Simple Summary
Chronic subdural hematoma is a common condition, especially in older adults, and the accurate measurement of its size on CT scans is essential for guiding treatment decisions. In this study, we analyzed 257 patients and found that manual measurements of hematoma volume are highly reliable between different radiologists and correlate well with a simple estimation formula. These results show that precise volume measurement is both feasible and highly consistent, providing a robust reference for developing and validating future automated tools that may assist radiologists in clinical practice.
Abstract
Background: Accurate volumetry and imaging characterization of chronic subdural hematoma (cSDH) are essential for prognostication and treatment planning, but manual assessment is time-consuming and therefore underutilized. Methods: We retrospectively analyzed preoperative non-contrast CT (NCCT) scans of 257 patients undergoing first-time surgery for uni- or bilateral cSDH. Hematoma volumes were measured manually using a semi-automated area-outlining tool on every second axial slice and compared with the volumes estimated through the ABC/2 formula. Hematoma attenuation patterns and components were categorized, and interrater reliability was assessed for volume, maximum diameter, and imaging features using intraclass correlation coefficients (ICCs) and Cohen’s κ. Results: A total of 339 hematomas were evaluated. Manual and ABC/2 volume measurements correlated strongly (R2 = 0.83, ICC [3, 1] = 0.90). The interrater agreement for manual volumetry was excellent (ICC [2, 1] = 0.96). Agreement was also excellent for maximum diameter (ICC [2, 1] > 0.9) and good for midline shift assessment (κ = 0.81). Agreement was moderate for the identification of fresh clots, trabeculations, and laminations (κ = 0.62–0.72) but poor for general attenuation patterns (κ = 0.44). Conclusions: The manual volumetry of cSDH is feasible and highly reproducible between raters of different experience levels. These results provide a robust reference standard for the validation of automated volumetry tools and support the implementation of quantitative hematoma assessment in future clinical trials and routine care.
1. Introduction
Chronic subdural hematoma (cSDH) is among the most common neurosurgical conditions [1,2,3]. The incidence is rising due to an aging population and an increased use of antithrombotic medication [4,5]. Endovascular treatment of cSDH has become a rapidly growing field of interest owing to reports of reduced recurrence rates compared to standard treatments [6,7,8] and refined models on the underlying pathological mechanisms behind cSDH and its recurrence [9,10,11]. In addition to a mounting volume of non-randomized data [12,13,14,15], randomized controlled trials (RCTs) recently showed a lower risk of hematoma recurrence or progression leading to reoperation than surgery alone [16,17].
Several additional randomized trials are underway, and eMMA may have the potential to become the first-line treatment for cSDH with mild symptoms [18].
Neuroimaging is a cornerstone in the management of cSDH for diagnosis, prognostication, and the evaluation of treatment effect [19,20]. Non-contrast Computed Tomography (NCCT) is the most used modality due to its availability, speed, and diagnostic accuracy [19].
With the increasing focus on endovascular treatment, imaging parameters are increasingly relevant for patient selection, treatment planning, and follow-up, including early prognostication, for example, by identifying small increases in hematoma volume.
In addition to hematoma volume [21], the risk of recurrence after conventional neurosurgical treatments has also been associated with cortical atrophy [22] hematoma volume overall attenuation patterns and the presence of septations, laminations, and fresh blood components [23]. Several different radiological classifications of cSDH have been proposed for predicting recurrence risk; one of the more commonly used is the one proposed by Nakaguchi [11].
Precise cSDH volume measurements in clinical routines are cumbersome and time-consuming; therefore, surrogate measurements, such as the maximum width in coronal or transversal planes, are common substitutes in clinical practice. The ABC/2 formula has been proposed for cSDH volume assessment [24]. However, the ABC/2 formula assumes an ellipsoid shape not typical for cSDH, which is characteristically crescent-shaped; moreover, it does not include falcine or tentorial components.
Automated delineation tools have been shown to accurately measure the volume of intracerebral hemorrhages (ICHs) better than the ABC/2 formula [25,26] and may facilitate the accurate volumetry of cSDH hematomas in clinical trials and clinical routine care.
The aim of this study was to characterize a consecutive cohort of patients undergoing surgery for cSDH using detailed hematoma volumetry and imaging assessments and to evaluate the interrater reliability for manual volume measurements and attenuation features, as well as the agreement between manual volumetry and the ABC/2 formula.
2. Materials and Methods
2.1. Study Design
This is a diagnostic accuracy study on a retrospective patient population. The STROBE guidelines for reporting on cross-sectional studies were observed.
2.2. Study Setting
The study was conducted at the Skåne University Hospital, the only healthcare institution in the Scania region of south Sweden providing neurosurgical care, serving a population of approximately 2 million people.
2.3. Participant Selection
From hospital records, all patients with an ICD-10 code of AAD10 who underwent first-time surgery for uni- or bilateral CSDH during 2015 and 2016 were considered for inclusion in the study. The patient’s personal identification numbers were used to search the regional Picture Archiving and Communications System (PACS) for relevant preoperative CT examinations. The regional PACS does include the majority of hospitals in the catchment area. Patients younger than 18 years, patients with acute SDH wrongly coded, and patients without access to preoperative head NCCT images in PACS were all excluded. The final study population included 257 patients with preoperative NCCT images in relation to first-ever uni- or bilateral CSDH surgery (Figure 1).
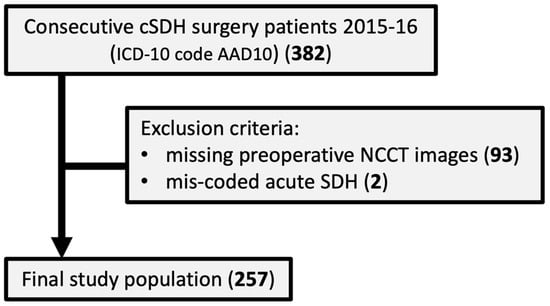
Figure 1.
Flow chart describing the study population.
The study was approved by the Swedish Ethical Review Authority (2024–03485–01), and individual informed consent was waived by the authority.
2.4. Data Collection
Patient age and sex were extracted from the Swedish personal identification number, and date of surgery was collected from the hospital medical records. These data were used to find relevant imaging in the hospital Picture Archive and Communication System (PACS).
2.5. Collection of Radiological Data
Preoperative NCCT images were identified for each patient. If multiple preoperative examinations were available, the examination performed closest in time before surgery was used for analysis.
2.6. Radiological Assessment
All imagery measurements and assessments were performed according to a prespecified reporting protocol, following assessment alignment between readers on >20 cases outside the study population.
NCCT imagery was assessed within the hospital PACS, IDS7 version v 24.2 (Sectra AB, Linköping, Sweden). Stacks of 4 or 5 mm thick reconstructed images without any overlap were generated from thin slice raw examination data. Care was taken to be as precise in delineation as possible, frequently shifting between parenchymal (center: 30/40, width: 80) and bone (center: 600, width: 2000) window settings. Where difficulties arose in separating hematoma from brain parenchyma, coronal and sagittal reconstructions and the coordinate guidance system within the IDS7 were used for increased accuracy in delineation.
2.7. Hematoma Volumetry
Manual volumetry was performed by outlining the hematoma borders on every second axial slice using the IDS7 area-outlining tool. Total volume was calculated by multiplying the annotated area by the slab thickness and by 2. The time to perform manual volume measurement was measured for raters 1 and 3. This measurement technique was used as a reference standard (gold standard) for subsequent volume measurements.
Volume measurement was also estimated using the ABC/2 formula (also known as the TADA formula) [27,28]. Maximum hematoma length and width on the axial slice with the largest hematoma extent were multiplied by the number of involved slices and by slice thickness, then divided by 2.
2.8. Hematoma Characterization
All hematomas were assessed according to the Nakaguchi classification [11]. A general hematoma attenuation pattern of hypo-, iso-, or hyperattenuating in relation to the adjacent gray brain matter was denoted. Additionally, the presence of fresh hematoma components, laminations, and trabeculations was recorded.
2.9. Uni- and Bilateral Hematomas
For patients with bilateral hematomas, both sides’ hematomas were treated as separate observations.
2.10. Radiological Assessors
One neuroradiologist with more than 8 years’ experience assessed all images with both hematoma volume measurement techniques and hematoma characterization (Reader 1). A second neuroradiologist with more than 30 years’ experience assessed 20% of images according to volume measurement 1 (Reader 2). One general radiology resident with approximately 3 years’ experience assessed the same 20% of images according to volume measurement technique 1 and hematoma characterization (Reader 3).
2.11. Statistical Analysis
Descriptive statistics are presented as counts (percentages, %) for categorical variables and medians (interquartile range, IQR) for continuous variables.
Agreement between manual hematoma volume measurement and the ABC/2 method for the calculation of hematoma volume was assessed using a scatter plot with linear regression and a quantile–quantile plot.
The intraclass correlation coefficients were assessed using a two-way mixed effects model. Limits of agreement were defined as the mean bias ± 1.96 SD in the Bland–Altman analyses. For categorical variables, interrater agreements were quantified using Cohen’s κ with 95% confidence intervals. The thresholds for interpretation of the interrater agreement measures (ICC and Cohen’s κ statistic) were “poor” below 0.50, “moderate” between 0.50 and 0.75, “good” between 0.75 and 0.90, and “excellent” above 0.90.
All analyses were performed in Stata/SE version 15.1. Statistical tests were two-sided and significance level was set at p < 0.05. Agreement analyses were performed using the kappa program in Stata.
3. Results
3.1. Description of Study Population
A flow chart of the study population is shown in Figure 1.
The majority of patients were men (180/257, 70.0%), and the median age was 75 years (1 standard deviation (SD) = 12.3; range 11–96).
Of the 257 included patients, 97 (37.7%) had left-sided hematomas, 78 (30.4%) right-sided, and 82 (31.9%) bilateral, yielding a total of 339 hematomas. Radiological characteristics of the hematomas (all, left-sided, and right-sided) are shown in Table 1, and typical radiological hematoma features are illustrated in Figure 2 and Figure 3.

Table 1.
Radiological characteristics of 339 chronic subdural hematomas among 257 subjects with first-time operation during 2015 and 2016.
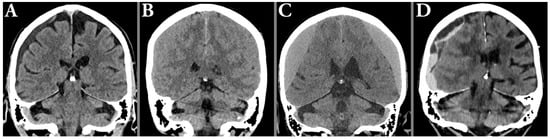
Figure 2.
Illustration of typical radiological hematoma attenuation types; hypoattenuation (A), isoattenuating (B), hyperattenuating (C) in relation to the gray matter, and mixed attenuation (D), typically seen in acute-on-chronic hematomas.
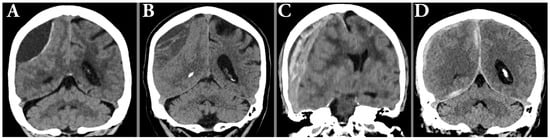
Figure 3.
Illustration of typical radiological hematoma features such as hypoattenuating hematoma with membrane (A), hypoattenuating hematoma with trabeculations (B), laminated acute-on-chronic with midline shift (C), and hematoma with parafalcine and tentorial components (D).
3.2. Comparison of Manual Measurements of Hematoma Volume with the ABC/2 Method
An illustration of the manual volume measurements is shown in Figure 4.
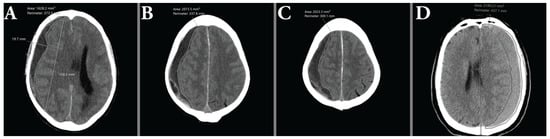
Figure 4.
(A–C) show consecutive area measurements made on a hypoattenuating hematoma as part of the ABC/2 method. (D) illustrates the difficulties of measuring isoattenuating hematomas, due to the indistinct demarcation between brain parenchyma and hematoma.
Figure 5A shows a scatter plot with a regression line for the manual volume measurements compared to the ABC/2 calculated volume for 339 hematomas by rater 1, and Figure 5B shows the Q-Q plot. There was a strong correlation between the manual and the ABC/2 method (beta = 0.80, 95% CI: 0.76–0.84; p = 6.0 × 10−133; R2 = 83.3%).
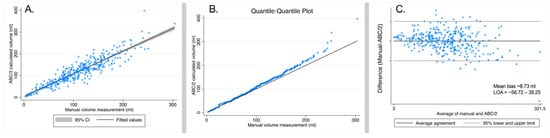
Figure 5.
(A) Scatter plot of manual volume measurement compared to ABC/2 calculated volumes for 339 hematomas in 257 subjects by rater 1. (B) Quantile–quantile (QQ) plot of manual volume measurements compared to ABC/2 calculated volumes for 339 hematomas in 257 subjects. (C) Bland–Altman plot for the agreement between manual volume measurements and the ABC/2 calculated volumes of 339 hematomas in 257 subjects. The mean bias (average difference between methods and limits of agreement (LOA = bias ± 1.96 × SD)) is shown in the graph.
The agreement between the manual and ABC/2 calculated volume measurements (intraclass correlation using a two-way fixed effects model) showed an ICC [3, 1] of 0.90 (95% CI: 0.88–0.92; p < 0.0001). The Bland–Altman plot for the agreement between the manual and the ABC/2 method is shown in Figure 5C.
3.3. Interrater Reliability of Manual Measurements of Hematoma Volume Based on Three Independent Readers
Among the 339 hematomas, 70 hematomas were manually assessed to calculate hematoma volume by three independent raters. The median time to complete manual volume measurement was 5:47 (IQR 4:02–8:34) for rater 1 and 10:00 (IQR 9:05–12:18) for rater 3.
The ICC [2, 1] (interrater reliability using a two-way mixed effects model) assessing the global agreement between all three raters was 0.9594 (95% CI: 0.9404–0.9732; p < 0.0001). Figure 6 shows the Bland–Altman plots for agreement between rater 1 compared to rater 2 and between rater 1 compared to 3, respectively.
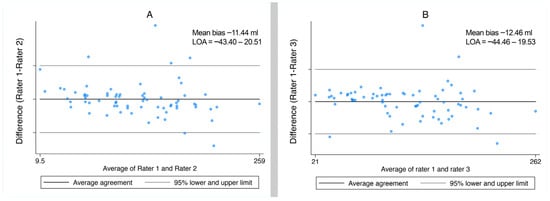
Figure 6.
Bland–Altman plots for agreement for manual volume measurements by rater 1 compared to rater 2 (A) and by rater 1 compared to rater 3 (B). The mean bias (average difference between methods and limits of agreement (LOA = bias ± 1.96 × SD)) is shown in the graph.
3.4. Interrater Reliability of Radiological Features of Hematomas
We examined the interrater reliability of two independent raters for radiological features of CSDH. The ICC for hematoma volume and maximum diameter in the coronal plane and transversal plane showed an excellent interrater agreement (ICC [1, 3] > 0.9). For categorical variables, including general attenuation patterns, various hematoma components, and midline shift to the opposite side of the hematoma, we calculated Cohen’s kappa to assess the between-rater reliability. Among the investigated features, the general attenuation patterns showed only a poor agreement (Cohen’s kappa = 0.44, 95% CI: 0.29–0.60). The corresponding reliability measures for hematoma components, including the presence of fresh clots, trabeculations, or laminations within the hematoma, were moderate (Cohen’s kappa between 0.62 and 0.72). The best agreement was seen for the assessment of midline shift to the opposite side of the hematoma, where the interrater Cohen’s kappa value was 0.81 (95% CI: 0.69–0.92) (Table 2), indicating good agreement.

Table 2.
Agreement of radiological features of 70 hematomas assessed by two independent raters (rater 1 and 3).
4. Discussion
Neuroimaging, primarily NCCT, is the imaging cornerstone in the management of cSDH for diagnosis, prognostication, and the evaluation of treatment response [19,20].
With improvements in scanner technology and image analysis tools, including AI-based tools [29,30,31], the diagnostic yield from NCCT may increase considerably and provide key imaging features that are relevant for prognostication [11,23]. Hematoma volume is of particular importance, as it is of prognostic importance in many conditions [32,33,34], as well as for CSDH [21].
Our results in this study, with a consecutively included population, a high regional high coverage, and multi-rater design, demonstrate that precise manual volume measurement is feasible and highly reproducible between readers of varying experience levels. Reliable cSDH volume measurement may be a key outcome measure in future trials, as well as in clinical routine care, to prognosticate clinical success or failure and select patients for additional follow-up or treatments, and in all these use-cases the variability of the measurement is crucial. The low interrater variability observed in this study supports the use of manual volumetry as a reliable reference standard going forward. This is encouraging, since exact manual volumetry is often perceived as being due to a similar attenuation of the brain and the hematoma.
Volumetry data from well-annotated consecutive cSDH-cohorts may also play an important role in validating automated image analysis tools for cSDH volume measurements, which are likely to become available in the near future.
Similarly, we show a high interrater agreement for the assessment of qualitative imaging features important for the prognostication of CSDH, such as the trabeculation or lamination of the hematoma.
Limitations
This study has several limitations. First, its retrospective, single-center design and the clinically selected cohort of patients undergoing cSDH surgery may introduce selection bias and limit the generalizability of the findings. Second, although volumetric measurements were performed by multiple readers, the absence of a formal interrater agreement analysis restricts our ability to quantify observer variability. Finally, because the cohort reflects only surgically treated patients, the findings may not be fully applicable to individuals managed conservatively.
5. Conclusions
In this study, we demonstrate that the manual measurement of cSDH volume is both technically feasible and highly reproducible across readers with different levels of radiological experience. This consistency highlights the robustness of volumetric assessment as an objective indicator of disease burden. Beyond its methodological reliability, cSDH volume offers clinically meaningful information that surpasses traditional qualitative assessments.
Author Contributions
Conceptualization, M.D. and J.W.; methodology, M.D. and J.W.; software, M.D.; validation, E.H. and B.M.H.; formal analysis, M.D., E.H., B.M.H., and J.W.; investigation, M.D., E.H., and B.R.; resources, J.W.; data curation, E.H. and B.M.H.; writing—original draft preparation, M.D.; writing—review and editing, M.D., E.H., B.R., B.M.H., and J.W.; visualization, M.D. and J.W.; supervision, B.M.H. and J.W.; project administration, B.M.H. and J.W.; funding acquisition, B.M.H. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Region Skane, grant number 47455, and Skane University hospital funds, grant number 96437 and 96438.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Swedish Ethical Review Authority (2023-00387-01 on 2 February 2023), and individual informed consent was waived by the authority.
Informed Consent Statement
Individual informed consent was waived by the Swedish Ethical Review Authority (2023-00387-01 on 2 February 2023) due to the retrospective nature of the study population.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
The authors wish to thank Isabel Drake for statistical expertise as well as all patients and colleagues at Skåne University hospital.
Conflicts of Interest
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JW is a founder and shareholder of Uman Sense AB and has been an invited speaker for Siemens Healthineers, Balt group, and Medtronic Inc. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| cSDH | Chronic subdural hematoma |
| eMMA | Embolization of the Middle Meningeal Artery |
| ICH | Intracerebral hemorrhage |
| ICC | Intraclass correlation coefficients |
| IQR | Interquartile range |
| NCCT | Non-contrast CT |
| PACS | Picture Archiving and Communications System |
| QQ plot | Quantile–quantile plot |
| SDH | Subdural hematoma |
References
- Almenawer, S.A.; Farrokhyar, F.; Hong, C.; Alhazzani, W.; Manoranjan, B.; Yarascavitch, B.; Parnian, A.; Benedicto, B.; Kesava, R.; Naresh, M.; et al. Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann. Surg. 2014, 259, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Feghali, J.; Yang, W.; Huang, J. Updates in Chronic Subdural Hematoma: Epidemiology, Etiology, Pathogenesis, Treatment, and Outcome. World Neurosurg. 2020, 141, 339–345. [Google Scholar] [CrossRef]
- Henry, J.; Amoo, M.; Kissner, M.; Deane, T.; Zilani, G.; Crockett, M.T.; Javadpour, M. Management of Chronic Subdural Hematoma: A Systematic Review and Component Network Meta-analysis of 455 Studies with 103,645 Cases. Neurosurgery 2022, 91, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Balser, D.; Farooq, S.; Mehmood, T.; Reyes, M.; Samadani, U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J. Neurosurg. 2015, 123, 1209–1215. [Google Scholar] [CrossRef]
- Rauhala, M.; Luoto, T.M.; Huhtala, H.; Iverson, G.L.; Niskakangas, T.; Ohman, J.; Helén, P. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J. Neurosurg. 2019, 132, 1147–1157. [Google Scholar] [CrossRef]
- Ironside, N.; Nguyen, C.; Do, Q.; Ugiliweneza, B.; Chen, C.J.; Sieg, E.P.; James, R.F.; Ding, D. Middle meningeal artery embolization for chronic subdural hematoma: A systematic review and meta-analysis. J. Neurointerv. Surg. 2021, 13, 951–957. [Google Scholar] [CrossRef]
- Sattari, S.A.; Yang, W.; Shahbandi, A.; Feghali, J.; Lee, R.P.; Xu, R.; Jackson, C.; Gonzalez, L.F.; Tamargo, R.J.; Huang, J.; et al. Middle Meningeal Artery Embolization Versus Conventional Management for Patients with Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. Neurosurgery 2023, 92, 1142–1154. [Google Scholar] [CrossRef]
- Shakir, M.; Irshad, H.A.; Alidina, Z.; Shaikh, T.; Ashfaq, D.; Ali, Z.; Pirzada, S.; Qureshi, A.I.; Thomas, A.; Kan, P.; et al. Middle meningeal artery embolization alone versus combined with conventional surgery in the management of chronic subdural hematoma: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2024, 246, 108580. [Google Scholar] [CrossRef]
- Edlmann, E.; Giorgi-Coll, S.; Whitfield, P.C.; Carpenter, K.L.H.; Hutchinson, P.J. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J. Neuroinflamm. 2017, 14, 108. [Google Scholar] [CrossRef]
- Moshayedi, P.; Liebeskind, D.S. Middle Meningeal Artery Embolization in Chronic Subdural Hematoma: Implications of Pathophysiology in Trial Design. Front. Neurol. 2020, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Nakaguchi, H.; Tanishima, T.; Yoshimasu, N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J. Neurosurg. 2001, 95, 256–262. [Google Scholar] [CrossRef]
- Ban, S.P.; Hwang, G.; Byoun, H.S.; Kim, T.; Lee, S.U.; Bang, J.S.; Han, J.H.; Kim, C.-Y.; Kwon, O.-K.; Oh, C.W. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma. Radiology 2018, 286, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Shotar, E.; Meyblum, L.; Premat, K.; Lenck, S.; Degos, V.; Grand, T.; Cortese, J.; Pouvelle, A.; Pouliquen, G.; Mouyal, S.; et al. Middle meningeal artery embolization reduces the post-operative recurrence rate of at-risk chronic subdural hematoma. J. Neurointerv. Surg. 2020, 12, 1209–1213. [Google Scholar] [PubMed]
- Dian, J.; Linton, J.; Shankar, J.J. Risk of recurrence of subdural hematoma after EMMA vs surgical drainage—Systematic review and meta-analysis. Interv. Neuroradiol. 2021, 27, 577–583. [Google Scholar]
- Kan, P.; Maragkos, G.A.; Srivatsan, A.; Srinivasan, V.; Johnson, J.; Burkhardt, J.K.; Robinson, T.M.; Salem, M.M.; Chen, S.; Riina, H.A.; et al. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: A Multi-Center Experience of 154 Consecutive Embolizations. Neurosurgery 2021, 88, 268–277. [Google Scholar] [CrossRef]
- Liu, J.; Ni, W.; Zuo, Q.; Yang, H.; Peng, Y.; Lin, Z.; Li, Z.; Wang, J.; Zhen, Y.; Luo, J.; et al. Middle Meningeal Artery Embolization for Nonacute Subdural Hematoma. N. Engl. J. Med. 2024, 391, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Knopman, J.; Mokin, M.; Hassan, A.E.; Harbaugh, R.E.; Khalessi, A.; Fiehler, J.; Gross, B.A.; Grandhi, R.; Tarpley, J.; et al. Adjunctive Middle Meningeal Artery Embolization for Subdural Hematoma. N. Engl. J. Med. 2024, 391, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.; Ullberg, T.; Nittby, H.; Marklund, N.; Wassélius, J. Swedish trial on embolization of middle meningeal artery versus surgical evacuation in chronic subdural hematoma (SWEMMA)-a national 12-month multi-center randomized controlled superiority trial with parallel group assignment, open treatment allocation and blinded clinical outcome assessment. Trials 2022, 23, 926. [Google Scholar] [PubMed]
- Miah, I.P.; Tank, Y.; Rosendaal, F.R.; Peul, W.C.; Dammers, R.; Lingsma, H.F.; Hertog, H.M.D.; Jellema, K.; van der Gaag, N.A. Radiological prognostic factors of chronic subdural hematoma recurrence: A systematic review and meta-analysis. Neuroradiology 2021, 63, 27–40, Erratum in Neuroradiology 2021, 63, 159–160. [Google Scholar] [CrossRef]
- Neshige, S.; Kuriyama, M.; Ota, S. Diffusion-weighted imaging findings predictive of postoperative recurrence of chronic subdural hematoma. J. Neurol. Sci. 2024, 467, 123324. [Google Scholar] [CrossRef]
- Bartek, J., Jr.; Sjåvik, K.; Kristiansson, H.; Ståhl, F.; Fornebo, I.; Förander, P.; Jakola, A.S. Predictors of Recurrence and Complications After Chronic Subdural Hematoma Surgery: A Population-Based Study. World Neurosurg. 2017, 106, 609–614. [Google Scholar] [CrossRef]
- Charehsaz, A.; Vayisoglu, T.; Uyaniker, Z.A.; Cekic, E.; Ozturk, E.; Isikay, A.I.; Hanalioglu, S. Relative Cortical Atrophy Index as a Strong Predictor of Recurrence After Surgery for Chronic Subdural Hematoma. Neurosurgery 2024, 95, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, M.E.; Kadioglu, H.H. Identification of Radiological and Clinical Factors that Increase the Risk of Chronic Subdural Hematoma Recurrence. Turk. Neurosurg. 2023, 33, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Turner, R.C.; Josiah, D.; Knotts, C.; Bhatia, S. Do Age and Anticoagulants Affect the Natural History of Acute Subdural Hematomas? Arch. Emerg. Med. Crit. Care 2016, 1, 1010. [Google Scholar]
- MacIntosh, B.J.; Liu, Q.; Schellhorn, T.; Beyer, M.K.; Groote, I.R.; Morberg, P.C.; Poulin, J.M.; Selseth, M.N.; Bakke, R.C.; Naqvi, A.; et al. Radiological features of brain hemorrhage through automated segmentation from computed tomography in stroke and traumatic brain injury. Front. Neurol. 2023, 14, 1244672. [Google Scholar] [CrossRef]
- Hillal, A.; Ullberg, T.; Ramgren, B.; Wassélius, J. Computed tomography in acute intracerebral hemorrhage: Neuroimaging predictors of hematoma expansion and outcome. Insights Imaging 2022, 13, 180. [Google Scholar] [CrossRef]
- Reddy, P.; Priya, P.S. ABC/2 formula versus computer-assisted analysis in calculating intra-cranial haemorrhage volume on computed tomographic imaging. Comput. Med. Biomech. Biomed. Eng. Imaging Vis. 2024, 12, 2327416. [Google Scholar] [CrossRef]
- Tada, A.; Hisada, K.; Suzuki, T.; Kadoya, S. Measurement volume of intracranial hematoma by computed tomography (author’s transl). No Shinkei Geka 1981, 9, 251–256. (In Japanese) [Google Scholar] [PubMed]
- Petrov, A.; Kashevnik, A.; Haleev, M.; Ali, A.; Ivanov, A.; Samochernykh, K.; Rozhchenko, L.; Bobinov, V. AI-Based Approach to One-Click Chronic Subdural Hematoma Segmentation Using Computed Tomography Images. Sensors 2024, 24, 721. [Google Scholar] [CrossRef]
- Kellogg, R.T.; Vargas, J.; Barros, G.; Sen, R.; Bass, D.; Mason, J.R.; Levitt, M. Segmentation of Chronic Subdural Hematomas Using 3D Convolutional Neural Networks. World Neurosurg. 2021, 148, e58–e65. [Google Scholar] [CrossRef]
- Fang, C.; Ji, X.; Pan, Y.; Xie, G.; Zhang, H.; Li, S.; Wan, J. Combining Clinical-Radiomics Features with Machine Learning Methods for Building Models to Predict Postoperative Recurrence in Patients with Chronic Subdural Hematoma: Retrospective Cohort Study. J. Med. Int. Res. 2024, 26, e54944. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Rueckel, J.; Döpfert, J.; Ling, W.X.; Opalka, J.; Brem, C.; Hesse, N.; Ingenerf, M.; Koliogiannis, V.; Solyanik, O.; et al. Artificial intelligence-based rapid brain volumetry substantially improves differential diagnosis in dementia. Alzheimers Dement. 2024, 16, e70037. [Google Scholar] [CrossRef]
- Kral, J.; Cabal, M.; Kasickova, L.; Havelka, J.; Jonszta, T.; Bar, M. Machine learning volumetry of ischemic brain lesions on CT after thrombectomy-prospective diagnostic accuracy study in ischemic stroke patients. Neuroradiology 2020, 62, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, A.K.; Hong, S.; Bretzner, M.; Schirmer, M.D.; Regenhardt, R.W.; Arsava, E.M.; Donahue, K.; Nardin, M.; Dalca, A.; Giese, A.-K.; et al. Association of Stroke Lesion Pattern and White Matter Hyperintensity Burden with Stroke Severity and Outcome. Neurology 2022, 99, e1364–e1379. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).