Modeling the Water Source Ecosystem in the Middle Route of the South-to-North Water Diversion Project: Implications for Management and Conservation
Abstract
1. Introduction
2. Materials and Methods
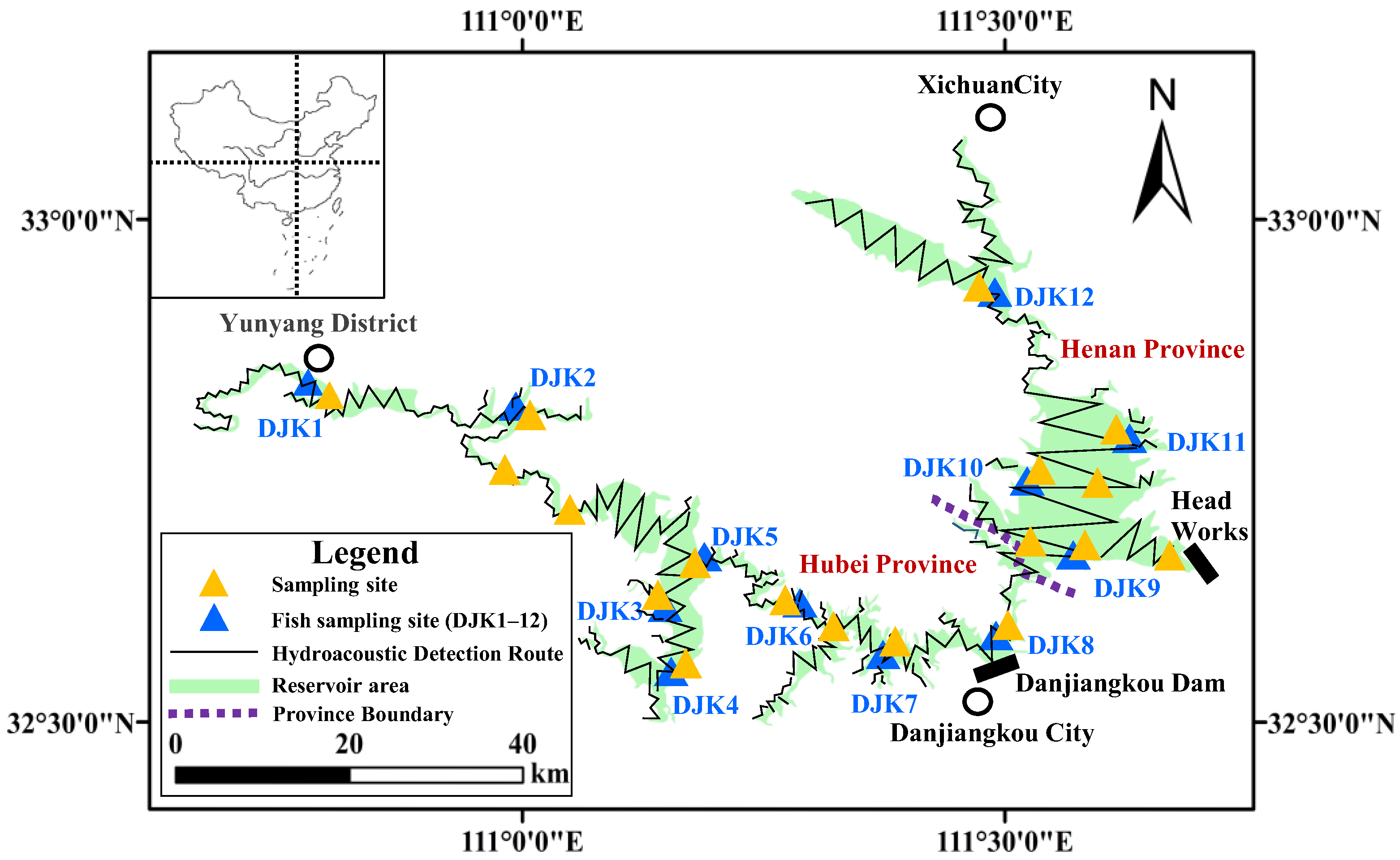
2.1. Study Area and Data Collection
2.2. Model Principles
2.3. Functional Group Definition
2.4. Sources of Basic Model Parameters
- (1)
- Biomass (B)
- (2)
- P/B Coefficient
- (3)
- Q/B Coefficient
- (4)
- Diet Composition Matrix (DC)
2.5. Model Balancing and Optimization
3. Results
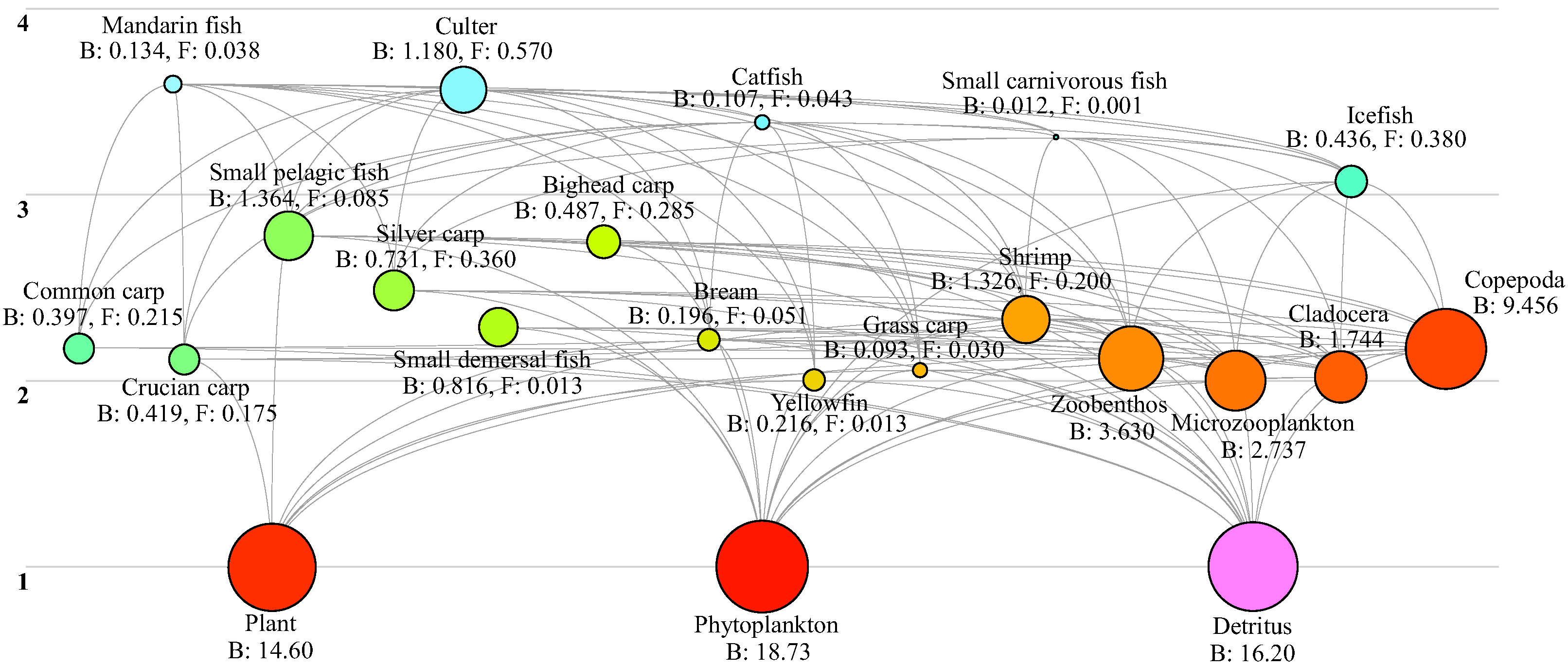
3.1. Food Web Structure
3.1.1. Food Web Composition
3.1.2. Ecotrophic Efficiency
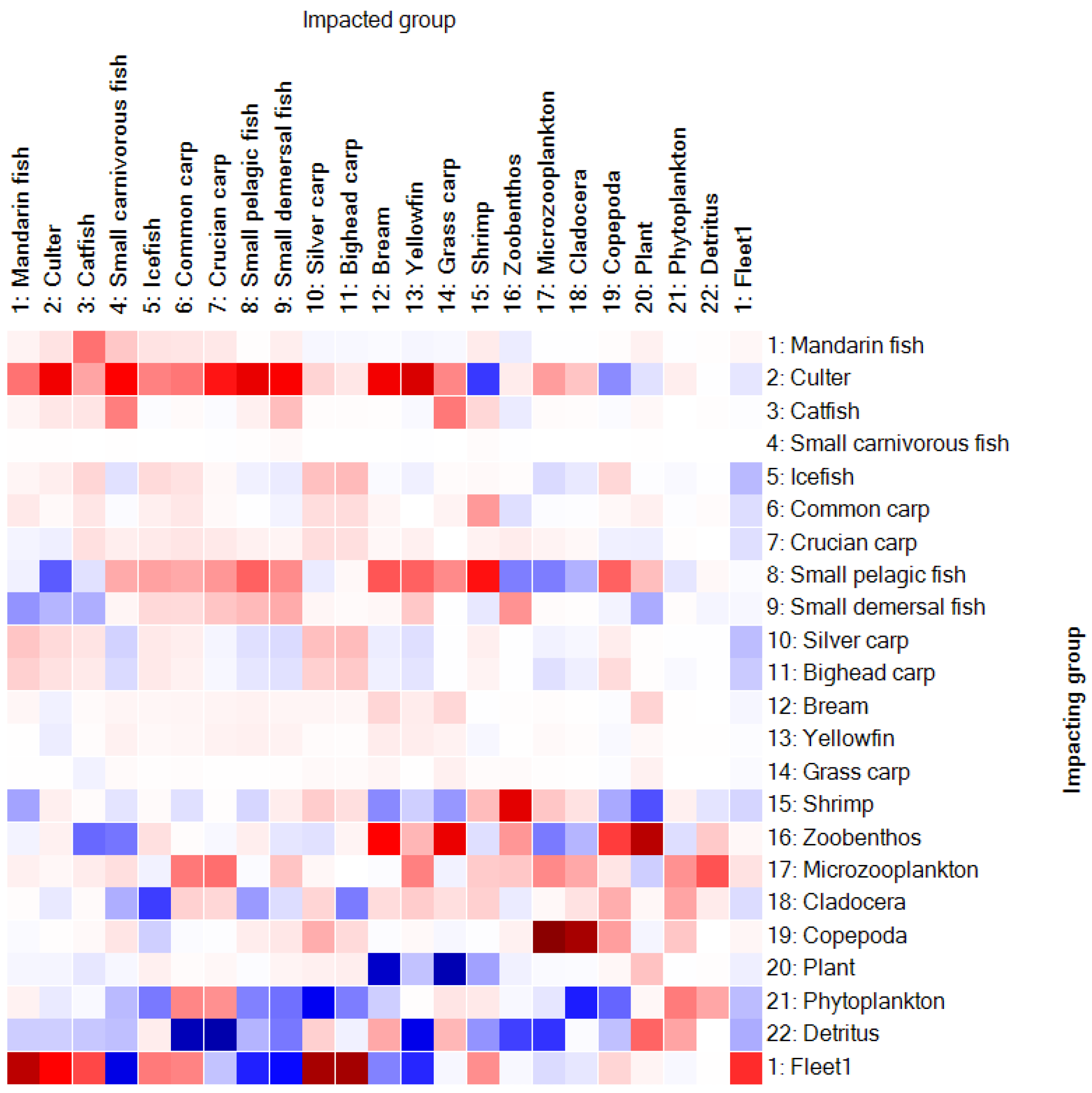
3.1.3. Mixed Trophic Impacts
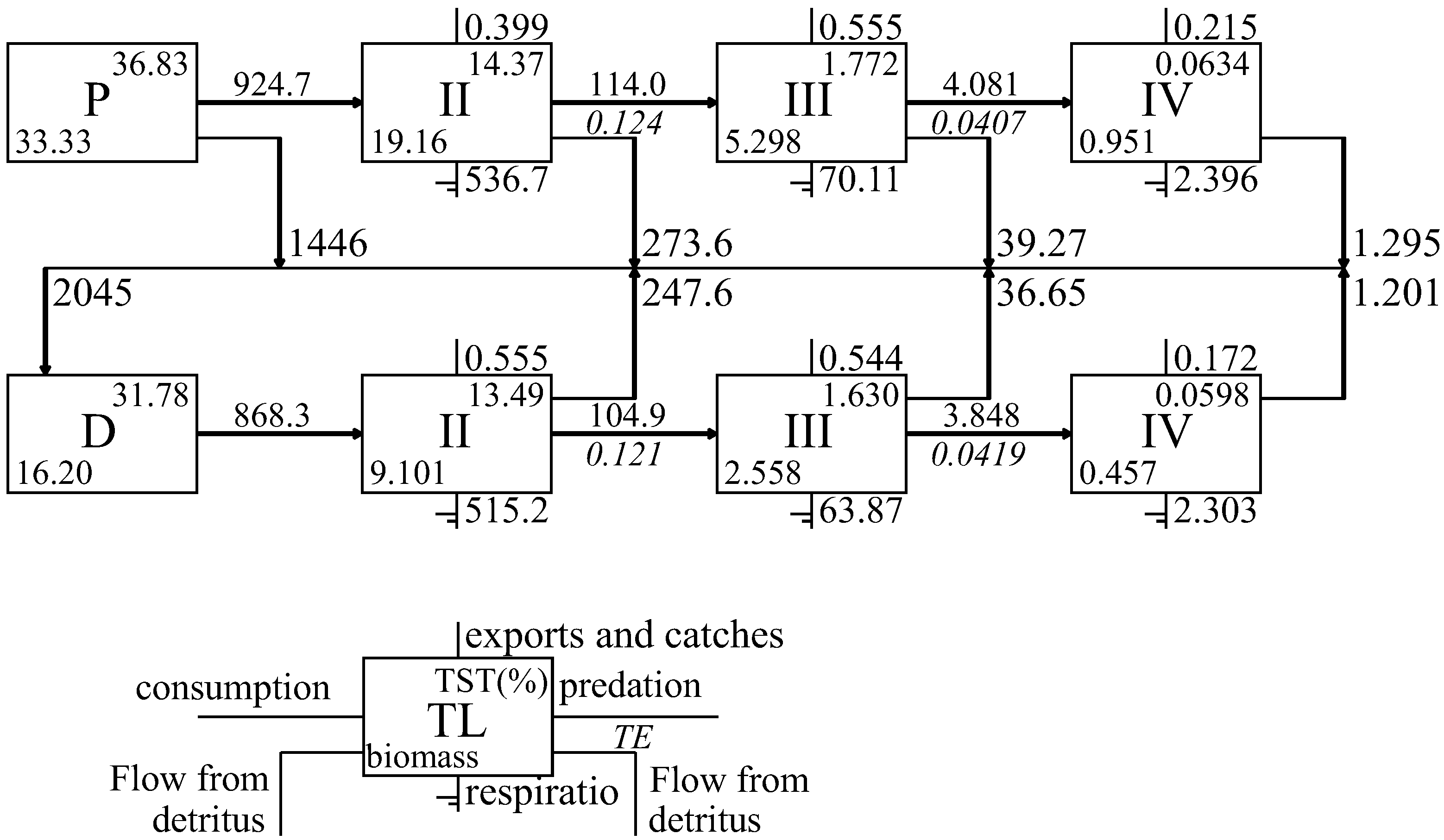
3.2. Characteristics of Energy Flow
3.3. Overall Ecosystem Characteristics
4. Discussion
4.1. Food Web Structure and Ecological Impacts of the Introduced N. taihuensis
4.2. Energy Flow Characteristics
4.3. Ecosystem Assessment
4.4. Management and Conversation Recommendations
- (1)
- Enhance Targeted Removal of N. taihuensis
- (2)
- Control Indigenous Zooplanktivorous Fish Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Y.; Gu, K.D.; Wang, X.Y.; Zhang, J.A.; Duan, J.Y.; Hu, Z.; Liu, Q.G. Food web structure and ecosystem functions of the water source in the middle route of China’s South-to-North Water Diversion Project. Fishes 2024, 9, 202. [Google Scholar] [CrossRef]
- Fu, J.J.; Wang, M.L.; Wang, B.H.; Xie, D.X. Operational management of Danjiangkou hydro-complex and its heightening works. Yangtze River 2015, 46, 14–16. [Google Scholar]
- Yin, W.; Wang, C.; Wang, L.; Xin, X.K.; Liu, G. Spatial and temporal distribution characteristics and influencing factors of total phosphorus in Danjiangkou Reservoir. Yangtze River 2023, 54, 1–7. [Google Scholar]
- Pan, Y.D.; Guo, S.J.; Li, Y.Y.; Yin, W.; Qi, P.C.; Shi, J.W.; Hu, L.Q.; Li, B.; Bi, S.G.; Zhu, J.Y. Effects of water level increase on phytoplankton assemblages in a drinking water reservoir. Water 2018, 10, 256. [Google Scholar] [CrossRef]
- Bai, J.P.; Huang, G.; Jiang, C.J.; Zhang, W.C.; Wang, Q.D.; Yao, L.G. Characteristics and historical changes of the fish assemblage in the Danjiangkou Reservoir. Biodivers. Sci. 2020, 28, 1202–1212. [Google Scholar] [CrossRef]
- Huang, G.; Cao, J.Q.; Heng, W.J.; Lei, H.; Tan, H.W.; Xiong, M.T.; Li, C.G.; Chen, F.; Chen, Z.T.; Han, X.Y.; et al. The characteristics of spatio-temporal distributions of fish resources in the Danjiangkou Reservoir based on hydroacoustic assessment. J. Lake Sci. 2025, 37, 963–975. [Google Scholar] [CrossRef]
- Yang, Z.W.; Li, Z.J.; Liu, J.S.; Zhang, T.L.; Ye, S.W.; Zhang, H. A comparative study on reproductive characteristics of different spawning stocks of the icefish (Neosalanx taihuensis) in the Danjiangkou Reservoir. Freshw. Fish. 2012, 42, 58–62. [Google Scholar]
- Xiong, M.T.; Li, R.J.; Zhang, T.L.; Liao, C.S.; Yu, G.L.; Yuan, J.; Liu, J.S.; Ye, S.W. Zooplankton Compositions in the Danjiangkou Reservoir, a Water Source for the South-to-North Water Diversion Project of China. Water 2022, 14, 3253. [Google Scholar] [CrossRef]
- Xiao, Y.N.; Cheng, J.H.; Mo, X.C.; Li, Y.R.; Liu, X.J.; Bi, S. Spatio-temporal variation of phytoplankton community and its relationship with environmental factor in Danjiangkou Reservoir. J. Lake Sci. 2023, 35, 821–832. [Google Scholar] [CrossRef]
- Chi, S.Y.; Zhao, X.F.; Gao, S.B.; Zhang, A.J.; Hu, J.; Li, S.X.; Hu, J.X.; Dong, F.Y. The spatial distribution pattern of autumn macroinvertebrates in relation to environmental factors in Danjiangkou Reservoir. Acta Ecol. Sin. 2021, 41, 1229–1241. [Google Scholar] [CrossRef]
- Hui, J.; Fang, D.D.; He, H.Z.; Ma, C.; Zhang, Q.Q.; Liu, D.Z. Water ecological health evaluation of Danjiangkou Reservoir based on fish biological integrity index. Henan Shuichan 2024, 4, 32–35+44. [Google Scholar]
- Coll, M.; Akoglu, E.; Arreguín-Sánchez, F.; Fulton, E.A.; Gascuel, D.; Heymans, J.J.; Libralato, S.; Mackinson, S.; Palomera, I.; Piroddi, C.; et al. Modelling dynamic ecosystems: Venturing beyond boundaries with the Ecopath approach. Rev. Fish Biol. Fish. 2015, 25, 413–424. [Google Scholar] [CrossRef]
- Craig, J.K.; Link, J.S. It is past time to use ecosystem models tactically to support ecosystem-based fisheries management: Case studies using Ecopath with Ecosim in an operational management context. Fish Fish. 2023, 24, 381–406. [Google Scholar] [CrossRef]
- Cremona, F.; Järvalt, A.; Bhele, U.; Timm, H.; Seller, S.; Haberman, J.; Zingel, P.; Agasild, H.; Nõges, P.; Nõges, T. Relationships between fisheries, foodweb structure, and detrital pathway in a large shallow lake. Hydrobiologia 2018, 820, 145–163. [Google Scholar] [CrossRef]
- Yao, Y.J.; Mao, Z.G.; Gu, X.H.; Zeng, Q.F.; Chen, H.H.; Wang, Y.Y.; Jeppesen, E. Influence of fishery management on trophic interactions and biomass fluxes in Lake Taihu based on a trophic mass-balance model exercise on a long-term data series. Ecol. Indic. 2024, 158, 111343. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Q.D.; Du, X.; Liu, H.; Feng, K.; Ye, S.W.; Yuan, J.; Liu, J.S.; Li, Z.J.; De Silva, S.S. Modeling trophic interactions and impacts of introduced icefish (Neosalanx taihuensis Chen) in three large reservoirs in the Yangtze River basin, China. Hydrobiologia 2020, 847, 3637–3657. [Google Scholar] [CrossRef]
- Liu, Z.M.; Feng, M.Q.; Yang, R.J.; Liu, G.G. Ecological system characteristics and ecological capacity of Hypophthalmichthys molitrix and Aristichthys nobilis in the Zhangze Reservoir based on Ecopath model. Acta Hydrobiol. Sin. 2024, 48, 1553–1565. [Google Scholar]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Calculation Methods for Carrying Capacity of Stock Enhancement and Aquaculture in Large Water Bodies: SC/T 1149-2020; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Palomares, M.L.; Pauly, D. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature and salinity. Mar. Freshw. Res. 1998, 49, 447–453. [Google Scholar] [CrossRef]
- Tang, J.F.; Xiao, X.Z.; Wang, Y.C.; Hu, S.; Wang, Y. Ecosystem structure and function of the main channel of the middle route of south-to-north water diversion project. China Environ. Sci. 2020, 40, 5391–5402. [Google Scholar]
- Parnell, A. Simmr: A Stable Isotope Mixing Model. 2023. Available online: https://CRAN.R-project.org/package=simmr (accessed on 14 April 2023).
- Wang, K.; Zhu, K.H.; Guo, Y.L.; Hu, J.; Gu, B.H. Reconstruction of consumer dietary sources based on stable isotopes. Acta Hydrobiol. Sin. 2022, 46, 767–777. [Google Scholar]
- Post, D.M. The long and short of food-chain length. Trends Ecol. Evol. 2002, 17, 269–277. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Link, J.S. Adding rigor to ecological network models by evaluating a set of pre-balance diagnostics: A plea for PREBAL. Ecol. Model. 2010, 221, 1580–1591, Erratum in Ecol. Model. 2016, 337, 348–349. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: A User’s Guide; Fisheries Centre, University of British Columbia: Vancouver, BC, Canada, 2005; Available online: https://www.vliz.be/imisdocs/publications/395938.pdf (accessed on 5 November 2025).
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Christensen, V. Ecosystem maturity—Towards quantification. Ecol. Model. 1995, 77, 3–32. [Google Scholar] [CrossRef]
- Zhang, S.F.; Shen, J.Z.; Wan, C.Y.; Ji, F.F.; Hu, L.; Qiu, L.H.; Peng, L.G.; Zhu, W. Effects of Neosalanx taihuensis feeding on zooplankton community structure in Xiaojiang River. J. Hydroecol. 2024, 45, 138–147. [Google Scholar]
- Fang, Y.H.; Wang, W.J.; Wang, C.; Chen, F.; Zheng, H.T.; Zhang, Q.; Jian, D. Investigation and Analysis of Plankton Community in the Danjiangkou Reservoir in 2019. In Proceedings of the 2019 Academic Annual Conference of the Chinese Hydraulic Engineering Society (Part V), Yichang, China, 22–24 October 2019; China Water & Power Press: Beijing, China, 2019; pp. 14–22. [Google Scholar]
- Wang, L.; Chen, J.; Su, H.J.; Ma, X.F.; Wu, Z.X.; Shen, H.; Yu, J.; Wu, Y.; Ding, G.Y.; Xie, P. Is zooplankton body size an indicator of water quality in (sub)tropical reservoirs in China? Ecosystems 2022, 25, 308–319. [Google Scholar] [CrossRef]
- Havens, K.E.; Beaver, J.R.; Manis, E.E.; East, T.L. Inter-lake comparisons indicate that fish predation, rather than high temperature, is the major driver of summer decline in Daphnia and other changes among cladoceran zooplankton in subtropical Florida lakes. Hydrobiologia 2015, 750, 57–67. [Google Scholar] [CrossRef]
- Shurin, J.B.; Gruner, D.S.; Hillebrand, H. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proc. R. Soc. B 2006, 273, 1–9. [Google Scholar] [CrossRef]
- Cui, W.H.; Xu, B.Q.; Dong, X.Q.; Yang, J.S.; Li, M.; Zhang, D.P.; Li, S.F.; Lv, Z.B.; Li, F.; Ren, Z.H. Comparative study of the characteristics of the energy flow and food web structure in the Laizhou Bay ecosystem based on the Ecopath and LIM-MCMC models. Front. Mar. Sci. 2025, 12, 1572355. [Google Scholar] [CrossRef]
- Lindeman, R.L. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Ulanowicz, R.E. Growth and Development: Ecosystem Phenomenology; Springer: New York, NY, USA, 1986; pp. 104–110. [Google Scholar]
- Liu, M.; Shu, M.X.; Lian, Q.P.; Guo, A.H.; Zhou, D.; Zou, S.B.; Yuan, J.L.; Chen, G.M. Assessment of ecosystem characteristics and fishery carbon sink potential of Qianxiahu Reservoir based on trophic level and carbon content methods. Fishes 2024, 9, 438. [Google Scholar] [CrossRef]
- Qiu, L.H.; Qiu, Y.H.; Peng, L.G.; Shen, J.Z.; Li, G.Y.; Li, J.W. Enhancing fishery management in Tanghe Reservoir, China: Insights from food web structure and ecosystem analysis. Water 2024, 16, 200. [Google Scholar] [CrossRef]
- Yu, J.; Liu, J.R.; Wang, L.; Wu, Z.X.; Yu, Z.M.; Liu, M.L.; Han, Y.C.; Xie, P. Analysis on the ecosystem structure and function of Lake Qiandao based on Ecopath model. Acta Hydrobiol. Sin. 2021, 45, 308–317. [Google Scholar]
- Nielsen, C.O.; Odum, E.P.; Odum, H.T. Fundamentals of ecology. Ecology 1960, 41, 400–401. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.S.; Zhang, G.; Liu, Z.J.; Liu, M.; Xie, S.G. Chemical compositions and energy densities of female Neosalanx taihuensis with ovaries at different developmental stages. Acta Hydrobiol. Sin. 2013, 37, 444–449. [Google Scholar]
- Chara-Serna, A.; Maxson, K.A.; Anderson, A.M.; Zalay, B.D.; Casper, A.F. Zooplankton as an Indicator of the Ecosystem Response to Bigheaded Carp Suppression via Commercial Harvest in the Illinois River (2010–2017); Illinois Natural History Survey Technical Report 2018 (37); Illinois Natural History Survey: Champaign, IL, USA, 2019. [Google Scholar]
| NO. | Functional Group | Dominant Species Composition |
|---|---|---|
| 1 | Mandarin fish | Siniperca chuatsi (91.30%), Siniperca kneri (8.70%) |
| 2 | Culter | Cultrichthys erythropterus (5.42%), Culter alburnus (10.15%), Culter mongolicus (75.80%), Culter oxycephaloides (8.62%) |
| 3 | Catfish | Pelteobagrus fulvidraco (31.70%), Pelteobagrus vachelli (35.59%), Pelteobaggrus nitidus (32.71%) |
| 4 | Small carnivorous fish | Opsariichthys bidens (17.98%), Rhinogobius giurinus (82.02%) |
| 5 | Icefish | Neosalanx taihuensis |
| 6 | Common carp | Cyprinus carpio (99.29%), Cyprinus carpio var. specularis (0.71%) |
| 7 | Crucian carp | Carassius auratus |
| 8 | Small pelagic fish | Hemiculter leucisculus (74.77%), Hemiculter bleekeri (13.62%), Pseudolaubuca sinensis (11.61%) |
| 9 | Small demersal fish | Pseudobrama simoni (41.65%), Squalidus argentatus (23.63%), Saurogobio dabryi (13.70%), Acheilognathus macropterus (21.02%) |
| 10 | Silver carp | Hypophthalmichthys molitrix |
| 11 | Bighead carp | Aristichthys nobilis |
| 12 | Bream | Parabramis pekinensis (32.41%), Megalobrama skolkovii (10.50%), Megalobrama amblycephala (57.09%) |
| 13 | Yellowfin | Xenocypris microlepis (86.72%), Xenocypris davidi (9.66%), Distoechodon tumirostris (3.62%) |
| 14 | Grass carp | Ctenopharyngodon idellus |
| 15 | Shrimp | Macrobrachium nipponense |
| 16 | Zoobenthos | Zoobenthos |
| 17 | Microzooplankton | Microzooplankton |
| 18 | Cladocera | Cladocera |
| 19 | Copepoda | Copepoda |
| 20 | Plant | Terrestrial plants, aquatic plants |
| 21 | Phytoplankton | Phytoplankton |
| 22 | Detritus | Suspended particulate organic matter, surface sediment, humus |
| NO. | Functional Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mandarin fish | |||||||||||||||||||
| 2 | Culter | 0.02 | ||||||||||||||||||
| 3 | Catfish | 0.03 | ||||||||||||||||||
| 4 | Small carnivorous fish | 0.002 | 0.001 | 0.002 | ||||||||||||||||
| 5 | Icefish | 0.091 | 0.062 | 0.010 | 0.010 | |||||||||||||||
| 6 | Common carp | 0.052 | 0.039 | 0.010 | ||||||||||||||||
| 7 | Crucian carp | 0.085 | 0.093 | 0.016 | ||||||||||||||||
| 8 | Small pelagic fish | 0.212 | 0.458 | 0.181 | 0.120 | |||||||||||||||
| 9 | Small demersal fish | 0.265 | 0.223 | 0.257 | 0.180 | |||||||||||||||
| 10 | Silver carp | |||||||||||||||||||
| 11 | Bighead carp | |||||||||||||||||||
| 12 | Bream | 0.012 | 0.055 | 0.011 | ||||||||||||||||
| 13 | Xenocypridines | 0.013 | 0.052 | 0.008 | ||||||||||||||||
| 14 | Grass carp | 0.008 | 0.009 | 0.030 | ||||||||||||||||
| 15 | Shrimp | 0.210 | 0.005 | 0.209 | 0.190 | 0.080 | 0.015 | 0.040 | ||||||||||||
| 16 | Zoobenthos | 0.003 | 0.266 | 0.280 | 0.020 | 0.062 | 0.087 | 0.020 | 0.100 | 0.050 | 0.220 | |||||||||
| 17 | Microzooplankton | 0.010 | 0.080 | 0.120 | 0.060 | 0.110 | 0.090 | 0.010 | 0.020 | 0.025 | 0.020 | 0.120 | ||||||||
| 18 | Cladocera | 0.130 | 0.410 | 0.250 | 0.140 | 0.090 | 0.327 | 0.020 | 0.030 | 0.050 | 0.050 | |||||||||
| 19 | Copepoda | 0.080 | 0.470 | 0.280 | 0.145 | 0.070 | 0.273 | 0.030 | 0.040 | 0.040 | ||||||||||
| 20 | Submerged plant | 0.050 | 0.050 | 0.018 | 0.635 | 0.120 | 0.720 | 0.160 | 0.120 | |||||||||||
| 21 | Phytoplankton | 0.020 | 0.110 | 0.245 | 0.610 | 0.170 | 0.160 | 0.160 | 0.050 | 0.100 | 0.120 | 0.425 | 0.710 | 0.470 | ||||||
| 22 | Detritus | 0.853 | 0.893 | 0.162 | 0.310 | 0.120 | 0.140 | 0.145 | 0.720 | 0.180 | 0.430 | 0.645 | 0.575 | 0.270 | 0.360 | |||||
| Sum | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Functional Group | Trophic Level | Biomass (t km−2) | P/B (year−1) | Q/B (year−1) | EE |
|---|---|---|---|---|---|
| Mandarin fish | 3.59 | 0.134 | 0.78 | 3.72 | 0.35 |
| Culter | 3.56 | 1.18 | 1.26 | 3.49 | 0.41 |
| Catfish | 3.39 | 0.107 | 1.12 | 6.10 | 0.57 |
| Small carnivorous fish | 3.31 | 0.012 | 1.24 | 5.87 | 0.43 |
| Icefish | 3.07 | 0.436 | 2.37 | 7.25 | 0.66 |
| Common carp | 2.18 | 0.397 | 2.02 | 8.50 | 0.51 |
| Crucian carp | 2.12 | 0.419 | 2.11 | 8.78 | 0.63 |
| Small pelagic fish | 2.78 | 1.364 | 2.21 | 16.10 | 0.73 |
| Small demersal fish | 2.48 | 0.816 | 2.13 | 15.50 | 0.69 |
| Silver carp | 2.28 | 0.731 | 1.5 | 7.52 | 0.43 |
| Bighead carp | 2.74 | 0.487 | 1.4 | 8.93 | 0.48 |
| Bream | 2.07 | 0.196 | 1.68 | 17.13 | 0.66 |
| Xenocypridines | 2.00 | 0.216 | 1.71 | 16.25 | 0.45 |
| Grass carp | 2.06 | 0.093 | 1.65 | 12.20 | 0.58 |
| Shrimp | 2.34 | 1.326 | 2.50 | 12.56 | 0.51 |
| Zoobenthos | 2.12 | 3.63 | 4.00 | 50.00 | 0.43 |
| Microzooplankton | 2.00 | 2.737 | 50.00 | 200.00 | 0.94 |
| Cladocera | 2.02 | 1.744 | 40.00 | 150.00 | 0.96 |
| Copepoda | 2.17 | 9.456 | 20.00 | 100.00 | 0.16 |
| Submerged plant | 1.00 | 15.60 | 2.00 | - | 0.89 |
| Phytoplankton | 1 | 18.73 | 125 | - | 0.38 |
| Detritus | 1 | 16.2 | - | - | 0.42 |
| Parameters | Units | Value |
|---|---|---|
| Total system throughput (TST) | t km−2 year−1 | 6439.88 |
| Total consumption (TC) | t km−2 year−1 | 2020.173 |
| Total exports (TE) | t km−2 year−1 | 1181.705 |
| Total respiration (TR) | t km−2 year−1 | 1190.745 |
| Sum of all flows into detritus (TD) | t km−2 year−1 | 2047.257 |
| Total production (TP) | t km−2 year−1 | 2797.843 |
| Total net primary production (TPP) | t km−2 year−1 | 2372.45 |
| Total biomass (excluding detritus) (TB) | t km−2 | 59.81 |
| Mean trophic level of the catch (MTLC) | 2.79 | |
| TPP/TR | 1.99 | |
| Connectance index (CI) | 0.259 | |
| System omnivory index (SOI) | 0.127 |
| Parameters | Units | DJKR (2022~2023) | ZZR (2023~2023) [17] | QXHR (2022) [39] | THR (2021~2022) [40] | SBYR (2016~2017) [16] | GHYR (2016~2017) [16] | GBZR (2016~2017) [16] | QDHR (2016) [41] |
|---|---|---|---|---|---|---|---|---|---|
| Total system throughput (TST) | t km−2 year−1 | 6439.88 | 53,531.34 | 13,232.99 | 21,350.240 | 8498.40 | 11,101.11 | 7137.50 | 24,698.27 |
| Total consumption (TC) | t km−2 year−1 | 2020.173 | 15,839.9 | 4493.812 | 6820.278 | 3525.70 | 4549.58 | 1317.84 | 5047.78 |
| Total exports (TE)) | t km−2 year−1 | 1181.705 | 13,933.94 | 2485.766 | 5364.935 | 502.15 | 817.79 | 2184.19 | 8453.85 |
| Total respiration (TR) | t km−2 year−1 | 1190.745 | 4645.56 | 2958.194 | 1984.388 | 2608.87 | 3352.30 | 972.05 | 1536.95 |
| Sum of all flows into detritus (TD) | t km−2 year−1 | 2047.257 | 19,111.94 | 3295.221 | 7180.641 | 1861.67 | 2381.35 | 2663.42 | 9659.69 |
| Total production (TP) | t km−2 year−1 | 2797.843 | 19,770.48 | 5192.688 | 7719.199 | 3322.71 | 4457.44 | 3238.47 | 10,243.53 |
| TPP/TR | 1.99 | 4.00 | 1.54 | 3.70 | 1.19 | 1.24 | 3.25 | 6.51 | |
| Connectance index (CI) | 0.259 | 0.267 | 0.270 | 0.299 | 0.256 | 0.234 | 0.236 | 0.263 | |
| System omnivory index (SOI) | 0.127 | 0.149 | 0.200 | 0.145 | 0.112 | 0.089 | 0.102 | 0.131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Yuan, T.; Lei, H.; Guo, C.; Chen, Z.; Xiong, M.; Li, C.; Chen, W.; Zhang, L.; Wang, Y.; et al. Modeling the Water Source Ecosystem in the Middle Route of the South-to-North Water Diversion Project: Implications for Management and Conservation. Fishes 2025, 10, 576. https://doi.org/10.3390/fishes10110576
Huang G, Yuan T, Lei H, Guo C, Chen Z, Xiong M, Li C, Chen W, Zhang L, Wang Y, et al. Modeling the Water Source Ecosystem in the Middle Route of the South-to-North Water Diversion Project: Implications for Management and Conservation. Fishes. 2025; 10(11):576. https://doi.org/10.3390/fishes10110576
Chicago/Turabian StyleHuang, Geng, Ting Yuan, Huan Lei, Chao Guo, Zetao Chen, Mantang Xiong, Chenguang Li, Wei Chen, Lequn Zhang, Yuqi Wang, and et al. 2025. "Modeling the Water Source Ecosystem in the Middle Route of the South-to-North Water Diversion Project: Implications for Management and Conservation" Fishes 10, no. 11: 576. https://doi.org/10.3390/fishes10110576
APA StyleHuang, G., Yuan, T., Lei, H., Guo, C., Chen, Z., Xiong, M., Li, C., Chen, W., Zhang, L., Wang, Y., & Chen, F. (2025). Modeling the Water Source Ecosystem in the Middle Route of the South-to-North Water Diversion Project: Implications for Management and Conservation. Fishes, 10(11), 576. https://doi.org/10.3390/fishes10110576






