Abstract
We present a literature review which summarizes the data supporting one of the alternative perspectives on the effect of alcohol consumption on cognitive aging—the possible positive effect of low to moderate drinking. Some of the main theories about aging, the mechanisms of brain aging, and the pathogenesis of cognitive decline and dementia are briefly described. In this context, the putative mechanisms of the protective action of non-alcoholic components in alcoholic beverages or low doses of ethanol against oxidative stress, inflammation, mitochondrial dysfunction, brain insulin resistance, and production of amyloid-β peptides are presented. The review article does not favor the data selected and highlighted, but it aims at inspiring more interest and further research on the topic.
Introduction
Aging is defined as an age-related progressive inability of the body’s internal and genetic mechanisms to defend, maintain and repair themselves in order to continue to function effectively [1]. As a result, many harmful changes accumulate in the cells and tissues with age. The cell loss and dysfunction that occur during normal aging lead to impaired organ functioning and inter-organ communication in the physiological systems and the body as a whole. The declining biological functions decrease resistance and adaptability, while the risk of diseases and death increases [2,3].
The outlined life perspective logically raises two existential questions: 1) whether the inevitable can be delayed and 2) how to live the allotted time. From a scientific point of view, these issues belong to the field of the so-called Anti-Aging Medicine [1]. Numerous studies have focused on different means of slowing down aging and prolonging life. There have been many attempts to find optimal diets and lifestyles that contribute to healthy longevity. Inevitably, when it comes to nutrition, the question of alcohol consumption arises [4]. This literature review focuses on the currently available data about the beneficial effects of moderate alcohol drinking as an important component of “successful” aging—cognitive integrity. Understanding the mechanisms of the potentially beneficial effects of moderate alcohol use implies knowledge of normal brain aging.
Discussions
The process of aging
The research on age-related physiological changes and their causes reveals aging as an extremely complex and multifactorial process. There are over 300 theories about aging, which should not be considered as mutually exclusive, but rather complementary in an attempt to explain the signs of the normal aging process [5,6].
According to the theory of free radicals, aging is the result of the accumulation of endogenous reactive oxygen species (ROS), and the associated oxidative stress [7]. The mitochondrial theory of aging focuses on ROS-induced mitochondrial DNA damage [8,9]. The well-known link between inflammation and oxidative stress relates the inflammatory hypothesis of aging to the free radical theory of aging [10]. Overproduction or the uncontrolled release of reactive oxygen and nitrogen species and the reduced capacity of endogenous antioxidant mechanisms are the major causative factors of tissue inflammation. The dysfunction in the regulatory pathways, occurring with age, upsets the balance between anti- and proinflammatory agents in favor of the latter, and maintains chronic inflammation. The immune theory of aging suggests that a network of cellular and molecular mechanisms, including DNA repair systems, enzymatic and non-enzymatic antioxidant systems, the production of heat-shock proteins, etc. controls the aging process indirectly [11]. All these mechanisms limit the negative effects of various physical, chemical, biological, etc. stressors and increase life expectancy.
In addition, the neuroendocrine-immune hypothesis draws attention to the interaction between the nervous, endocrine, and immune systems including different neurotransmitters, neuropeptides, and cytokines [8]. According to the waste accumulation theory, the inability to effectively remove the non-degradable by-products of metabolism with subsequent accumulation of metabolic wastes contributes to cell aging [6]. Examples of extracellular deposits are cholesterol-containing plaques in blood vessels, and protein polymers, such as β-amyloid, in the central nervous system (CNS) (amyloid plaques). In summary, the current theories on aging demonstrate that different processes contribute to aging; they interact and simultaneously work at different levels of functional organization [12].
Brain aging and cognitive functions
As people advance in age, the efficiency of brain functions, in particular the cognitive ones, are negatively altered. By definition, cognitive functions encompass those mental processes through which knowledge is acquired and the understanding of the world occurs—perception, attention, memorization, reasoning, evaluation, problem-solving, decision making, creativity, and speech [13,14]. Observations show that the age-related decline in cognitive functions in healthy people begins in the third decade of life and continues throughout life. [4].
Aging is an irreversible process that depends on genetic and environmental factors and involves complicated metabolic and molecular mechanisms. The cardiometabolic mechanism reveals that the vascular pathology associated with obesity, type 2 diabetes, and the metabolic syndrome, contributes to cognitive decline and dementia in adults. However, the problems are not limited to reduced brain perfusion. An interesting aspect of the metabolic mechanisms is the defective insulin sensitivity and signaling in the brain [15,16,17]. Insulin regulates the energy metabolism of neurons, as well as their differentiation, growth, survival, synaptic plasticity, and neurotransmission. In the hippocampus, it contributes to the long-term potentiation, thus affecting learning and memory. Therefore, brain insulin resistance will disturb the above-mentioned processes. Recently, researchers have proposed the phrase “type 3 diabetes” for Alzheimer’s disease, because of the shared molecular and cellular characteristics between insulin resistance and cognitive decline in the elderly [18].
Vitamin D is another pleiotropic regulator, which is involved in the neurodegenerative processes that occur with aging. Vitamin D deficiency, which is common in elderly people, is considered a risk factor for cognitive decline and dementia [19,20,21]. Vitamin D plays a neurotrophic and neuroprotective role by stimulating the synthesis of neurotrophic agents such as nerve growth factor and neurotrophins in astrocytes and oligodendrocytes [22,23]; it regulates calcium homeostasis in the brain [24,25], it modulates brain neurotransmitters, and displays anti-inflammatory activity by suppressing the pro-inflammatory cytokines in the brain [26,27]. Moreover, vitamin D has an antioxidant activity and inhibits the generation of ROS, lipid peroxidation, and the inactivation of some antioxidant enzymes [28].
The free radical theory of aging postulates that free radical reactions contribute to aging. The brain is one of the most metabolically active organs, where a delicate balance is maintained between the mechanisms of production of free radicals and the mechanisms of brain defense [29]. The moderate production of ROS by mitochondria, described as a physiological level of oxidative stress, is known to up-regulate the program of mitochondrial biogenesis and the antioxidant capacity of the brain, and thus works in favor of brain protection. However, the accumulated oxidative damage and the deterioration of the mitochondrial function in the aging brain causes changes in cellular architecture. Therefore, it is logical to assume that the uncontrolled production of free radicals is a major factor in the loss of neuronal homeostasis and the development of neurodegenerative diseases [30].
Neuroinflammatory processes also contribute to brain aging. Experimental data from animal models with Alzheimer’s disease and Parkinson’s disease indicate that systemic inflammation initiates an exacerbated immune response in the CNS through the local innate immune system, the microglial cells. Microglia become easily susceptible to secondary inflammatory stimulation, which can elicit an excessive inflammatory response [31]. The sustained activation of microglia changes their functions and induces the increased secretion of proinflammatory cytokines and neurotoxic factors that contribute to the systemic inflammation and the progression of neurodegenerative diseases [32,33].
Sometimes, it is difficult to distinguish between the normal changes in the aging brain and the incipient neurodegenerative processes. Alterations in brain tissue can precede the occurrence of clinical signs and/or neuropathological lesions by years and can be detected in elderly individuals who do not show cognitive decline [34].
Therefore, a lot of effort has been invested in elucidating the pathogenesis of degenerative brain diseases and identifying reliable biomarkers for the diagnosis of preclinical and prodromal stages of dementia. Studies over the past 30 years have shown that the pathogenesis of Alzheimer’s disease involves a plethora of events related to the impaired production, degradation, and clearance of amyloid-β protein (Aβ). The initial events of this plethora include the overproduction of amyloid-β precursor protein (AβPP) caused by rare mutations in the AβPP, PSEN1 or PSEN2 genes, malfunctioning of Aβ-degrading proteases, and impaired Aβ clearance from the brain due to inefficient transport mechanisms [6,35].
The imbalance between production and clearance leads to an accumulation of excessive amounts of Aβ, which are thought to initiate a sequence of pathological changes such as loss of synapses and neurons, impaired glucose uptake, oxidative damage, the impairment of brain energy metabolism, tau hyperphosphorylation, and the formation of neurofibrillary tangles, amyloid plaque deposition and, ultimately, neurodegeneration. This complex cascade of pathological events continues in the course of the development of Alzheimer’s disease, and leads to the accumulation of structural and functional damage to the brain, causing the key symptoms of the disease [35]. In this context, in addition to the established diagnostic biomarkers of Alzheimer’s disease—cerebrospinal fluid Aβ42, total tau (tTau) and phosphorylated tau181 (pTau181) [36], the possibilities of detecting soluble forms of the amyloid precursor protein (APP) in cerebrospinal fluid and blood are also evaluated [37].
Brain-imaging techniques (PET, MRI) also present a high potential for the structural-topographic assessment of pathological changes [4]. Recently, views on the pathogenesis of Alzheimer’s disease have become more complex with the established role of oxidative damage associated with an increased production of ROS, the loss of mitochondrial function, altered metal homeostasis, and decreased antioxidant protection, which may affect the production and accumulation of Aβ and hyperphosphorylated Tau protein. The latter can become components of a vicious circle that can exacerbate mitochondrial dysfunction and ROS production [38].
The prevention and delay of cognitive aging through nutritional (food and drink) strategies
To find the “fountain of youth” has been the everlasting dream of humankind. Through hygiene, vaccines, antibiotics, insulin, and high-tech interventions, advanced medicine has led to increased life expectancy over the last century. This is currently a global trend. According to the data from the European Commission and Eurostat, by the year 2060, the elderly population (aged 65 and above) is expected to grow from 17.4% to almost 30% [4], while for the population over the age of 80, the increase is expected to increase fivefold [39]. This inevitably raises the question of the quality of life of this growing share of the human population, which largely depends on full cognition. As part of the efforts of “anti-aging” medicine, optimal means, interventions, and approaches for slowing down cognitive aging and the prevention of neurodegenerative diseases are sought [40]. However, it must be acknowledged that preventive methods have a limited capacity and that there are currently no means of stopping or reversing the aging process in humans. This gives rise to tendencies to develop complex strategies for maintaining cognitive health.
Preclinical and clinical studies on healthy individuals or people in the early stages of cognitive decline have shown the potential of nutrition to exert a beneficial effect on cognitive functions [4,41]. It is well known, not only in the scientific community, that long-term adherence to the Mediterranean diet (rich in fruits, vegetables, nuts, olive oil, moderately saturated with fish and poorer in red meat) is associated with better cognition in adults and reduced risk of dementia or Alzheimer’s disease [38,42,43]. Moderate drinking of mainly red wine is commonly reported as a component of the Mediterranean diet. Wine is not the only alcoholic beverage that is present on the table of different nations. This increases the scientific interest in the potential health benefits (including mental) of moderate alcohol consumption worldwide [44].
The interest is not only global, but also ancient. The Sumerian cuneiform and Egyptian papyri, dating back to 2200 BC, contain prescriptions for wine-based medicines [38,45]. In ancient Greece, Hippocrates (460-370 BC) considered wine to be part of a healthy diet and even prescribed it as a medicine for some diseases [45]. Speaking of ancient Greece, it is interesting to quote the poet Ebulus, who presents views on moderate wine consumption in one of his plays: “For moderation, three kiliks (three cups) are placed: one for health, the first to be emptied, the second for love and pleasure, and the third for sleep.” [46]. Historical data about the suitability of wine for the treatment of dementia, in particular, can be found in the first printed book on wine, written by the physician and theologian Arnaldus de Villa Nova in the 14th century [47]. The views and practices of ancient and medieval physicians do not seem unfounded, because studies and literature reviews from the late 20th and early 21st centuries provide evidence that moderate alcohol use in middle age and older age can be considered, albeit cautiously, protective against dementia in old age. An analysis of the results of the epidemiological studies published between 1998 and 2008 shows that light to moderate drinking is associated with a 38% lower risk of dementia and a 32% lower risk of developing Alzheimer’s disease [48,49].
Similar conclusions were reached by the meta-analysis of 15 studies, which revealed that light to moderate alcohol consumption in adulthood reduces the risk of dementia by 47% compared to abstainers. Solfrizzi and co-authors monitored alcoholic patients with mild cognitive dysfunction, which is considered an early symptom of Alzheimer’s disease. According to the observation, consuming up to 15g of alcohol per day reduces the rate of progression to dementia by approximately 85% [50]. Impressive in its scale is the prospective study by Zhang and co-workers, the results of which have been published recently [51]. A nationally representative sample of 1,887 participants (with a mean age of 61.8 years) assessed the association of alcohol consumption and age-related decline in cognitive function, using multiple cognitive measurements, which have been carried out for 12 years. The data show that low to moderate alcohol use is associated with better general cognition and better results in specific cognitive domains—word recall, mental status, and vocabulary. Low to moderate drinking is also associated with a slower rate of cognitive decline in these domains.
The findings are consistent with another study conducted among people living in a community (with a mean age of 73.2 years), where regular moderate alcohol consumption was associated with better cognitive function as compared to abstainers. Some of the prospective population-based studies have found a J-shaped, others a U-shaped curve in the link between alcohol consumption and the risk of cognitive dysfunction and dementia, which is considered evidence for the potential beneficial effects of moderate alcohol consumption [38,41,51,52,53,54,55,56]. This includes the cumulative effects of moderate alcohol consumption in youth and middle age—according to the data summarized by Stockley in 2015. Compared to abstainers, people who regularly consume moderate amounts of alcohol display higher scores in terms of attention stability, information processing speed, immediate or delayed word reproduction, recognition memory, working memory, and other cognitive dimensions.
The topic of the type of alcohol consumed is worth considering, along with the potentially beneficial effects of moderate alcohol consumption. Moussa et al. (2015) report that long-term moderate alcohol use does neither exacerbate nor accelerate the natural course of age-related decline in cognitive function [53]. Similar conclusions are drawn by Huang et al. (2002) [54] who, in a prospective study of 402 residents of the Kungsholmen district in Stockholm, aged 75 and above, found that light to moderate alcohol consumption (wine, beer, or liquor) was associated with a reduced incidence of dementia and Alzheimer’s disease. They hypothesize that this association may reflect a potentially protective effect of light to moderate alcohol consumption on the development of dementia and Alzheimer’s disease; this effect may be attributed to alcohol per se, and cannot be related to the type of alcoholic beverages consumed.
However, based on the data from other publications, it can be assumed that “In vino veritas. i.e. In wine, there is truth”. Neafsey and Collins, assessing the correlation between alcohol consumption and age-related decline in cognitive function, have found evidence for a beneficial effect of moderate alcohol consumption [55]. The analysis (based on the data from 14 countries) showed that wine has a more pronounced protective effect than beer and hard alcohol.
Another prospective study on 2,613 men and women aimed at assessing the association between alcohol consumption (total and different types of beverages) and cognitive decline in middle age (assuming that slowing the rate of cognitive decline leads to the preservation of cognitive functioning in old age) [56]. The global cognitive function and specific domains such as memory, speed, and flexibility were assessed twice at 5-year intervals. It has been found that moderate intake of red wine is associated with the smallest decline in global cognitive function, memory, and cognitive flexibility (especially in women), while moderate use of other alcoholic beverages (more common in men) does not correlate with the rate of cognitive decline.
These findings support the assumption that the non-alcoholic substances in red wine are most likely responsible for the cognitive preserving effect (see the next section). The beneficial effect of wine is also confirmed by the results of a study including 980 individuals aged 65 and above without dementia at baseline [57]. After 4 years of follow-up, 260 people developed dementia, (199 of them developed Alzheimer’s disease). The analysis of alcohol use and habits of the persons led the authors to the conclusion that the consumption of up to three servings of wine daily (33 g of alcohol) is associated with a lower risk of developing Alzheimer’s disease. According to a study assessing the association between average alcoholic intake and cognitive performance in 15,807 patients, daily alcohol consumption of less than 40 g for women and 80 g or less for men is associated with a decreased probability of cognitive impairment [58].

Table 1.
Excerpts from the cited reference list regarding drinking categories/doses and beneficial/not harmful/harmful effects of alcohol.
Table 1.
Excerpts from the cited reference list regarding drinking categories/doses and beneficial/not harmful/harmful effects of alcohol.
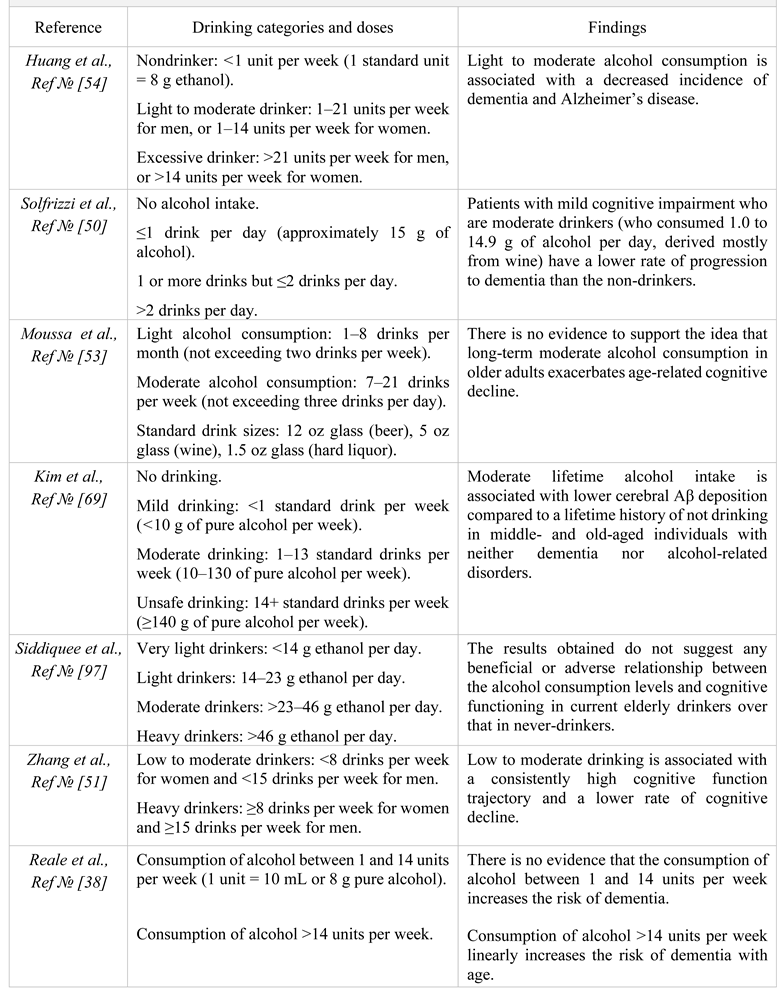 |
Biological mechanisms of the potential protective effect of alcoholic beverages on cognitive aging and dementia
Although there is evidence that the moderate consumption of any type of alcoholic beverage may have a protective effect on cognitive aging, wine is particularly suitable for discussing the biological mechanisms of this effect due to its rich composition of biologically active substances. More than 500 compounds have been identified in the fruit and wine of Vitis vinifera (grapevine) [41]. Wine contains alcohol, the largest amount being ethanol. The concentration of ethanol in table wines usually varies between 8 and 15 volume percent [59]. The main difference between wine and other alcoholic beverages is that wine contains phenolic compounds similar to those found in fruits, vegetables, and tea, the consumption of which is associated with a lower incidence of mild cognitive dysfunction, dementia, and other cerebrovascular neurodegenerative diseases. Wine contains two main classes of phenolic compounds: flavonoids and non-flavonoids. Their relative content depends on several factors such as the grape variety, climatic conditions, soil type and the type of cultivation, wine production technology, and wine aging. The main polyphenols in wine are flavanols, flavonols, anthocyanins, and resveratrol. Flavonoids include catechin, epicatechin, proanthocyanidins, flavones, and anthocyanins. Myricetin, kaempferol, rutin, and quercetin are representatives of flavonols. Delphinidin-3-glucoside, cyanidin-3-glucoside, and malvidin-3-glucoside are the most common anthocyanins in wine [38]. Some of the most important flavonoids in wine are anthocyanins, flavanols (catechins or flavan-3-ols), and flavonols (quercetin and myricetin); the group also includes proanthocyanidins, dimers, and oligomers of catechin and epicatechin and their gallic acid esters. Non-flavonoids are different classes of compounds that are substituted phenols. These include benzoic acid derivatives (vanillic and gallic), benzaldehydes (vanillin and syringe aldehyde), cinnamic acids (para-coumaric, ferulic, caffeic), and cinnamon aldehydes (mustard aldehyde) [60]. Non-phenolic compounds that contribute to the antioxidant activity of wine are ascorbic acid and sulfur dioxide [61]. Their natural content in wine is very small, but they are often added in the winemaking process. Sulfur dioxide inactivates the enzyme polyphenol oxidase [62], which is responsible for the oxidation of phenolic compounds, and thus contributes to the preservation of the polyphenolic content and antioxidant activity of wine.
Resveratrol is an important compound in wine with beneficial health effects. It is a natural phytoalexin that is produced by some plants in response to damage [63]. Resveratrol belongs to the class of organic compounds (stilbenes) and it is found in nature in two isoforms (i.e. cis- and trans-), the trans isoform being biologically active. Both isoforms are synthesized in the skin of grapes and reach maximum concentration before ripening. Stilbene synthase, the key enzyme in resveratrol biosynthesis, is activated by exogenous factors such as stress, UV light, chemical signals, and pathogens. Red grapes and red wine contain about 3 to 10 times more resveratrol than white ones, with an average trans-resveratrol content of 1.9–1.7 mg/L. According to our data, the highest polyphenolic content is found in red wines (average of 567 ± 32 mg/L), followed by rosé (323 ± 84 mg/L) and white wines (281 ± 42 mg/L) [64]. The data from animal studies reveal that phenolic compounds derived from grapes and wine are absorbed, cross the blood-brain barrier, and accumulate in the brain. Alcohol also plays an important role by promoting their absorption and thus increasing their bioavailability [65].
At present, it is difficult to state whether the possible health benefits of wine are due to ethanol, its micro-components, or to their synergistic effect. The complex beneficial effect of phenolic compounds and ethanol on the risk of cardiovascular and cerebrovascular diseases can be associated with changes in the lipid profile, hemostasis, and circulation [66]. In particular, an ethanol-induced increase in HDL-cholesterol concentration (which is inversely correlated with the risk of cardiovascular disorder) has been reported. Light alcohol intake was associated with an increased activity of tissue plasminogen activator and decreased levels of plasma coagulation factors such as fibrinogen, proconvertin, and von Willebrand factor [67,68]. All these changes are associated with a reduced risk of atherosclerosis, Alzheimer’s disease, and vascular dementia. The results obtained from human studies reveal that moderate alcohol intake is protective against vascular changes and atrophy in the brain, and ischemic brain injury [69]. From the above data, it can be concluded, that the main ingredients of wine will work against dementia and in favor of the cognitive functions, by counteracting atherosclerosis and protecting the brain from ischemia.
As previously mentioned, brain aging and cognitive decline are associated with oxidative stress, inflammation, mitochondrial dysfunction, and brain insulin resistance. In this regard, it is important to note that resveratrol can lower ROS by down-regulating enzymes such as xanthine oxidase, involved in the production of ROS and up-regulating endogenous antioxidant enzymes (superoxide dismutase, glutathione peroxidase, catalase, heme oxygenase) [70,71]. Resveratrol can counteract the production of mitochondrial ROS, resulting in increased mitochondrial activity and bioenergetic efficiency. It is considered that the inhibition of the ATP synthase by resveratrol might play a significant role in the pathophysiology of neurological disorders and aging [72]. Resveratrol modulates the activity of sirtuin 1 (SIRT1) and thus the regulation of p53, a key protein involved in DNA repair [73]. As a ligand of leukotriene A4 hydrolase, resveratrol exhibits anti-inflammatory and antioxidant activity [74]. The data on the ability of its analogs to reduce insulin resistance by improving energy homeostasis is intriguing given the reduced insulin effectiveness, accompanying brain aging [75]. Experiments have demonstrated the neuroprotective effects of resveratrol, as well as the positive effects on cognitive functions, learning, memory, and depressive state [76,77].
Red wine is a rich source of polyphenolic antioxidants such as flavonols (quercetin) and flavonoids (anthocyanins). Acting as an antioxidant, quercetin is capable of effectively removing ROS [78] and may have a protective effect in Alzheimer’s disease and oxidative stress-related neurodegenerative diseases. Quercetin exerts neuroprotective effects against the toxic molecules by modulating the mechanisms of cell death, increasing the resistance of neurons to oxidative stress, inhibiting the inducible nitric oxide synthase (iNOS), regulating cyclooxygenase-2 (COX-2), and exerting the anti-inflammatory activity [38]. The anthocyanins also exhibit antioxidant and anti-inflammatory effects by inhibiting inflammatory enzymes such as iNOS and COX-1 [79]. Oligonol, extracted from grape seeds and part of Vitis vinifera must also be added to the polyphenol list with antioxidant and anti-inflammatory effects [38,80]. Since oxidative stress and inflammation are linked to neurodegeneration, the polyphenols in wine may exert protective effects against neurodegenerative diseases.
What are the beneficial effects of wine ingredients in the pathogenesis of Alzheimer’s disease? This neurodegenerative disease is characterized by amyloid-β (Aβ) deposits and the accumulation of plaques in the brain. Recently, Kim et al. (2020) have published some data that moderate alcohol consumption throughout life is associated with lower Aβ deposition in the brain as compared to abstainers in middle-aged and elderly individuals [69]. Aβ peptide is generated by proteolytic cleavage of amyloid precursor protein (APP) by alpha, beta, and gamma secretases. Unlike alpha-secretase, which cleaves APP to the non-toxic amyloid alpha, toxic Aβ is derived by the sequential action of beta and gamma secretases. The most common isoforms that are produced are Aβ40 and Aβ42. In experiments with transgenic mice Tg2576 (an animal model of Alzheimer’s disease), it was found that the administration of red wine in doses equivalent to two standard drinks leads to non-amyloidogenic processing of APP and thus prevents Aβ production. The levels of amyloidogenic Aβ1-40 and Aβ1-42 in the neocortex and the hippocampus of mice were decreased, while the alpha-secretase activity was increased [81]. Resveratrol in red wine has been shown to have beneficial effects on the pathogenesis of Alzheimer’s disease by affecting some molecular mechanisms of the amyloid cascade. It lowers intracellular Aβ levels by inhibiting the formation of Aβ fibrils and promoting autophagic and lysosomal degradation of Aβ, it reduces tau-phosphorylation and deposition, as well as Aβ-induced ROS production [41,82]. Other polyphenols also demonstrated beneficial effects on the pathogenesis of Alzheimer’s disease: quercetin inhibits Aβ synthesis, and epigallocatechin-3-gallate modulates APP processing by acting on the non-amyloidogenic α-secretase pathway and counteracting Aβ-induced oxidative stress [38]. Aβ aggregation has been recognized as a necessary condition for Aβ-neurotoxicity. In vitro and animal studies have shown that grape-seed derived polyphenolics (containing mostly catechin and epicatechin) prevent Aβ oligomerization and reduce the cognitive impairment in a model with Alzheimer’s disease [83].
Protein kinase C (PKC) is a family of protein kinase enzymes, involved in signal transduction pathways; it is also important in memory processes and learning [84]. PKC modulates APP processing and can reduce the accumulation of pathogenic Aβ in the brain [85]. Resveratrol and epigallocatechin-3-gallate have been reported to protect hippocampal neurons from Aβ-induced toxicity by activating PKC [41].
Studies reveal changes in specific neurotransmitter systems with normal aging and more deficits in dementia. Severe loss of cholinergic neurons in the nucleus basalis is a feature of Alzheimer’s disease [86]. There is a reason to believe that the impaired cholinergic neurotransmission may also contribute to the formation of amyloid fibrillar plaques. In this regard, it is worth noting that ethanol (in low concentrations) can stimulate the release of acetylcholine in the hippocampus with a positive effect on cognitive functions [66]. This may explain the observation that drinking small amounts of alcohol improves the memory of the events that occurred before consumption in healthy individuals [87]. There is also evidence that acetylcholine levels may be positively affected by catechins (epigallocatechin-3-gallate), which act as acetylcholinesterase inhibitors [88]. The inhibition of acetylcholinesterase contributes to the restoration of cholinergic neurotransmission and appears to prevent the aggregation of amyloid-β peptides and the formation of amyloid fibrillar plaques [89].
A study on the effect of ethanol on the kinetics of β-amyloid aggregation in vitro revealed that ethanol prevents the formation of stable Aβ dimers in vitro, thus protecting the cells maintained in culture [90]. The research of Bate and Williams on the effects of ethanol on cultured cortical and hippocampal neurons provides evidence that moderate alcohol consumption prevents dementia in Alzheimer’s disease. Ethanol administered in physiologically relevant concentrations of 0.02–0.08% protected neurons against the synapse damage induced by Aβ42, a neurotoxin responsible for memory defects occurring in Alzheimer’s disease [91].
While looking for approaches to counteract the neurodegenerative processes in the brain associated with oxidative damage as a result of high ROS production (generated, for example, by neurotoxic agents such as Aβ), scientists have recently discovered the potential of neurotrophins [92]. The brain-derived neurotrophic factor (BDNF) is known to be a key regulator of neuronal plasticity and development. Upon binding to its receptor, BDNF activates a transduction-signaling pathway that is common to the process of stimulating cell proliferation and/or inhibiting the apoptosis cascade. Accordingly, it is interesting to point out that moderate alcohol consumption increases BDNF levels [93]. Regarding neurotrophins, vitamin D is worth being mentioned again, because it exerts its multifaceted neuroprotective effects by regulating the synthesis of neurotrophins. Several studies on humans presented data that alcohol consumption is positively associated with vitamin D status and reduced risk of vitamin D deficiency [94,95,96]. The mechanism by which alcohol can affect serum vitamin D concentrations is unclear at the moment, but the effects should not be overlooked when talking about alcohol consumption, neuroprotection, and cognitive well-being.
Highlights
- ✔
- Prospective population-based studies have revealed a J-shaped or a U-shaped curve in the link between alcohol consumption and the risk of cognitive dysfunction and dementia, which is considered evidence for the potential beneficial effects of moderate alcohol consumption.
- ✔
- The more common opinion is that red wine has a more pronounced protective effect than other alcoholic beverages.
- ✔
- The main ingredients of wine act against dementia and in favor of the cognitive functions because phenolic compounds and ethanol exhibit anti-inflammatory and antioxidant activity, and reduce insulin resistance, inhibit Aβ synthesis and lower the intracellular Aβ levels, tau-phosphorylation, and amyloid plaque deposition, thus counteracting the Aβ-induced synaptic damage.
Conclusions
The data presented in this article reveal only a small part of the discussion on the potentially beneficial effects of moderate alcohol consumption on cognitive aging and dementia, and the biological mechanisms of the beneficial effects of alcohol and alcoholic beverages. Further research is needed to confirm the data cited and to refute the other common opinions (supported by reports in the scientific literature) about the lack of beneficial effects of moderate alcohol consumption on the cognitive functions in the aging process [97,98,99,100].
Conflicts of Interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- Arora, B.P. Anti-aging medicine. Indian J Plast Surg 2008, 41, S130–S133. [Google Scholar] [CrossRef][Green Version]
- Lipsitz, L.A. Aging as a process of complexity loss. In Complex Systems Science in Biomedicine; Deisboeck, T.S., Kresh, J.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 641–654. ISBN 978-0-387-33532-2. [Google Scholar]
- Libertini, G. Aging Definition. In Encyclopedia of Gerontology and Population Aging; Gu, D., Dupre, M.E., Eds.; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res Rev 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, Z.A. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990, 65, 375–398. [Google Scholar] [CrossRef]
- Sergiev, P.V.; Dontsova, O.A.; Berezkin, G.V. Theories of aging: An ever-evolving field. Acta Naturae 2015, 7, 9–18. [Google Scholar] [CrossRef]
- Harman, D. The free radical theory of aging. Antioxid Redox Signal 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. Clin Interv Aging 2007, 2, 401–412. [Google Scholar] [PubMed]
- Jin, K. Modern biological theories of aging. Aging Dis. 2010, 1, 72–74. [Google Scholar]
- Cesari, M.; Kritchevsky, S.B.; Leeuwenburgh, C.; Pahor, M. Oxidative damage and platelet activation as new predictors of mobility disability and mortality in elders. Antioxid Redox Signal. 2006, 8, 609–619. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Valensin, S.; Bonafe, M.; Paolisso, G.; Yashin, A.I.; Monti, D.; De Benedictis, G. The network and remodeling theories of aging: Historical background and new perspectives. Exp Gerontol. 2000, 35, 879–896. [Google Scholar] [CrossRef]
- Anderson, L.W.; Krathwohl, D.R. A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Objectives; Longman Publishing: New York, NY, USA, 2001. [Google Scholar]
- Dietrich, A. The cognitive neuroscience of creativity. Psychon Bull Rev. 2004, 11, 1011–1026. [Google Scholar] [CrossRef]
- Craft, S.; Cholerton, B.; Baker, L.D. Insulin and Alzheimer’s disease: Untangling the web. J Alzheimers Dis. 2013, 33 (Suppl. S1), S263–S275. [Google Scholar] [CrossRef]
- Calvo-Ochoa, E.; Arias, C. Cellular and metabolic alterations in the hippocampus caused by insulin signaling dysfunction and its association with cognitive impairment during aging and Alzheimer’s disease: Studies in animal models. Diabetes Metab Res Rev 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.; McNeilly, A.; Sutherland, C. Insulin resistance in the brain: Anold-age or new-age problem? Biochem Pharmacol 2012, 84, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Thirumala, V.; Hemachandra, P. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.; Shoaib, A.; Gorthy, G.; Grossberg, G.T. The role of vitamin D in cognitive disorders in older adults. US Neurology 2018, 14, 41–46. [Google Scholar] [CrossRef]
- Rimmelzwaan, L.M.; van Schoor, N.M.; Lips, P.; Berendse, H.W.; Eekhoff, E.M. Systematic review of the relationship between vitamin D and Parkinson’s disease. J Parkinsons Dis 2016, 29, 29–37. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Vitamin D deficiency is associated with increased risk of Alzheimer’s disease and dementia: Evidence from meta-analysis. Nutr J. 2015, 14, 76. [Google Scholar] [CrossRef]
- Baas, D.; Prüfer, K.; Ittel, M.E.; Kuchler-Bopp, S.; Labourdette, G.; Sarliève, L.L.; Brachet, P. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000, 59–68. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wu, J.N.; Cherng, T.L.; Hoffer, B.J.; Chen, H.H.; Borlongan, C.V.; Wang, Y. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res 2001, 904, 67–75. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS ONE 2011, 6, e17553. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001, 21, 98–108. [Google Scholar] [CrossRef]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Banerjee, A.; Khemka, V.K.; Ganguly, A.; Roy, D.; Ganguly, U.; Chakrabarti, S. Vitamin D and Alzheimer’s disease: Neurocognition to therapeutics. Int J Alzheimers Dis. 2015, 2015, 192747. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beauchet, O. Vitamin D-mentia: Randomized clinical trials should be the next step. Neuroepidemiology. 2011, 37, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Munshi, S.; Banerjee, K.; Thakurta, I.G.; Sinha, M.; Bagh, M.B. Mitochondrial dysfunction during brain aging: Role of oxidative stress and modulation by antioxidant supplementation. Aging Dis 2011, 2, 242–256. [Google Scholar]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat Rev Neurol 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Perry, V.H. The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain Behav Immun 2004, 18, 407–413. [Google Scholar] [CrossRef]
- Perry, V.H.; Cunningham, C.; Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 2007, 7, 161–167. [Google Scholar] [CrossRef]
- Giaccone, G.; Arzberger, T.; Alafuzoff, I.; Al-Sarraj, S.; Budka, H.; Duyckaerts, C.; Falkai, P.; Ferrer, I.; Ironside, J.W.; Kovacs, G.G.; et al. New lexicon and criteria for the diagnosis of Alzheimer’s disease. Lancet Neurol 2011, 10, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Jack Jr, C.R.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Perneczky, R.; Alexopoulos, P.; Kurz, A. Soluble amyloid precursor proteins and secretases as Alzheimer’s disease biomarkers. Trends Mol Med 2014, 20, 8–15. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of wine consumption with Alzheimer’s disease. Nutrients 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Butler, R.N.; Fossel, M.; Harman, M.; Heward, C.B.; Olshansky, S.J.; Perls, T.T.; Rothman, D.J.; Rothman, S.M.; Warner, H.R.; West, M.D.; et al. Is there an Antiaging Medicine? J Gerontol A Biol Sci Med Sci 2002, 57, B333–B338. [Google Scholar] [CrossRef][Green Version]
- Stockley, C.S. Wine consumption, cognitive function and dementias—A relationship? Nutr Aging 2015, 3, 125–137. [Google Scholar] [CrossRef]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary patterns and risk of dementia: A systematic review and meta-analysis of cohort studies. Mol Neurobiol 2016, 53, 6144–6154. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Hoang, T.; Sidney, S.; Steffen, L.M.; Jacobs DRJr Shikany, J.M.; Wilkins, J.T.; Yaffe, K. Dietary patterns during adulthood and cognitive performance in midlife: The CARDIA study. Neurology 2019, 92, e1589–e1599. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J. (Ed.) The Oxford Companion to Wine, 3rd ed.; Oxford University Press: Oxford, UK, 2006; p. 433. [Google Scholar]
- Liappas, J.A.; Lascaratos, J.; Fafouti, S.; Christodoulou, G.N. Alexander the Great’s relationship with alcohol. Addiction 2003, 98, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, T.; Crouvisier-Urion, K.; Lagorce, A.; Ballester, J.; Geoffroy, A.; Roullier-Gall, C.; Chanut, J.; Gougeon, R.D.; Schmitt-Kopplin, P.; Bellat, J.P. Wine aging: A bottleneck story. NPJ Sci Food. 2019, 3, 14. [Google Scholar] [CrossRef]
- Peters, R.; Peters, J.; Warner, J.; Beckett, N.; Bulpitt, C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age Ageing 2008, 37, 505–512. [Google Scholar] [CrossRef]
- Anstey, K.J.; Mack, H.A.; Cherbuin, N. Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. Am J Geriatr Psychiatry 2009, 17, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; D’Introno, A.; Colacicco, A.M.; Capurso, C.; Gagliardi, G.; Santamato, A.; Baldassarre, G.; Capurso, A.; Panza, F. Lifestyle-related factors, alcohol consumption, and mild cognitive impairment. J Am Geriatric Soc 2007, 55, 1679–1681. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, L.; Miles, T.; Shen, Y.; Cordero, J.; Qi, Y.; Liang, L.; Li, C. Association of low to moderate alcohol drinking with cognitive functions from middle to older age among US adults. JAMA Netw Open 2020, 3, e207922. [Google Scholar] [CrossRef]
- Richard, E.L.; Kritz-Silverstein, D.; Laughlin, G.A.; Fung, T.T.; Barrett-Connor, E.; McEvoy, L.K. Alcohol intake and cognitively healthy longevity in community-dwelling adults: The Rancho Bernardo Study. J Alzheimers Dis 2017, 59, 803–814. [Google Scholar] [CrossRef]
- Moussa, M.N.; Simpson, S.L.; Mayhugh, R.E.; Grata, M.E.; Burdette, J.H.; Porrino, L.J.; Laurienti, P.J. Long-term moderate alcohol consumption does not exacerbate age-related cognitive decline in healthy, community-dwelling older adults. Front Aging Neuroscience. 2015, 6, 341. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, C.; Winblad, B.; Fratiglioni, L. Alcohol consumption and incidence of dementia in a community sample aged 75 years and older. J Clin Epidemiol 2002, 55, 959–964. [Google Scholar] [CrossRef]
- Neafsey, E.J.; Collins, M.A. Moderate alcohol consumption and cognitive risk. Neuropsychiatr Dis Treat. 2011, 7, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Nooyens, A.C.J.; Bueno-de-Mesquita, H.B.; van Gelder, B.M.; van Boxtel, M.P.J.; Verschuren, W.M.M. Consumption of alcoholic beverages and cognitive decline at middle age: The Doetinchem Cohort Study. Br J Nutr 2014, 111, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.X.; Siddiqui, M.; Shea, S.; Mayeux, R. Alcohol intake and risk of dementia. J Am Geriatric Soc 2004, 52, 540–546. [Google Scholar] [CrossRef]
- Zuccala, G.; Onder, G.; Pedone, C.; Cesari, M.; Landi, F.; Bernabei, R.; Cocchi, A. Dose-related impact of alcohol consumption on cognitive function in advanced age: Results of a multicenter survey. Alcohol Clin Exp Res 2001, 25, 1743–1748. [Google Scholar] [CrossRef]
- Bao, Q.; Zhao, H.; Han, S.; Zhang, C.; Hasi, W. Surface-enhanced Raman spectroscopy for rapid identification and quantification of Flibanserin in different kinds of wine. Anal Methods 2020, 12, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Cassino, C.; Gianotti, V.; Bonello, F.; Tsolakis, C.; Cravero, M.; Osella, D. Antioxidant composition of a selection of Italian red wines and their corresponding free-radical scavenging ability. J Chem. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Barril, C.; Clark, A.C.; Scollary, G.R. Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Anal Chim Acta. 2012, 732, 186–193. [Google Scholar] [CrossRef]
- Coetzee, C.; Lisjak, K.; Nicolau, L.; Kilmartin, P.; du Toit, W.J. Oxygen and sulfur dioxide additions to Sauvignon blanc must: Effect on must and wine composition. Flavour Fragr J 2013, 28, 155–167. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Calvello, R.; Panaro, M.A. Reviewing the role of resveratrol as a natural modulator of microglial activities. Curr Pharm Des 2015, 21, 5277–5291. [Google Scholar] [CrossRef]
- Todorova, M.N.; Pasheva, M.G.; Kiselova-Kaneva, Y.D.; Ivanova, D.G.; Galunska, B.T. Phenolics content and antioxidant activity of beverages on the Bulgarian market—Wines, juices and compotes. Bulg Chem Commun. 2018, 50, 164–168. [Google Scholar]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How much wine do you have to drink to stay healthy? Adv Nutr 2016, 7, 706–718. [Google Scholar] [CrossRef]
- Wiegmann, C.; Mick, I.; Brandl, E.J.; Heinz, A.; Gutwinski, S. Alcohol and dementia—What is the link? A systematic review. Neuropsychiatr Dis Treat. 2020, 16, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Tabengwa, E.M.; Grenett, H.E.; Benza, R.L.; Abou-Agag, L.H.; Tresnak, J.K.; Wheeler, C.G.; Booyse, F.M. Ethanol-induced up-regulation of the urokinase receptor in cultured human endothelial cells. Alcohol Clin Exp Res 2001, 25, 163–170. [Google Scholar]
- Lee, K.W.; Lip, G.Y.H. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: A systematic review. Arch Intern Med 2003, 163, 2368–2392. [Google Scholar] [CrossRef]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Ko, K.; Jeon, S.Y.; Sohn, B.K.; Lee, J.Y.; Kim, Y.K.; Shin, S.A.; et al. Association of moderate alcohol intake with in vivo amyloid-beta deposition in human brain: A cross-sectional study. PLoS Med 2020, 17, e1003022. [Google Scholar] [CrossRef] [PubMed]
- Gerszon, J.; Rodacka, A.; Puchała, M. Antioxidant properties of resveratrol and its protective effects in neurodegenerative diseases. Adv Cell Biol. 2014, 4, 97–117. [Google Scholar] [CrossRef]
- Desquiret-Dumas, V.; Gueguen, N.; Leman, G.; Baron, S.; Nivet-Antoine, V.; Chupin, S.; Chevrollier, A.; Vessières, E.; Ayer, A.; Ferré, M. Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells. J Biol Chem 2013, 288, 36662–36675. [Google Scholar] [CrossRef]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Davies, D.R.; Mamat, B.; Magnusson, O.T.; Christensen, J.; Haraldsson, M.H.; Mishra, R.; Pease, B.; Hansen, E.; Singh, J.; Zembower, D.; et al. Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. J Med Chem 2009, 52, 4694–4715. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Ge, L.; Liu, L.; Liu, H.; Liu, S.; Xue, H.; Wang, X.; Yuan, L.; Wang, Z.; Liu, D. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol. 2015, 768, 49–57. [Google Scholar] [CrossRef]
- Ge, J.F.; Xu, Y.Y.; Li, N.; Zhang, Y.; Qiu, G.L.; Chu, C.H.; Wang, C.Y.; Qin, G.; Chen, F.H. Resveratrol improved the spatial learning and memory in subclinical hypothyroidism rat induced by hemi-thyroid electrocauterization. Endocr J 2015, 62, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Chervenkov, T.; Gerova, D.; Galunska, B.; Enchev, V. Theoretical and experimental evaluation of antioxidant potential of natural bioflavonoids rutin and quercetin: PP8C-9. FEBS J 2008, 275, 377. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Bahijri, S.M.; Ajabnoor, G.; Hegazy GAAlsheikh, L.; Moumena, M.Z.; Bashanfar, B.M.; Alzahrani, A.H. Supplementation with oligonol, prevents weight gain and improves lipid profile in overweight and obese saudi females. Curr Nutr Food Sci 2018, 14, 164–170. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates A beta neuropathology in a mouse model of Alzheimer’s disease. FASEB J 2006, 20, 2313–2320. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J Biol Chem 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.H.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci 2008, 28, 6388–6392. [Google Scholar] [CrossRef]
- Sweatt, J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 2004, 14, 311–317. [Google Scholar] [CrossRef]
- Kim, T.; Hinton, D.J.; Choi, D.S. Protein kinase C-regulated aβ production and clearance. Int J Alzheimers Dis. 2011, 2011, 857368. [Google Scholar] [CrossRef] [PubMed]
- Mega, M.S. The cholinergic deficit in Alzheimer’s disease: Impact on cognition, behaviour and function. Int J Neuropsychopharmacol 2000, 3, 3–12. [Google Scholar] [CrossRef]
- Fadda, F.; Rossetti, Z.L. Chronic ethanol consumption: From neuroadaptation to neurodegeneration. Progress Neurobiol 1998, 56, 385–431. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.J.; Aldunate, R.; Inestrosa, N.C. Peripheral binding site is involved in the neurotrophic activity of acetylcholinesterase. Neuroreport 1999, 10, 3621–3625. [Google Scholar] [CrossRef]
- Heymann, D.; Stern, Y.; Cosentino, S.; Tatarina-Nulman, O.; Dorrejo, J.N.; Gu, Y. The association between alcohol use and the progression of Alzheimer’s disease. Curr Alzheimer Res 2016, 13, 1356–1362. [Google Scholar] [CrossRef]
- Bate, C.; Williams, A. Ethanol protects cultured neurons against amyloid-β and α-synuclein-induced synapse damage. Neuropharmacology 2011, 61, 1406–1412. [Google Scholar] [CrossRef]
- Habtemariam, S. The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: New pharmacological concepts for old and new drugs. Neural Regen Res 2018, 13, 983–984. [Google Scholar] [CrossRef]
- Logrip, M.L.; Barak, S.; Warnault, V.; Ron, D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015, 1628 Pt A, 60–67. [Google Scholar] [CrossRef]
- van Grootheest, G.; Milaneschi, Y.; Lips, P.T.A.M.; Heijboer, A.C.; Smit, J.H.; Penninx, B.W.J.H. Determinants of plasma 25-hydroxyvitamin D levels in healthy adults in the Netherlands. Neth J Med 2014, 72, 533–540. [Google Scholar]
- Gorter, E.A.; Krijnen, P.; Schipper, I.B. Vitamin D deficiency in adult fracture patients: Prevalence and risk factors. Eur J Trauma Emerg Surg 2016, 42, 369–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tardelli, V.S.; Lago, M.P.P.D.; Silveira, D.X.D.; Fidalgo, T.M. Vitamin D and alcohol: A review of the current literature. Psychiatry Res. 2017, 248, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, A.T.; Kadota, A.; Fujiyoshi, A.; Miyagawa, N.; Saito, Y.; Suzuki, H.; Kondo, K.; Yamauchi, H.; Ito, T.; Segawa, H.; et al. Alcohol consumption and cognitive function in elderly Japanese men. Alcohol. 2020, 85, 145–152. [Google Scholar] [CrossRef]
- Velikova, M.; Stoyanov, Z. Alcohol and the cognitive functions of the aging brain. Journal of the Union of Scientists—Varna. Medicine and Ecology Series 2019, 24, 61–65. [Google Scholar] [CrossRef]
- Topiwala, A.; Allan, C.L.; Valkanova, V.; Zsoldos, E.; Filippini, N.; Sexton, C.; Mahmood, A.; Fooks, P.; Singh-Manoux, A.; Mackay, C.E.; et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ. 2017, 357, j2353. [Google Scholar] [CrossRef]
- Albu, C.V.; Pădureanu, V.; Boldeanu, M.V.; Bumbea, A.M.; Enescu, A.Ş.; Albulescu, D.M.; Siloși, C.A.; Enescu, A. Vascular neurocognitive disorders and the vascular risk factors. J Mind Med Sci 2018, 5, 7–5. [Google Scholar] [CrossRef]
© 2021 by the author. 2021 Margarita Velikova, Bistra Galunska, Raya Dimitrova, Zlatislav Stoyanov