Isolation, Maintenance and Differentiation of Primary Tracheal Basal Cells from Adult Rhesus Macaque
Abstract
1. Introduction
2. Experimental Design
Materials
- PureCol (Advanced Biomatrix, 5005-100ML, San Diego, USA)
- DNAse (Sigma DN25-100MG, Merck KGaA, Darmstadt, Germany)
- Protease (Sigma P5147-100MG, Merck KGaA, Darmstadt, Germany)
- DMEM/F12 (Gibco, 11330-032, Dublin, Ireland)
- Pneumacult-Ex (Stemcell Technologies, 05008, Cambridge, USA)
- Pneumacult-ALI (Stemcell Technologies, 05001, Cambridge, USA)
- Phosphate-buffered saline (PBS) (Gibco, 14190-144, Dublin, Ireland)
- Phosphate-buffered saline (PBS) (containing CaCl and MgCl) (Gibco, 14040-133, Dublin, Ireland)
- 3D Matrigel (Matrigel Matrix Basement Membrane, Corning, 356231, New York, USA)
3. Procedure
3.1. Coating: Time for Completion 02:30 h
- PAUSE Coated plates can be stored up to 2 weeks at 4 °C.
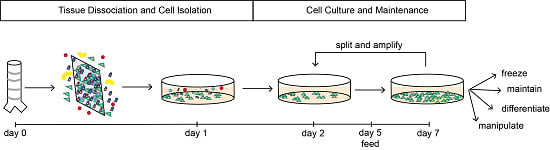
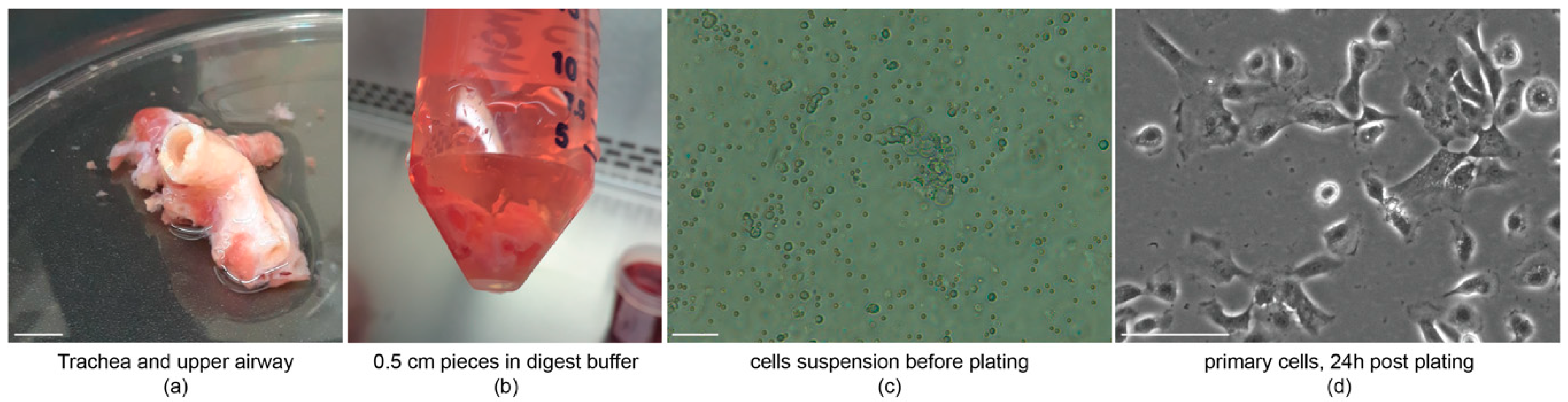
3.2. Tissue Dissociation and Cell Isolation: Time for Completion 20 h
- CRITICAL STEP Scraping should be done carefully, not cutting into the underlying stroma. We recommend holding a corner of the tissue with forceps and then stroking with the scalpel in a single direction, with a movement similar to spreading butter on toast. Discard remains.
3.3. Culture and Maintenance: Time for Completion 4 to 5 days
- CRITICAL STEP Do not filter the resuspended pellet, BCs will attach, non-basal cells will not and can be washed off the next day. Cell yield is markedly increased if not filtered.
- OPTIONAL STEP After the first passage cells do not need to be passaged onto coated dishes (see Section 3.1). For the first passage, after flow sorting, cell manipulations and after thawing collagen plates are an essential requirement.
- OPTIONAL STEP as few as 0.1 × 106 cells can be plated in a 10 cm dish, initial growth will be slower. If even lower numbers of cells are plated, conditioned medium is highly recommended (see Section 5).
3.4. Manipulation and Differentiation: Time for Completion-Variable
3.4.1. Differentiation on Air–Liquid Interphase (ALI): Time for Completion 14 days
- CRITICAL STEP Gradual change from Pneumacult-Ex to Pneumacult-ALI is highly recommended as cell layers tended to rupture or detach if an immediate change is performed. Gradual change results in more even, healthier cultures. For optimal culture health, media should be changed every 48 h at this point, until in 100% Pneumacult-ALI.
3.4.2. Differentiation in Tracheosphere Assay: Time for Completion 14 days
- CRITICAL STEP Matrigel polymerizes quickly once at room temperature. We recommend using cold media, and doing all steps on ice. We usually use ice cold pipette tips for this step. Avoid air bubbles in the 3D Matrigel.
- CRITICAL STEP The tracheospheres should not be moved to 100% Pneumacult-ALI. They are ultimately cultured in 30% Pneumacult-Ex and 70% Pneumacult-ALI medium. Sphere morphology is better in in this 30:70 medium compared to 100% Pneumacult-ALI.
- OPTIONAL STEP For tracheosphere cultures at clonal density (5–10 cells/µL), the cells need to be cultured in a minimum of 30% conditioned Pneumacult-Ex Medium (see Section 5). They will take longer to form spheres (usually 10 days). The transition into differentiation medium should not be done before 14 days in vitro.
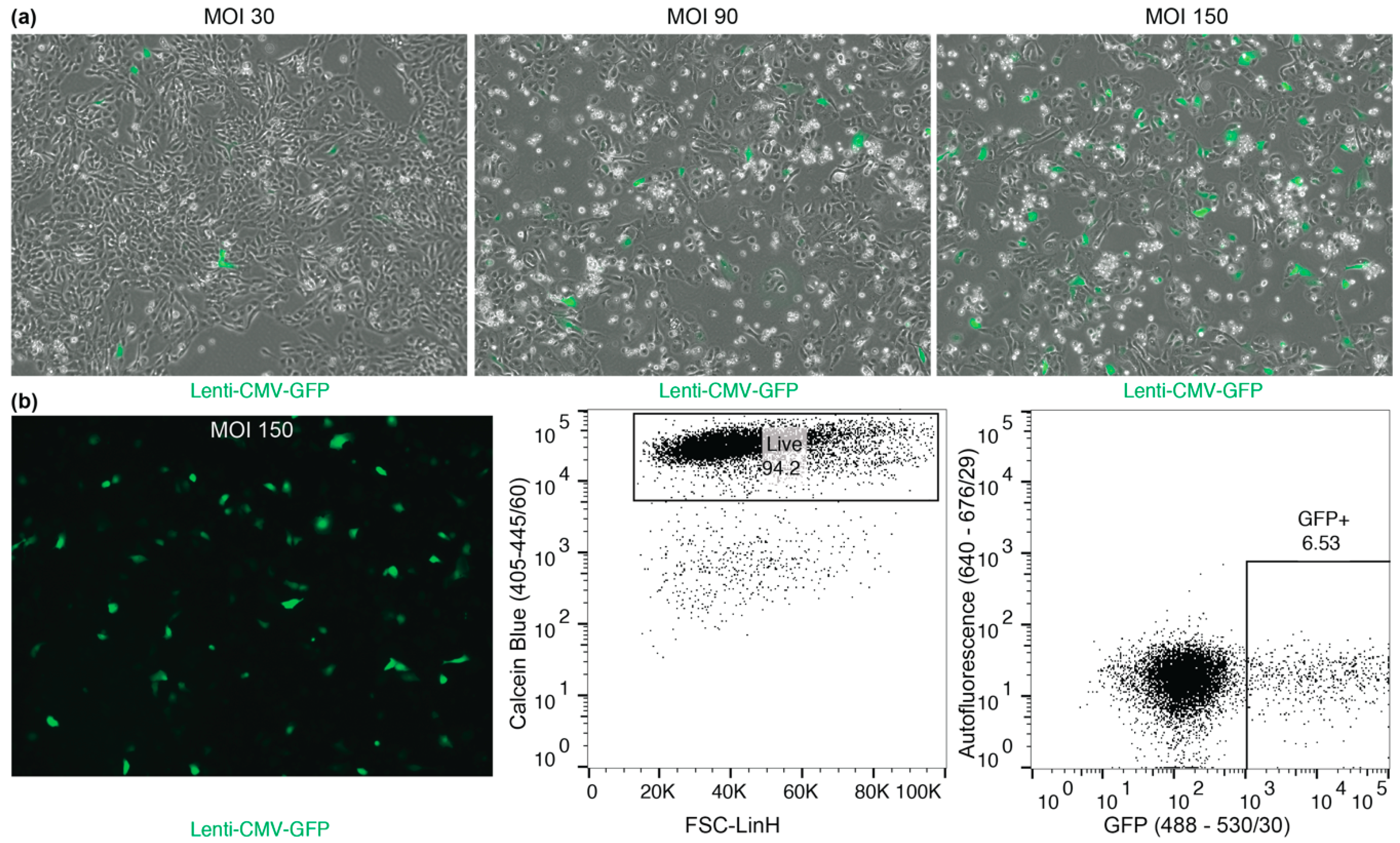
3.4.3. Transduction of Macaque BC Cultures: Time for Completion 72 h
- OPTIONAL STEP Do not change medium before infection but use fresh medium to prepare virus to increase viral uptake.
- CRITICAL STEP Infected macaque BCs should be used within 7–14 days for experiments because transgene silencing reduced GFP expression. Even after sorting expression can be reduced by up to 60% within 14 days. In order to avoid silencing and establishing stable lines, Lenti-SIV virus constructs should be used [7].
- PAUSE GFP+ infected cells can be frozen (see Section 3.5) and thawed at a later time point.
3.4.4. Sample Preparation for Flow Cytometric Analysis or Purification
Flow Cytometric Analysis: Time for Completion 2 h
- PAUSE Fixed and ethanol permeabilized cells can be stored in 90% ethanol for up to 5 days at −20 °C.
Purification by Flow Sorting: Time for Completion 30 min
- CRITICAL STEP Collection in conditioned medium containing Y-27632 and the usage of freshly prepared collagen-coated plates resulted in better recovery of the sorted cells.
3.5. Freezing, Long Term Storage and Thawing
3.5.1. Freezing and Long-Term Storage: Time for Completion 30 min
3.5.2. Thawing: Time for Completion 30 min
- CRITICAL STEP To increase the cell viability and yield after thawing, PBS should be used cold and medium should be warmed no higher than room temperature.
4. Expected Results
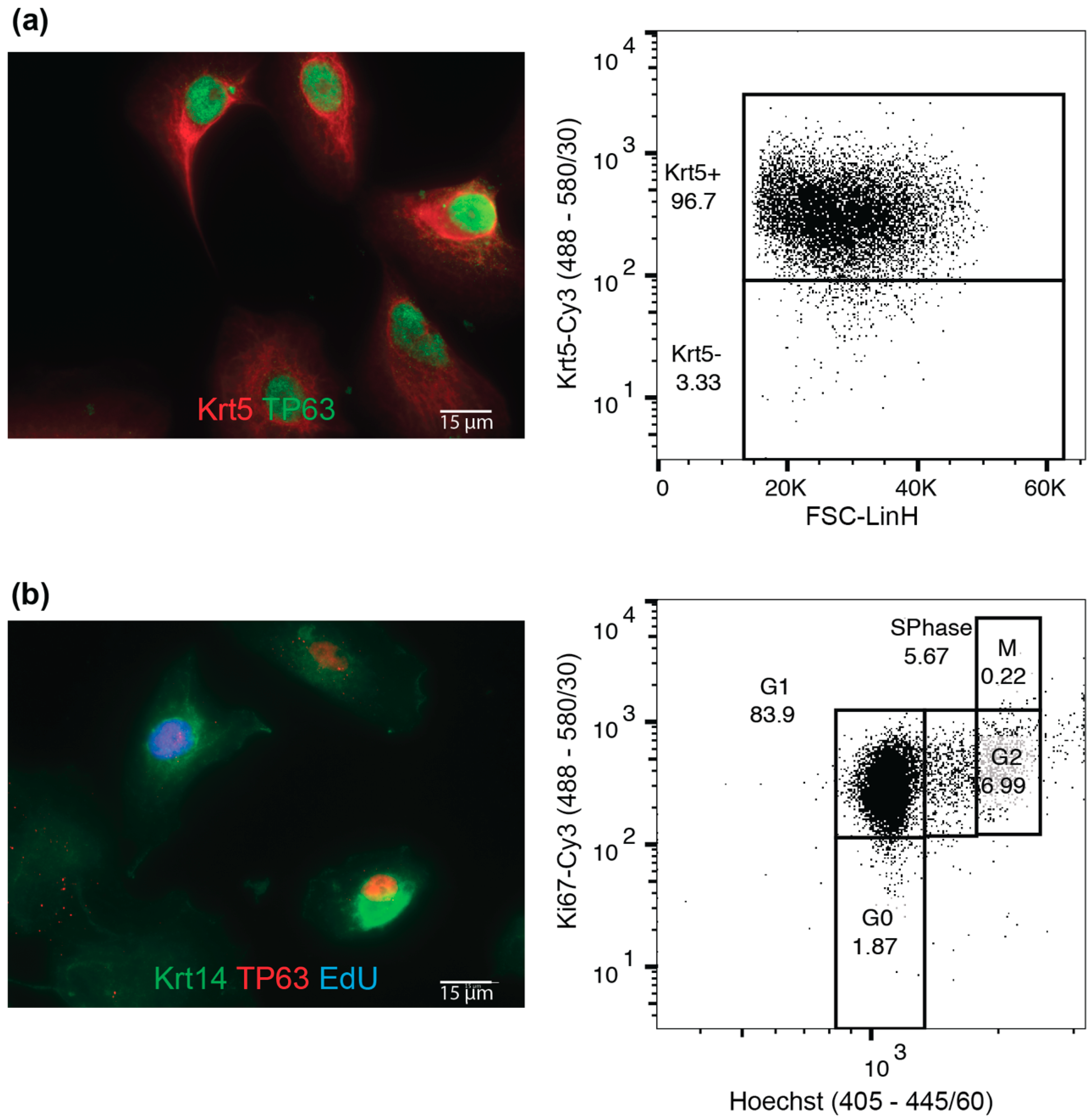
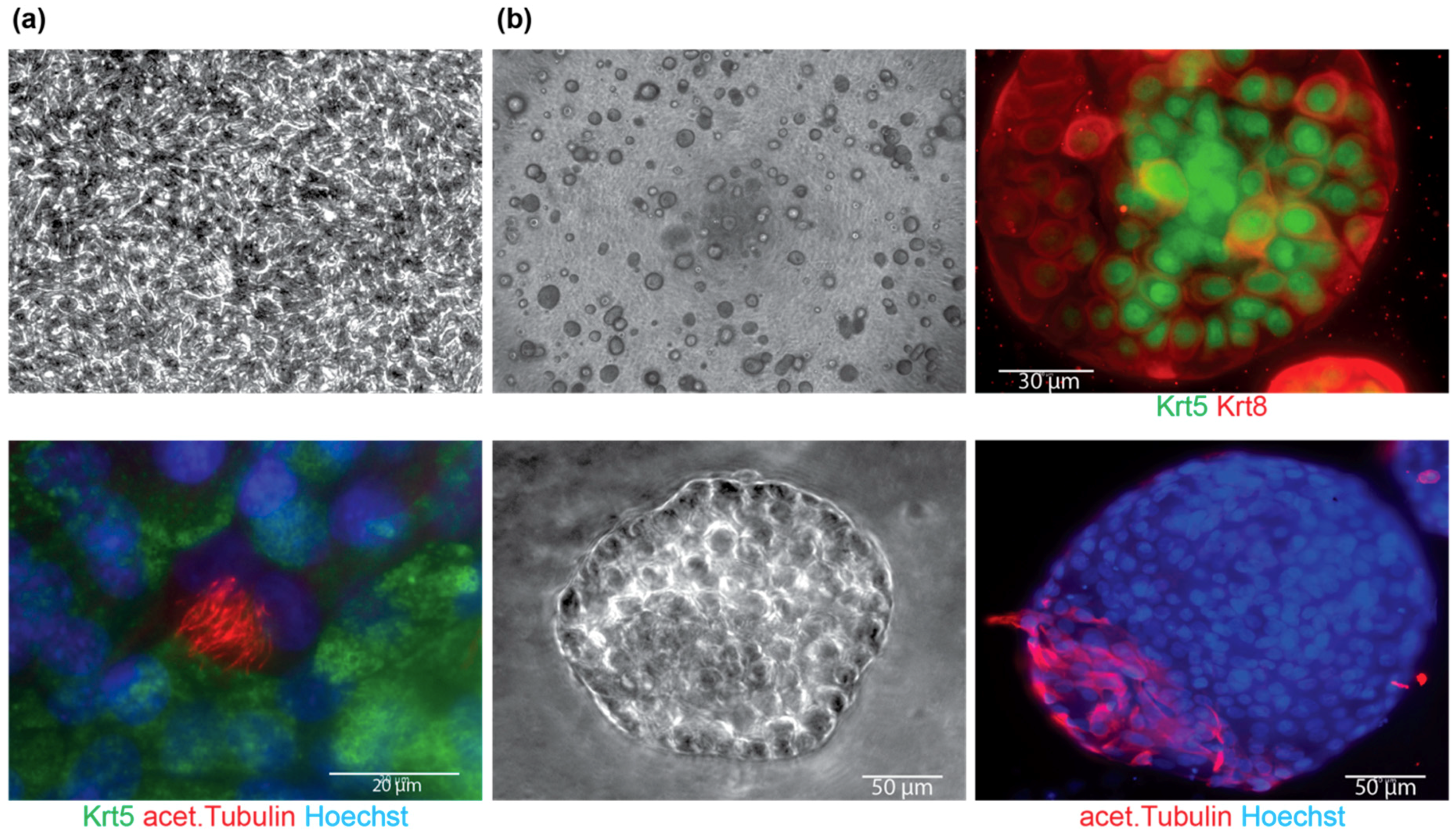
4.1. Tissue Dissociation, Cell Isolation and Maintenance
4.2. Differentiation
4.3. Macaque BC Culture Manipulations
4.4. Anticipated Yields and Outcomes
| Expected Yield | |
|---|---|
| Cell number from first dissociation | 1.6 ± 0.2 × 106 cells total (n = 3, nexp = 1) |
| Viability from first dissociation | >85% viability (n = 3, nexp = 1) |
| Cell number per 10 cm dish | 3.5 ± 1.8 × 106 cells total |
| Viability from later passages | >95% viability |
| Passage number till abnormal phenotypes occur | 8.3 ± 3.5 passages |
| Time to form spheres | 4.6 ± 0.6 days |
| Time to see cobblestone morphology on ALI | 2.6 ± 1.2 days |
| Recovery and viability after thawing | 67.7% ± 8.8% recovery, >65% viability |
| Cells in S-Phase (by EdU) | 15.1% ± 4.5% |
| Average number of Krt5+ cells | 99.2% ± 0.3% |
| Average number of Krt5+p63+ cells | 89.7% ± 4.8% |
4.5. Troubleshooting
| Problem | Potential Cause | Solution |
|---|---|---|
| Low cell number from tissue |
|
|
| Low cell number from first dissociation |
|
|
| Low viability from first dissociation |
|
|
| Low cell numbers after few passages and/or low viability |
|
|
| Poor attachment after dissociation |
|
|
| Low proliferation |
|
|
| Low number of Krt5+ cells |
|
|
| Abnormal Morphology |
|
|
| Poor recovery/viability after thawing |
|
|
5. Reagents Setup
5.1. Coating Medium
5.2. Wash Buffer
5.3. Dissociation Buffers
5.3.1. 10× Protease Mix
5.3.2. Digest Buffer
5.4. Pneumacult-Ex and Pneumacult-ALI
5.4.1. Pneumacult-Ex
5.4.2. Pneumacult-ALI
5.5. Conditioned Medium
5.6. Flow Cytometry Buffer
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rock, J.R.; Hogan, B.L. Epithelial Progenitor Cells in Lung Development, Maintenance, Repair, and Disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L.M. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Vinarsky, V.; Tata, P.R.; Brazauskas, K.; Choi, S.H.; Crooke, A.K.; Zhang, B.; Solomon, G.M.; Turner, B.; Bihler, H.; et al. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 2016, 19, 217–231. [Google Scholar] [CrossRef]
- Pardo-Saganta, A.; Law, B.M.; Tata, P.R.; Villoria, J.; Saez, B.; Mou, H.; Zhao, R.; Rajagopal, J. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 2015, 16, 184–197. [Google Scholar] [CrossRef]
- Pardo-Saganta, A.; Law, B.M.; Tata, P.R.; Villoria, J.; Saez, B.; Mou, H.; Zhao, R.; Rajagopal, J. Yap Tunes Airway Epithelial Size and Architecture by Regulating the Identity, Maintenance, and Self-Renewal of Stem Cells. Dev. Cell 2014, 30, 151–165. [Google Scholar]
- Fulcher, M.L.; Gabriel, S.; Burns, K.A.; Yankaskas, J.R.; Randell, S.H. Well-differentiated human airway epithelial cell cultures. In Human Cell Culture Protocols; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Robert, E.D.; Kuramoto, K. Sources and methods to obtain stem and progenitor cells Large Animal Models for Stem and Progenitor Cell Analysis. Blood 2005, 22A. [Google Scholar] [CrossRef]
- Scalise, G.; Mazaheri, M.R.; Kremastinou, J.; Howard, C.R.; Sorensen, K.; Zuckerman, A.J. Transmission of Hepatitis B to the Rhesus Monkey. J. Med. Primatol. 1978, 7, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G.; Randell, S.H.; Engelhardt, J.F.; Voynow, J.; Sunday, M.E. Airway Epithelial Cells: Current Concepts and Challenges. Proc. Am. Thorac. Soc. 2008, 5, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Mostoslavsky, G.; Fabian, A.J.; Rooney, S.; Alt, F.W.; Mulligan, R.C. Complete correction of murine Artemis immunodeficiency by lentiviral vector-mediated gene transfer. Proc. Natl. Acad. Sci. USA 2006, 103, 16406–16411. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engler, A.E.; Mostoslavsky, G.; Miller, L.; Rock, J.R. Isolation, Maintenance and Differentiation of Primary Tracheal Basal Cells from Adult Rhesus Macaque. Methods Protoc. 2019, 2, 79. https://doi.org/10.3390/mps2040079
Engler AE, Mostoslavsky G, Miller L, Rock JR. Isolation, Maintenance and Differentiation of Primary Tracheal Basal Cells from Adult Rhesus Macaque. Methods and Protocols. 2019; 2(4):79. https://doi.org/10.3390/mps2040079
Chicago/Turabian StyleEngler, Anna E., Gustavo Mostoslavsky, Lisa Miller, and Jason R. Rock. 2019. "Isolation, Maintenance and Differentiation of Primary Tracheal Basal Cells from Adult Rhesus Macaque" Methods and Protocols 2, no. 4: 79. https://doi.org/10.3390/mps2040079
APA StyleEngler, A. E., Mostoslavsky, G., Miller, L., & Rock, J. R. (2019). Isolation, Maintenance and Differentiation of Primary Tracheal Basal Cells from Adult Rhesus Macaque. Methods and Protocols, 2(4), 79. https://doi.org/10.3390/mps2040079