anti-parasitic effect. M. fructus, the fruit of the Rosaceae plum, undergoes a low-temperature baking process after summer harvesting, resulting in a yellowish-brown flesh and a shriveled, blackened peel. Historically, M. fructus has been utilized as a folk medicine. Recently, as a herbaceous plant with minimal side effects and environmental friendliness, it has garnered increasing attention from scholars. The herb is enriched with various active substances, including organic acids, phenolic compounds, and flavonoids, which are also the key components of M. fructus []. Modern pharmacological investigations have revealed the multifaceted benefits of M. fructus, encompassing anti-inflammatory, antibacterial, free radical scavenging, and anti-parasitic properties. A previous study noted that the extract of M. fructus exhibited antibacterial activity against oral pathological bacteria, with a MIC (minimum inhibitory concentration) ranging from 0.15625 to 0.0003 g/mL []. Further, Debnath’s in vitro research demonstrated the extract’s proficiency in scavenging free radicals, nitrite activity, and inhibiting lipid oxidation []. Chen reported antibacterial activity against Streptococcus mutans [], while Yildiz’s experiment indicated that the addition of citric acid to tilapia’s diet significantly improved its resistance to Cryptocaryon irritans []. Additionally, Hibiscus syriacus extract, which is similarly rich in organic acids and phenols, effectively controls scuticociliatosis in turbot []. Although direct cytotoxicity on shrimp hemocytes or tissues was not evaluated here, indirect evidence from our assays and aquaculture reviews suggests low risk; future studies will incorporate targeted host safety assessments (e.g., MTT hemocyte viability or tissue histology) []. Collectively, these studies underscore the extensive application prospects and immense potential of M. fructus in both human medicine and aquaculture. It is not only harmless to the environment but also beneficial to animals.
The ciliates were observed under a microscope following exposure to M. fructus extract. The results demonstrated significant morphological alterations in the surface cilia of M. sinensis, including breakage of tail cilia and disintegration of the surface membrane and cilia. However, the mechanism underlying the effects of M. fructus was not investigated in this study. Nevertheless, a plausible explanation for the lethal action of M. fructus on M. sinensis is that organic acids and phenolic compounds present in M. fructus exert inhibitory effects on ciliates by targeting their surface membrane. Generally, the surface membrane plays a crucial role in maintaining homeostasis and survival within hosts for parasites, making it an ideal target for anti-parasitic drugs [,,]. The active components within M. fructus can penetrate this surface membrane and induce morphological changes leading to dysfunction within parasite bodies, thereby exhibiting anti-parasitic activity. In a similar investigation involving Gyrodactylus kobayashii treated with ketones from Turmeric rhizome, extensive damage to the membrane was observed using electron microscopy []. Senna has been shown to alter the permeability of tapeworms’ surface membranes, resulting in irreversible damage []. Another possible explanation is that ciliates obtain nutrients from culture media through their mouthparts while simultaneously ingesting extracts from M. fructus into their bodies, causing organelle failure, ultimately leading to death. Similar studies have revealed cell disorders occurring in parasites upon exposure to polyphenol extracts derived from neem leaves, which promote parasite death []. Additionally, Artemisia argyi induces membrane damage, as well as the destruction of renal tubes and fallopian tubes among parasites [].
5. Conclusions
This study identified Metanophrys sinensis as the cause of scuticociliatosis in Litopenaeus vannamei, confirming its virulence, with LD50 values of 6638 individuals/mL at 24 h and 4258 individuals/mL at 48 h. The parasite affects both external and internal organs, posing a significant threat to shrimp health.
A key finding is that Mume fructus extract effectively controls M. sinensis with a 0.5% concentration, achieving complete eradication within three hours. This provides a promising alternative to chemical treatments, offering an environmentally friendly solution for aquaculture disease management.
These results emphasize the potential of herbal medicine in sustainable aquaculture practices. Future research should focus on understanding the mechanisms of herbal extracts, testing their long-term efficacy in field conditions, and exploring additional herbal treatments to prevent and control parasitic diseases in aquaculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10120604/s1. Table S1: 23 kinds of experimental herbals.; Table S2: Population growth parameters of the ciliate isolated from Litopenaeus vannamei.
Author Contributions
Methodology, J.F., J.W. and Z.L.; validation, Y.X.; formal analysis, Z.L., H.C. and J.W.; investigation, H.C.; resources, M.G., J.F. and Z.L.; data curation, M.G., Y.X. and H.C.; writing—original draft, Y.X.; writing—review & editing, M.G., X.J. and R.M.; visualization, X.J.; supervision, S.X. and R.M.; project administration, M.G., S.X. and R.M.; funding acquisition, M.G., J.F. and S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fund of the Key Laboratory of South China Sea Fishery Resource Exploitation and Utilization, Ministry of Agriculture and Rural Affairs, P.R. China (FREU2021-02); the Ningbo Public Welfare Program (Science and Technology Commissioner) (2024S209); the Ningbo Public Benefit Science and Technology Program Project (2021S076); and the K. C. Wong Magna Fund of Ningbo University.
Institutional Review Board Statement
The committee on the Ethics of Animal Experiments of Ningbo University (No. SYXK20190005) was established in 2019, but the committee authorities were only for rabbits, mice, and rats, not including aquatic animals. In this study, shrimps were obtained from a commercial fish farm, and all experimental operations involving animals complied with the requirements of the governing regulation for the use of experimental animals in Zhejiang Province (Zhejiang provincial government order No. 263, released on 17 August 2009, effective from 1 October 2010) and were performed according to the Experimental Animal Management Law of China and met the animal operation standards of the industry.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, R.; Wu, Y.; Li, G.; Zhao, S.; Li, L.; Fang, W. Pharmacokinetics of sarafloxacin hydrochloride in the pacific white shrimp, Litopenaeus vannamei, following multiple-dose oral administration. Aquaculture 2022, 548, 737662. [Google Scholar] [CrossRef]
- Dong, P.; Guo, H.; Wang, Y.; Wang, R.; Chen, H.; Zhao, Y.; Wang, K.; Zhang, D. Gastrointestinal microbiota imbalance is triggered by the enrichment of Vibrio in subadult Litopenaeus vannamei with acute hepatopancreatic necrosis disease. Aquaculture 2021, 533, 736199. [Google Scholar] [CrossRef]
- Klongklaew, N.; Praiboon, J.; Tamtin, M.; Srisapoome, P. Chemical composition of a hot water crude extract (HWCE) from Ulva intestinalis and its potential effects on growth performance, immune responses, and resistance to white spot syndrome virus and yellowhead virus in Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immun. 2021, 112, 8–22. [Google Scholar]
- Landers, S.C.; Lee, R.F.; Walters, T.L.; Walker, A.N.; Powell, S.A.; Patel, M.K.; Frischer, M.E. Hyalophysa lynni n. sp.(Ciliophora, Apostomatida), a new pathogenic ciliate and causative agent of shrimp black gill in penaeid shrimp. Eur. J. Protistol. 2020, 73, 125673. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Leiro, J.; Lamas, J. Reprint of “Fish immunity to scuticociliate parasites”. Dev. Comp. Immunol. 2014, 43, 280–289. [Google Scholar] [CrossRef]
- Sahoo, P.; Pattanayak, S.; Paul, A.; Sahoo, M.; Kumar, P.R.; Panda, D.; Pillai, B. First record of Metanophrys sinensis (Protozoa: Ciliophora: Scuticociliatida) from India causing large scale mortality in a new host Macrobrachium rosenbergii larvae. J. Fish Dis. 2018, 41, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Debnath, P.P.; Stentiford, G.D.; Bateman, K.S.; Salin, K.R.; Bass, D. Diseases of the giant river prawn Macrobrachium rosenbergii: A review for a growing industry. Rev. Aquac. 2022, 15, 738–758. [Google Scholar] [CrossRef]
- Muznebin, F.; Alfaro, A.C.; Webb, S.C. Occurrence of Perkinsus olseni and other parasites in New Zealand black-footed abalone (Haliotis iris). N. Z. J. Mar. Freshw. Res. 2023, 57, 261–281. [Google Scholar] [CrossRef]
- Pan, X.; Li, W.; Warren, A.; Dong, Z. Tetrahymena pyriformis infection in the tiger barb, Puntius tetrazona: Parasite characterization and pathology of infected fish. Aquaculture 2022, 549, 737725. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Scuticociliatosis and its recent prophylactic measures in aquaculture with special reference to South Korea: Taxonomy, diversity and diagnosis of scuticociliatosis: Part I Control strategies of scuticociliatosis: Part II. Fish Shellfish Immun. 2010, 29, 15–31. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Scarfe, A.D.; Palić, D. Aquaculture biosecurity: Practical approach to prevent, control, and eradicate diseases. Aquac. Health Manag. 2020, 75–116. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; MMS, C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Mamta; Rao, R.; Wani, K.A. Status of organochlorine and organophosphorus pesticides in wetlands and its impact on aquatic organisms. Environ. Claims J. 2019, 31, 44–78. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Effendi, I.; Yoswaty, D.; Syawal, H.; Austin, B.; Lyndon, A.R.; Kurniawan, R.; Wahyuni, S.; Al-Harbi, A. The Use of Medicinal Herbs in Aquaculture Industry: A Review. Curr. Asp. Pharm. Res. Dev. 2022, 7, 7–20. [Google Scholar]
- Wangkahart, E.; Wachiraamonloed, S.; Lee, P.-T.; Subramani, P.A.; Qi, Z.; Wang, B. Impacts of Aegle marmelos fruit extract as a medicinal herb on growth performance, antioxidant and immune responses, digestive enzymes, and disease resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immun. 2022, 120, 402–410. [Google Scholar] [CrossRef]
- Huang, H.-T.; Lee, P.-T.; Liao, Z.-H.; Chen, H.-Y.; Nan, F.-H. Effects of Phyllanthus amarus extract on nonspecific immune responses, growth, and resistance to Vibrio alginolyticus in white shrimp Litopenaeus vannamei. Fish Shellfish Immun. 2020, 107, 1–8. [Google Scholar]
- Othmen, Z.O.B.; Jerbi, M.A.; Timoumi, R.; Besbes, R.; Haouas, Z.; Achour, L.; Elazomi, A.; Zaet, A.; Zourgui, L.; Kacem, A. Liver histopathological and oxidative stress assessment by a combination of formaldehyde and oxytetracycline in sea bass (Dicentrarchuslabrax L). Open Vet. J. 2024, 14, 630. [Google Scholar] [CrossRef]
- Yıldız, F.; Kaptaner, B. Effects of Formaldehyde on Cytotoxicity and Antioxidant Defense/Oxidative Stress Biomarkers in Hepatocytes Isolated from Rainbow Trout (Oncorhynchus mykiss). Biol. Bull. 2025, 52, 209. [Google Scholar] [CrossRef]
- Zhou, S.; Li, W.X.; Wang, Y.Q.; Zou, H.; Wu, S.G.; Wang, G.T. Anthelmintic efficacies of three common disinfectants and extracts of four traditional Chinese medicinal plants against Gyrodactylus kobayashii (Monogenea) in goldfish (Carassius auratus). Aquaculture 2017, 466, 72–77. [Google Scholar] [CrossRef]
- Bai, D.; RLi, R.; Xing, K.; Guo, Y.; Chen, C.; Qiao, X.; Mao, H.; Zhu, G. In vitro antibacterial activity of herbal medicines and combinations of herbal medicines and antibiotics against Edwardsiella tarda. Isr. J. Aquac.–Bamidgeh 2009, 61, 27–34. [Google Scholar] [CrossRef]
- Mao, W.W.; Zhao, Z.C.; Li, G.H.; Guo, W.L.; Ding, Z.H.; Wang, S.F.; Sun, Y.; Cao, Z.J.; Li, J.L.; Zhou, Y.C. In vitro screening and in vivo evaluation of antiparasitic phytochemicals against Cryptocaryon irritans in pompano, Trachinotus ovatus. J. World Aquac. Soc. 2022, 53, 1084–1100. [Google Scholar] [CrossRef]
- Dan, X.; Li, A.; Lin, X.; Teng, N.; Zhu, X. A standardized method to propagate Cryptocaryon irritans on a susceptible host pompano Trachinotus ovatus. Aquaculture 2006, 258, 127–133. [Google Scholar] [CrossRef]
- Babko, R.; Kuzmina, T. Ciliata (protista, ciliophora) of epiphyton of higher aquatic plants in a small river. Hydrobiol. J. 2004, 40, 17. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, Y.; Li, T.; Song, W.; Wang, Y.; Wu, T.; Zhang, G.; Liu, Y.; Ma, H.; Song, W. Biodiversity of freshwater ciliates (Protista, Ciliophora) in the Lake Weishan Wetland, China: The state of the art. Mar. Life Sci. Technol. 2022, 4, 429–451. [Google Scholar] [CrossRef]
- Gao, F.; Fan, X.; Yi, Z.; Strüder-Kypke, M.; Song, W. Phylogenetic consideration of two scuticociliate genera, Philasterides and Boveria (Protozoa, Ciliophora) based on 18 S rRNA gene sequences. Parasitol. Int. 2010, 59, 549–555. [Google Scholar] [CrossRef]
- Pan, X.; Fan, X.; Al-Farraj, S.A.; Gao, S.; Chen, Y. Taxonomy and morphology of four “ophrys-related” scuticociliates (Protista, Ciliophora, Scuticociliata), with the description of a new genus, Paramesanophrys gen. nov. Eur. J. Taxon. 2016, 191, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, K.; Chen, X.; Wei, M.; Yu, X.; Su, H.; Gan, P.; Yu, K. Inactivation of ciliate Uronema marinum under UV/peroxydisulfate advanced disinfection system in marine water. Sep. Purif. Technol. 2023, 305, 122563. [Google Scholar] [CrossRef]
- Álvarez-Pellitero, M.; Palenzuela, O.; Padrós, F.; Sitjà-Bobadilla, A.; Riaza, A.; Silva, R.; Arán, J. Histophagous scuticociliatids (Ciliophora) parasitizing turbot Scophthalmus maximus: Morphology, in vitro culture and virulence. Folia Parasitol. 2004, 51, 177–187. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, M.; Ma, H.; Al-Rasheid, K.A.; Hu, X. Morphology and small-subunit rRNA gene sequences of two novel marine ciliates, Metanophrys orientalis spec. nov. and Uronemella sinensis spec. nov.(Protista, Ciliophora, Scuticociliatia), with an improved diagnosis of the genus Uronemella. Int. J. Syst. Evol. Microbiol. 2013, 63, 3515–3523. [Google Scholar] [CrossRef]
- Aoki, M.M.; Kisiala, A.B.; Rahman, T.; Morrison, E.N.; Emery, R.N. Cytokinins are pervasive among common in vitro culture media: An analysis of their forms, concentrations and potential sources. J. Biotechnol. 2021, 334, 43–46. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Gao, Z.; Jiang, H.; Wang, X.; Mai, K.; He, G. Establishment and characterization of a fibroblast-like cell line from the muscle of turbot (Scophthalmus maximus L.). Fish Physiol. Biochem. 2019, 45, 1129–1139. [Google Scholar] [CrossRef]
- Kang, H.-S.; Whang, I.; Cho, J.-K. First report of Paranophrys marina (Protozoa, Ciliophora, Scuticociliatia) isolated from olive flounder Paralichthys olivaceus in Korea: Morphological and phylogenetic analysis. Fish Pathol. 2021, 34, 47–53. [Google Scholar]
- Lee, J.H.; Park, J.J.; Choi, J.H.; Kang, S.Y.; Kang, Y.J.; Park, K. Effects of clioquinol on the scuticociliatosis-causing protozoan Miamiensis avidus in olive flounder Paralichthys olivaceus. J. Fish Dis. 2018, 41, 451–462. [Google Scholar] [CrossRef]
- Pérez-Bustamante, I.S.; Cáceres-Martínez, J.; Cruz-Flores, R.; Vásquez-Yeomans, R. First record of dark leathery surface of geoduck clams in Panopea globosa. J. Invertebr. Pathol. 2021, 180, 107543. [Google Scholar] [CrossRef]
- Plunket, L.; Hidu, H. The role of Uronema marinum (Protozoa) in oyster hatchery production. Aquaculture 1978, 15, 219–225. [Google Scholar] [CrossRef]
- Gong, X.-P.; Tang, Y.; Song, Y.-Y.; Du, G.; Li, J. Comprehensive review of phytochemical constituents, pharmacological properties, and clinical applications of Prunus mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Wong, R.W.; Hägg, U.; Chen, Y.; Herath, T.D.; Lakshman Samaranayake, P.; Kao, R. Prunus mume extract exhibits antimicrobial activity against pathogenic oral bacteria. Int. J. Paediatr. Dent. 2011, 21, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Bak, J.P.; Samad, N.B.; Jin, H.L.; Lee, B.R.; Lim, B.O. Antioxidant activity of mume fructus extract. J. Food Biochem. 2012, 36, 224–232. [Google Scholar] [CrossRef]
- Chen, Y.; Wong, R.W.; Seneviratne, C.; Hägg, U.; Mcgrath, C.; Samaranayake, L.; Kao, R. The antimicrobial efficacy of Fructus mume extract on orthodontic bracket: A monospecies-biofilm model study in vitro. Arch. Oral Biol. 2011, 56, 16–21. [Google Scholar] [CrossRef]
- Yildiz, H.Y.; Yilmaz, B.H. Dietary citric acid decreased the theront number of Cryptocaryon irritans (Ciliata) in seawater-adapted tilapia (Oreochromis niloticus). J. Fish Dis. 2024, 47, e13881. [Google Scholar] [CrossRef]
- Carvalho, A.; Domingues, I.; Carvalho, C.; Silva, A.M.; Soares, A.M.; Marques, C.R. In vitro antiprotozoal activity of Hibiscus sabdariffa extract against a ciliate causing high mortalities in turbot aquaculture. Biology 2023, 12, 912. [Google Scholar] [CrossRef]
- Woo, S.J. Application of Medicinal Herbs and Plants to Control Ciliate Infection in Finfish for Sustainable Aquaculture: A Review. Rev. Aquac. 2025, 17, e70063. [Google Scholar] [CrossRef]
- Coyne, R.S.; Hannick, L.; Shanmugam, D.; Hostetler, J.B.; Brami, D.; Joardar, V.S.; Johnson, J.; Radune, D.; Singh, I.; Badger, J.H. Comparative genomics of the pathogenic ciliate Ichthyophthirius multifiliis, its free-living relatives and a host species provide insights into adoption of a parasitic lifestyle and prospects for disease control. Genome Biol. 2011, 12, R100. [Google Scholar] [CrossRef]
- Kelly, F.D.; Sanchez, M.A.; Landfear, S.M. Touching the surface: Diverse roles for the flagellar membrane in kinetoplastid parasites. Microbiol. Mol. Biol. Rev. 2020, 84, e00079-19. [Google Scholar] [CrossRef] [PubMed]
- Marana, M.H.; Al-Jubury, A.; Mathiessen, H.; Buchmann, K. Lipopeptide surfactant killing of Ichthyophthirius multifiliis: Mode of action. Aquac. Rep. 2023, 30, 101562. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Tu, X.; Ling, F.; Wang, G. Efficacy and antiparasitic mechanism of curdione from Curcuma zedoaria against Gyrodactylus kobayashii in goldfish. Aquaculture 2020, 523, 735186. [Google Scholar] [CrossRef]
- Kundu, S.; Roy, S.; Nandi, S.; Ukil, B.; Lyndem, L.M. Senna alexandrina Mill. induced ultrastructural changes on Hymenolepis diminuta. J. Parasit. Dis. 2017, 41, 147–154. [Google Scholar] [CrossRef]
- Mukherjee, N.; Mukherjee, S.; Saini, P.; Roy, P.; Babu, S.P.S. Antifilarial effects of polyphenol rich ethanolic extract from the leaves of Azadirachta indica through molecular and biochemical approaches describing reactive oxygen species (ROS) mediated apoptosis of Setaria cervi. Exp. Parasitol. 2014, 136, 41–58. [Google Scholar] [CrossRef]
- Beshay, E. Therapeutic efficacy of Artemisia absinthium against Hymenolepis nana: In vitro and in vivo studies in comparison with the anthelmintic praziquantel. J. Helminthol. 2018, 92, 298–308. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).