The Importance of Cardiac T2* Magnetic Resonance Imaging for Monitoring Cardiac Siderosis in Thalassemia Major Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Magnetic Resonance Imaging Interpretation
2.3. Echocardiography
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Borgna-Pignatti, C.; Rugolotto, S.; De Stefano, P.; Zhao, H.; Cappellini, M.D.; Del Vecchio, G.C.; Romeo, M.A.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004, 89, 1187–1193. [Google Scholar]
- Olivieri, N.F.; Brittenham, G.M. Iron-chelating therapy and the treatment of thalassemia. Blood 1997, 89, 739–761. [Google Scholar] [CrossRef] [PubMed]
- Borgna-Pignatti, C.; Cappellini, M.D.; De Stefano, P.; Vecchio, G.C.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; Piga, A.; Romeo, M.A.; Zhao, H.; et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood 2006, 107, 3733–3737. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Khan, M.; Darlison, M. Survival in beta thalassaemia major in the UK: Data from the UK Thalassaemia Register. Lancet 2000, 355, 2051–2052. [Google Scholar] [CrossRef]
- Tanner, M.A.; Galanello, R.; Dessi, C.; Smith, G.C.; Westwood, M.A.; Agus, A.; Pibiri, M.; Nair, S.V.; Walker, J.M.; Pennell, D.J. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J. Cardiovasc. Magn. Reson. 2008, 10, 12. [Google Scholar] [CrossRef]
- Davis, B.A.; O’Sullivan, C.; Jarritt, P.H.; Porter, J.B. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood 2004, 104, 263–269. [Google Scholar] [CrossRef]
- Westwood, M.A.; Anderson, L.J.; Maceira, A.M.; Shah, F.T.; Prescott, E.; Porter, J.B.; Wonke, B.; Walker, J.M.; Pennell, D.J. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J. Magn. Reson. Imaging 2007, 25, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Holden, S.; Davies, B.; Prescott, E.; Charrier, C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Otto-Duessel, M.; Aguilar, M.; Nick, H.; Nelson, M.D.; Coates, T.D.; Pollack, H.; Moats, R. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation 2005, 112, 535–543. [Google Scholar] [CrossRef]
- Westwood, M.A.; Firmin, D.N.; Gildo, M.; Renzo, G.; Stathis, G.; Markissia, K.; Vasili, B.; Pennell, D.J. Intercentre reproducibility of magnetic resonance T2*measurements of myocardial iron in thalassaemia. Int. J. Cardiovasc. Imaging 2005, 21, 531–538. [Google Scholar] [CrossRef]
- Krittayaphon, R.; Viprakasit, V.; Saiviroonporn, P.; Siritanaratkul, N.; Siripornpitak, S.; Meekaewkunchorn, A.; Kirawittaya, T.; Sripornsawan, P.; Jetsrisuparb, A.; Srinakarin, J.; et al. prevalence and predictors of cardiac and liver iron overload in patients with thalassemia: A multicenter study based on real-world data. Blood Cells Mol. Dis. 2017, 66, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chaosuwannakit, N.; Makarawate, P. The value of magnetic resonance imaging in evaluation of myocardial and liver iron overload in a thalassaemia endemic population: A report from Northeastern Thailand. Pol. J. Radiol. 2019, 84, e262–e268. [Google Scholar] [CrossRef]
- Saiviroonporn, P.; Viprakasit, V.; Boonyasirinant, T.; Khuhapinant, A.; Wood, J.C.; Krittayaphong, R. Comparison of the region-based and pixel-wise methods for cardiac T2*analysis in 50 transfusion dependent Thai thalassemia patients. J. Comput. Assist. Tomogr. 2011, 35, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Celutkiene, J.; Spoletini, I.; Coats, A.J.; Chioncel, O. Left ventricular function monitoring in heart failure. Eur. Heart J. Suppl. 2019, 21, 17–19. [Google Scholar] [CrossRef]
- Douglas, P.S.; Garcia, M.J.; Haines, D.E.; Lai, W.W.; Manning, W.J.; Patel, A.R.; Picard Michael, H.; Polk, D.M.; Ragosta, M.; Ward, R.P.; et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society. J. Am. Coll. Cardiol. 2011, 57, 1126–1166. [Google Scholar]
- Barosi, G.; Arbustini, E.; Gavazzi, A.; Grasso, M.; Pucci, A. Myocardial iron grading by endomyocardial biopsy. A clinico-pathologic study on iron overloaded patients. Eur. J. Haematol. 1989, 42, 382–388. [Google Scholar] [CrossRef]
- Carpenter, J.P.; He, T.; Kirk, P.; Roughton, M.; Anderson, L.J.; Noronha, S.V.; Sheppard, M.N.; Porter, J.B.; Walker, J.M.; Wood, J.C.; et al. On T2*magnetic resonance and cardiac iron. Circulation 2011, 123, 1519–1528. [Google Scholar] [CrossRef]
- Westwood, M.A.; Sheppard, M.N.; Awogbade, M.; Ellis, G.; Stephens, A.D.; Pennell, D.J. Myocardial biopsy and T2*magnetic resonance in heart failure due to thalassaemia. Br. J. Haematol. 2005, 128, 2. [Google Scholar] [CrossRef]
- Wing-Yang, A.; Winnie, W.L.; Winnie, W.C.; Hui-Leung, Y.; Alvin, S.L.; Rever, C.L.; Chan, H.M.-H.; Lee, H.K.-K.; Law, M.-F.; Liu, H.S.Y.; et al. A cross-sectional magnetic resonance imaging assessment of organ specific hemosiderosis in 180 thalassemia major patients in Hong Kong. Haematologica 2008, 93, 784–785. [Google Scholar]
- Chirnomas, S.D.; Geukes-Foppen, M.; Barry, K.; Braunstein, J.; Kalish, L.A.; Neufeld, E.J.; Powell, A.J. Practical implications of liver and heart iron load assessment by T2*-MRI in children and adults with transfusion-dependent anemias. Am. J. Hematol. 2008, 83, 781–783. [Google Scholar] [CrossRef]
- Daar, S.; Pathare, A.V.; Jain, R.; Al Zadjali, S.; Pennell, D.J. T2*cardiovascular magnetic resonance in the management of thalassemia patients in Oman. Haematologica 2009, 94, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Brown, G.; Leung, M.; Bavishi, K.; Taylor, J.; Lioyd, J.; Lee, S.-H.; Tay, L.; Worthley, S. Observational study of iron overload assessed by magnetic resonance imaging in an adult population of transfusion dependent patients with beta thalassemia: Significant association between low cardiac T2* < 10 ms and the occurrence of cardiac events. Int. Med. J. 2010, 40, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.L.; Kim, H.Y.; Thompson, A.A.; Quinn, C.T.; Mueller, B.U.; Odame, I.; Giardina, P.J.; Vichinsky, E.P.; Boudreaux, J.M.; Cohen, A.R.; et al. Chelation use and iron burden in North American and British thalassemia patients: A report from the Thalassemia Longitudinal Cohort. Blood 2012, 119, 2746–2753. [Google Scholar] [CrossRef]
- Noetzli, L.J.; Carson, S.M.; Nord, A.S.; Coates, T.D.; Wood, J.C. Longitudinal analysis of heart and liver iron in thalassemia major. Blood 2008, 112, 2973–2978. [Google Scholar] [CrossRef]
- Anderson, L.J.; Westwood, M.A.; Holden, S.; Davis, B.; Prescott, E.; Wonke, B.; Porter, J.B.; Malcolm Walker, J.; Pennell, D.J. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: A prospective study using T2*cardiovascular magnetic resonance. Br. J. Haematol. 2004, 127, 348–355. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Normal (n = 98) | Marginal (n = 5) | Mild to Moderate (n = 12) | Severe (n = 4) | p-Value |
|---|---|---|---|---|---|
| Mean age (years) | 27.9 ± 13.1 | 28.1 ± 6.8 | 37.7 ± 11.6 | 37.8 ± 9.7 | 0.14 |
| Male gender, n (%) | 54 (55.1) | 3 (60) | 7 (58.3) | 3 (75) | 0.83 |
| Thalassemia type | |||||
| Homozygous beta thalassemia, n (%) | 43 (43.9) | 2 (40) | 5 (41.7) | 2 (50) | 0.88 |
| Beta thalassemia/HbE, n (%) | 55 (56.1) | 3 (60) | 7 (58.3) | 2 (50) | 0.81 |
| History of splenectomy, n (%) | 36 (36.7) | 3 (60) | 3 (25) | 4 (100) | 0.01 * |
| Mean pre-transfusion hemoglobin level (g/dl) | 9.5 ± 0.3 | 9.4 ± 0.3 | 9.4 ± 0.4 | 9.4 ± 0.4 | 0.84 |
| Mean baseline ferritin level (mg/dl) | 2523.8 ± 1561.4 | 4741.9 ± 2184.6 | 3535.4 ± 2113.6 | 4189.1 ± 2172.6 | 0.06 |
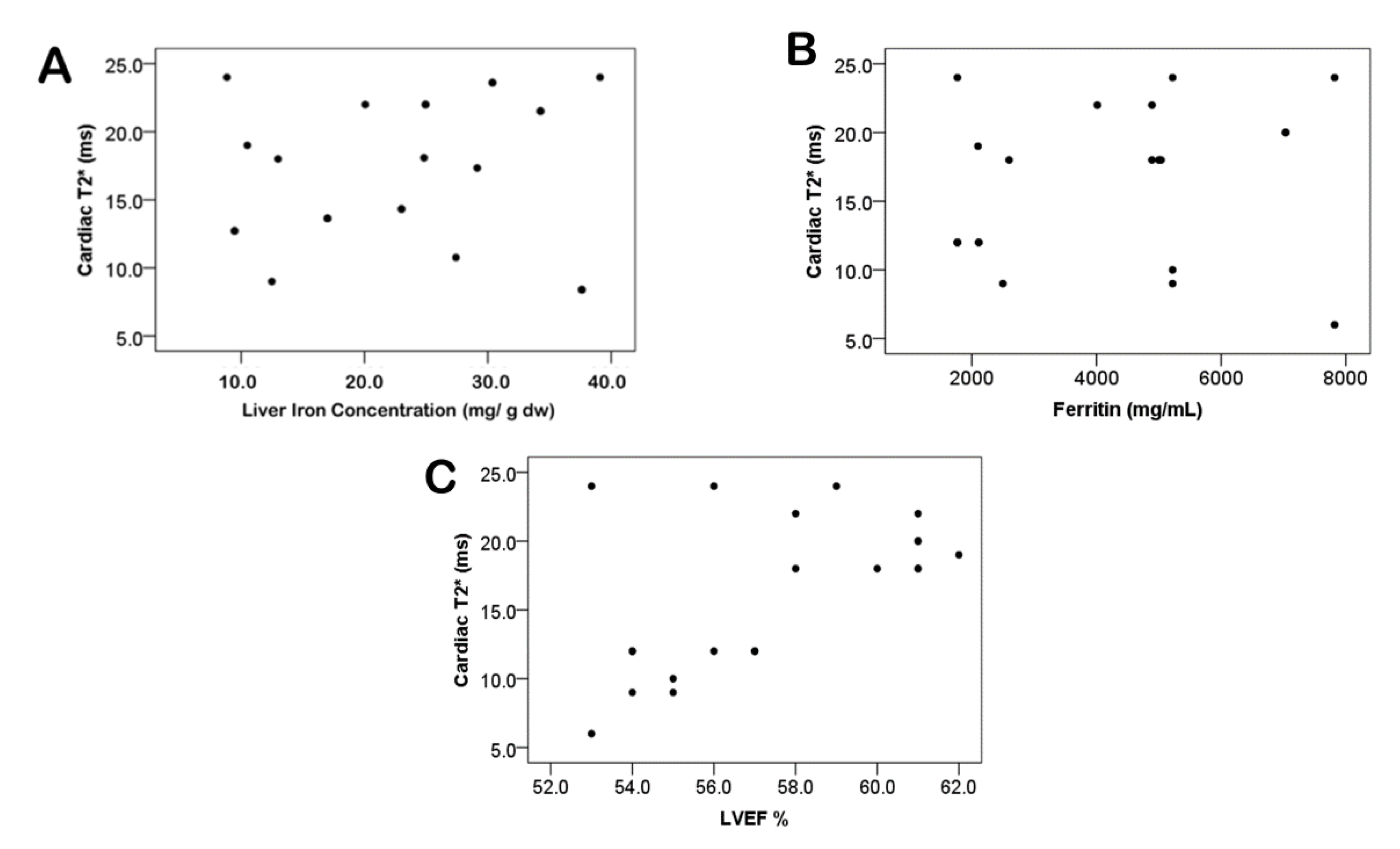
| Median baseline LIC (mg/g dry weight) | 19.2 ± 5.5 | 25.3 ± 7.7 | 21.6 ± 13.3 | 23.9 ± 6.5 | 0.16 |
| Mean LVEF (%) | 63.6 ± 14.5 | 59.5 ± 13.2 | 61.9 ± 13.6 | 58.3 ± 11.1 | 0.69 |
| Cardiac T2 * | Baseline | One Year | Three Year | Five Year | F | p-Value |
|---|---|---|---|---|---|---|
| Normal (n = 98) | 43.1 ± 7.2 | 42.2 ± 6.6 | 43.4 ± 6.1 | 43.6 ± 5.6 | 194 | 0.59 |
| Marginal (n = 5) | 23.2 ± 1.2 | 31.8 ± 5.8 | 31.6 ± 3.9 | 33.5 ± 1.6 | 8 | <0.001 * |
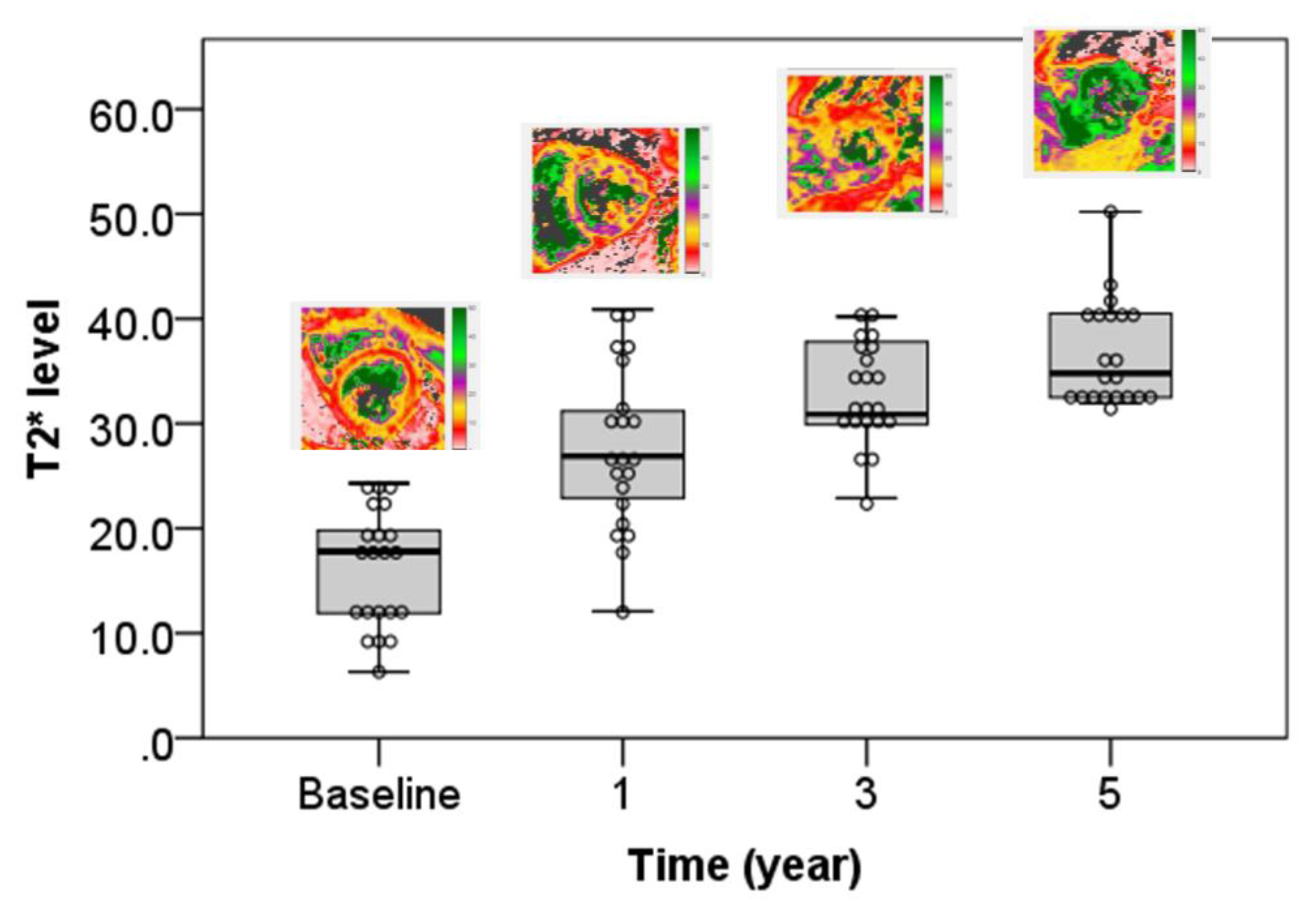
| Mild to moderate (n = 12) | 15.8 ± 3.4 | 29.2 ± 6.4 | 34.2 ± 4.6 | 38.9 ± 5.5 | 22 | <0.001 * |
| Severe (n = 4) | 8.5 ± 1.5 | 17.1 ± 3.4 | 30.3 ± 6.1 | 33.9 ± 1.9 | 6 | <0.001 * |
| Parameters | Time | F | p-Value | |||
|---|---|---|---|---|---|---|
| Baseline | One Year | Three Year | Five Year | |||
| T2 * | 16.24 ± 5.63 | 27.57 ± 7.72 | 32.86 ± 4.77 | 36.62 ± 4.99 | 68.126 | <0.001 * |
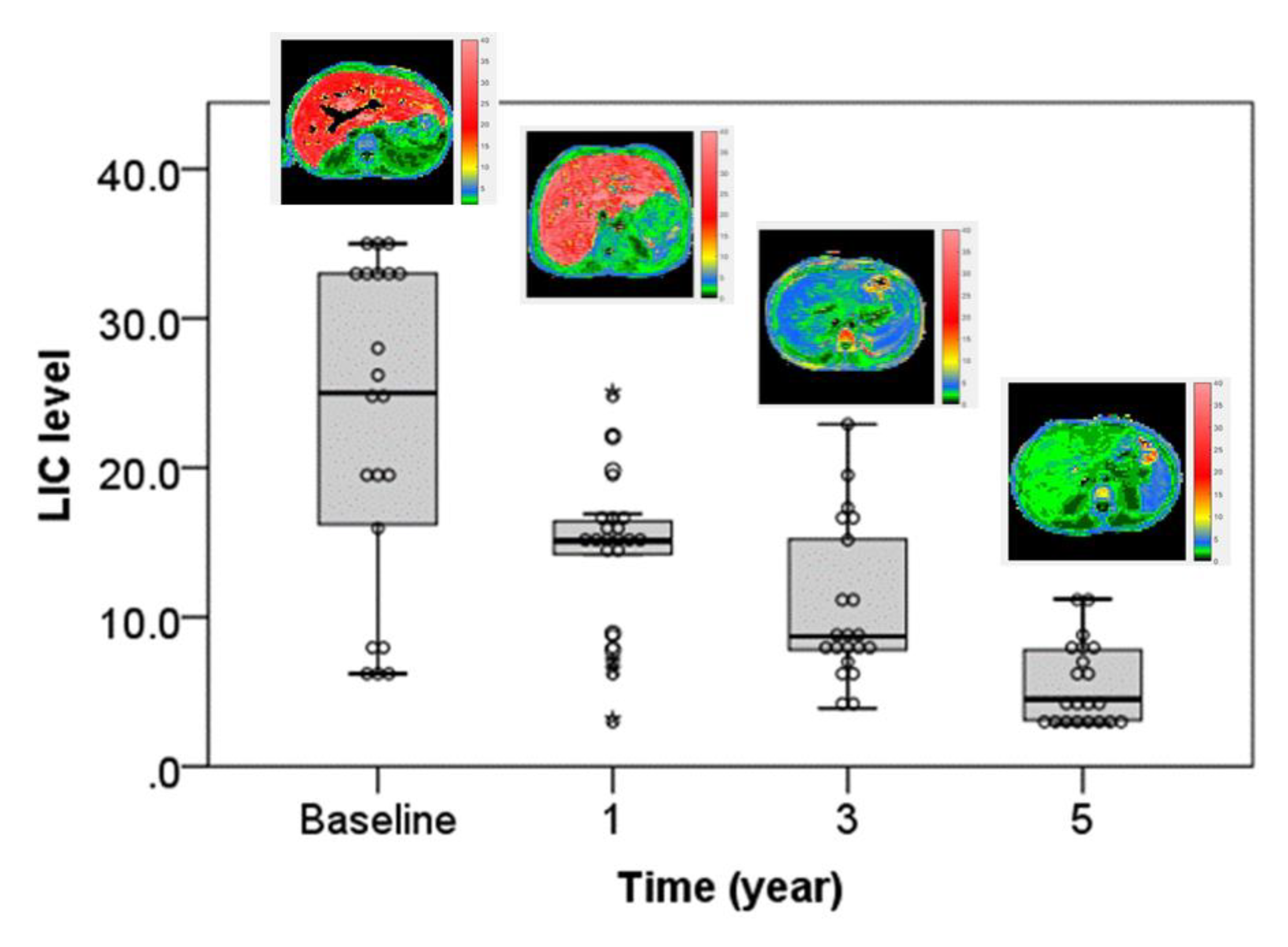
| LIC | 22.86 ± 10.92 | 14.33 ± 5.25 | 10.71 ± 5.33 | 5.43 ± 2.77 | 40.515 | <0.001 * |
| Ferritin | 4175 ± 2120 | 3034 ± 1438 | 2425 ± 901 | 1546 ± 691 | 17.953 | <0.001 * |
| LVEF | 57.43 ± 3.04 | 59.38 ± 4.48 | 60.19 ± 3.93 | 60.57 ± 3.41 | 16.789 | 0.61 |
| Patients with Cardiac Siderosis (n = 21) | Patients without Cardiac Siderosis (n = 98) | p-Value | |
|---|---|---|---|
| Iron chelation therapy | |||
| 11 (52.4) | 28 (28.6) | 0.36 |
| 4 (19) | 20 (20.4) | 0.89 |
| 2 (9.6) | 49 (50) | 0.0007 * |
| Combination therapy, n (%) | 4 (19) | 1 (1) | 0.0002 * |
| Volume of red cell transfusion (unit/year) | 19.6 ± 6.9 | 11.1 ± 7.8 | <0.0001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaosuwannakit, N.; Makarawate, P.; Wanitpongpun, C. The Importance of Cardiac T2* Magnetic Resonance Imaging for Monitoring Cardiac Siderosis in Thalassemia Major Patients. Tomography 2021, 7, 130-138. https://doi.org/10.3390/tomography7020012
Chaosuwannakit N, Makarawate P, Wanitpongpun C. The Importance of Cardiac T2* Magnetic Resonance Imaging for Monitoring Cardiac Siderosis in Thalassemia Major Patients. Tomography. 2021; 7(2):130-138. https://doi.org/10.3390/tomography7020012
Chicago/Turabian StyleChaosuwannakit, Narumol, Pattarapong Makarawate, and Chinnadol Wanitpongpun. 2021. "The Importance of Cardiac T2* Magnetic Resonance Imaging for Monitoring Cardiac Siderosis in Thalassemia Major Patients" Tomography 7, no. 2: 130-138. https://doi.org/10.3390/tomography7020012
APA StyleChaosuwannakit, N., Makarawate, P., & Wanitpongpun, C. (2021). The Importance of Cardiac T2* Magnetic Resonance Imaging for Monitoring Cardiac Siderosis in Thalassemia Major Patients. Tomography, 7(2), 130-138. https://doi.org/10.3390/tomography7020012





