Abstract
Sucralose stands as the most common non-nutritive sweetener; however, its metabolic effects have sparked significant controversy over the years. We aim to examine the effects of sucralose daily intake on glycemia, subjective appetite, and gut microbiota (GM) changes in subjects with overweight or obesity. In this randomized, crossover, and controlled trial, 23 participants with a body mass index between 25 kg/m2 and 39.9 kg/m2 will be assigned to one of two interventions to receive either sucralose (2 mg/kg/day equivalent to 40% of the acceptable daily intake) or glucose (control) for 4 weeks, each phase separated by a 4-week washout period. The glycemic response will be determined during a meal tolerance test, subjective appetite will be evaluated using a visual analog scale, and GM changes will be analyzed by next-generation sequencing of the bacterial rRNA 16S gene from fecal samples. All measures will be performed before and after intervention periods. We hypothesize that sucralose supplementation induces changes in glycemic response, subjective appetite, and gut microbiota in overweight and obese participants. This protocol was approved by the Ethics Committee of the UJAT (No. 0721) and was registered in the Australian New Zealand Clinical Trials Registry (ACTRN12621001531808).
1. Introduction
Global obesity and overweight rates have risen sharply, becoming a major health concern due to their association with type 2 diabetes (T2D) and cardiovascular disease. Mexico ranks second in obesity prevalence among the nations belonging to the Organization for Economic Cooperation and Development (OECD), with 36.9% of its population affected [1]. Non-nutritive sweeteners (NNS) have been implemented as substitutes for sugar to reduce caloric intake and prevent body weight gain. NNS can be artificially produced as sucralose, aspartame, acesulfame K, or saccharin or can be obtained from natural sources, such as steviol glycosides or monk fruit [2].
Sucralose is an artificial NNS derived from sucrose, which has a sweetening capacity 600 times greater than sucrose and has an acceptable daily intake (ADI) estimated value of 5 mg/kg/day according to the Food and Drug Administration (FDA) [3]. The use of sucralose is widely recommended among individuals with obesity and T2D. However, experimental and clinical trials have reported undesirable effects on glucose and energy metabolism, which can be attributed to three potential mechanisms: activation of T1R2/T1R3 sweet taste receptors, affecting insulin and incretin release; triggering a cephalic response that may increase caloric intake; and interaction with gut microbiota (GM), potentially causing dysbiosis. In the latter case, sucralose may disrupt GM composition, increasing intestinal permeability and allowing lipopolysaccharides (LPS) to enter the bloodstream, leading to a low-grade inflammatory response (metabolic endotoxemia) that could induce insulin resistance [4].
In humans, the effects of realistic doses of sucralose on glucose metabolism have been mostly assessed in healthy subjects. Suez et al. showed a clear alteration in glycemic response after two weeks of consuming sucralose (1.8 mg/kg BW/day equivalent to 34% ADI, FDA), which was correlated with GM alterations [5]. Romo-Romo et al. reported an increase in insulin resistance estimated by the HOMA-IR index after only two weeks of sucralose consumption (2.25 mg/kg BW/day equivalent to 45% ADI, FDA) [6]. Recently, Mendez-García et al. (2022) found an altered glycemic response related to changes in GM composition after sucralose intake (0.65 mg/kg BW/day equivalent to 13% ADI, FDA) for 10 weeks [7]. Conversely, other studies in humans do not identify the harmful effects of sucralose on glycemic response or GM [2,8,9]. Thus, it is unclear whether sucralose can induce metabolic alterations modulated by the microbiota modifications.
Particularly, most previous studies examining glycemic response and appetite modifications to sucralose administration have been largely limited to studies in animal models or individuals with normal body weight [10,11,12,13,14,15,16,17]. However, subjects with increased body weight represent an important group due to their increased risk of developing metabolic diseases such as diabetes, hypertension, and heart disease. For this reason, we will conduct a crossover, randomized, and controlled study in subjects with overweight or obesity. The primary outcome of the study will be the glycemic and insulinemic responses, and the secondary outcomes will include pre-post intervention changes in fasting parameters of glucose, insulin, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides. Additionally, pre-post changes in anthropometric measures, blood pressure, GM composition, and subjective appetite will be considered. The knowledge gained in this study about the potential metabolic alterations induced by sucralose will be crucial in guiding recommendations for its use, especially given its widespread presence in the food industry.
2. Experimental Design
2.1. Participants
A total of 23 adults of both sexes will be recruited. During the screening process, subjects will assist with an interview with a healthcare provider to determine anthropometric indexes, blood pressure, fasting glucose determination, and medical check-ups. Participants will fulfill the following inclusion criteria: both genders, aged 18 to 50, overweight or obese (25–39.9 kg/m2), with fasting glucose < 125 mg/dl, and with a maintained stable weight in the previous three months. Patients receiving antibiotics, probiotics, or prebiotics without the ability to give informed consent, pregnant or breastfeeding women, smokers, or alcoholics who have allergies to sucralose or food intolerance, and those with heart, liver, gastrointestinal, or renal disorders will not be included. Patients who have sensitivity or discomfort related to the high sweetness of the test beverage and patients who need to receive treatments with antibiotics, probiotics, or other treatments with drugs that interfere with carbohydrate metabolism or microbiota integrity during the experimental period will be excluded.
2.2. Interventions
A dose of 2 mg/kg/day of commercial sucralose (Golden Hills) will be administered to participants in the sucralose intervention arm for 4 weeks. This dose is equivalent to 40% of the acceptable daily intake (5 mg/kg/day, FDA). Sucralose will be provided to participants in commercial sachets for mixing into their habitual beverages, to be consumed with their three main daily meals. The sucralose dose will be adjusted according to the body weight using an Excel fact sheet based on its concentration in the sachets (2% m/m in glucose). The control group will receive only pure glucose (dextrose), according to the amount of glucose present in the sachets administered in the sucralose arm. The glucose will be weighed and provided to the patients in separate sachets. Adherence will be monitored by personal phone calls and messages via WhatsApp; also, a daily food record format will be given to the participants to provide the number of sachets consumed during the test days. Later, low-NNS intake will remain during the experiment, according to the researcher’s recommendations.
2.3. Meal Tolerance Test
A 2 h MTT will be performed before and after each intervention phase to assess glycemic and insulinemic responses, as well as subjective appetite. Prior to the MTT day, it will be requested that subjects not consume alcohol and do not perform intense exercise during 24 h. On test days, after 12 h fasting, subjects will be appointed to the research center at 7:00 a.m., and they will first complete a 100 mm visual analog scale (VAS) self-reported paper questionnaire (time point 0) to measure subjective appetite [18]. Then, an intravenous catheter will be inserted into the antecubital vein of each subject, and a fasting blood sample will be taken (time point 0) for biochemical determinations. After, subjects will be given a standardized breakfast that consists of a nutritional supplement (Simisure; Modern Strategies; Mexico) and a 22 g Kellogg’s Rice Krispies bar (Kellogg’s Company, Battle Creek, MI, USA). The breakfast’s nutritional distribution will be added up to a total of 210 kcal, 7 g from protein, 38.5 g from carbohydrates, and 3 g from lipids. Blood samples will be collected after this breakfast at 15, 30, 60, 90, and 120 min. Glucose and insulin concentrations will be determined in these blood samples, and the VAS questionnaire will be applied 5 min before each sampling. During the test period, subjects will stay at the research center and will be free to read, use electronic devices, watch television, or engage in conversation.
2.4. Subjective Appetite
To assess appetite sensation, a validated VAS will be administered before and after each intervention phase. VAS consists of 100-mm-long lines. Each line´s endpoints are labeled with words indicating the most intense positive and negative appetite sensations. Hunger, satiety, fullness, and prospective food consumption will be assessed. Questions will be asked as follows: (1) How hungry do you feel? (2) How satisfied do you feel? (3) How full do you feel? (4) How much food do you think you could eat?
2.5. Anthropometric Measurements and Blood Pressure
Body weight and composition will be measured using a body composition analyzer based on bioelectrical impedance (Tanita, TBF-300A, Mexico City, Mexico). Height will be measured using a portable stadiometer (Seca® 214). BMI will be determined using weight and height measurements, and the classification will be made following the World Health Organization (WHO) guidelines: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obesity (30.0 kg/m2). A retractable non-stretching tape measure will be used for waist circumference (WC) and hip circumference (HC) measurements. WC will be measured halfway between the bottom rib and iliac crest, while HC will be taken at the widest part of the buttocks. These measurements will determine the waist-hip ratio. Blood pressure will be measured twice using an Omron HEM-7120 professional portable blood pressure monitor. The two measurements of the blood pressure will be taken within one minute, as recommended in the Clinical Practice Guidelines on the Management of Hypertension [19]. The final reading will be based on the average of two readings recorded. Both the anthropometric and blood pressure measurements will be taken before and after each intervention phase.
2.6. Biochemical Determinations
Blood samples from the antecubital vein will be collected before and after each intervention phase to determine glucose, insulin, triglycerides, TC, and HDL-C. Serum samples that will not be immediately analyzed will be stored at −70 °C until further analysis. Glucose, cholesterol, HDL-C, and triglyceride determinations will be performed using a Spinreact, Spin200E Clinical Chemistry Autoanalyzer. Insulin will be measured by chemiluminescent microparticle immunoassay assay (CMIA) using a Wiener Lab CLIA-900 System, Rosario, Argentine. To estimate insulin resistance (IR), the homeostatic model assessment index (HOMA-IR) will be used. This is calculated by multiplying fasting glucose (mg/dL) and insulin (μU/mL) concentrations, then dividing by 405 [20].
2.7. Gut Microbiota Composition
The fecal samples will be collected for gut microbiota analysis before and after each intervention phase. Microbial DNA extraction from fecal samples will be performed using the QIAamp® Fast DNA Stool Mini Kit (QIAGEN, Hilden, GE, USA) (Cat No. 51604) according to the manufacturer’s instructions. DNA concentrations will be measured with the Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The 16S ribosomal RNA (rRNA) gene will be amplified by polymerase chain reaction (PCR). The PCR products will be visualized using agarose gels.
3. Procedure
3.1. Ethical Aspects
Research will be conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved on 22 April 2021 by the Ethics Committee of the Universidad Juárez Autónoma de Tabasco (UJAT) (No. 0724), and it was registered on 10 April 2021 at the Australian New Zealand Clinical Trials Registry (ACTRN12621001531808). This trial will take place at the División Académica de Ciencias de la Salud (DACS) at UJAT. Informed consent will be obtained from participants by trained research coordinators., who will explain the study’s aims, potential risks, and participants’ right to withdraw from the experiment at any time. Personal information from enrolled participants will be collected and stored confidentially. As part of the informed consent process, participants will be informed that the study protocol includes the collection of blood and fecal samples.
3.2. Trial Design
This study is a crossover, randomized controlled trial (RCT) to determine the effect of daily intake of sucralose on glycemic response, subjective appetite, and GM in subjects with overweight or obesity. Participants will be invited to attend the laboratory for preliminary measurements and baseline data after providing written informed consent. Following this, they will be randomly allocated into one of two treatment groups to receive either sucralose or glucose (control) daily for four weeks, each phase separated by a four-week washout period. An MTT, subjective appetite assessment, and stool collection will be carried out before and after each intervention phase (four measurement points). A flow diagram of the study protocol is shown in Figure 1, and the participant timeline actions are presented in Table 1.
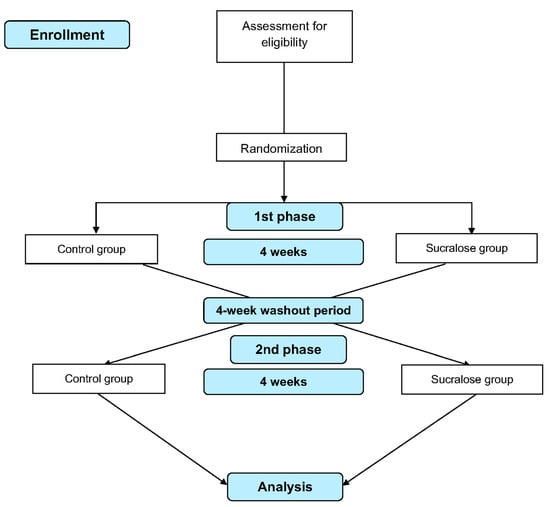
Figure 1.
Flow diagram of the study design.

Table 1.
Schedule of enrollment, interventions, and assessments, according to the SPIRIT 2013 statement. During the intervention periods, all the assessments were performed a day before and after interventions in the respective phases.
3.3. Recruitment
Trial participants who are overweight and obese will be screened and recruited by researchers at the DACS of the UJAT from Villahermosa, Tabasco, Mexico. Moreover, information flyers via social media containing the details of the trial will be posted at respective institutions, hospitals, and other public places. The researchers will enroll patients by the inclusion and exclusion criteria. Patient recruitment started in September 2023. A computer-based online random number generator (www.random.org) will be used to allocate participants to the respective interventions, either sucralose or glucose. Randomized treatments will be supplied in sealed envelopes.
3.4. Safety Assessment
Each participant will be assessed for any adverse events during the clinical study. Sucralose consumption may cause mild discomfort related to its high sweetness power; however, this will depend on the sensitivity of the participant. At the site of the venous puncture, a bruise and/or a sensation of mild pain may appear. Some unexpected side reactions may appear, which vary in each subject, and these may not necessarily be caused by the treatments. In case of discomfort or adverse effects, participants will be treated by a healthcare provider.
3.5. Data Collection and Management
The research staff will interview subjects to gather demographic information and other baseline characteristics of the study group, including NNS consumption frequency, anthropometric measures, and medical history (comorbidities, medications, smoking). All participants will receive medical and nutritional sessions during and following the study. At no time will the participants be identified by the publications or presentations derived from this study, and each of the data provided will be handled with absolute confidentiality. Stool samples will be used exclusively for genetic analysis of the bacteria. The genetic information of the participants will not be studied or analyzed.
3.6. Statistical Analysis
Sample size calculation was performed using NCSS and PASS v. 11.0 software. To detect a difference of 10% in the primary outcome variable (glycemic response AUC) and considering a power of 80% and a type 1 error of 5% in a crossover design, a sample size of 19 participants was calculated. However, to allow for a dropout rate of 20%, 24 participants will be considered. Four samples per participant will be analyzed: two baselines and two endpoints. The primary analysis will be performed using GraphPad Prism v. 7.0 and QIIME Software. The D’Agostino and Pearson tests will be used to determine Gaussian distributions. Differences in fasting biochemical markers will be assessed using a one-way analysis of variance (ANOVA) in combination with Tukey’s post hoc test. Time-course data will be analyzed by two-way repeated measures (RM) ANOVA to assess the effects of treatment, time, and the interaction of treatment and time. The intervention´s effect on GM community dissimilarity will be analyzed with permutational multivariate analysis of variance (PERMANOVA) on the Bray–Curtis distance and visualized with Principal Component Analysis (PCoA). ANOVA, or the Kruskal–Wallis test, will be used to compare alpha diversity using the Shannon index. The Firmicutes/Bacteroidetes ratio (F/B ratio) will be calculated from relative abundances. A p-value < 0.05 will be considered significant for all analyses.
4. Expected Results
The present study will investigate the effects of realistic doses of sucralose (40% ADI) on metabolic markers, appetite, and GM changes in subjects with overweight or obesity. The problem of obesity has spread globally, not just confined to developed countries. Over the past decades, there have been predictions that if the current trend continues, most of the adult population worldwide will be overweight or obese by 2030 [21]. In this context, non-nutritive sweeteners have been implemented to lower calorie consumption and, consequently, mitigate the risk of increased body weight [22]. However, some studies have demonstrated its participation in the development of metabolic alterations.
Some studies have focused on assessing the effects of sucralose on fasting glucose or glycemic and insulinemic responses in animal models [14,23,24,25,26,27] and in healthy or with T1D/T2D subjects [2,6,7,9,13,15,28,29,30,31]. Other studies show evidence of changes in appetite parameters from a single-dose consuming sucralose [10,12,17,32,33,34]. However, the limited evidence evaluating the effects of sucralose consumption on metabolic markers, appetite, and GM in overweight or obese subjects is inconclusive [35,36,37,38]. This study aims to provide crucial information to this population by evaluating some different factors that have been separately studied under varying conditions, mainly in healthy individuals. This planned study has several strengths. First, notably, this is an RCT that will evaluate metabolic markers and appetite associated with GM composition as an explanatory variable in subjects with overweight or obesity. Second, a crossover trial will be used to minimize inter-individual variability since each participant acts as his own control. One advantage of this design is that fewer participants compared to parallel trials are needed to achieve the same level of significance in terms of type I and II errors. Third, this study involves realistic doses of sucralose. Fourth, this is a long-term study, as the interventions will consist of four weeks, separated by a four-week washout period. Overall, the findings of this study will assist health professionals in making more informed recommendations regarding the consumption of commercial sweeteners and food products containing sucralose.
Author Contributions
Conceptualization, J.L.B.-C. and V.O.-H.; methodology, Z.R.-L., M.R.-G., J.D.M., C.G.G.-P. and C.S.A.-V.; software, M.C.M.-L. and C.G.-V.; validation, J.L.B.-C., M.C.M.-L. and C.G.G.-P.; investigation, V.O.-H., Z.R.-L. and J.C.D.-Z.; writing—original draft preparation, Z.R.-L., J.D.M., M.R.-G., I.E.J.-R. and C.S.A.-V.; visualization, J.C.D.-Z., I.E.J.-R. and C.G.-V.; supervision, J.L.B.-C. and M.R.-G.; project administration, J.L.B.-C. All authors have contributed substantially to the protocol in revising it critically for important intellectual content and final approval of the version to be published. All the authors also agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The project was approved by the Ethics Committee of the Universidad Juarez Autónoma de Tabasco (No. 0724) and registered by the Australian New Zealand Clinical Trials Registry (ACTRN12621001531808).
Informed Consent Statement
Written informed consent will be obtained from the participants before participation in the study.
Data Availability Statement
No new data were created or analyzed in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shamah-Levy, T.; Romero-Martínez, M.; Cuevas-Nasu, L.; Méndez Gómez-Humaran, I.; Antonio Avila-Arcos, M.; Rivera-Dommarco, J.A. The Mexican national health and nutrition survey as a basis for public policy planning: Overweight and obesity. Nutrients 2019, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Y.; Friel, J.; Mackay, D. The effects of non-nutritive artificial sweeteners, aspartame and sucralose, on the gut microbiome in healthy adults: Secondary outcomes of a randomized double-blinded crossover clinical trial. Nutrients 2020, 12, 3408. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.A.; Roberts, A.; Nestmann, E.R. Critical review of the current literature on the safety of sucralose. Food Chem. Toxicol. 2017, 106, 324–355. [Google Scholar] [CrossRef] [PubMed]
- Shum, B.; Georgia, S. The effects of non-nutritive sweetener consumption in the pediatric populations: What we know, what we don’t, and what we need to learn. Front Endocrinol. 2021, 12, 625415. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328. [Google Scholar] [CrossRef]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; Brito-Córdova, G.X.; Gómez-Díaz, R.A.; Almeda-Valdes, P. Sucralose decreases insulin sensitivity in healthy subjects: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 485–491. [Google Scholar] [CrossRef]
- Méndez-García, L.A.; Bueno-Hernández, N.; Cid-Soto, M.A.; De León, K.L.; Mendoza-Martínez, V.M.; Espinosa-Flores, A.J.; Carrero-Aguirre, M.; Esquivel-Velázquez, M.; León-Hernández, M.; Viurcos-Sanabria, R. Ten-week sucralose consumption induces gut dysbiosis and altered glucose and insulin levels in healthy young adults. Microorganisms 2022, 10, 434. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Thomson, P.; Santibanez, R.; Aguirre, C.; Galgani, J.E.; Garrido, D.; Garrido, D. Short-term impact of sucralose consumption on the metabolic response and gut microbiome of healthy adults. Br. J. Nutr. 2019, 122, 856–862. [Google Scholar] [CrossRef]
- Brown, A.W.; Brown, M.M.B.; Onken, K.L.; Beitz, D.C. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr. Res. 2011, 31, 882–888. [Google Scholar] [CrossRef]
- Contreras-Chavez, G.G.; Estrada, J.A.; Contreras, I. Changes in appetite regulation-related signaling pathways in the brain of mice supplemented with non-nutritive sweeteners. J. Mol. Neurosci. 2021, 71, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Ford, H.E.; Peters, V.; Martin, N.M.; Sleeth, M.L.; Ghatei, M.A.; Frost, G.S.; Bloom, S.R. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur. J. Clin. Nutr. 2011, 65, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Lertrit, A.; Srimachai, S.; Saetung, S.; Chanprasertyothin, S.; Chailurkit, L.-o.; Areevut, C.; Katekao, P.; Ongphiphadhanakul, B.; Sriphrapradang, C. Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: A randomized, double-blind, placebo-controlled trial. Nutition 2018, 55, 125–130. [Google Scholar] [CrossRef]
- Ramos-García, M.; Ble-Castillo, J.L.; García-Vázquez, C.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; Olvera-Hernández, V.; Genis-Mendoza, A.D.; Córdova-Uscanga, R.; Álvarez-González, C.A.; Díaz-Zagoya, J.C. Effects of non-nutritive sweeteners on energy intake, body weight and postprandial glycemia in healthy and with altered glycemic response rats. Foods 2021, 10, 958. [Google Scholar] [CrossRef]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; López-Carrasco, M.G.; Guillén-Pineda, L.E.; Brito-Córdova, G.X.; Gómez-Díaz, R.A.; Gómez-Pérez, F.J.; Almeda-Valdes, P. Sucralose consumption over 2 weeks in healthy subjects does not modify fasting plasma concentrations of appetite-regulating hormones: A randomized clinical trial. J. Acad. Nutr. Diet 2020, 120, 1295–1304. [Google Scholar] [CrossRef]
- Temizkan, S.; Deyneli, O.; Yasar, M.; Arpa, M.; Gunes, M.; Yazici, D.; Sirikci, O.; Haklar, G.; Imeryuz, N.; Yavuz, D. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur. J. Clin. Nutr. 2015, 69, 162–166. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, B.R.; Bound, M.J.; Checklin, H.L.; Bellon, M.; Little, T.J.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am. J. Clin. Nutr. 2012, 95, 78–83. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Weber, M.A.; Schiffrin, E.L.; White, W.B.; Mann, S.; Lindholm, L.H.; Kenerson, J.G.; Flack, J.M.; Carter, B.L.; Materson, B.J.; Ram, C.V.S. Clinical practice guidelines for the management of hypertension in the community: A statement by the American Society of Hypertension and the International Society of Hypertension. J. Hypertens. 2014, 32, 3–15. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Chapter 1—Worldwide epidemic of obesity. In Obesity and Obstetrics, 2nd ed.; Mahmood, T.A., Arulkumaran, S., Chervenak, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- Yunker, A.G.; Alves, J.M.; Luo, S.; Angelo, B.; DeFendis, A.; Pickering, T.A.; Monterosso, J.R.; Page, K.A. Obesity and sex-related associations with differential effects of sucralose vs sucrose on appetite and reward processing: A randomized crossover trial. JAMA Netw. Open 2021, 4, e2126313. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Tien, C.-H.; Chen, Y.-H.; Garrido, D.; Farzi, A.; Herzog, H.; Fan, H.-Y.; Chen, Y.-C. Effect of sucralose intake on human and mouse/rat gut microbiota composition: A systematic review and meta-analysis. Food Rev. Int. 2023, 40, 1265–1275. [Google Scholar] [CrossRef]
- Pino-Seguel, P.; Moya, O.; Borquez, J.C.; Pino-de la Fuente, F.; Díaz-Castro, F.; Donoso-Barraza, C.; Llanos, M.; Troncoso, R.; Bravo-Sagua, R. Sucralose consumption ameliorates high-fat diet-induced glucose intolerance and liver weight gain in mice. Front. Nutr. 2022, 9, 979624. [Google Scholar] [CrossRef]
- Qian, C.; Qi, Y.; Feng, R.; Yang, M.; Zhang, M.; Liu, W.; Rayner, C.K.; Ma, J. Sucralose can improve glucose tolerance and upregulate expression of sweet taste receptors and glucose transporters in an obese rat model. Eur. J. Nutr. 2021, 60, 1809–1817. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Yang, M.; Qian, C.; Liu, Y.; Qi, Y.; Feng, R.; Yang, M.; Liu, W.; Ma, J. Low doses of sucralose alter fecal microbiota in high-fat diet-induced obese rats. Front. Nutr. 2021, 8, 787055. [Google Scholar] [CrossRef]
- Zheng, Z.; Xiao, Y.; Ma, L.; Lyu, W.; Peng, H.; Wang, X.; Ren, Y.; Li, J. Low dose of sucralose alter gut microbiome in mice. Front. Nutr. 2022, 9, 848392. [Google Scholar] [CrossRef]
- Bueno-Hernández, N.; Esquivel-Velázquez, M.; Alcántara-Suárez, R.; Gómez-Arauz, A.Y.; Espinosa-Flores, A.J.; de León-Barrera, K.L.; Mendoza-Martínez, V.M.; Sánchez Medina, G.A.; León-Hernández, M.; Ruiz-Barranco, A. Chronic sucralose consumption induces elevation of serum insulin in young healthy adults: A randomized, double blind, controlled trial. Nutr. J. 2020, 19, 32. [Google Scholar] [CrossRef]
- Grotz, V.L.; Henry, R.R.; McGill, J.B.; Prince, M.J.; Shamoon, H.; Trout, J.R.; Pi-Sunyer, F.X. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J. Am. Diet. Assoc. 2003, 103, 1607–1612. [Google Scholar] [CrossRef]
- Grotz, V.L.; Pi-Sunyer, X.; Porte, D., Jr.; Roberts, A.; Trout, J.R. A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul. Toxicol. Pharmacol. 2017, 88, 22–33. [Google Scholar] [CrossRef]
- Reyna, N.Y.; Cano, C.; Bermudez, V.J.; Medina, M.T.; Souki, A.J.; Ambard, M.; Nunez, M.; Ferrer, M.A.; Inglett, G.E. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. Am. J. Ther. 2003, 10, 438–443. [Google Scholar] [CrossRef]
- Brown, R.J.; Walter, M.; Rother, K.I. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 2009, 32, 2184–2186. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, L.A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am. J. Physiol. Gastrointest. Liver. Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chang, J.; Checklin, H.L.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br. J. Nutr. 2010, 104, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.A.; Mattes, R.D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am. J. Clin. Nutr. 2019, 109, 1288–1301. [Google Scholar] [CrossRef]
- Nichol, A.D.; Salame, C.; Rother, K.I.; Pepino, M.Y. Effects of sucralose ingestion versus sucralose taste on metabolic responses to an oral glucose tolerance test in participants with normal weight and obesity: A randomized crossover trial. Nutrients 2019, 12, 29. [Google Scholar] [CrossRef]
- Villaño, D.; Masoodi, H.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P. Stevia, sucralose and sucrose added to a maqui-Citrus beverage and their effects on glycemic response in overweight subjects: A randomized clinical trial. Food Sci. Technol. 2021, 144, 111173. [Google Scholar] [CrossRef]
- Zafrilla, P.; Masoodi, H.; Cerdá, B.; García-Viguera, C.; Villaño, D. Biological effects of stevia, sucralose and sucrose in Citrus–maqui juices on overweight subjects. Food Funct. 2021, 12, 8535–8543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).