Housing European Ground Squirrels (Spermophilus citellus) for an Ex Situ Conservation Program
Abstract
1. Introduction
2. Materials and Methods
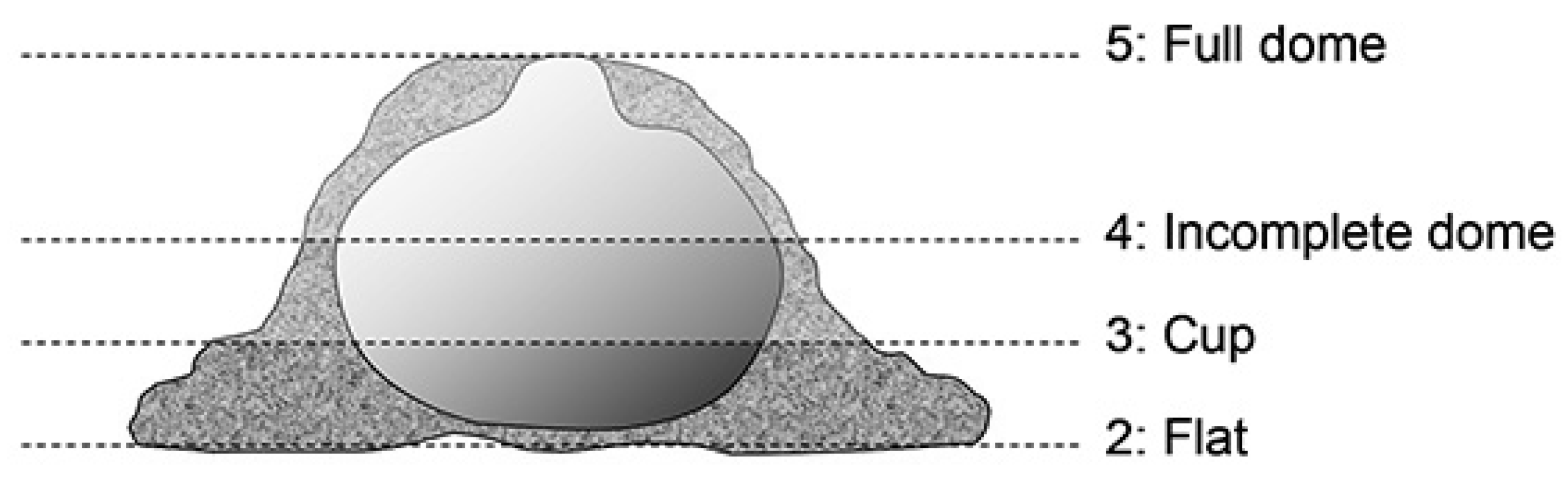
2.1. Nest Material Selection Test
2.2. Feed Preference Test
- ○
- Commercially available rabbit feed (Agroszász Ltd.) = Rabbit feed;
- ○
- Versele-laga Cuni Adult Complete rabbit feed = Complete;
- ○
- Versele-laga Nature Cuni rabbit feed = Nature.
Statistical Analysis
3. Results
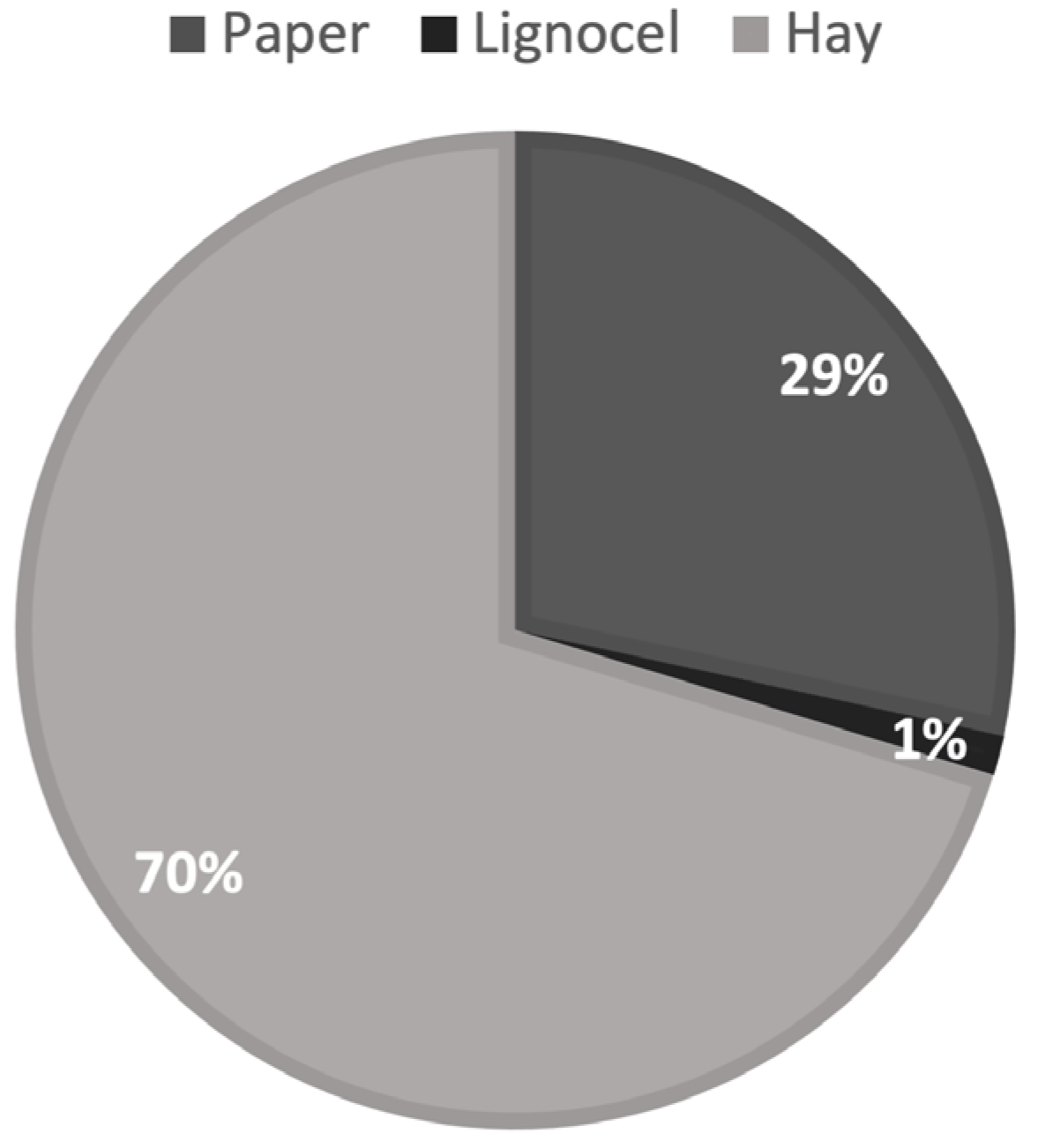
3.1. Result of Selection of Nest Material
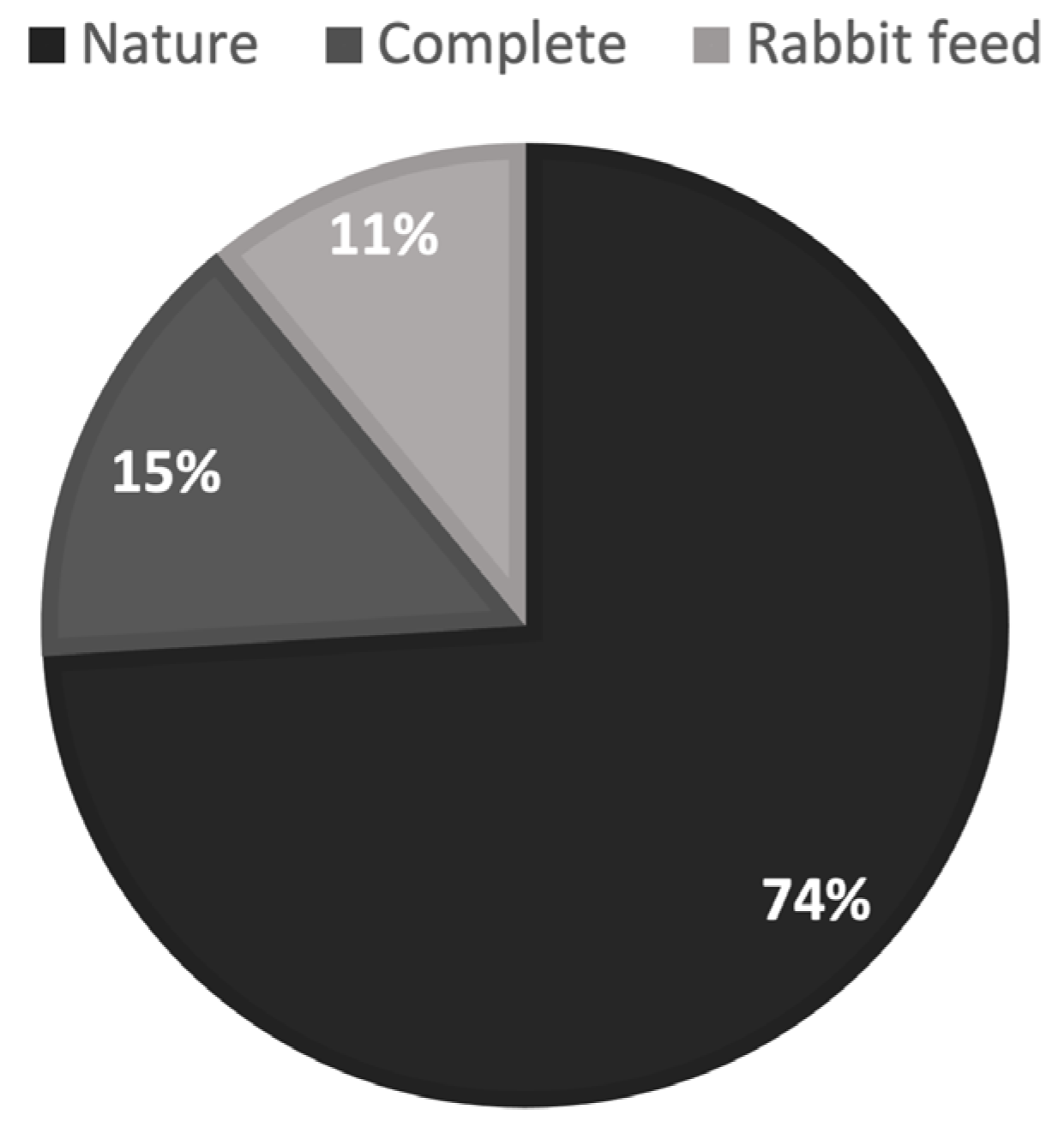
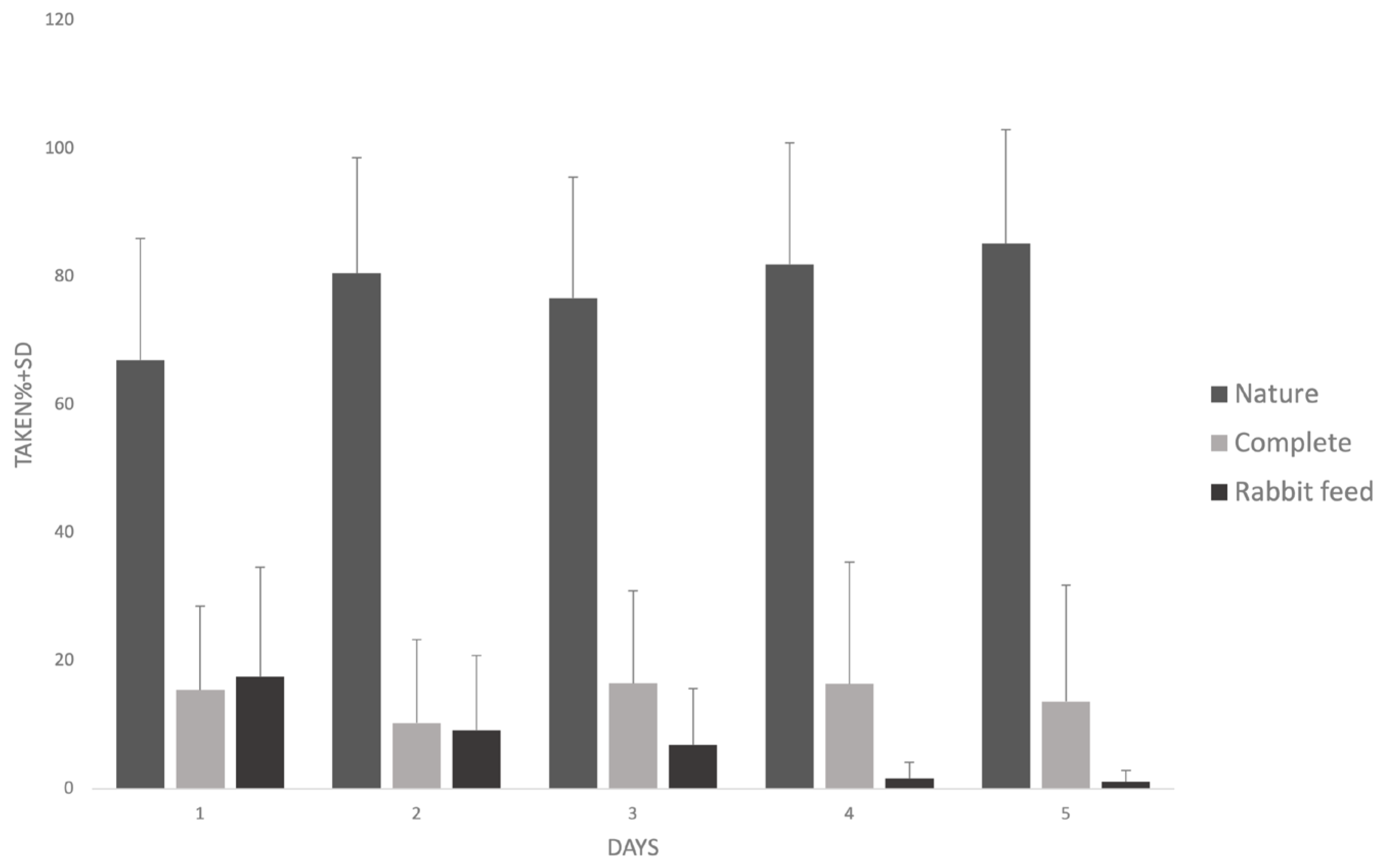
3.2. Results of a Feed Preference Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, D. Building the European union’s Natura 2000 network. Nat. Conserv. 2012, 1, 11–26. [Google Scholar] [CrossRef]
- Szabó, G.; Zimmermann, Z. Földalatti ökoszisztéma-mérnök fajok szerepe a gyepek fenntartásában. Gyepgazd. Közl. 2012, 10, 49–55. [Google Scholar] [CrossRef]
- Lindtner, P.; Gömöryová, E.; Gömöry, D.; Stašiov, S.; Kubovčík, V. Development of physico-chemical and biological soil properties on the European ground squirrel mounds. Geoderma 2019, 339, 85–93. [Google Scholar] [CrossRef]
- Laundré, J.W. Effect of ground squirrel burrows on plant productivity in a cool desert environment. J. Range Manag. 1998, 51, 638–643. [Google Scholar] [CrossRef]
- Váczi, O.; Katona, K.; Altbäcker, V. A bugacpusztai ürgepopuláció tér-és időbeli mintázata. Vadbiológia 1996, 5, 141–148. [Google Scholar]
- Cserkész, T. Az Ürge (Spermophilus citellus) Gyakoriságának Változása Magyarországon 1950 és 2017 között. LIFE13NAT/HU/000183 A Kerecsensólyom és a Parlagi sas Táplálékbázisának Megőrzése a Kárpát-Medencében LIFE+NATURE Project A1: Budapest, Hungary; European Commision: Brussels, Belgium, 2018; p. 25.
- Altbacker, V. Szaporodási Stratégiák Vizsgálata az Ürgénél, OTKA Zárójelentés, Budapest; Tanszék: Göd, Hungary, 2007. [Google Scholar]
- Lovassy, S. Magyarország Gerinces Állatai és Gazdasági Vonatkozásaik; Királyi Magyar Természettudományi Társulat: Budapest, Hungary, 1927; p. 895. [Google Scholar]
- Coroiu, C.; Krystufek, B.; Vohralík, V.; Zagorodnyuk, I. Spermophilus citellus. In 2008 IUCN Red List of Threatened Species (2008); IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Matějů, J.; Řicanova, Š.; Ambros, M.; Kala, B.; Hapl, E.; Matějů, K. Reintroductions of the European Ground Squirrel (Spermophilus citellus) in Central Europe (Rodentia: Sciuridae). Lynx Ser. Nova 2010, 41, 175–191. [Google Scholar]
- Herzig-Straschil, B.; Schmelzer, E. The European Ground Squirrel (Spermophilus citellus) in Burgenland, Austria: 1950ies to 2013. In Proceedings of the 5th European Ground Squirrel Meeting, Rust, Austria, 2–5 October 2014; University of Vienna: Rust, Austria, 2014. [Google Scholar]
- Matějů, J.; Schnitzerová, P.; Uhlíková, J.; Větrovcová, J. Eleven years of European ground squirrel monitoring in the Czech Republic. In Proceedings of the 5th European Ground Squirrel Meeting, Rust, Austria, 2–5 October 2014; University of Vienna: Rust, Austria, 2014. [Google Scholar]
- Váczi, O.; Altbacker, V. Füves repülőterek ürge állományának felmérése. Term. Véd. Közl. 1999, 8, 205–214. [Google Scholar]
- Janák, M.; Marhoul, P.; Matějů, J. Action Plan for the Conservation of the European Ground Squirrel Spermophilus citellus in the European Union; European Commission: Ispra, Italy, 2013; p. 61.
- Hulova, S.; Sedlacek, F. Population genetic structure of the European ground squirrel in the Czech Republic. Conserv. Genet. 2008, 9, 615–625. [Google Scholar] [CrossRef]
- Váczi, O. The Effects of Abiotic Environmental Factors on Spatiotemporal Activity Pattern of the European Ground Squirrel (Spermophilus citellus). Ph.D. Thesis, Eötvös Loránd University, Budapest, Hungary, 2005. [Google Scholar]
- Christie, S. Why keep tigers in zoos? In Tigers of the World: The Science, Politics and Conservation of Panthera tigris; Academic Press: Cambridge, UK, 2010; pp. 205–214. [Google Scholar]
- Maunder, M.; Byers, O. The IUCN Technical Guidelines on the Management of Ex Situ Populations for Conservation: Reflecting major changes in the application of ex situ conservation. Oryx 2005, 39, 95–98. [Google Scholar] [CrossRef][Green Version]
- Bethge, P.; Munks, S.; Otley, H.; Nicol, S. Platypus burrow temperatures at a subalpine Tasmanian lake. Proc. Linn. Soc. N. S. W. 2004, 125, 273–276. [Google Scholar]
- Geiser, F. Reduction of metabolism during hibernation and daily torpor in mammals and birds—Temperature effect or physiological inhibition? J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1988, 158, 25–37. [Google Scholar] [CrossRef]
- Houston, A.I.; Mcnamara, J.M. A theoretical investigation of the fat reserves and mortality levels of small birds in winter. Ornis Scand. 1993, 24, 205–219. [Google Scholar] [CrossRef]
- Lovegrove, B.G.; Raman, J.; Perrin, M.R. Heterothermy in elephant shrews, Elephantulus spp. (Macroscelidea): Daily torpor or hibernation? J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2001, 171, 1–10. [Google Scholar] [CrossRef]
- McCafferty, D.J.; Moncrieff, J.B.; Taylor, I.R. Winter microclimate of field voles (Microtus agrestis) in SW Scotland. J. Therm. Biol. 2003, 28, 397–401. [Google Scholar] [CrossRef]
- Lamprecht, I.; Schmolz, E. Thermal investigations of some bird nests. Thermochim. Acta 2004, 415, 141–148. [Google Scholar] [CrossRef]
- Millesi, E.; Huber, S.; Everts, L.G.; Dittami, J.P. Reproductive decisions in female European ground squirrels: Factors affecting reproductive output and maternal investment. Ethology 1999, 105, 163–175. [Google Scholar] [CrossRef]
- Sherman, P.W.; Runge, M.C. Demography of a population collapse: The northern Idaho ground squirrel (Spermophilus brunneus brunneus). Ecology 2002, 83, 2816–2831. [Google Scholar] [CrossRef]
- Smith, G.W.; Johnson, D.R. Demography of a Townsend ground squirrel population in southwestern Idaho. Ecology 1985, 66, 171–178. [Google Scholar] [CrossRef]
- Dobson, F.S.; Risch, T.S.; Murie, J.O. Increasing returns in the life history of Columbian ground squirrels. J. Anim. Ecol. 1999, 68, 73–86. [Google Scholar] [CrossRef]
- Risch, T.S.; Michener, G.R.; Dobson, F.S. Variation in litter size: A test of hypotheses in Richardson’s ground squirrels. Ecology 2007, 88, 306–314. [Google Scholar] [CrossRef]
- Győri-Koósz, B.; Katona, K.; Altbacker, V. Az ürge étrendjének vizsgálata legelt és kaszált gyepterületeken. Magy. Apróvad Közl. 2013, 11, 215–225. [Google Scholar]
- Herzig-Straschil, B. Nahrung und Nahrungserwerb des Ziesels. Acta Theriol. 1976, 21, 131–139. [Google Scholar] [CrossRef]
- Gedeon, C.I.; Markó, G.; Németh, I.; Nyitrai, V.; Altbäcker, V. Nest material selection affects nest insulation quality for the European ground squirrel (Spermophilus citellus). J. Mammal. 2010, 91, 636–641. [Google Scholar] [CrossRef][Green Version]
- Szenczi, P.; Bánszegi, O.; Dúcs, A.; Gedeon, C.I.; Markó, G.; Németh, I.; Altbäcker, V. Morphology and function of communal mounds of overwintering mound-building mice (Mus spicilegus). J. Mammal. 2011, 92, 852–860. [Google Scholar] [CrossRef]
- Bilkó, Á.; Petróczi, I.; Bárdos, B.; Nagy, I.; Altbacker, V. Composition of the wild rabbit nest and its implication for domestic rabbit breeding. Appl. Sci. 2022, 12, 1915. [Google Scholar] [CrossRef]
- Gaskill, B.N.; Karas, A.Z.; Garner, J.P.; Pritchett-Corning, K.R. Nest building as an indicator of health and welfare in laboratory mice. JoVE J. Vis. Exp. 2013, 82, e51012. [Google Scholar]
- Redman, P.; Selman, C.; Speakman, J.R. Male short-tailed field voles (Microtus agrestis) build better insulated nests than females. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1999, 169, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Mcgowan, A.; Sharp, S.P.; Hatchwell, B.J. The structure and function of nests of long-tailed tits, Aegithalos caudatus. Funct. Ecol. 2004, 18, 578–583. [Google Scholar] [CrossRef]
- Bárdos, B.; Nagy, I.; Gerencsér, Z.; Altbacker, V. Nest material preference of wild mouse species in laboratory housing. Appl. Sci. 2022, 12, 5750. [Google Scholar] [CrossRef]
- Neely, C.L.; Pedemonte, K.A.; Boggs, K.N.; Flinn, J.M. Nest building behavior as an early indicator of behavioral deficits in mice. J. Vis. Exp. 2019, 152, 60139. [Google Scholar]
- Bárdos, B.; Kövér, G.; Szabó, A.; Gerencsér, Z.; István, N. Feed preference and feeding behavior of different mouse species in laboratory housing. Acta Agr. Kapos. 2022, 26, 17–26. [Google Scholar] [CrossRef]
- Merriman, D.K.; Lahvis, G.; Jooss, M.; Gesicki, J.A.; Schill, K. Current practices in a captive breeding colony of 13-lined ground squirrels (Ictidomys tridecemlineatus). Lab Anim. 2012, 41, 315–325. [Google Scholar] [CrossRef]
- Vaughan, D.K.; Gruber, A.R.; Michalski, M.L.; Seidling, J.; Schlink, S. Capture, care, and captive breeding of 13-lined ground squirrels, Spermophilus tridecemlineatus. Lab Anim. 2006, 35, 33–40. [Google Scholar] [CrossRef]
- Eshelman, B.D.; Jenkins, S.H. Food selection by Belding’s ground squirrels in relation to plant nutritional features. J. Mammal. 1989, 70, 846–852. [Google Scholar] [CrossRef]
- Armstrong, D.G.; McEvoy, T.G.; Baxter, G.; Robinson, J.J.; Hogg, C.O.; Woad, K.J.; Webb, R.; Sinclair, K.D. Effect of dietary energy and protein on bovine follicular dynamics and embryo production in vitro: Associations with the ovarian insulin-like growth factor system. Biol. Reprod. 2001, 64, 1624–1632. [Google Scholar] [CrossRef]
- Luo, M.S. Food habits research of Daurian ground squirrel. Acta Zool. Sin. 1975, 21, 62–70. [Google Scholar]
- Zhang, Z.Q.; Li, K.S. The study of feeding and breeding of Daurian ground squirrel in laboratory Ⅱ. Chi. J. Vect. Biol. Cont. 1988, 4, 232–235. [Google Scholar]
- Sun, X.; Gao, Y.; Wang, Q.; Jiang, S.; Guo, S.; Liu, K. The artificial feeding, breeding and research on hibernation bouts of the Daurian ground squirrel (Spermophilus dauricus). Acta Theriol. Sin. 2012, 32, 356–361. [Google Scholar]
- Frank, C.L. Short-term variations in diet fatty acid composition and torpor by ground squirrels. J. Mammal. 2002, 83, 1013–1019. [Google Scholar] [CrossRef]
- Frank, C.L.; Storey, K.B. The effect of total unsaturate content on hibernation. In Adaptations to the Cold: Tenth International Hibernation Symposium; University of New England Press: New Hampshire, UK, 1996; p. 211. [Google Scholar]
- Kenagy, G.J. Energy allocation for reproduction in the golden-mantled ground squirrel. Symp. Zool. Soc. Lond. 1987, 57, 259–273. [Google Scholar]
- Kenagy, G.J.; Barnes, B.M. Seasonal reproductive patterns in four coexisting rodent species from the Cascade Mountains, Washington. J. Mammal. 1988, 69, 274–292. [Google Scholar] [CrossRef]
- Frank, C.L. Polyunsaturate content and diet selection by ground squirrels (Spermophilus lateralis). Ecology 1994, 75, 458–463. [Google Scholar] [CrossRef]
- Straus, A.; Hoffmann, I.E.; Millesi, E. Effects of nutritional factors on juvenile development in male European ground squirrels (Spermophilus citellus). Mammal. Biol. 2006, 72, 354–363. [Google Scholar] [CrossRef]
- Nyitrai, V.; Németh, I.; Altbäcker, V. A külső hőmérséklet hatása a közönséges ürge (Spermophilus citellus) hibernációjára. Állattan. Közl. 2005, 90, 63–74. [Google Scholar]
- Kalotás, Z. Kedves kis rágcsálónk: Az ürge. Természet Világa 2015, 146, 11. [Google Scholar]
| Feed Name | Feed Structure and Diameter | Components |
|---|---|---|
| Commercially available rabbit feed (Agroszász Ltd.) | granulated (3.3 mm) | grass pellets, |
| lucerne, | ||
| extracted sunflower semolina, carrot slices, | ||
| wheat, | ||
| wheat bran, | ||
| oats, | ||
| barley, | ||
| full-fat soy, | ||
| additives. | ||
| Versele-laga Cuni Adult Complete rabbit feed | granulated (5.4 mm) | grass pellets, |
| carrot, | ||
| vegetable protein extracts, | ||
| linseed, | ||
| fructo-oligosaccharides, | ||
| marigold, | ||
| yucca. | ||
| Versele-laga Nature Cuni rabbit feed | mixture | grass pellets, |
| green peas, | ||
| carrot, | ||
| parsnip, | ||
| apple, | ||
| animal protein, | ||
| oils and fats, | ||
| fructo-oligosaccharides, | ||
| mannan oligosaccharides, | ||
| marigold, | ||
| chlorella algae, | ||
| yucca. |
| Nutrient | Commercially Available Rabbit Feed (Agroszász Ltd.) | Versele-Laga Cuni Adult Complete Rabbit Feed | Versele-Laga Nature Cuni Rabbit Feed |
|---|---|---|---|
| Crude protein (%) | 16 | 14 | 14.7 |
| Crude fat (%) | 3.3 | 3 | 3.5 |
| Crude fibre (%) | 15 | 20 | 16.5 |
| Crude ash (%) | 7 | 7.5 | 8.3 |
| Moisture content (%) | 7.4 | 7.5 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárdos, B.; Altbacker, V.; Török, H.K.; Nagy, I. Housing European Ground Squirrels (Spermophilus citellus) for an Ex Situ Conservation Program. Methods Protoc. 2024, 7, 18. https://doi.org/10.3390/mps7020018
Bárdos B, Altbacker V, Török HK, Nagy I. Housing European Ground Squirrels (Spermophilus citellus) for an Ex Situ Conservation Program. Methods and Protocols. 2024; 7(2):18. https://doi.org/10.3390/mps7020018
Chicago/Turabian StyleBárdos, Boróka, Vilmos Altbacker, Henrietta Kinga Török, and István Nagy. 2024. "Housing European Ground Squirrels (Spermophilus citellus) for an Ex Situ Conservation Program" Methods and Protocols 7, no. 2: 18. https://doi.org/10.3390/mps7020018
APA StyleBárdos, B., Altbacker, V., Török, H. K., & Nagy, I. (2024). Housing European Ground Squirrels (Spermophilus citellus) for an Ex Situ Conservation Program. Methods and Protocols, 7(2), 18. https://doi.org/10.3390/mps7020018






