Abstract
Very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency is a long-chain fatty acid oxidation disorder that manifests as either a severe phenotype associated with cardiomyopathy, a hypoglycemic phenotype, or a myopathic phenotype. As the hypoglycemic phenotype can cause sudden infant death, VLCAD deficiency is included in newborn screening (NBS) panels in many countries. The tetradecenoylcarnitine (C14:1) level in dried blood specimens is commonly used as a primary marker for VLCAD deficiency in NBS panels. Its ratio to acetylcarnitine (C2) and various other acylcarnitines is used as secondary markers. In Japan, tandem mass spectrometry-based NBS, initially launched as a pilot study in 1997, was introduced to the nationwide NBS program in 2013. In the present study, we evaluated levels of acylcarnitine with various chain lengths (C18 to C2), free carnitine, and their ratios in 175 infants who tested positive for VLCAD deficiency with C14:1 and C14:1/C2 ratios. Our analyses indicated that the ratios of C14:1 to medium-chain acylcarnitines (C10, C8, and C6) were the most effective markers in reducing false-positive rates. Their use with appropriate cutoffs is expected to improve NBS performance for VLCAD deficiency.
1. Introduction
Very-long-chain acyl-CoA dehydrogenase (VLCAD), an enzyme involved in fatty acid β-oxidation, is bound to the mitochondrial inner membrane and converts long-chain acyl-CoA supplied by carnitine palmitoyltransferase II into 2-enoyl-CoA with corresponding chain lengths. Since the first cases were reported in 1993 [1,2,3], VLCAD deficiency has been clinically classified into three phenotypes: (1) severe phenotype, characterized by cardiomyopathy associated with hypoglycemia developing in the neonatal and early infantile periods; (2) hypoglycemic phenotype, which mainly manifests during infancy and early childhood, provoking hypoketotic hypoglycemia, Reye-like encephalopathy, and, in more severe cases, cardiac arrest; and (3) myopathic phenotype, characterized by recurrent rhabdomyolysis with onset in adolescence or later [4]. The hypoglycemic phenotype can cause sudden infant death; therefore, it is included in newborn screening (NBS) panels in many countries.
Tetradecenoylcarnitine (C14:1) in dried blood specimens (DBSs) is commonly used as the primary marker for VLCAD deficiency in NBS [5,6]. Positive results are confirmed by analyzing serum or plasma acylcarnitine levels, which are usually more sensitive to detecting fatty acid oxidation disorders. Analysis of organic acid levels in urine may reveal nonketotic dicarboxylic aciduria, commonly observed during the catabolic state in patients with various fatty acid oxidation disorders. Abnormal findings in metabolite analysis are confirmed by measuring VLCAD activity or β-oxidation ability in blood mononuclear cells or skin fibroblasts and the genetic analysis of ACADVL. The number of ACADVL variants has greatly increased since introducing tandem mass spectrometry (MS/MS)-based NBS, including many novel variants of unknown clinical significance, for which quick and reliable evaluation of enzymatic function is essential [7,8,9].
NBS for VLCAD deficiency using C14:1 is associated with high false-positive rates [10]. Therefore, the ratio of C14:1 to acetylcarnitine (C2) has long been used as a secondary marker to improve specificity [1]. The utility of the C14:1 ratio to other acylcarnitines such as C16, C16-OH, C12, and C12:1 has also been reported [8,11,12,13,14,15,16]. In Japan, MS/MS-based NBS was launched as a pilot study in 1997 and introduced to the nationwide NBS program in 2013, which includes C14:1 and C14:1/C2 as markers for VLCAD deficiency [17]. We and our colleagues have offered confirmatory tests for NBS-positive infants and observed many patients with mild phenotypes that remain asymptomatic without medical management, and normal infants with false-positive test results [18,19]. In the present study, we evaluated these markers’ performance compared to various acylcarnitines and their ratios on a larger scale to identify more effective NBS markers for VLCAD deficiency.
2. Materials and Methods
2.1. Research Subjects
A total of 214 infants, who tested positive for VLCAD deficiency in NBS and were subsequently evaluated for VLCAD activity, were eligible for the present study. Among the infants showing impaired VLCAD activity, those with biallelic ACADVL variants were enrolled as patients with VLCAD deficiency (Group A). Infants who were heterozygous for ACADVL variants, including tentative cases, were enrolled as carriers (Group B). Those not confirmed by genetic analysis were excluded. Infants with normal-level VLCAD activity were enrolled as healthy infants (Group C). The utilities of levels of various acylcarnitines and free carnitine (C0) and their ratios in DBSs as NBS markers for VLCAD deficiency were evaluated by comparing the NBS data of these groups.
2.2. NBS Test for VLCAD Deficiency
DBSs for NBS were generally collected on postnatal day 4 or 5 according to the official protocol used since NBS for amino acid disorders began in 1977. DBSs collected using uniform filter paper (Advantec Toyo Kaisha, Tokyo, Japan) were analyzed by flow-injection electrospray-ionization MS/MS following a previously described protocol [20] with some modifications. During the pilot study conducted from 1997 to 2012, a C14:1 level of ≥0.4 nmol/mL and a C14:1/C2 ratio of ≥0.013 were used to identify newborns at risk of VLCAD deficiency. Both cutoff values corresponded to the 99.5th percentile of values in healthy newborns. Since 2013, nationwide NBS tests have been assigned to 35 regional laboratories, where the cutoff values were adjusted. The mean (±standard deviation) cutoffs used were 0.34 ± 0.06 nmol/mL and 0.013 ± 0.004 for the C14:1 level and C14:1/C2 ratio, respectively. Regarding MS/MS devices and sample preparation kits, including stable-isotope-labeled internal standards, products from several manufacturers were used in various combinations (MS/MS devices from AB Sciex, MA, USA; Waters, MA, USA; Shimadzu, Kyoto, Japan; internal standards from Siemens, Munich, Germany; PerkinElmer, MA, USA; Cambridge Isotope Laboratory, MA, USA; Sekisui Medical, Tokyo, Japan). Interlaboratory variations were evaluated regularly by external quality control tests for precision levels in MS/MS analysis, managing within ±15% of a reference value offered by the Quality Control Committee of the Japanese Society for Neonatal Screening [21].
2.3. Confirmatory Tests for VLCAD Deficiency
Generally, the serum acylcarnitine profile of infants with positive NBS results for VLCAD deficiency was determined by the same MS/MS method as for DBSs, using supernatant obtained after centrifugation of the mixture of serum and internal standard solution. Samples were obtained within one month after birth in most cases. In addition, the enzymatic activity of VLCAD was measured, as described in previous reports [18,22]. A high-performance liquid chromatography system (Shimadzu, Kyoto, Japan) was used to detect the production of 2-hexadecenoyl-CoA from palmitoyl-CoA and ferrocenium hexafluorophosphate (Sigma-Aldrich, Saint Louis, MO, USA), catalyzed by a crude lysate of peripheral lymphocytes isolated from NBS-positive infants. Lymphocytes were sonicated in 0.4% taurodeoxycholic acid (Sigma-Aldrich, Saint Louis MO, USA) solution to disrupt the mitochondrial membrane and liberate the enzyme. The CoA-derivatives were detected by ultraviolet spectrophotometry at 260 nm. The mean (±standard deviation) VLCAD activity in 54 normal control adults was 149.9 ± 57.1 pmol/min/106 cells.
For infants with impaired VLCAD activity, further genetic analysis was performed according to the methods described in a previous report [23] with some modifications after receiving informed consent from the infants’ parents. Genomic DNA was extracted from peripheral leukocytes. All exons and flanking intron regions containing ACADVL were amplified using a polymerase chain reaction, and the products were analyzed by direct sequencing. In some infants, gene panel sequencing provided by a national health insurance scheme targeting fatty acid oxidation disorders [24] was performed in Kazusa DNA Research Institute (Kisarazu, Chiba, Japan) according to the preference of the pediatrician in charge.
2.4. Receiver Operating Characteristic Analysis
Receiver operating characteristic (ROC) curves were generated to evaluate the sensitivity and specificity of various acylcarnitines and their ratios as markers for VLCAD deficiency. ROC analysis was performed using R and RStudio statistical programs (version 4.2.2; The R Foundation, Vienna, Austria). The Youden index was used to determine the optimal cutoff value for each marker.
3. Results
3.1. Diagnosis of NBS Positivity and Levels of Currently Used Markers
The characteristics of infants enrolled in the present study with positive NBS results for VLCAD deficiency are summarized in Table 1. Detailed results of NBS and confirmatory tests for each infant are listed in Supplementary Table S1.

Table 1.
Comparison between NBS-positive infants and symptomatic patients according to the classifications used in the present study.
Group A included 95 infants with biallelic ACADVL variants that were categorized according to their VLCAD activity: <20% (Group A-1: N-01 to N-73), 20–40% (Group A-2: N-74 to N-92), and >40% (Group A-3: N-93 to N-95). Group B included 39 infants heterozygous for ACADVL variants that were categorized according to a VLCAD activity: 20–40% (Group B-1: N-96 to N-118) and >40% (Group B-2: N-119 to N-134). Group C included 41 infants with a VLCAD activity of >70% (N-135 to N-175). In this group, VLCAD activity was defined as normal without confirmation by ACADVL sequencing in all but one infant (N-136).
Figure 1 shows the levels of C14:1 and C14:1/C2 in DBSs, C14:1 levels in serum, and VLCAD activity in the study groups. None of these markers could clearly distinguish Group A from Groups B or C.
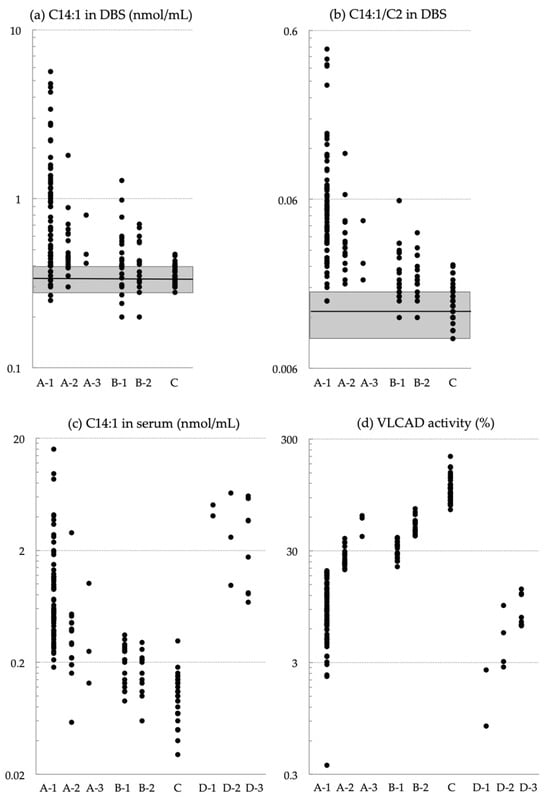
Figure 1.
(a,b) Levels of (a) C14:1 and (b) the C14:1/C2 ratio in dried blood specimens (DBSs). (c,d) Levels of (c) C14:1 in serum and (d) VLCAD activity in lymphocytes. Groups are NBS-positive infants with biallelic ACADVL variants and a VLCAD activity of <20% (A-1), 20–40% (A-2), and >40% (A-3); infants heterozygous for ACADVL variants with a VLCAD activity of 20–40% (B-1), and >40% (B-2). Infants with a VLCAD activity of >70% without confirmation by ACADVL sequencing (C). Shaded squares in (a,b) indicate the following cutoff ranges (mean ± standard deviation) across 35 regional laboratories (0.34 ± 0.06 nmol/mL for C14:1 and 0.013 ± 0.004 for the C14:1/C2 ratio). Group D includes patients with VLCAD deficiency symptoms diagnosed after clinical onset. The patients were categorized by severe phenotype (D-1), hypoglycemic phenotype (D-2), and myopathic phenotype (D-3).
For reference, we enrolled 14 symptomatic patients as Group D (Table 1 and Figure 1), which included patients with severe phenotype (Group D-1: S-01, S-02) and those with hypoglycemic (D-2: S-03 to S-06) and myopathic phenotypes (D-3: S-07 to S-14). These patients were born before the nationwide implementation of NBS and were not enrolled in the pilot study for MS/MS-based NBS. The confirmatory test results for each symptomatic patient are listed in Supplementary Table S1.
3.2. Comparing the C14:1 Level and C14:1/C2 Ratio to Various Acylcarnitines in DBSs
Supplementary Table S2 shows individual acylcarnitine levels (C18:1, C18, C16, C16-OH, C14:1, C14, C12, C10, C8, C6, C4, C3, and C2) and C0 in DBSs of newborns in the present study. Supplementary Figure S1 shows scatter plots of the data. Although none of the measured parameters could clearly differentiate between infants with and without VLCAD deficiency (Group A versus C), ROC analysis revealed that the area under the ROC curve (AUC) with the optimal cutoff was highest for a decrease in C8 (0.954), followed by decreases in C10 (0.938), C6 (0.928), C2 (0.877), and an increase in C14:1 (0.881) (Table 2). These results confirmed that the currently used C14:1 level is an appropriate primary marker for VLCAD deficiency. They also suggested that medium-chain acylcarnitines may be better denominators than C2 in improving the sensitivity and specificity of C14:1.

Table 2.
ROC analysis of specific acylcarnitine levels and free carnitine in dried blood specimens of newborns.
Supplementary Figure S2 shows scatter plots of C14:1 ratios to specific acylcarnitines and C0. According to the ROC analysis (Table 3), the C14:1/C8 ratio had the highest AUC (0.999), followed by the C14:1/C6 (AUC, 0.997), C14:1/C10 (AUC, 0.992), C14:1/C16-OH (AUC, 0.991), and C14:1/C2 (AUC, 0.978) ratios. Regarding sensitivity, the C14:1/C8 ratio exhibited the highest sensitivity (0.989), followed by the C14:1/C6 and C14:1/C10 ratios with sensitivities of 0.986 and 0.978, respectively. The specificities were 1.000 for all three ratios. The ROC curves of the C14:1/C10, C14:1/C8, and C14:1/C6 ratios are shown in Figure 2. The box plots comparing these ratios with the C14:1/C2 ratio and C14:1 level are shown in Figure 3.

Table 3.
ROC analysis of C14:1 ratios to specific acylcarnitines and C0 in dried blood specimens of newborns.
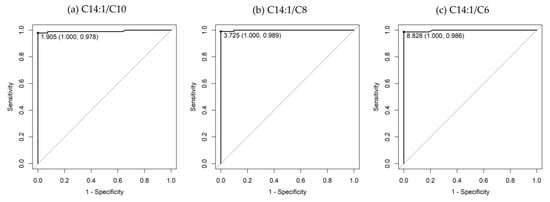
Figure 2.
ROC curves of (a) C14:1/C10, (b) C14:1/C8, and (c) C14:1/C6 ratios. The area under the ROC curve for the C14:1/C10, C14:1/C8, and C14:1/C6 ratios are 0.992 (95% confidence interval, 0.9784–1), 0.999 (95% confidence interval, 0.9967–1), and 0.997 (95% confidence interval, 0.9915–1), respectively.
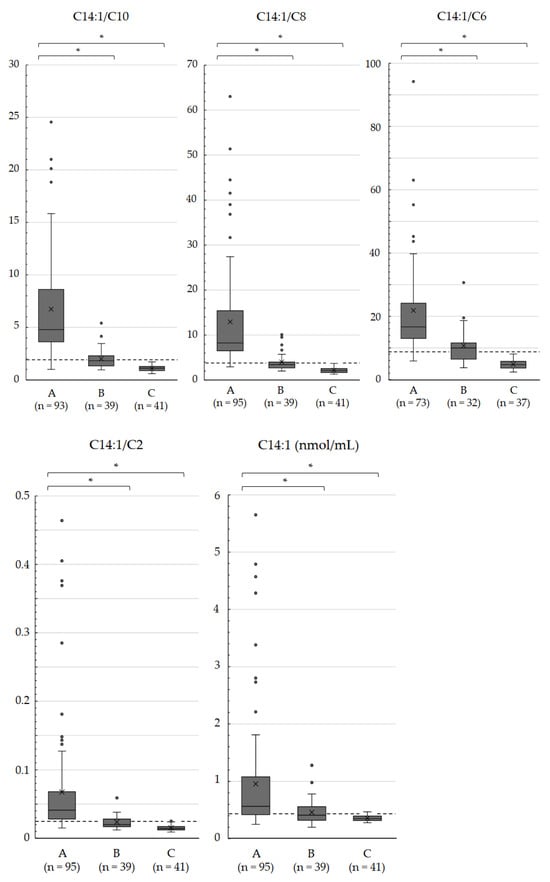
Figure 3.
Box plots of the C14:1/C10, C14:1/C8, C14:1/C6, and C14:1/C2 ratios and C14:1 levels in dried blood specimens of newborns. Infants with biallelic ACADVL variants A, infants heterozygous for ACADVL variants B, and infants with a VLCAD activity of >70% without confirmation by ACADVL sequencing C. Dashed lines indicate the optimal cutoffs shown by the ROC analysis. Horizontal lines and cross marks within the boxes indicate median values and mean values, respectively. * Significant differences were indicated using Welch’s t-test (p < 0.01).
4. Discussion
False-positive results in NBS can cause unnecessary anxiety for parents; therefore, markers that strike the best balance between sensitivity and specificity must be pursued. In the present study, we compared the levels and ratios of various acylcarnitines and C0 in the DBSs of 175 infants. Increased C14:1 levels exhibited the highest AUC values among long-chain acylcarnitines, which accumulate in patients with VLCAD deficiency. We also found that the AUC was higher for decreases in the medium-chain acylcarnitines C10, C8, and C6. As expected from these results, further analysis revealed that the AUCs for the C14:1/C10, C14:1/C8, and C14:1/C6 ratios were higher than those for the C14:1 level and C14:1/C2 ratio. The AUC for the C14:1/C16-OH ratio was also higher than that for the C14:1/C2 ratio. However, the C16-OH levels in newborn DBSs were too low for accurate measurements compared to other acylcarnitines included in NBS. The C14:1/C10, C14:1/C8, and C14:1/C6 ratios are promising markers, which is reasonable given the substrate specificity of VLCAD [25].
Considering the clinical symptoms of VLCAD deficiency and the availability of effective medical management, preventing false-negative results should be prioritized. Table 4 summarizes performance of the C14:1 level and the C14:1/C2, C14:1/C10, C14:1/C8, and C14:1/C6 ratios with their optimal cutoffs applied to the NBS data of infants in Groups A and C. The C14:1/C10 ratio yielded false-negative results in two patients, including one patient in Group A-2 (N-79) and another in Group A-3 (N-95). The C14:1/C8 and C14:1/C6 ratios yielded false-negative results in only one patient (N-95) who exhibited the highest VLCAD activity in Group A (62.0%). Our study cohort included infants with an indeterminate diagnosis (Group B) whose data were excluded from the ROC analysis. Our evaluation, specifically in Group B, indicated that the positivity rates based on the C14:1/C10, C14:1/C8, and C14:1/C6 ratios were 0.565, 0.478, and 0.571, respectively, in Group B-1 and 0.188, 0.250, and 0.455, respectively, in Group B-2. Assuming that patients with potential compound heterozygosity were more likely to be included in Group B-1 than in Group B-2, these ratios may also help detect this subset of patients with the lowest increase in false-positive rates.

Table 4.
Application of the C14:1 level and C14:1/C2, C14:1/C10, C14:1/C8, and C14:1/C6 ratios to NBS data using optimal cutoffs determined by ROC analysis.
Few studies outside Japan have recommended the C14:1 ratio to medium-chain acylcarnitines as NBS markers for VLCAD deficiency. Liebig et al. indicated that the C14:1/C8 and C14:1/C4 ratios were helpful, albeit without concrete data [7]. Boneh et al. detected six patients with VLCAD deficiency among 189,000 newborns and found that the C14:1 and C14 levels and C14:1/C10 ratio were elevated in these patients’ DBSs. However, the authors did not mention other acylcarnitines or their ratios [26]. Spiekerkoetter et al. presented data on a second-tier parameter [(C14 + C14:1 + C14:2)/(C8 + C6)] in two patients identified by NBS; however, this parameter had limited utility [8]. One study from China used the C14:1/C8 ratio as an NBS marker for VLCAD deficiency; however, details on its performance, such as its false-positive rate or evidence for choosing this marker, were not disclosed [27].
One major limitation of our study was the lack of reference values for NBS markers in VLCAD deficiency, except those for the currently used C14:1 level and C14:1/C2 ratio. The C14:1/C10, C14:1/C8, and C14:1/C6 ratio performances must be evaluated using nationwide NBS data. In addition, DBS sampling is conducted on postnatal day 4 or 5 in Japan, while it is scheduled between 24 and 48 h after birth in many countries [28] because the accumulation of fatty acylcarnitines in newborn blood rapidly decreases after feeding initiation. Therefore, our findings should be further validated according to specific countries’ NBS protocols.
5. Conclusions
In Japan, NBS for VLCAD deficiency, which was initially launched as a pilot study in 1997, has become a nationwide healthcare service since 2013. We confirmed VLCAD deficiency in 95 out of 175 infants based on NBS. The currently used C14:1 level and C14:1/C2 ratio markers are associated with high false-positive rates. Therefore, we performed an extensive evaluation to compare acylcarnitine levels of various chain lengths, ranging from C2 to C18, and C0. We also compared ratios of C14:1 to other acylcarnitines and C0 in the DBSs of 175 infants. Our analyses revealed that the C14:1/C10, C14:1C8, and C14:1/C6 ratios were most effective based on lower false-positive rates. Our future plans include retrospective and prospective nationwide studies to evaluate their performance. We ultimately aim to set appropriate cutoffs and reduce false-positive rates for VLCAD deficiency in Japan based on NBS results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijns10010015/s1. Table S1: Results of newborn screening and confirmatory tests of the infants enrolled in the present study and the diagnostic data of symptomatic patients acquired after the clinical onset used as reference; Table S2: Concentrations of acylcarnitines and free carnitine in dried blood specimens of newborns; Figure S1: Levels of various acylcarnitines and free carnitine in dried blood specimens of newborns; Figure S2: Ratios of C14:1 to various acylcarnitines and free carnitine in dried blood specimens of newborns.
Author Contributions
Project administration, funding acquisition, measurement of VLCAD activity, and original draft preparation, G.T.; genetic analysis, J.A., K.H., M.T., R.K. and F.S.; genetic analysis and funding acquisition, H.S.; acylcarnitine analysis of dried blood and serum specimens, M.Y. and Y.S.; supervision, S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Health and Labour Sciences Research Grants for (1) Health Research on Children, Youth and Families (grant no: H29-Sukoyaka–Shitei-001 to Go Tajima as chief investigator) and (2) Research on Rare and Intractable Diseases (grant no: 23FC1033 to Kimitoshi Nakamura as chief investigator) from the Ministry of Health, Labour, and Welfare of Japan, and by the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (grant no: JP20ek0109482 to Hideo Sasai as chief investigator and JP23ek0109636 to Takashi Hamazaki as chief investigator).
Institutional Review Board Statement
The study was conducted in accordance with the ethical standards of relevant committees on human experimentation (institutional and national) and the Helsinki Declaration of 1975 as revised in 2000. The study was approved by the Ethics Committee of the National Center for Child Health and Development (protocol no. 1497, approved on 28 June 2017; protocol no. 1544, approved on 22 August 2017; protocol no. 1603, approved on 26 October 2017; and protocol no. 1815, approved on 8 May 2018). Approval for enzymatic and genetic studies was also obtained from the Ethics Committees of Hiroshima University, the National Hospital Organization Kure Medical Center and Chugoku Cancer Center, the University of Fukui, and Gifu University.
Informed Consent Statement
Informed consent to perform confirmatory tests for VLCAD deficiency and publish the data was obtained from the parents of all infants involved in this study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We thank Ryoji Fujiki, Junichi Hosokawa, and Osamu Ohara (Kazusa DNA Research Institute, Kisarazu, Chiba, Japan) for conducting gene panel sequencing; Saki Fujihara and Chiyoko Yoshii (Hiroshima City Medical Association Clinical Laboratory, Hiroshima, Japan) for conducting NBS tests for the infants enrolled in this study; Keiko Konomura (National Institute of Public Health, Wako, Saitama, Japan), Nobuyuki Ishige (Tokyo Health Service Association, Tokyo, Japan), and Junji Hanai (Hokkaido Pharmaceutical Association Public Health Examination Center, Sapporo, Japan) for supporting statistical analyses; and Eri Hoshino (National Center for Child Health and Development, Tokyo, Japan) for English proofreading. Quality control tests for regional NBS laboratories were managed by Haruko Kitazawa, Takako Maeda, Akiko Shimura, and Kimiko Ozawa (National Center for Child Health and Development, Tokyo, Japan). Direct sequencing of ACADVL was performed at the Analysis Center of Life Science, Hiroshima University, at the Institute for Clinical Research, National Hospital Organization Kure Medical Center and Chugoku Cancer Center, and the Research Institute of National Center for Child Health and Development.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bertrand, C.; Largillière, C.; Zabot, M.T.; Mathieu, M.; Vianey-Saban, C. Very-long-chain acyl-CoA dehydrogenase deficiency: Identification of new inborn error of mitochondrial fatty acid oxidation in fibroblasts. Biochim. Biophys. Acta 1993, 1180, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Uchida, Y.; Kelley, R.I.; Marble, M.; Hofman, K.; Tonsgard, J.H.; Rhead, W.J.; Hashimoto, T. A novel disease with deficiency of mitochondrial very-long-chain acyl-CoA dehydrogenase. Biochem. Biophys. Res. Commun. 1993, 191, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Indo, Y.; Coates, P.M.; Hashimoto, T.; Tanaka, K. Identification of very-long-chain acyl-CoA dehydrogenase deficiency in three patients previously diagnosed with long-chain acyl-CoA dehydrogenase deficiency. Pediatr. Res. 1993, 34, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Andresen, B.S.; Olpin, S.; Poorthuis, B.J.; Scholte, H.R.; Vianey-Saban, C.; Wanders, R.; Ijlst, L.; Morris, A.; Pourfarzam, M.; Bartlett, K.; et al. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am. J. Hum. Genet. 1999, 64, 479–494. [Google Scholar] [CrossRef]
- McHugh, D.; Cameron, C.A.; Abdenur, J.E.; Abdulrahman, M.; Adair, O.; Al Nuaimi, S.A. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: A worldwide collaborative project. Genet. Med. 2011, 13, 230–254. [Google Scholar] [CrossRef] [PubMed]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Liebig, M.; Schymik, I.; Mueller, M.; Wendel, U.; Mayatepek, E.; Ruiter, J.; Strauss, A.W.; Wanders, R.J.; Spiekerkoetter, U. Neonatal screening for very long-chain acyl-coA dehydrogenase deficiency: Enzymatic and molecular evaluation of neonates with elevated C14:1-carnitine levels. Pediatrics 2006, 118, 1065–1069. [Google Scholar] [CrossRef]
- Spiekerkoetter, U.; Haussmann, U.; Mueller, M.; Ter Veld, F.; Stehn, M.; Santer, R.; Lukacs, Z. Tandem mass spectrometry screening for very long-chain acyl-CoA dehydrogenase deficiency: The value of second-tier enzyme testing. J. Pediatr. 2010, 157, 668–673. [Google Scholar] [CrossRef]
- Hesse, J.; Braun, C.; Behringer, S.; Matysiak, U.; Spiekerkoetter, U.; Tucci, S. The diagnostic challenge in very-long chain acyl-CoA dehydrogenase deficiency (VLCADD). J. Inherit. Metab. Dis. 2018, 41, 1169–1178. [Google Scholar] [CrossRef]
- Diekman, E.; de Sain-van der Velden, M.; Waterham, H.; Kluijtmans, L.; Schielen, P.; van Veen, E.B.; Ferdinandusse, S.; Wijburg, F.; Visser, G. The newborn screening paradox: Sensitivity vs. overdiagnosis in VLCAD deficiency. JIMD Rep. 2015, 27, 101–106. [Google Scholar] [CrossRef]
- Merritt, J.L., 2nd; Vedal, S.; Abdenur, J.E.; Au, S.M.; Barshop, B.A.; Feuchtbaum, L.; Harding, C.O.; Hermerath, C.; Lorey, F.; Sesser, D.E.; et al. Infants suspected to have very-long chain acyl-CoA dehydrogenase deficiency from newborn screening. Mol. Genet. Metab. 2014, 111, 484–492. [Google Scholar] [CrossRef]
- Merinero, B.; Alcaide, P.; Martín-Hernández, E.; Morais, A.; García-Silva, M.T.; Quijada-Fraile, P.; Pedrón-Giner, C.; Dulin, E.; Yahyaoui, R.; Egea, J.M.; et al. Four years’ experience in the diagnosis of very long-chain acyl-CoA dehydrogenase deficiency in infants detected in three Spanish newborn screening centers. JIMD Rep. 2018, 39, 63–74. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Gao, A.; Wang, Q.; Ma, J.; Li, H.; Wang, T. New ratios for performance improvement for identifying acyl-CoA dehydrogenase deficiencies in expanded newborn screening: A retrospective study. Front. Genet. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Osawa, Y.; Kobayashi, H.; Hasegawa, Y.; Fukuda, S.; Yamaguchi, S.; Taketani, T. Serum C14:1/ C12:1 ratio is a useful marker for differentiating affected patients with very long-chain acyl-CoA dehydrogenase deficiency from heterozygous carriers. Mol. Genet. Metab. Rep. 2019, 21, 100535. [Google Scholar] [CrossRef]
- Remec, Z.I.; Groselj, U.; Drole Torkar, A.; Zerjav Tansek, M.; Cuk, V.; Perko, D.; Ulaga, B.; Lipovec, N.; Debeljak, M.; Kovac, J.; et al. Very long-chain acyl-CoA dehydrogenase deficiency: High incidence of detected patients with expanded newborn screening program. Front. Genet. 2021, 12, 648493. [Google Scholar] [CrossRef]
- Upadia, J.; Noh, G.; Lefante, J.J.; Andersson, H.C. Biochemical and molecular characteristics among infants with abnormal newborn screen for very-long-chain acyl-CoA dehydrogenase deficiency: A single center experience. Mol. Genet. Metab. Rep. 2023, 37, 101002. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Taketani, T. Management and diagnosis of mitochondrial fatty acid oxidation disorders: Focus on very-long-chain acyl-CoA dehydrogenase deficiency. J. Hum. Genet. 2019, 64, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Kobayashi, H.; Tajima, G.; Hara, K.; Yamada, K.; Fukuda, S.; Hasegawa, Y.; Aisaki, J.; Yuasa, M.; Hata, I.; et al. The frequencies of very long-chain acyl-CoA dehydrogenase deficiency genetic variants in Japan have changed since the implementation of expanded newborn screening. Mol. Genet. Metab. 2022, 136, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.; Awano, H.; Nishida, K.; Fujioka, K.; Nishiyama, A.; Miyake, O.; Iijima, K. False positive cases of elevated tetradecenoyl carnitine in newborn mass screening showed significant loss of body weight. Mol. Genet. Metab. Rep. 2020, 24, 100634. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Hata, I.; Kikawa, Y.; Mayumi, M.; Tanaka, Y.; Sudo, M.; Kado, N. Modifications in electrospray tandem mass spectrometry for a neonatal-screening pilot study in Japan. J. Chromatogr. B Biomed. Sci. Appl. 1999, 731, 97–103. [Google Scholar] [CrossRef]
- Hanai, J. Current status and future tasks of quality assurance in newborn screening. Jpn. J. Neonatal Screen. 2023, 33, 31–40. [Google Scholar]
- Tajima, G.; Sakura, N.; Shirao, K.; Okada, S.; Tsumura, M.; Nishimura, Y.; Ono, H.; Hasegawa, Y.; Hata, I.; Naito, E.; et al. Development of a new enzymatic diagnosis method for very-long-chain acyl-CoA dehydrogenase deficiency by detecting 2-hexadecenoyl-CoA production and its application in tandem mass spectrometry-based selective screening and newborn screening in Japan. Pediatr. Res. 2008, 64, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Hasegawa, Y.; Murayama, K.; Ogawa, M.; Hasegawa, T.; Kawai, M.; Sakata, N.; Yoshida, K.; Yarita, H.; Imai, K.; et al. A new diagnostic test for VLCAD deficiency using immunohistochemistry. Neurology 2004, 62, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Nakama, M.; Sasai, H.; Kubota, M.; Hasegawa, Y.; Fujiki, R.; Okuyama, T.; Ohara, O.; Fukao, T. Novel HADHB mutations in a patient with mitochondrial trifunctional protein deficiency. Hum. Genome Var. 2020, 7, 10. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Vreken, P.; den Boer, M.E.J.; Wijburg, F.A.; van Gennip, A.H.; IJlst, L. Disorders of fatty acyl-CoA β-oxidation. J. Inherit. Metab. Dis. 1999, 22, 442–487. [Google Scholar] [CrossRef] [PubMed]
- Boneh, A.; Andresen, B.S.; Gregersen, N.; Ibrahim, M.; Tzanakos, N.; Peters, H.; Yaplito-Lee, J.; Pitt, J.J. VLCAD deficiency: Pitfalls in newborn screening and confirmation of diagnosis by mutation analysis. Mol. Genet. Metab. 2006, 88, 166–170. [Google Scholar] [CrossRef]
- Tong, F.; Chen, T.; Jiang, P.; Yang, R.; Zhao, Z.; Shu, Q. Analysis of ACADVL gene variations among nine neonates with very long-chain acyl-CoA dehydrogenase deficiency. Zhonghua Yi Xue Chuan Xue Za Zhi 2019, 36, 310–313. (In Chinese) [Google Scholar]
- Baker, J.J.; Burton, B.K. Diagnosis and clinical management of long-chain fatty-acid oxidation disorders: A review. touchREVIEWS Endocrinol. 2021, 17, 108–111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).