Abstract
The past several decades have borne witness to several breakthroughs and paradigm shifts within the field of cardiovascular medicine, but one component that has remained constant throughout this time is the need for accurate animal models for the refinement and elaboration of the hypotheses and therapies crucial to our capacity to combat human disease. Numerous sophisticated and high-throughput molecular strategies have emerged, including rational drug design and the multi-omics approaches that allow extensive characterization of the host response to disease states and their prospective resolutions, but these technologies all require grounding within a faithful representation of their clinical context. Over this period, our lab has exhaustively tested, progressively refined, and extensively contributed to cardiovascular discovery on the basis of one such faithful representation. It is the purpose of this paper to review our porcine model of chronic myocardial ischemia using ameroid constriction and the subsequent myriad of physiological and molecular–biological insights it has allowed our lab to attain and describe. We hope that, by depicting our methods and the insight they have yielded clearly and completely—drawing for this purpose on comprehensive videographic illustration—other research teams will be empowered to carry our work forward, drawing on our experience to refine their own investigations into the pathogenesis and eradication of cardiovascular disease.
1. Introduction
Of all pathophysiologic processes, there is none as deadly and none as ubiquitous as heart disease. This is true in the United States, where the most recent statistics collected by the American Heart Association and the Centers for Disease Control indicate that over 20 million Americans are afflicted with coronary heart disease (CHD); that a myocardial infarction occurs every 40 s; and that the age-adjusted death rate of heart disease is both higher than any other case and increasing in recent years [1,2]. Internationally, the data describe a similarly preeminent role for cardiovascular disease, with over 9 million deaths attributed to ischemic heart disease (IHD) recorded in 2021 alone and implicating this as the leading cause of death worldwide by a margin of nearly 2.5 [3]. This has translated in the United States to the performance of approximately 482,000 percutaneous and 202,000 surgical corrective procedures for coronary disease annually. Despite this substantial procedural volume, there are currently a large proportion of patients afflicted with CHD who lack revascularization options; this population has been estimated to reach as high as 1.8 million patients in North America alone [4]. The unparalleled breadth and the severity of the physiologic impact of heart disease constitute mandates to continue in the pursuit of improved and novel therapies [5,6,7,8,9,10,11,12,13,14]. The scientific community has responded robustly to this call, with the National Institutes of Health spending over USD 1 billion annually for the last 14 years on heart-disease-related research [15]. Although this is a small fraction of the total economic burden of heart disease, which has been estimated as lost income annually in the United States alone of USD 203.3 billion [16], it has nevertheless yielded numerous profoundly impactful advances in the prevention, diagnosis, and management of cardiovascular pathology [17].
As this necessary work continues, it is essential that it be conducted in a manner optimally suited to the translatability of significant basic scientific discoveries to the clinical setting; unfortunately, there have been many potential therapies that have failed to realize their preclinical promise in clinical trials [18]. Although the reason for this failure of translation is certainly multifactorial, one prominent cause is the failure of preclinical models to fully recapitulate the nuances of human cardiac anatomy, pathophysiology, and comorbidities. Such models, most commonly murine (e.g., ligation of the left anterior descending coronary artery to produce myocardial infarction), are an indispensable part of cardiovascular research given the technical, financial, and ethical challenges inherent in the use of larger organisms and have undoubtedly produced many significant insights into the fundamental pathobiology of heart disease [19]; still, the translational success that is urgently needed for the millions of patients suffering with these disease processes demands the discovery and utilization of the most faithful models possible.
In the case of IHD, which represents the major contributor to the overall burden of cardiovascular disease, swine are considered to represent the most suitable model organism, with suitability construed as potential for extrapolation to patients and in conformity to a number of criteria defined in the literature:
- Faithful representation of the disease process as it occurs in humans;
- Provision of experimental latitude over the location and chronicity of the induced ischemic insult;
- The induced insult is physiologically measurable and the results are reproducible;
- The model admits of the development of comorbidities usually associated with IHD in humans, such as metabolic syndrome;
- Amenability of the model to post-ischemic interventions, e.g., systemic or intracardiac administration of drugs, nanoparticles, and extracellular vesicles;
- The model is logistically feasible to administer in terms of technical difficulty, time required by the protocol, and cost associated with these considerations [20].
Pig models satisfy these criteria chiefly because of their close anatomic, physiologic, metabolic, and proteomic analogy with human cardiovascular disease. Specifically, the pig heart relative to its total body weight is similar to the proportions seen in humans; ventricular electromechanical function is similar between swine and humans; and, importantly, swine possess similar coronary arterial anatomy and pathophysiology to humans [21,22] but do not have a substantial collateral circulation, permitting the reliable, reproducible induction of ischemia through use of experimental means to occlude porcine coronary arteries. Moreover, swine share with humans the tendency to develop atherosclerotic plaques with age and to develop atherosclerotic lesions in response to dietary modification and hyperglycemia [23]. The logistical advantages provided by swine are also worthy of consideration, as they reach maturity more rapidly than other animal models and breed year-round, facilitating regular availability for scientific purposes [24].
It should also be noted that several additional species have been utilized throughout the years to study IHD, with varying degrees of conformity to the principles articulated above. One such species is the guinea pig, which has been utilized in the study of cardiovascular disease due to similarities in electrophysiological characteristics and cholesterol metabolism with humans [25]. Although this has permitted electrophysiologic insights to be derived from such models [26,27], the robust endogenous collateral coronary network possessed by guinea pigs differs from humans in providing the species with a corresponding bulwark against ischemic disease and thus limits their applicability to the study of chronic myocardial ischemia [28]. Another species utilized in small animal models is the rabbit, the hearts of which possess coronary anatomical and electrophysiological similarity to the hearts of humans, including a paucity of collateral circulation and a corresponding reliability of infarct induction [29]. Given this, as well as the analogous development of atherosclerotic lesions in rabbits to the process as it occurs in humans, it is not surprising that these animals have been used for many decades in cardiovascular research [30,31,32]. These considerations, in conjunction with the increased phylogenetic proximity of rabbits to humans, may warrant consideration of use of this organism as a bridge between small- and large-animal models of IHD [33]. Historically, the large animal of choice was the canine [34,35], and hemodynamic and size similarities to humans support this option, while the rich collateralization of the dog heart in conjunction with ethical objections argue against it [36].
Taken together, these considerations have proven compelling for many research teams invested in translational efforts to ameliorate the impact of CHD and have culminated in the consideration of swine as the most attractive model organism for the study of cardiovascular disease [36]. In the case of our research group, a porcine model of chronic myocardial ischemia has been in use, with elaboration aimed at maximizing experimental efficacy and facility of administration, for nearly three decades. The model over this time has proven to be extraordinarily fruitful, providing insights into a vast network of molecular underpinnings of the myocardial response to ischemic disease that have, in turn, served as the basis for numerous substantial advances in the pharmacologic armamentarium against ischemic insults. These achievements are summarized in tabular form below (Table 1), and references are provided for further elaboration of the state of the experimental model at the time it was used to produce them. It exceeds the scope of this protocol paper to fully delineate the alterations our model has undergone over the course of its development, but readers are invited to review the references included in Table 1 for additional explanation of features that do not appear in the contemporary configuration described below.

Table 1.
Although not comprehensive, this table provides a representative depiction of the insights achieved through use of our model; it also describes the variation of many key experimental components available to investigators aiming to design analogous protocol.
2. Experimental Design
As is readily apparent through the many modulations we have made to our protocol over the past several decades, the endpoints relevant to the study of ischemic cardiac disease may be successfully attained through a multitude of approaches; consider, for instance, the use of ultrasound, cardiac MRI, or pressure–volume loops for the assessment of myocardial functional responses to treatment. In this example, as well as across the other modifiable steps in our protocol, there are virtues and drawbacks to each possibility. With this section, we will present the essential components of our protocol together with rationales for their selection over potential alternatives, with the aim to aid researchers in understanding why the protocol delineated in the subsequent section exists in its current form [52,53,54,55,56]. It is our hope that this discussion will allow other teams to adapt our methods to their unique scientific goals and available resources.
2.1. Establishment of Chronic Myocardial Ischemia
Although our lab has employed a similar model to study acute myocardial infarction in the past, our current focus entails the induction of chronic myocardial ischemia using the surgical placement of an ameroid constrictor device (Research Instruments SW, Escondito, CA, USA). This technique, which has been employed by many investigative teams for over 60 years [57,58,59], generates a condition in the affected myocardial territory known as hibernating myocardium, in which blood flow and function are depressed, but the tissue remains viable and possesses reserve that may manifest with treatment aimed at amelioration of ischemia [60]. In addition to the clinical relevance of simulating the pathophysiology of the human coronary circulation when subjected to gradual atherosclerotic occlusion, this approach also entails the tolerability advantage of sparing a myocardium relatively deficient in epicardial collaterals from the sudden and total cessation of blood flow produced by ligation [61]. Ameroid constrictors consist of two major components: an outer, nondeformable rim of plastic or metal such as stainless steel or titanium and an inner ring composed of hygroscopic casein. When placed around the LCx, this inner casein ring undergoes gradual centripetal swelling, yielding correspondingly gradual coronary occlusion and ischemia to the affected myocardial territory [62]. This process produces reliable, rapid reductions in flow through the device over the first few postoperative days, with progression to complete occlusion occurring over the following four weeks, over which the collateral circulation develops. Placement of the constrictor over the LCx, which is the smallest of the three major coronary arteries in swine, confers the additional advantage of minimizing the extent of ventricular myocardium affected by ischemia to approximately 20%, which may mitigate the extent of sudden cardiac deaths due to dysrhythmia or infarction produced by this model [63,64]. This, in turn, permits survival of experimental animals for a duration sufficient to demonstrate differential degrees of pathophysiologic change produced by treatment. The duration of ischemia utilized in our protocol of 4 weeks is consistent with results obtained in human studies, in which early revascularization (<35 days) produced improved function in the ventricular myocardium, while waiting longer than this failed to do so [65]. Placement should be proximal to the first obtuse marginal branch of the LCx in order to produce a consistent ischemic region of sufficient size for subsequent tissue studies, and ameroid size should be tailored to that of the artery as visualized intraoperatively [61].
There are multiple alternatives to ameroid constriction utilized in the literature, including the production of fixed stenosis using silk ties or the serial placement of flow probes and hydraulic occluders [66,67,68]. Although these methods provide the advantages of precise calibration of the degree of stenosis and a reduction in myocardial blood flow at rest that may be desirable for studies focused on flow modulation, they are hampered both by occasionally high mortality and by increased technical demand [64]. In the case of hydraulic coronary occlusion, dissection of the vessel for 1–2 cm to accommodate serial placement of a flow probe and the occluder is needed, whereupon a cuff placed over the vessel and gradually inflated to the desired degree of stenosis. Similarly, silk tie occlusion involves dissection of an appropriate length of vessel free, whereupon a needle is placed over and parallel to the artery. Silk suture is then used to occlude the artery to the diameter of the needle after the needle is removed. A multitude of needles may be needed for this in order to progressively occlude the artery to achieve flow values consistent with a pre-specified target [66]. Advantages seen with such methods include inherent standardization of rate and degree of occlusion, including the option to modulate stenosis postoperatively in the case of hydraulic occluders if the device is externalized, while disadvantages include technical demand and comparative unfamiliarity in the field [64]. In contrast to the sophisticated equipment needed for and technical complexities of studies such as these, induction of chronic myocardial ischemia by means of an ameroid constrictor as described below is technically straightforward; consistently effective as assessed by measurement of differential myocardial blood flow; productive of a low mortality rate of approximately 20%; and, given its status as the most commonly employed model, optimal for comparison with historical results and across investigative teams [69].
2.2. Analysis of Myocardial Blood Flow Using Isotopic Microspheres
Following the placement of the ameroid constrictor device, confirmatory assessment of the affects thereof on coronary blood flow is necessary to confirm induction of ischemia and thus to validate any changes in the ischemic myocardium produced by treatment provided to animals in the experimental groups. To accomplish this, our group uses isotope-labeled microspheres (BioPhysics Assay Laboratory, Inc., [BioPAL], Worcester, MA, USA). The associated procedure involves the injection of any of ten available solutions of stable isotope microspheres, measuring approximately 15 μm and supplied at a concentration of 2.5 million microspheres per mL. Our lab routinely uses gold-labeled microspheres to map the ischemic territory, lutetium microspheres to determine blood flow at rest, and samarium microspheres for blood flow during rapid pacing; we also maintain a supply of several other isotopes, including europium, lanthanum, and ytterbium, should repetition of the assay become necessary. Briefly, gold microspheres are injected into the left atrial appendage during mechanical occlusion of the LCx with a vessel loop immediately prior to ameroid placement. Because the vessel is occluded, subsequent quantification of microsphere content in the myocardial territory supplied by the LCx will reveal a relative paucity of gold microspheres when compared with territories supplied by the other epicardial coronary arteries. Similarly, when lutetium and samarium are injected into the left atrial appendage during the subsequent harvest surgery, myocardial blood flow can be calculated, provided a constant rate of pump-mediated blood withdrawal (6.67 mL/min using the Harvard Apparatus, Holliston, MA, USA) according to the following equation: myocardial blood flow = (reference blood flow [mL/min]/tissue weight [g]) × (tissue microsphere count/reference blood microsphere count). In other words, the ratio of microsphere concentration in the tissue to the withdrawn blood is used to derive the rate of blood flow to the myocardial tissue sections sent for analysis. Because different isotopes are injected at rest and during rapid pacing and analyzed separately, any change in blood flow produced by these conditions is discovered using this technique. Assessment of blood flow at rest using lutetium reveals any change in myocardial blood flow that may arise due to the administration of treatments. The additional step of using samarium to track changes during rapid pacing is necessary because the collateral circulation that develops over the course of treatment restores blood flow to the occluded region during the weeks over which treatment is administered but is insufficient in the context of exercise-induced stress [70]. This physiologic nuance is clinically significant as it reflects the onset of anginal pain with exercise in patients [71]. Synthesizing these procedures, a total of three isotopes are injected into all experimental animals across the two surgical procedures we perform: gold during the ameroid constrictor surgery and lutetium and samarium during the terminal myocardial harvest surgery. At the end of the latter, the heart is resected and the left ventricle is sectioned into sixteen ways by dividing it first into apical and basal sections at the midpapillary level and then into even circumferential sections. Of these sections, the basal free wall is most likely to have been most affected by ischemia, given that it houses the territory of the proximal LCx. For all animals, we verify this by sending samples from ten sections adjacent to the free wall for gold microsphere quantification. Because microspheres were injected during LCx occlusion, the most ischemic segment will be that in which there is the lowest count of gold microspheres. Relative perfusion to this section in particular, as determined by samarium and lutetium microspheres as above, represents a powerful means of quantifying the collateral augmentation produced by pro-angiogenic therapies in conjunction with immunohistochemical visualization of the vessels themselves [39].
In all cases, isotopic microsphere analysis is achieved using neutron activation, in which isotopes are rendered active following tissue collection and thus emit radiation that can be precisely quantified. Neutron activation entails exposure to a neutron field, precipitating brief gamma emission by microsphere atoms and subsequent spectroscopic analysis with quantification of disintegrations per minute. It does not require destruction of the tissue, raising the possibility of additional subsequent analysis if needed. This technique is highly sensitive, with the capacity to detect even a single microsphere embedded in analyzed tissue. Additionally, it confers the substantial advantage over the previous gold standard of radioactive microspheres of mitigating exposure of lab personnel to the consequent occupational hazards and over the optical technique previously used by our laboratory of obviating additional tissue processing [72,73,74]. Additional details of microsphere use pertaining to our protocol are provided in the subsequent Procedure section of this manuscript.
2.3. Quantifying Functional Changes in the Ischemic Myocardium
In an animal model designed to elucidate strategies for optimizing clinical outcomes in the context of advanced ischemic cardiac disease, there are no endpoints more import than those pertaining to cardiac function. There are a multitude of assessment modalities available for this purpose, including, in decreasing order according to invasiveness, pressure–volume loop recordings, intracoronary flow pressure and flow monitoring, cardiac MRI, and echocardiography [75]. Each of these techniques provides a unique perspective on the myocardial response to disease and therapeutics and may be combined within a single study should augmented analytic breadth be desired. Because of its ease of use and familiarity, echocardiography is often employed for myocardial functional assessment in swine models; geometric limitations imposed by the swine pectoral morphology may be overcome through use of a transesophageal probe [76]. Parameters possible to acquire using this technique include chamber dimensions and volumes as well as derivate assessments of ventricular function, such as ejection fraction and cardiac output. The ubiquity and facility with which echocardiography is performed provide the additional advantage of the iterability of this technique: serial echocardiograms can track changes in myocardial function over time after induction of ischemia and in response to treatment [69,77,78]. The related technique of sonomicrometry, which involves the direct implantation of piezoelectric crystals into the ventricular myocardium, allows for excellent resolution but lacks the portability of standard ultrasonographic analysis [79].
Another technique capable of providing remarkable resolution in myocardial functional analysis in swine is cardiac MRI, which provides the additional advantage of reducing operator dependence in the acquisition of data. MRI images can be acquired conveniently during the same anesthetic event as other surgical procedures and provide details regarding coronary patency, myocardial wall thickness and circumferential strain, blood flow, tissue viability, and ejection fraction. Like echocardiography, cardiac MRI can also be repeated in the interest of acquiring pre-surgical baseline data prior to the induction of ischemia, to which changes attributable to treatment can then be compared [80,81].
Although echocardiography and cardiac MRI clearly possess strong advantages and have been employed by our lab in the past (see Table 1), we no longer use these techniques and have arrived instead in recent years at pressure–volume loop catheter-based transduction of myocardial functional parameters as our preferred analytic method. This is because, while the desirability of longitudinal functional analysis should not be understated, the concurrent acquisition of pressure- and volume-based parameters permits a load-independent assessment of cardiac chamber mechanics that cannot be obtained by other means [82]. Direct left ventricular placement of a pressure–volume loop catheter, as practiced in our lab, currently allows, in conjunction with aortic pressure acquisition by means of a separate catheter placed by femoral arterial cutdown, a comprehensive panel of functional myocardial parameters, including stroke volume, stroke work, ejection fraction, cardiac output, the pressure–volume area and myocardial oxygen consumption, the end-systolic pressure–volume relationship (ESPVR) and contractility, and the end-diastolic pressure–volume relationship (EDPVR) and ventricular compliance. To obtain the ESPVR and EDPVR, modulation of ventricular preload is necessary for the generation of multiple values; this is uniquely possible with invasive pressure–volume loop acquisition and easily achieved in our model through use of a vessel loop to occlude the inferior vena cava (IVC) [83]. Notably, it is also possible to perform this procedure using different cannulation configurations, including using a closed-chest approach involving balloon occlusion of the IVC, ventricular catheterization by way of the femoral artery for pressure transduction, and acquisition of volume measurements with cardiac MRI [84]. Given that our protocol entails myocardial harvest for tissue analysis immediately following pressure–volume loop generation, however, our lab has not integrated these less invasive alternatives into our procedure.
3. Procedure
In this section, we describe in detail the procedural steps comprising the induction and subsequent analysis of our porcine model of chronic myocardial ischemia. The first subsection is devoted to induction through surgical placement of an ameroid constrictor, as discussed above, while the second subsection outlines the second analytic surgical procedure, in which pressure–volume loop acquisition and myocardial harvest are performed. Although the time between these procedures and the age of the swine on which they are performed vary according to the analytic objective of a specific protocol, we typically perform these procedures on juvenile swine (at between 5 and 12 weeks of age), with approximately 6 weeks elapsing between procedures. The use of juvenile animals is necessary to prevent immediate coronary occlusion following ameroid placement, while the interval between procedures allows both for ameroid closure and the effects of any treatment administered to ramify on cardiac functional and tissue parameters subsequently studied. In future experiments, we hope to utilize aged animals, as this may more optimally model the impaired capacity for neovascularization that occurs in the elderly patients most commonly afflicted with ischemic cardiac disease [80,85]. A brief summary of the variety of data generated by the means of this protocol is presented in the subsequent section; specific examples pertaining to protocols we have completed in the past are widely available in printed and digital media, as exemplified in the references provided in Table 1.
3.1. Ameroid Constrictor Placement (1–2 h)
3.1.1. Preoperative Phase
- Swine receive aspirin (10 mg/kg) 1 day preoperatively and continue on it 5 days postoperatively. This increases consistency in ischemic region development [86].
- Anesthesia is induced with intramuscular injections of telazol (4.4 mg/kg) and xylazine (2.2 mg/kg). Buprenorphine (0.03 mg/kg) and a fentanyl patch (4 ug/kg) are given prior to surgery for analgesia.
- Following an aseptic prep, a 20–22-gauge catheter is inserted percutaneously into a large auricular vein. This is connected to a drip with 0.9% saline at a rate of 5–10 mL/kg/h. Excede (ceftiofur, a broad-spectrum cephalosporin antibiotic) is administered as a single dose IM preoperatively for antimicrobial prophylaxis.
- Intubation is performed with a cuffed endotracheal tube to establish an airway for mechanical ventilation. Isoflurane 0.75–3.0% is used for maintenance anesthesia. The pig is prepped and draped in the usual sterile fashion using an iodoform antibiotic solution and sterile cloth drapes.
3.1.2. Intraoperative Phase
- With the animal positioned supine, lidocaine 2% (2–5 mg/kg) is administered intradermally prior to the skin incision.
- A mini left thoracotomy is then performed: a transverse incision is made along the ribs beginning at the left sternal border and extending laterally for approximately 6 cm. This incision is placed just inferiorly to the left axillary fold (Figure 1).
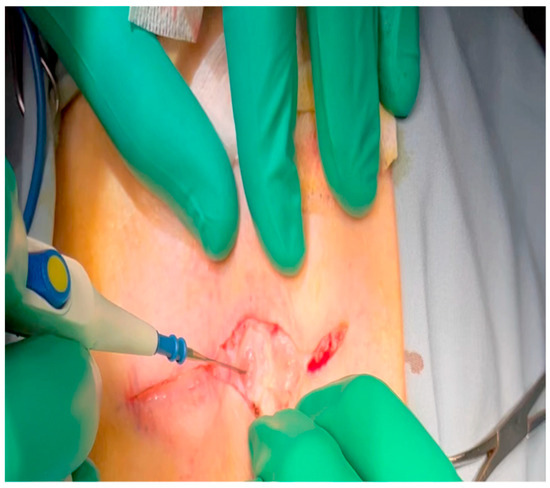 Figure 1. Electrocautery is used to make an incision through the subcutaneous tissue immediately inferior to the axillary crease.
Figure 1. Electrocautery is used to make an incision through the subcutaneous tissue immediately inferior to the axillary crease. - Electrocautery is used to cut through the skin and muscle of the chest wall. A Weitlaner retractor is used to retract the muscle. The intercostal muscles are divided just above the 3rd or 4th rib (Figure 2), and a Kelly clamp facilitates entry into the parietal pleura and protects the underlying intrathoracic structures as the incision is enlarged; finally, a rib spreader is placed to open the incision.
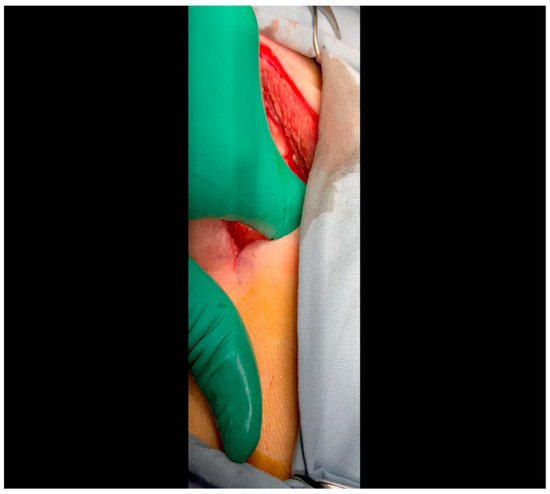 Figure 2. Rib spaces are counted to ensure entry into the thoracic cavity in a manner optimally suited for subsequent visualization of the LCx. This is achieved variably at the 3rd or 4th intercostal space.
Figure 2. Rib spaces are counted to ensure entry into the thoracic cavity in a manner optimally suited for subsequent visualization of the LCx. This is achieved variably at the 3rd or 4th intercostal space. - The pericardium is opened (Figure 3) with scissors. It is important to identify and protect the phrenic nerve.
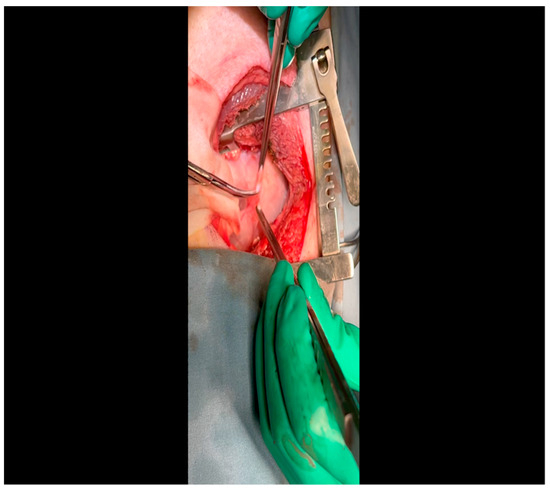 Figure 3. A pericardiotomy is made over the left atrial appendage; the assistant aids in this maneuver by tenting up the pericardial membrane above the underlying epicardial surface.
Figure 3. A pericardiotomy is made over the left atrial appendage; the assistant aids in this maneuver by tenting up the pericardial membrane above the underlying epicardial surface. - The lingula of the left lung is identified and pushed back into the chest cavity if needed to avoid obstruction of the visual field.
- A 3-0 silk suture secures the pericardium to the skin, elevating the heart into the surgical field and retracting the pericardium (Figure 4).
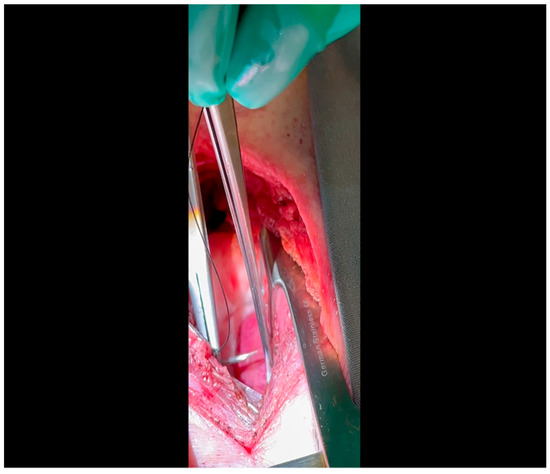 Figure 4. A fine silk stitch is driven first through the pericardium and then through the skin at the margins of the incision. This will elevate the heart into the field to approximate the LCx to the surgeon’s instruments.
Figure 4. A fine silk stitch is driven first through the pericardium and then through the skin at the margins of the incision. This will elevate the heart into the field to approximate the LCx to the surgeon’s instruments. - Twenty mL of blood is withdrawn from the left atrial appendage to be sent for baseline metabolic parameters prior to treatment, as needed. A Satinsky clamp is used to isolate the puncture (Figure 5), a 2-0 silk tie is placed around the clamp to ligate the puncture, and a small hemostat is applied to the tie pull the appendage out of the surgical view.
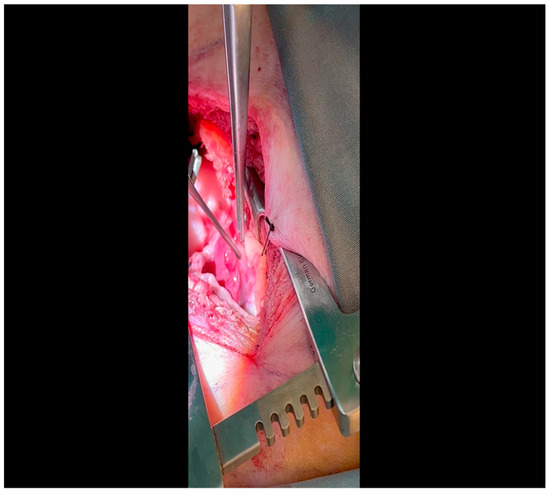 Figure 5. A Satinksy clamp is applied to the left atrial appendage at the site of puncture. A silk tie will be applied around this site, which will then serve the dual purposes of achieving hemostasis and aiding exposure.
Figure 5. A Satinksy clamp is applied to the left atrial appendage at the site of puncture. A silk tie will be applied around this site, which will then serve the dual purposes of achieving hemostasis and aiding exposure. - Using Metzenbaum scissors, a small nick is made in the epicardial fat overlying the left anterior descending/left circumflex artery junction. The left circumflex artery is identified (Figure 6) and dissected free. A silicone vessel loop is placed around the left circumflex artery (Figure 7).
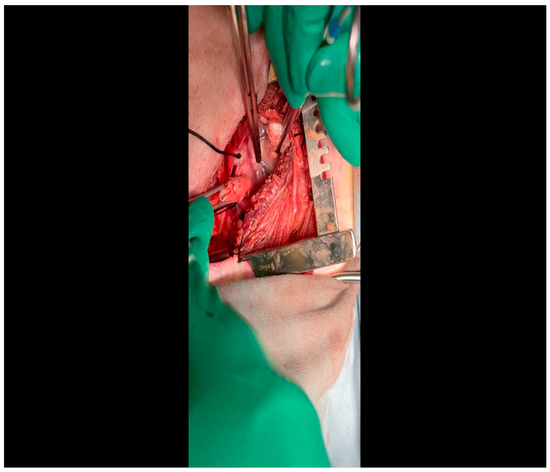 Figure 6. A silk tie retracts the left atrial appendage laterally; the assistant designates the presence of the LCx as it courses at the base of the atrioventricular groove.
Figure 6. A silk tie retracts the left atrial appendage laterally; the assistant designates the presence of the LCx as it courses at the base of the atrioventricular groove.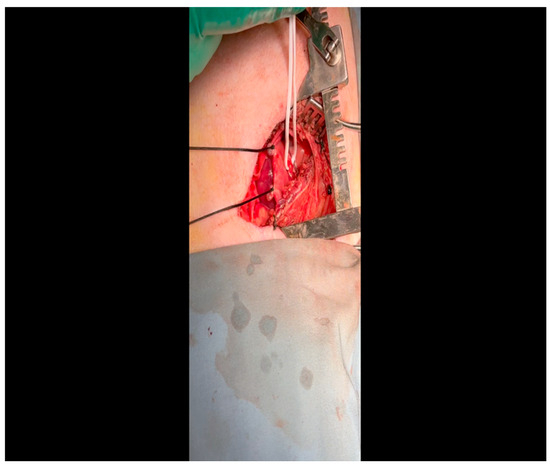 Figure 7. A vessel loop is placed around the LCx; an additional silk tie was applied to the left atrial appendage for optimal exposure.
Figure 7. A vessel loop is placed around the LCx; an additional silk tie was applied to the left atrial appendage for optimal exposure.  CRITICAL STEP. Gentle retractile pressure is applied to the vessel loops to occlude the distal left circumflex coronary artery for 2 min; the EKG is monitored concurrently for ST segment elevations, confirming that the coronary circulation is successfully occluded. During this time, 5 mL of gold microspheres is injected by an assistant into the left atrial appendage. The injection apparatus is constructed as follows: a fine-gauge butterfly needle is affixed to a three-way stopcock, to which two syringes are also connected. One syringe contains the microsphere solution, while the other contains saline. The saline is used to confirm position within the atrial cavity by visualizing blood in the butterfly tubing upon withdrawal and also for flushing the tubing of microspheres after the microsphere syringe has been emptied. Microsphere injection transpires over 30 s and is begun shortly after occlusion of the artery. After 2 min, the pressure on the vessel loops is released. Confirmation of occlusion is critical as this is the means by which the ischemic territory is subsequently mapped, as described above.
CRITICAL STEP. Gentle retractile pressure is applied to the vessel loops to occlude the distal left circumflex coronary artery for 2 min; the EKG is monitored concurrently for ST segment elevations, confirming that the coronary circulation is successfully occluded. During this time, 5 mL of gold microspheres is injected by an assistant into the left atrial appendage. The injection apparatus is constructed as follows: a fine-gauge butterfly needle is affixed to a three-way stopcock, to which two syringes are also connected. One syringe contains the microsphere solution, while the other contains saline. The saline is used to confirm position within the atrial cavity by visualizing blood in the butterfly tubing upon withdrawal and also for flushing the tubing of microspheres after the microsphere syringe has been emptied. Microsphere injection transpires over 30 s and is begun shortly after occlusion of the artery. After 2 min, the pressure on the vessel loops is released. Confirmation of occlusion is critical as this is the means by which the ischemic territory is subsequently mapped, as described above.- When the ST changes return to normal, the ameroid constrictor (1.5–2.5 cm, depending on size of the left circumflex artery, with 2.25 cm constituting the most commonly used device; see Figure 8) can be placed. Prior to attempting placement, it is essential to ensure that an adequate pocket is dissected: the proximal aspect of the vessel must be circumferentially freed from surrounding tissue, and the junction with the left main coronary artery should ideally be visualized. Small bridging veins may require clipping and division, and fine, non-absorbable suture (we use 6-0 polypropylene) should be made available in the event that vascular repair or ligation of venous bleeding is required. The device should be lubricated prior to placement to ease placement onto the artery. We have found that using Allis forceps to grasp the metallic outer casing of the ameroid permits a firm grip despite lubrication. An assistant may additionally use DeBakey forceps to retract surrounding epicardial tissue out from the intended ameroid pocket. Once an adequate pocket is visualized, the vessel loop is removed, and the ameroid is placed onto the artery. Forceps or a finger may be used to stabilize the ameroid while it is being placed, and the keyhole is rotated so that it faces outward (Figure 9). This will permit the device to remain in situ despite the beating of the heart (Figure 10), whereas the heartbeat may dislodge the device if it is facing in the opposite direction. If there is excessive manipulation of the left circumflex artery during this procedure, heparin will be given IV to help prevent thrombus formation. If there is spasm, topical nitroglycerin solution will be applied to the artery directly.
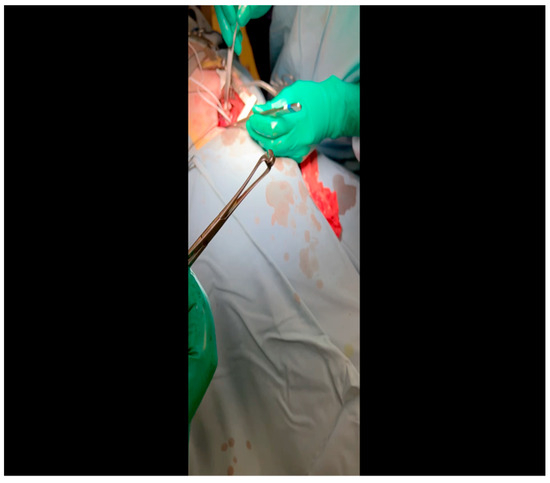 Figure 8. The ameroid constrictor is inspected to ensure that the outer metal and inner casein rings align. Lubricant may be applied to the ring to optimize the ease with which it is applied to the LCx.
Figure 8. The ameroid constrictor is inspected to ensure that the outer metal and inner casein rings align. Lubricant may be applied to the ring to optimize the ease with which it is applied to the LCx.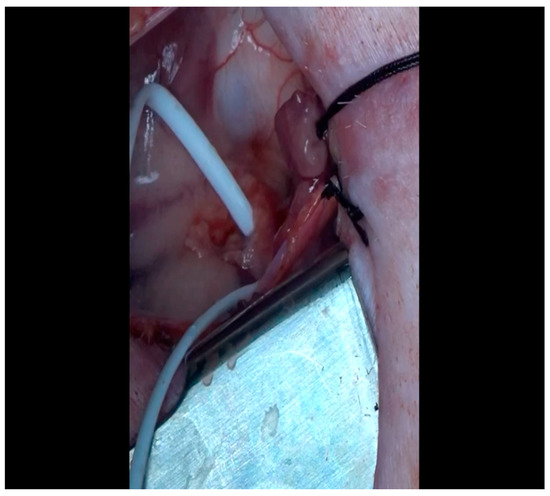 Figure 9. The vessel loop is removed and the lubricated ameroid constrictor is placed using Allis forceps around the vessel. The surgeon’s fingers and/or instruments are used to rotate the ameroid keyhole outward to prevent dislodgment due to the heartbeat.
Figure 9. The vessel loop is removed and the lubricated ameroid constrictor is placed using Allis forceps around the vessel. The surgeon’s fingers and/or instruments are used to rotate the ameroid keyhole outward to prevent dislodgment due to the heartbeat.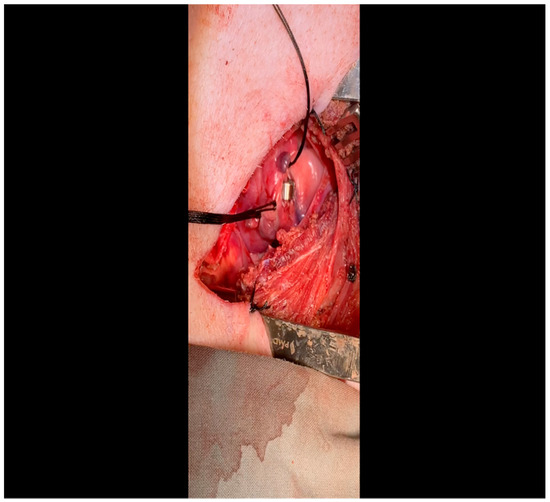 Figure 10. The ameroid constrictor is seen affixed to the LCx. Note that the keyhole is oriented outward as this minimizes the possibility of ameroid slippage prior to closure around the underlying vessel.
Figure 10. The ameroid constrictor is seen affixed to the LCx. Note that the keyhole is oriented outward as this minimizes the possibility of ameroid slippage prior to closure around the underlying vessel.
- 11.
- Prior to closure of the incision site, all aspects of the surgical field are carefully inspected for any bleeding and ligated as needed with suture or titanium hemostatic clips. If any injury to the lung is identified, it is repaired and appropriately tested to ensure no air leakage. These are uncommon but possible consequences of intrathoracic surgical procedures, and repairs are undertaken if any are indicated.
- 12.
- The pericardium is closed with 3-0 Vicryl sutures. The ribs are closed with 0 nylon suture on a blunt-tipped needle. A breath-hold technique should be employed while placing the final rib suture, and a red rubber tube should be placed within the suture line and attached to wall suction during closure to evacuate air. Finally, the muscle and subcutaneous tissue are closed in layers with 3-0 Vicryl and 4-0 Monocryl suture, respectively.
3.1.3. Postoperative Phase
- Isoflurane is weaned, and veterinary staff assist with recovering the animal postoperatively in a padded recovery cage with heat support. The animal is returned to the housing room once stable and ambulatory.
3.2. Perfusion and Pressure–Volume Loop Acquisition, Terminal Myocardial Harvest (1.5–3 h, Figure 11)
3.2.1. Preoperative Phase
- Anesthesia is induced, and the animal is positioned, prepped, and draped in standard sterile fashion in an identical manner to that described above for the previous procedure. As this is not a survival surgery, neither aspirin nor antibiotic prophylaxis are necessary.

Figure 11.
As this procedure has, to the knowledge of the authors, never been previously depicted, it is represented here in its entirety, rather than as isolated vignettes of critical steps as was performed for the ameroid constrictor procedure. Narration accompanies the video throughout to provide concise description of and rationale for all events while they are performed.
3.2.2. Intraoperative Phase
- Femoral arterial catheterization: after intubation and induction of general anesthesia, lidocaine is injected as above for the thoracotomy, but in this case, within the left or right inguinal crease, according to surgeon preference.
- A combination of sharp and blunt dissection using electrocautery and a right-angle clamp is utilized to expose the femoral artery, which will thus be cleared of surrounding tissue and encircled with vessel loops.
- Once the animal is heparinized (see below: the timing of this depends on progress made in the concurrently performed sternotomy by a co-surgeon), the Seldinger technique is used to obtain arterial access: an 18-gauge needle punctures the artery; a fine intravascular wire is placed through the needle; the needle is removed over the wire; and an appropriately sized dilator and sheath are advanced together over the wire to establish durable arterial access.
- Sternotomy, perfusion analysis, and pressure–volume loop acquisition: this procedure is initiated concurrently with femoral arterial catheterization by an additional surgeon. Electrocautery is used to incise the skin down to the bone between the sternal notch and xiphoid process.
- Blunt finger dissection is performed behind the sternum to permit space for a Lebsche knife, which is used to divide the sternum sharply down the midline. Use of a Lebsche knife is preferred over a sternal saw due to the sharp angulation and increased thickness of the superior portion of the porcine sternum; the knife permits readjustment as needed to divide through this region without risking damage to the underlying great vessels [87].
- The pericardium and pleura are dissected from the anterior chest wall with a combination of blunt and sharp dissection.
- A sternal retractor is placed to separate the rib cage and expose the heart.
- The pericardium is opened with Metzenbaum scissors.
- As pacing is needed to simulate a stressed heart and elucidate the physiologic consequences of collateral formation and the functional effects thereof, external pacemaker leads are attached to the left and right atria and tested to ensure capacity to pace the heart to 150 bpm as demonstrated by EKG.
- As IVC occlusion is needed to generate the ESPVR and EDPVR, a Satinsky clamp is used to encircle the intrathoracic IVC with a vessel loop for intermittent occlusion during pressure–volume loop acquisition.
- The animal is then heparinized, and femoral artery cannulation proceeds. The femoral arterial sheath is connected to the Harvard Apparatus withdrawal pump.
- Left atrial access is obtained using a fine-gauge butterfly needle attached to a three-way stopcock as performed for the ameroid constrictor surgery.
- Lutetium-labeled microspheres (5 mL) are then injected into the left atrium over 30 s while simultaneously withdrawing 10 mL of blood from the femoral artery over a period of 1.5 min at a rate of 6.67 mL/minute, as described above. This is performed at rest.
- Subsequently, samarium-labeled microspheres (5 mL) are injected into the left atrium and blood withdrawn from the femoral artery in the same fashion, but in this case during pacing at 150 bpm. Blood from Steps 13 and 14 is sent to BioPaL for perfusion analysis, along with left ventricular tissue samples (see below).
- Left ventricular apical access is obtained using Seldinger technique: a 3-0 polypropylene purse string suture is placed; an 18-gauge needle is positioned in the middle of the purse string; a wire is placed through the aperture and the needle removed; and a dilator and sheath are inserted and then tied in place using the polypropylene suture.
- A pressure–volume transduction catheter (Millar, Houston, TX, USA) will be inserted through this sheath, while another is inserted transfemorally. These catheters will be used to measure volumetric changes in the left ventricle and the pressure in the aorta. This procedure will be performed at resting heart rate, during IVC occlusion, and with pacing to 150 beats/min to yield a comprehensive survey of physiologic parameters for the assessment of treatment effects.
- After acquisition of pressure–volume data, the procedure is complete, and the surgeons proceed with myocardial resection and exsanguination under anesthesia, which is a method of euthanasia that is consistent with humane principles of animal care.
- All extraneous instruments, including electrocautery, suture, needles, hemostats, scissors, cannulation equipment, and pacemaker leads and clamps are removed from the field. The retractor remains in place to provide sufficient berth for myocardial resection.
- The superior vena cava (SVC) is dissected out from the surrounding tissue and the Kelly clamp. The IVC is already exposed and secured with a vessel loop from a previous step. A Satinsky clamp is placed to occlude the IVC, while a Kelly clamp occludes the SVC: this minimizes the effect of exsanguination on surgical site visibility.
- A #10 scalpel is used to remove all myocardial attachments, including the SVC, the IVC, the aorta, the pulmonary arteries, and the pulmonary veins.
- The resected heart is delivered from the thoracic cavity and brought to a separate table for sectioning. A series of sixteen circumferential sections defined in terms of proximity to the left anterior descending coronary artery are generated and snap-frozen in liquid nitrogen. Tissue from all other myocardial chambers, all cardiac valves, the great vessels, and a variety of peripheral organs are also resected and frozen for subsequent analysis.
4. Expected Results
See the references in Table 1 for a thorough overview of the discoveries underwritten by this model. In general, four categories of data are generated: 1. gold microsphere distribution results from samples of each of the 16 left ventricular sections taken to differentiate between ischemic and non-ischemic tissue for subsequent histologic, multi-omic, and other molecular analyses; 2. lutetium and samarium microsphere-mediated assessment of perfusion to all 16 ventricular sections, which can then be differentiated as ischemic or non-ischemic according to the aforementioned gold microsphere distribution results; 3. functional parameters derived from pressure–volume loop acquisition during the terminal myocardial harvest procedure as listed previously; and 4. a variety of molecular, histological, and multi-omic tissue analyses that serve the experimental objectives of the specific protocol [88,89,90,91,92]. As is clear from review of the results collected in Table 1, this model has proven extraordinarily fruitful over the past several decades of its operation in our lab; it is our sincere hope that this manuscript is employed to assist with the establishment of additional large-animal-based research programs designed to reveal the pathobiology of chronic myocardial ischemia. It is only with this foundation that cardiovascular research can continue its history of progress toward the generation of novel therapeutic strategies that will successfully ameliorate the impact of CHD on human morbidity and mortality.
Author Contributions
Conceptualization: C.R.S., D.D.H., M.B., and M.K. Methodology: J.F., M.R.A., and F.W.S.; Writing: C.R.S.; Visualization: C.R.S., D.D.H., M.B., M.K., S.A.S., and C.X.; Supervision: F.W.S. and J.F.; Project administration and funding acquisition: F.W.S., M.R.A., and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH T32HL160517 (C.R.S., D.D.H., M.B); the National Heart, Lung, and Blood Institute (NHLBI) 1F32HL160063-01 (S.S.); T32 GM065085 (C.X); NIH R01HL133624 and R56HL133624-05 (M.R.A.); and NIH R01HL46716 and R01HL128831 (F.W.S.).
Institutional Review Board Statement
Contemporary experiments described in this manuscript were approved by the Institutional Animal Care and Use Committee of the Rhode Island Hospital, and animals were cared for with assistance from the veterinary care team at Rhode Island Hospital. Experiments were designed and carried out in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank the veterinary staff at our institution for the ample and effective support they have provided for our many projects over the years of our operation.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001123 (accessed on 20 November 2023). [CrossRef]
- Mortality in the United States. 2020. Available online: https://stacks.cdc.gov/view/cdc/112079 (accessed on 20 November 2023).
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Povsic, T.J.; Henry, T.D.; Ohman, E.M. Therapeutic Approaches for the No-Option Refractory Angina Patient. Circ. Cardiovasc. Interv. 2021, 14, e009002. [Google Scholar] [CrossRef]
- Aribindi, S.; Jiang, J.; Shaikh, U.; Hidad, A. Cardiac Stem Cell–Derived Treatment for Ischemic Heart Disease: A Review. Georget. Med. Rev. 2023, 7. [Google Scholar] [CrossRef]
- Raftrey, B.; Williams, I.M.; Rios Coronado, P.E.; Fan, X.; Chang, A.H.; Zhao, M.; Roth, R.; Trimm, E.; Racelis, R.; D’Amato, G.; et al. Dach1 Extends Artery Networks and Protects against Cardiac Injury. Circ. Res. 2021, 129, 702–716. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants. 2020, 9, 1292. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Dimitroglou, Y.; Aggeli, C.; Theofilis, P.; Tsioufis, P.; Oikonomou, E.; Chasikidis, C.; Tsioufis, K.; Tousoulis, D. Novel Anti-Inflammatory Therapies in Coronary Artery Disease and Acute Coronary Syndromes. Life 2023, 13, 1669. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Roy, P.; Javadzadegan, A.; Moshfegh, A.; Fearon, W.F.; Ng, M.; Lowe, H.; Brieger, D.; Kritharides, L.; Yong, A.S. Remote Ischemic Preconditioning Acutely Improves Coronary Microcirculatory Function. J. Am. Heart Assoc. 2018, 7, e009058. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Trotta, M.C.; Sasso, F.C.; Sacra, C.; Carpinella, G.; Mauro, C.; Minicucci, F.; Calabrò, P.; Amico, M.D.; Ascenzo, F.D.; et al. SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis. Cardiovasc. Diabetol. 2023, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Massetti, M.; Testa, N.; Di Martino, L.; Castellano, G.; Turriziani, F.; Sasso, F.C.; Torella, M.; De Feo, M.; Santulli, G.; et al. Effects of Sodium-Glucose Transporter 2 Inhibitors (SGLT2-I) in Patients With Ischemic Heart Disease (IHD) Treated by Coronary Artery Bypass Grafting via MiECC: Inflammatory Burden, and Clinical Outcomes at 5 Years of Follow-Up. Front. Pharmacol. 2021, 12, 777083. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Everett, B.M. Novel Antiatherosclerotic Therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 538–545. [Google Scholar] [CrossRef]
- Allen, K.B.; Dowling, R.D.; DelRossi, A.J.; Realyvasques, F.; Lefrak, E.A.; Pfeffer, T.A.; Fudge, T.L.; Mostovych, M.; Schuch, D.; Szentpetery, S.; et al. Transmyocardial laser revascularization combined with coronary artery bypass grafting: A multicenter, blinded, prospective, randomized, controlled trial. J. Thorac. Cardiovasc. Surg. 2000, 119, 540–549. [Google Scholar] [CrossRef]
- RePORT. Available online: https://report.nih.gov/funding/categorical-spending#/ (accessed on 20 December 2023).
- Weintraub, W.S. The Economic Burden of Illness. JAMA Netw. Open 2023, 6, e232663. [Google Scholar] [CrossRef]
- Mehta, N.J.; Khan, I.A. Cardiology’s 10 Greatest Discoveries of the 20th Century. Tex. Heart Inst. J. 2002, 29, 164–171. [Google Scholar]
- Figtree, G.A.; Broadfoot, K.; Casadei, B.; Califf, R.; Crea, F.; Drummond, G.R.; Freedman, J.E.; Guzik, T.J.; Harrison, D.; Hausenloy, D.J.; et al. A Call to Action for New Global Approaches to Cardiovascular Disease Drug Solutions. Circulation 2021, 144, 159–169. [Google Scholar] [CrossRef]
- Jia, T.; Wang, C.; Han, Z.; Wang, X.; Ding, M.; Wang, Q. Experimental Rodent Models of Cardiovascular Diseases. Front. Cardiovasc. Med. 2020, 7, 588075. [Google Scholar] [CrossRef] [PubMed]
- Crisóstomo, V.; Maestre, J.; Maynar, M.; Sun, F.; Báez-Díaz, C.; Usón, J.; Sánchez-Margallo, F.M. Development of a Closed Chest Model of Chronic Myocardial Infarction in Swine: Magnetic Resonance Imaging and Pathological Evaluation. ISRN Cardiol. 2013, 2013, e781762. [Google Scholar] [CrossRef][Green Version]
- Establishing a Swine Model of Post-myocardial Infarction Heart Failure for Stem Cell Treatment. Available online: https://app.jove.com/t/60392/establishing-swine-model-post-myocardial-infarction-heart-failure-for (accessed on 20 November 2023).
- Tsang, H.G.; Rashdan, N.A.; Whitelaw, C.B.A.; Corcoran, B.M.; Summers, K.M.; MacRae, V.E. Large animal models of cardiovascular disease. Cell Biochem. Funct. 2016, 34, 113–132. [Google Scholar] [CrossRef]
- Santos, A.; Fernández-Friera, L.; Villalba, M.; López-Melgar, B.; España, S.; Mateo, J.; Mota, R.A.; Jiménez-Borreguero, J.; Ruiz-Cabello, J. Cardiovascular imaging: What have we learned from animal models? Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Murata, K.; Masumoto, H.; Osada, H.; Murata, K.; Masumoto, H. Large Animal Models in Cardiovascular Research. In Animal Models and Experimental Research in Medicine; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Dasagrandhi, D.; R, A.S.K.; Muthuswamy, A.; Lennox, A.M.; Jayavelu, T.; Devanathan, V.; Swaminathan, J.K. Ischemia/reperfusion injury in male guinea pigs: An efficient model to investigate myocardial damage in cardiovascular complications. Biomed. Pharmacother. 2018, 99, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Mendonca Costa, C.; Anderson, G.C.; Meijborg, V.M.F.; O’shea, C.; Shattock, M.J.; Kirchhof, P.; Coronel, R.; Niederer, S.; Pavlovic, D.; Dhanjal, T.; et al. The Amplitude-Normalized Area of a Bipolar Electrogram as a Measure of Local Conduction Delay in the Heart. Front. Physiol. 2020, 11, 465. [Google Scholar] [CrossRef]
- Lai, Z.F.; Nishi, K. Intracellular chloride activity increases in guinea pig ventricular muscle during simulated ischemia. Am. J. Physiol.-Heart Circ. Physiol. 1998, 275, H1613–H1619. [Google Scholar] [CrossRef] [PubMed]
- Verdouw, P.D.; van den Doel, M.A.; de Zeeuw, S.; Duncker, D.J. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc. Res. 1998, 39, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Genovés, P.; Arias-Mutis, Ó.J.; Parra, G.; Such-Miquel, L.; Zarzoso, M.; Del Canto, I.; Soler, C.; Díaz, A.; Blanch, E.; Alberola, A.; et al. Development and Long-Term Follow-Up of an Experimental Model of Myocardial Infarction in Rabbits. Animals 2020, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Harken, A.H.; Simson, M.B.; Haselgrove, J.; Wetstein, L.; Harden, W.R.; Barlow, C.H. Early ischemia after complete coronary ligation in the rabbit, dog, pig, and monkey. Am. J. Physiol. 1981, 241, H202–H210. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, K.S.; Park, J.L.; Tanhehco, E.J.; Booth, E.A.; Marks, R.M.; Lucchesi, B.R. Attenuation of interleukin-8 expression in C6-deficient rabbits after myocardial ischemia/reperfusion. J. Mol. Cell. Cardiol. 1998, 30, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.; Sayed, A.; Banach, M. Coenzyme Q10 Reduces Infarct Size in Animal Models of Myocardial Ischemia-Reperfusion Injury: A Meta-Analysis and Summary of Underlying Mechanisms. Front. Cardiovasc. Med. 2022, 9, 857364. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, Y.; Yan, H.; Niimi, M.; Wang, Y.; Liang, J. Principles and Applications of Rabbit Models for Atherosclerosis Research. J. Atheroscler. Thromb. 2018, 25, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, W.H.; Mehta, H.B.; Barrieux, A.; Guth, B.D.; Neeley, W.E.; Ross, J. Ischemia of the dog heart induces the appearance of a cardiac mRNA coding for a protein with migration characteristics similar to heat-shock/stress protein 71. Circ. Res. 1986, 59, 110–114. [Google Scholar] [CrossRef]
- Reffelmann, T.; Kloner, R.A. The “no-reflow” phenomenon: Basic science and clinical correlates. Heart 2002, 87, 162–168. [Google Scholar] [CrossRef]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.Q. Large Mammalian Animal Models of Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.D.; Sabe, S.A.; Xu, C.M.; Sabra, M.; Broadwin, M.; Malhotra, A.; Li, J.W.; Abid, M.R.; Sellke, F.W. Sodium-glucose co-transporter 2 inhibitor canagliflozin modulates myocardial metabolism and inflammation in a swine model for chronic myocardial ischemia. Surgery 2024, 175, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Broadwin, M.; Aghagoli, G.; Sabe, S.A.; Harris, D.D.; Wallace, J.; Lawson, J.; Ragayendran, A.; Fedulov, A.V.; Sellke, F.W. Extracellular vesicle treatment partially reverts epigenetic alterations in chronically ischemic porcine myocardium. Vessel. Plus 2023, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, L.A.; Potz, B.A.; Aboul Gheit, A.; Shi, G.; Stanley, M.; Zhang, Z.; Sodha, N.R.; Ahsan, N.; Abid, M.R.; Sellke, F.W. Extracellular Vesicles Promote Arteriogenesis in Chronically Ischemic Myocardium in the Setting of Metabolic Syndrome. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2019, 8, e012617. [Google Scholar] [CrossRef]
- Potz, B.A.; Sabe, A.A.; Elmadhun, N.Y.; Feng, J.; Liu, Y.; Mitchell, H.; Quesenberry, P.; Abid, M.R.; Sellke, F.W. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery 2015, 158, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Elmadhun, N.Y.; Lassaletta, A.D.; Chu, L.M.; Sellke, F.W. Metformin Alters The Insulin Signaling Pathway In Ischemic Cardiac Tissue In A Swine Model Of Metabolic Syndrome. J. Thorac. Cardiovasc. Surg. 2013, 145, 258–266. [Google Scholar] [CrossRef]
- Robich, M.P.; Osipov, R.M.; Nezafat, R.; Feng, J.; Clements, R.T.; Bianchi, C.; Boodhwani, M.; Coady, M.A.; Laham, R.J.; Sellke, F.W.; et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation 2010, 122 (Suppl. 11), S142–S149. [Google Scholar] [CrossRef]
- Boodhwani, M.; Sodha, N.R.; Mieno, S.; Ramlawi, B.; Xu, S.-H.; Feng, J.; Clements, R.T.; Ruel, M.; Sellke, F.W. Insulin treatment enhances the myocardial angiogenic response in diabetes. J. Thorac. Cardiovasc. Surg. 2007, 134, 1453–1460. [Google Scholar] [CrossRef]
- Boodhwani, M.; Nakai, Y.; Mieno, S.; Voisine, P.; Bianchi, C.; Araujo, E.G.; Feng, J.; Michael, K.; Li, J.; Sellke, F.W. Hypercholesterolemia Impairs the Myocardial Angiogenic Response in a Swine Model of Chronic Ischemia: Role of Endostatin and Oxidative Stress. Ann. Thorac. Surg. 2006, 81, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Voisine, P.; Bianchi, C.; Khan, T.A.; Ruel, M.; Xu, S.-H.; Feng, J.; Li, J.; Malik, T.; Rosinberg, A.; Sellke, F.W. Normalization of coronary microvascular reactivity and improvement in myocardial perfusion by surgical vascular endothelial growth factor therapy combined with oral supplementation of l-arginine in a porcine model of endothelial dysfunction. J. Thorac. Cardiovasc. Surg. 2005, 129, 1414–1420. [Google Scholar] [CrossRef][Green Version]
- Ruel, M.A.; Sellke, F.W.; Bianchi, C.; A Khan, T.; Faro, R.; Zhang, J.-P.; E Cohn, W. Endogenous myocardial angiogenesis and revascularization using a gastric submucosal patch. Ann. Thorac. Surg. 2003, 75, 1443–1449. [Google Scholar] [CrossRef]
- Bianchi, C.; Wakiyama, H.; Faro, R.; Khan, T.; McCully, J.D.; Levitsky, S.; Szabó, C.; Sellke, F.W. A novel peroxynitrite decomposer catalyst (FP-15) reduces myocardial infarct size in an in vivo peroxynitrite decomposer and acute ischemia-reperfusion in pigs. Ann. Thorac. Surg. 2002, 74, 1201–1207. [Google Scholar] [CrossRef]
- Sato, K.; Wu, T.; Laham, R.J.; Johnson, R.B.; Douglas, P.; Li, J.; Sellke, F.W.; Bunting, S.; Simons, M.; Post, M.J. Efficacy of intracoronary or intravenous VEGF165in a pig model of chronic myocardial ischemia. J. Am. Coll. Cardiol. 2001, 37, 616–623. [Google Scholar] [CrossRef]
- Harada, K.; Friedman, M.; Lopez, J.J.; Wang, S.Y.; Li, J.; Prasad, P.V.; Pearlman, J.D.; Edelman, E.R.; Sellke, F.W.; Simons, M. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am. J. Physiol. Circ. Physiol. 1996, 270, H1791–H1802. [Google Scholar] [CrossRef]
- Wang, S.Y.; Nunez, B.D.; Morgan, J.P.; Dai, H.B.; Ross, J.N.; Sellke, F.W. Cocaine and the porcine coronary microcirculation: Effects of chronic cocaine exposure and hypercholesterolemia. J. Cardiothorac. Vasc. Anesth. 1995, 9, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Grossman, W.; Friedman, M.; Edelman, E.R.; Prasad, P.V.; Keighley, C.S.; Manning, W.J.; Sellke, F.W.; Simons, M. Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J. Clin. Investig. 1994, 94, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.D.; Sabe, S.A.; Sabra, M.; Xu, C.M.; Malhotra, A.; Broadwin, M.; Banerjee, D.; Abid, M.R.; Sellke, F.W. Intramyocardial injection of hypoxia-conditioned extracellular vesicles modulates apoptotic signaling in chronically ischemic myocardium. JTCVS Open 2023, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Broadwin, M.; Harris, D.D.; Sabe, S.A.; Sengun, E.; Sylvestre, A.J.; Alexandrov, B.S.; Sellke, F.W.; Usheva, A. Impaired cardiac glycolysis and glycogen depletion are linked to poor myocardial outcomes in juvenile male swine with metabolic syndrome and ischemia. Physiol. Rep. 2023, 11, e15742. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Sabra, M.; Harris, D.D.; Malhotra, A.; Aboulgheit, A.; Stanley, M.; Abid, M.R.; Sellke, F.W. Canagliflozin Improves Myocardial Perfusion, Fibrosis, and Function in a Swine Model of Chronic Myocardial Ischemia. J. Am. Heart Assoc. 2023, 12, e028623. [Google Scholar] [CrossRef] [PubMed]
- Sabe, S.A.; Xu, C.M.; Potz, B.A.; Malhotra, A.; Sabra, M.; Harris, D.D.; Broadwin, M.; Abid, M.R.; Sellke, F.W. Comparative Analysis of Normoxia- and Hypoxia-Modified Extracellular Vesicle Therapy in Function, Perfusion, and Collateralization in Chronically Ischemic Myocardium. Int. J. Mol. Sci. 2023, 24, 2076. [Google Scholar] [CrossRef] [PubMed]
- Sabe, S.A.; Harris, D.D.; Broadwin, M.; Sabra, M.; Xu, C.M.; Banerjee, D.; Abid, M.R.; Sellke, F.W. Sitagliptin therapy improves myocardial perfusion and arteriolar collateralization in chronically ischemic myocardium: A pilot study. Physiol. Rep. 2023, 11, e15744. [Google Scholar] [CrossRef]
- Litvak, J.; Siderides, L.E.; Vineberg, A.M. The experimental production of coronary artery insufficiency and occlusion. Am. Heart J. 1957, 53, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.T.; Vatner, S.F. Mechanism of Impaired Myocardial Function During Progressive Coronary Stenosis in Conscious Pigs. Circ. Res. 1995, 76, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.M.; White, F.C.; Mathieu-Costello, O.; Guth, B.D.; Heusch, G.; Bloor, C.M.; Longhurst, J.C. Effects of left circumflex Ameroid constrictor placement on adrenergic innervation of myocardium. Am. J. Physiol.-Heart Circ. Physiol. 1987, 253, H1425–H1434. [Google Scholar] [CrossRef] [PubMed]
- Hocum Stone, L.; Wright, C.; Chappuis, E.; Messer, M.; Ward, H.B.; McFalls, E.O.; Kelly, R.F. Surgical Swine Model of Chronic Cardiac Ischemia Treated by Off-Pump Coronary Artery Bypass Graft Surgery. J. Vis. Exp. 2018, 133, 57229. [Google Scholar] [CrossRef]
- Keeran, K.J.; Jeffries, K.R.; Zetts, A.D.; Taylor, J.; Kozlov, S.; Hunt, T.J. A Chronic Cardiac Ischemia Model in Swine Using an Ameroid Constrictor. J. Vis. Exp. 2017, 128, 56190. [Google Scholar] [CrossRef]
- Anderson, T.S.; Rance, G.A.; Jiang, L.; Piggott, M.J.; Field, E.J.; Chanoit, G.P. Changes in chemical and ultrastructural composition of ameroid constrictors following in vitro expansion. PLoS ONE 2018, 13, e0207471. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, W.E. Ameroid constrictor: Uniform closure rates and a calibration procedure. J. Appl. Physiol. 1969, 27, 419–421. [Google Scholar] [CrossRef]
- Hughes, G.C.; Post, M.J.; Simons, M.; Annex, B.H. Translational Physiology: Porcine models of human coronary artery disease: Implications for preclinical trials of therapeutic angiogenesis. J. Appl. Physiol. 2003, 94, 1689–1701. [Google Scholar] [CrossRef]
- Beanlands, R.S.; Hendry, P.J.; Masters, R.G.; deKemp, R.A.; Woodend, K.; Ruddy, T.D. Delay in revascularization is associated with increased mortality rate in patients with severe left ventricular dysfunction and viable myocardium on fluorine 18-fluorodeoxyglucose positron emission tomography imaging. Circulation 1998, 98 (Suppl. S19), II51–II56. [Google Scholar]
- Chen, C.; Chen, L.; Fallon, J.T.; Ma, L.; Li, L.; Bow, L.; Knibbs, D.; McKay, R.; Gillam, L.D.; Waters, D.D. Functional and Structural Alterations With 24-Hour Myocardial Hibernation and Recovery After Reperfusion. Circulation 1996, 94, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Young, L.H.; Renfu, Y.; Russell, R.; Hu, X.; Caplan, M.; Ren, J.; Shulman, G.I.; Sinusas, A.J.; Gnudi, L.; Viberti, G.; et al. Low-Flow Ischemia Leads to Translocation of Canine Heart GLUT-4 and GLUT-1 Glucose Transporters to the Sarcolemma In Vivo. Circulation 1997, 95, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, L. Effect of acute subendocardial ischemia on ventricular refractory periods. Exp. Clin. Cardiol. 2007, 12, 63–66. [Google Scholar] [PubMed]
- Caillaud, D.; Calderon, J.; Réant, P.; Lafitte, S.; Dos Santos, P.; Couffinhal, T.; Roques, X.; Barandon, L. Echocardiographic analysis with a two-dimensional strain of chronic myocardial ischemia induced with ameroid constrictor in the pig. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 689–693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roth, D.M.; Maruoka, Y.; Rogers, J.; White, F.C.; Longhurst, J.C.; Bloor, C.M. Development of coronary collateral circulation in left circumflex Ameroid-occluded swine myocardium. Am. J. Physiol. 1987, 253 Pt 2, H1279–H1288. [Google Scholar] [CrossRef]
- Lopez, J.J.; Edelman, E.R.; Stamler, A.; Hibberd, M.G.; Prasad, P.; Thomas, K.A.; Disalvo, J.; Caputo, R.P.; Carrozza, J.P.; Douglas, P.S.; et al. Angiogenic potential of perivascularly delivered aFGF in a porcine model of chronic myocardial ischemia. Am. J. Physiol.-Heart Circ. Physiol. 1998, 274, H930–H936. [Google Scholar] [CrossRef]
- Reinhardt, C.P.; Dalhberg, S.; Tries, M.A.; Marcel, R.; Leppo, J.A. Stable labeled microspheres to measure perfusion: Validation of a neutron activation assay technique. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H108–H116. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Bassingthwaighte, J.B. Blood flow distributions by microsphere deposition methods. Cardiovasc. Res. 2000, 45, 13–21. [Google Scholar] [CrossRef]
- Hoffman, J.I. The history of the microsphere method for measuring blood flows with special reference to myocardial blood flow: A per-sonal memoir. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H705–H710. [Google Scholar] [CrossRef]
- Koudstaal, S.; Jansen of Lorkeers, S.J.; Gho, J.M.I.H.; van Hout, G.P.; Jansen, M.S.; Gründeman, P.F.; Pasterkamp, G.; Doevendans, P.A.; Hoefer, I.E.; Chamuleau, S.A. Myocardial Infarction and Functional Outcome Assessment in Pigs. J. Vis. Exp. 2014, 86, 51269. [Google Scholar] [CrossRef]
- Billig, S.; Zayat, R.; Ebeling, A.; Steffen, H.; Nix, C.; Hatam, N.; Schnöring, H.; Derwall, M. Transesophageal echocardiography in swine: Evaluation of left and right ventricular structure, function and myocardial work. Int. J. Cardiovasc. Imaging 2021, 37, 835–846. [Google Scholar] [CrossRef]
- Serial Assessment of Left Ventricular Remodeling and Function by Echo-Tissue Doppler Imaging after Myocardial Infarction in Streptozotocin-Induced Diabetic Swine—ClinicalKey. Available online: https://www-clinicalkey-com.revproxy.brown.edu/#!/content/playContent/1-s2.0-S0894731709001989?returnurl=null&referrer=null (accessed on 20 November 2023).
- Pasrija, C.; Quinn, R.W.; Alkhatib, H.; Tran, D.; Bernstein, D.; Rice, M.; Kotloff, E.; Morales, D.; D’ambra, M.N.; Vesely, M.R.; et al. Development of a Reproducible Swine Model of Chronic Ischemic Mitral Regurgitation: Lessons Learned. Ann. Thorac. Surg. 2021, 111, 117–125. [Google Scholar] [CrossRef]
- Coppola, B.A.; Omens, J.H. Use of Larger Species such as Dog and Pig as Model Systems to Study Cardiac Disease. Drug Discov. Today Dis. Models. 2009, 5, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.L.H.; Swingen, C.; Holley, C.; Wright, C.; Chappuis, E.; Ward, H.B.; McFalls, E.O.; Kelly, R.F. Magnetic resonance imaging assessment of cardiac function in a swine model of hibernating myocardium 3 months following bypass surgery. J. Thorac. Cardiovasc. Surg. 2017, 153, 582–590. [Google Scholar] [CrossRef]
- Crisostomo, V.; Baez, C.; Abad, J.L.; Sanchez, B.; Alvarez, V.; Rosado, R.; Gómez-Mauricio, G.; Gheysens, O.; Blanco-Blazquez, V.; Blazquez, R.; et al. Dose-dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart-derived cells. Stem Cell Res. Ther. 2019, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, G.; Batlivala, S.; Callahan, R.; Thatte, N.; Rockefeller, T.; Nawaytou, H.; Reddy, S.V.; Hussain, T.; Chabiniok, R.; Butts, R.; et al. Clinical Applications of Pressure-Volume Assessment in Congenital Heart Disease. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Kassiri, Z.; Virag, J.A.I.; de Castro Brás, L.E.; Scherrer-Crosbie, M. Guidelines for measuring cardiac physiology in mice. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H733–H752. [Google Scholar] [CrossRef] [PubMed]
- Seemann, F.; Bruce, C.G.; Khan, J.M.; Ramasawmy, R.; Potersnak, A.G.; Herzka, D.A.; Kakareka, J.W.; Jaimes, A.E.; Schenke, W.H.; O’brien, K.J.; et al. Dynamic pressure–volume loop analysis by simultaneous real-time cardiovascular magnetic resonance and left heart catheterization. J. Cardiovasc. Magn. Reson. 2023, 25, 1. [Google Scholar] [CrossRef]
- Hoenig, M.R.; Bianchi, C.; Rosenzweig, A.; Sellke, F.W. Decreased vascular repair and neovascularization with ageing: Mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr. Mol. Med. 2008, 8, 754–767. [Google Scholar] [CrossRef] [PubMed]
- O’Konski, M.S.; White, F.C.; Longhurst, J.; Roth, D.; Bloor, C.M. Ameroid constriction of the proximal left circumflex coronary artery in swine. A model of limited coronary collateral circulation. Am. J. Cardiovasc. Pathol. 1987, 1, 69–77. [Google Scholar]
- Shah, A.; Goerlich, C.E.; Pasrija, C.; Hirsch, J.; Fisher, S.; Odonkor, P.; Strauss, E.; Ayares, D.; Mohiuddin, M.M.; Griffith, B.P. Anatomical Differences Between Human and Pig Hearts and Their Relevance for Cardiac Xenotransplantation Surgical Technique. JACC Case Rep. 2022, 4, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Young, R.; Page, B.J.; Shen, X.; Tata, N.; Li, J.; Duan, X.; Fallavollita, J.A.; Canty, J.M. Reproducible Ion-Current-Based Approach for 24-Plex Comparison of the Tissue Proteomes of Hibernating versus Normal Myocardium in Swine Models. J. Proteome Res. 2014, 13, 2571–2584. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Fujita, J.; Kanazawa, H.; Kawaguchi, S.; Handa, N.; Yamada, Y.; Okuda, S.; Hishikawa, S.; Teratani, T.; Kunita, S.; et al. Cryoinjury-induced acute myocardial infarction model and ameroid constrictor-induced ischemic heart disease model in adult micro-mini pigs for preclinical studies. Transl. Med. Commun. 2017, 2, 1. [Google Scholar] [CrossRef]
- Johnson, L.L.; Schofield, L.; Donahay, T.; Bouchard, M.; Poppas, A.; Haubner, R. Radiolabeled RGD Peptides to Image Angiogenesis in Swine Model of Hibernating Myocardium. JACC Cardiovasc. Imaging 2008, 1, 500–510. [Google Scholar] [CrossRef]
- van den Wijngaard, J.P.H.M.; Schulten, H.; van Horssen, P.; ter Wee, R.D.; Siebes, M.; Post, M.J.; Spaan, J.A.E. Porcine coronary collateral formation in the absence of a pressure gradient remote of the ischemic border zone. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H1930–H1937. [Google Scholar] [CrossRef]
- Pearlman, J.D.; Laham, R.J.; Simons, M. Coronary Angiogenesis: Detection in Vivo with MR Imaging Sensitive to Collateral Neocirculation—Preliminary Study in Pigs. Radiology 2000, 214, 801–807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).