Comparative Analysis of CT Fluoroscopy Modes and Gastropexy Techniques in CT-Guided Percutaneous Radiologic Gastrostomy
Abstract
1. Introduction
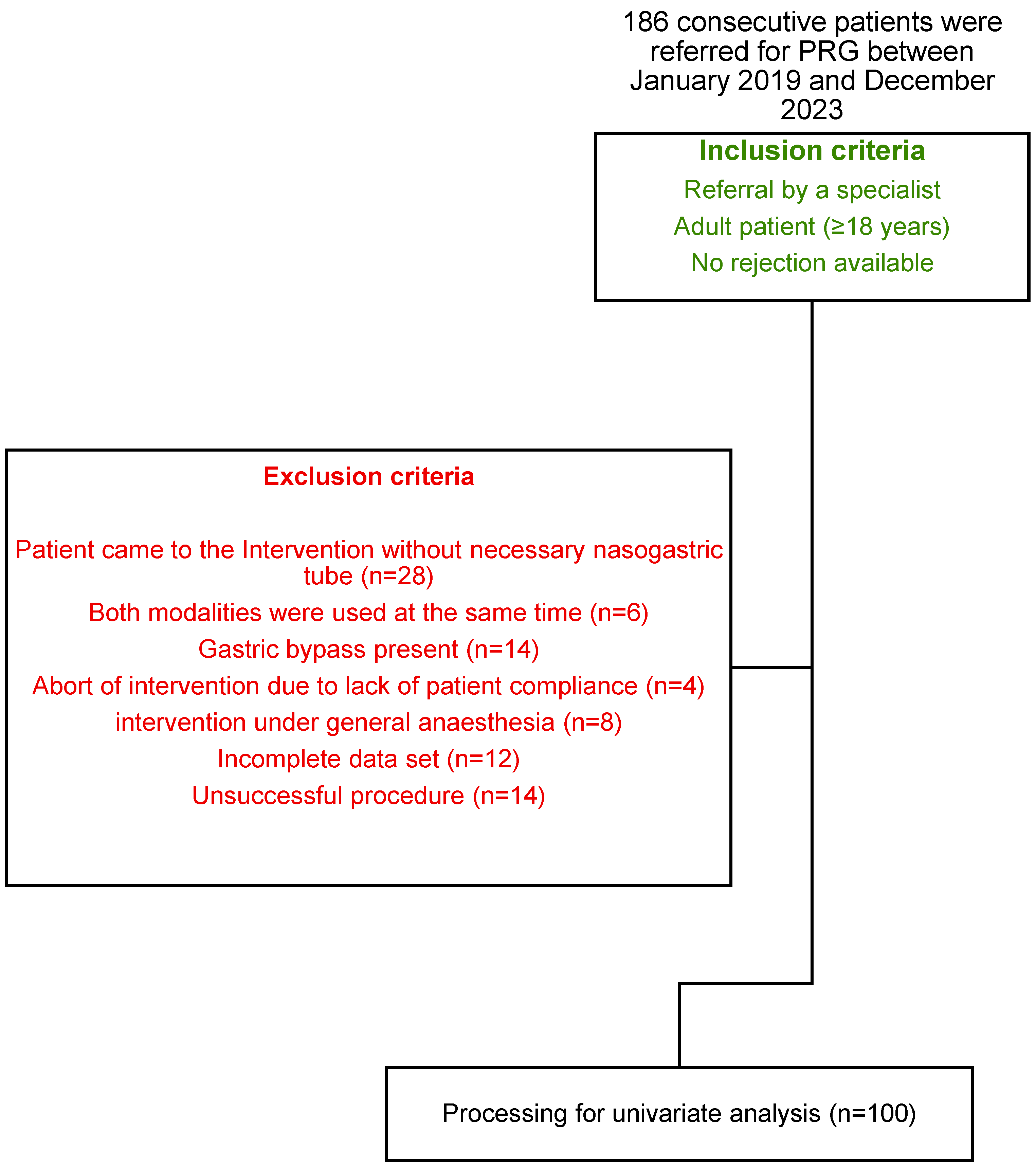
2. Materials and Methods
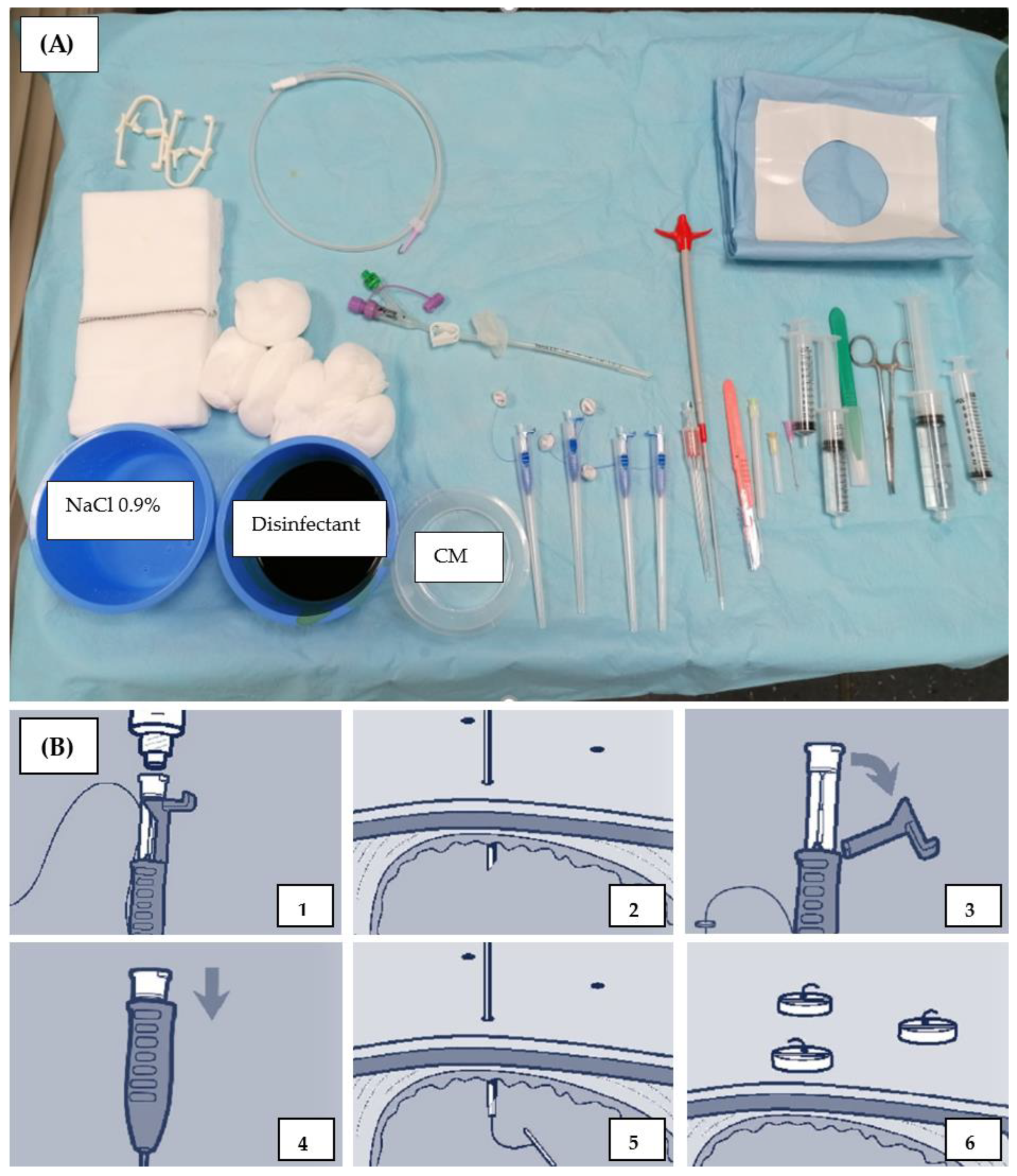
2.1. Baseline Assessment and Preparatory Measures
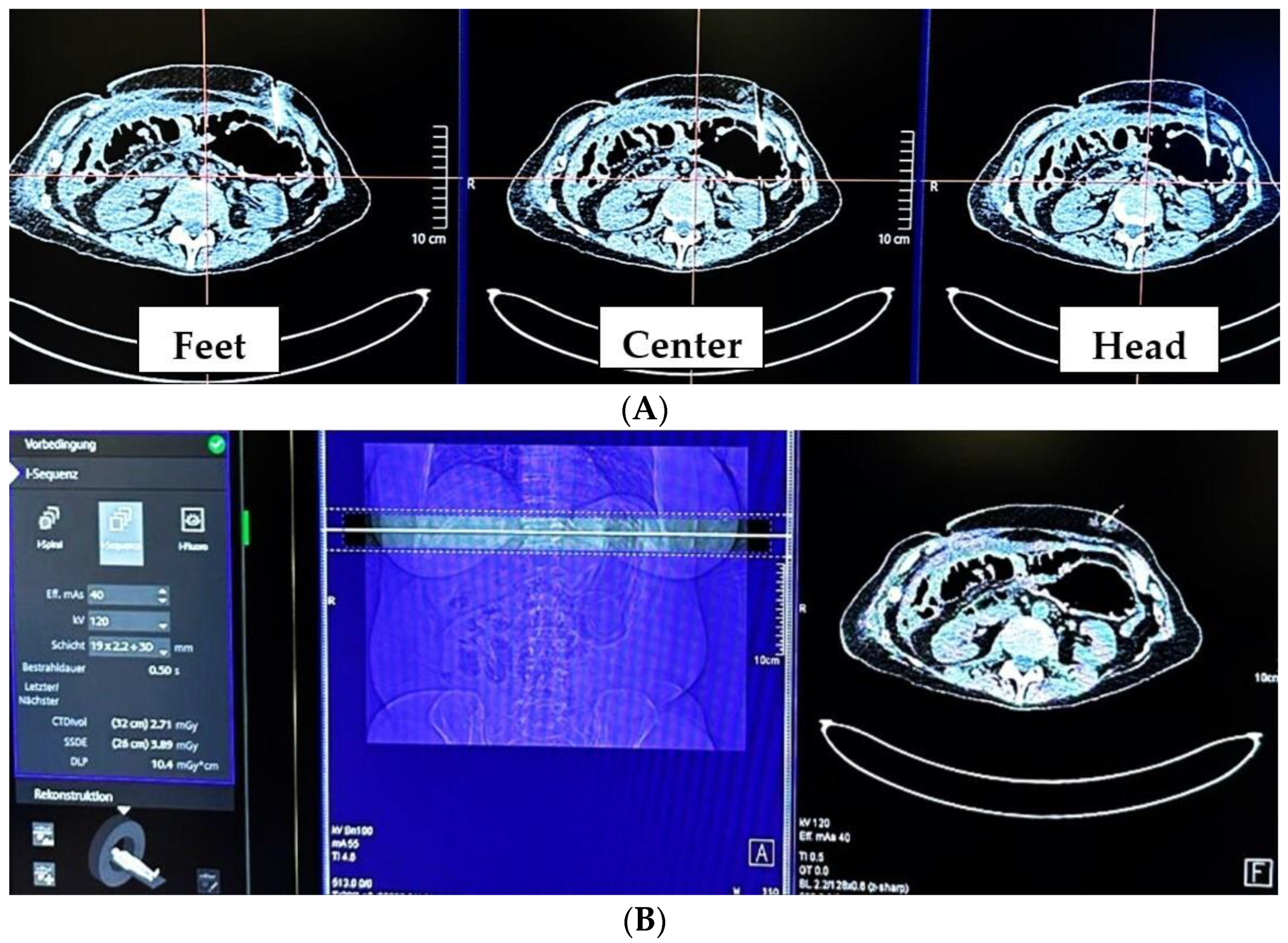
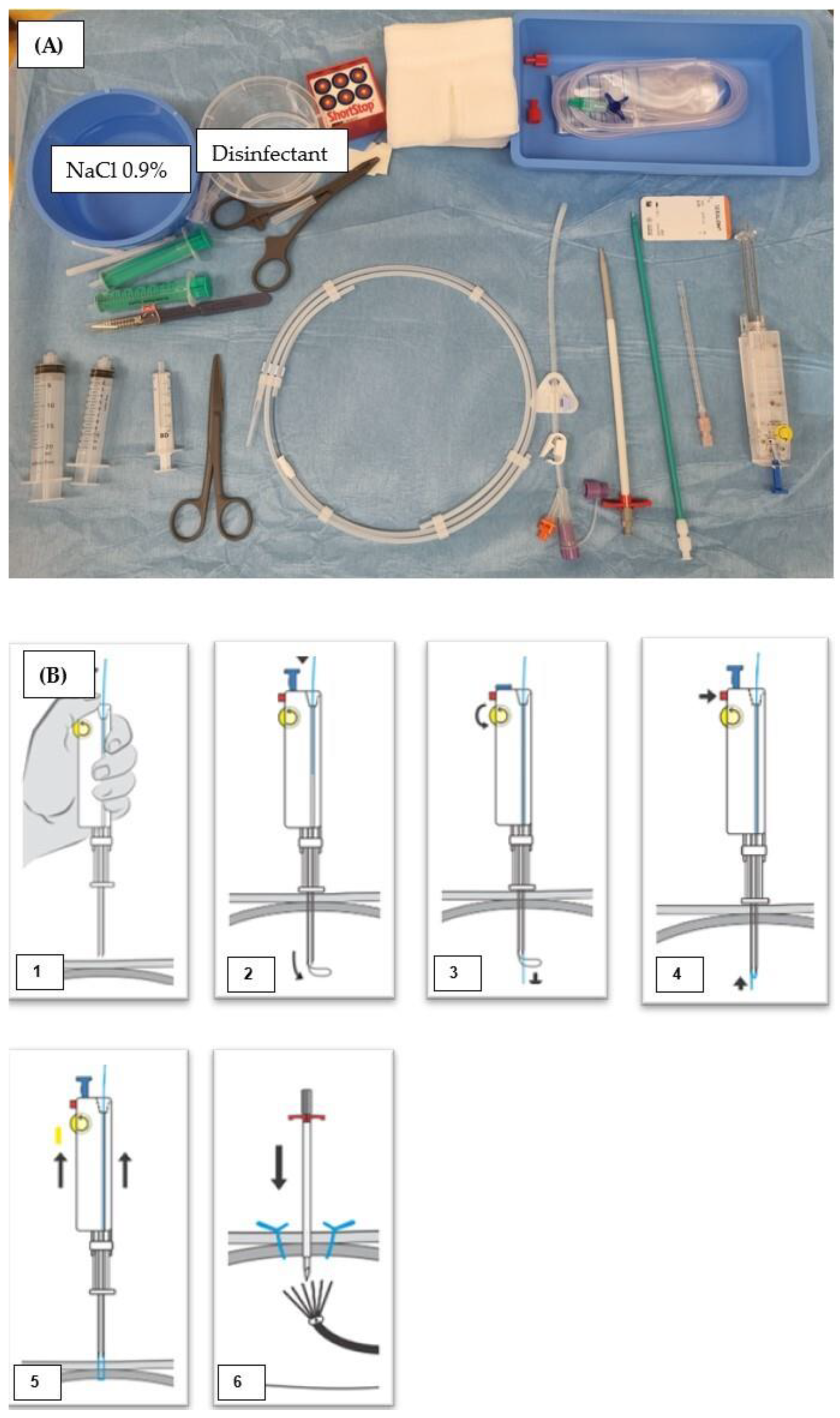
2.2. CT-Guided PRG Techniques at the Two Study Sites
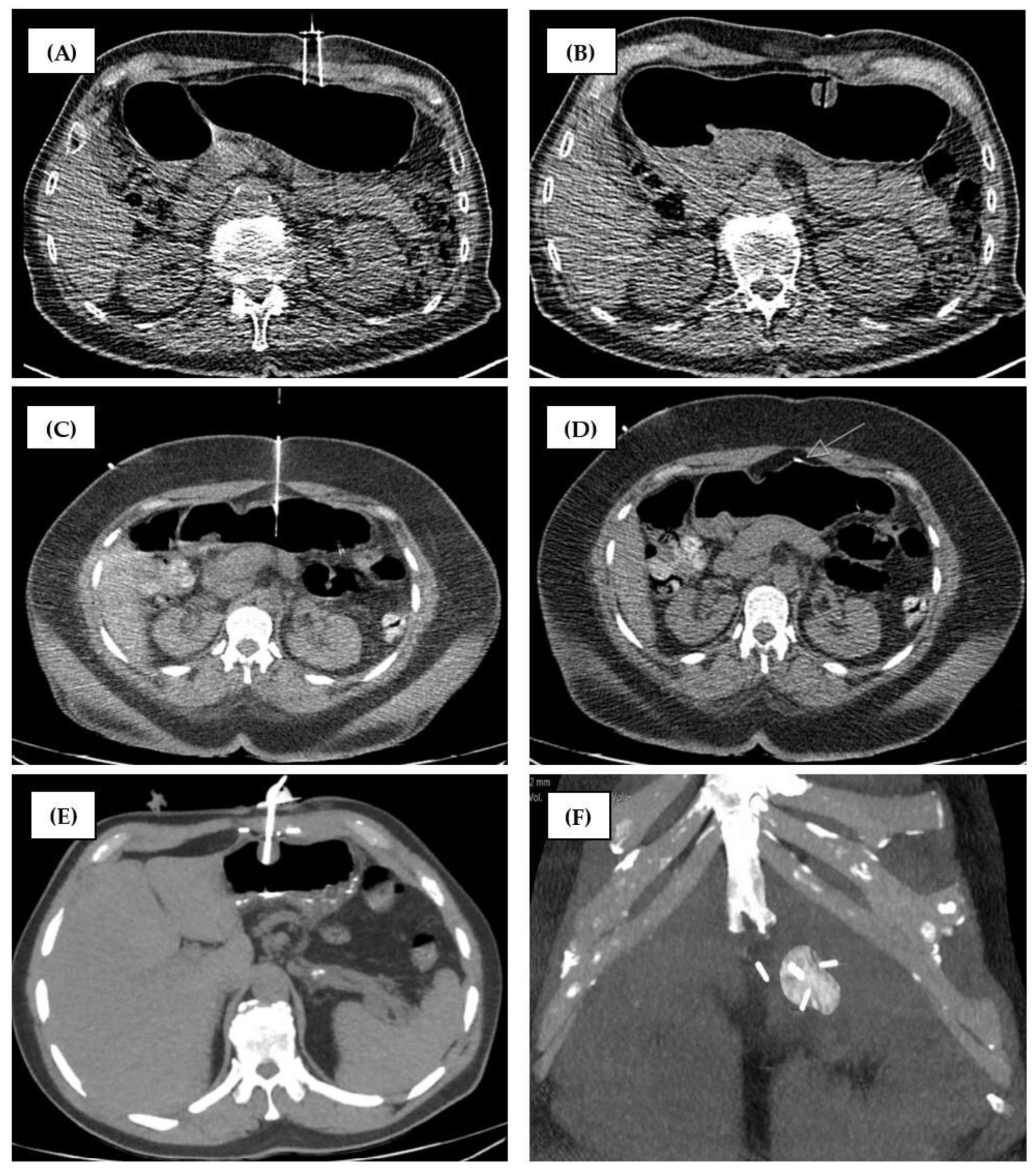
2.2.1. Group 1 (MS-CT BM and Retention Anchor Suture)
2.2.2. Group 2 (RT-CTF and Gastropexy Device)
2.3. Follow-Up
2.4. Data Evaluation
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Interventional Parameters
3.3. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mildenberger, P.; Oberholzer, K.; Kauczor, H.; Düber, C.; Kurz, S.; Schild, H.; Thelen, M. Radiologically assisted percutaneous gastro-/enterostomy--a retrospective analysis of 90 procedures. RöFo 1996, 165, 74–79. [Google Scholar] [PubMed]
- Deurloo, E.; Kool, L.S.; Kröger, R.; Van Coevorden, F.; Balm, A. Percutaneous radiological gastrostomy in patients with head and neck cancer. Eur. J. Surg. Oncol. 2001, 27, 94–97. [Google Scholar] [CrossRef]
- Lorentzen, T.; Nolsøe, C.; Adamsen, S. Percutaneous radiologic gastrostomy with a simplified gastropexy technique under ultrasonographic and fluoroscopic guidance: Experience in 154 patients. Acta Radiol. 2007, 48, 13–19. [Google Scholar] [CrossRef]
- Tamura, A.; Kato, K.; Suzuki, M.; Sone, M.; Tanaka, R.; Nakasato, T.; Ehara, S. CT-guided percutaneous radiologic gastrostomy for patients with head and neck cancer: A retrospective evaluation in 177 patients. Cardiovasc. Interv. Radiol. 2016, 39, 271–278. [Google Scholar] [CrossRef]
- Wollman, B.; d’Agostino, H. Percutaneous radiologic and endoscopic gastrostomy: A 3-year institutional analysis of procedure performance. Am. J. Roentgenol. 1997, 169, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Schneider, J.; Düber, C.; Pitton, M. Comparison of fluoroscopy-guided Pull-type percutaneous radiological gastrostomy (Pull-type-PRG) with conventional percutaneous radiological gastrostomy (Push-type-PRG): Clinical results in 253 patients. Eur. Radiol. 2011, 21, 2354–2361. [Google Scholar] [CrossRef]
- Brönnimann, M.P.; Hirzberger, L.; Keller, P.M.; Gsell-Albert, M. Antibacterial Effects of X-ray and MRI Contrast Media: An In Vitro Pilot Study. Int. J. Mol. Sci. 2023, 24, 3470. [Google Scholar] [CrossRef]
- Segger, L.; Auer, T.A.; Fleckenstein, F.N.; Fehrenbach, U.; Torsello, G.F.; Frisch, A.; Jonczyk, M.; Hamm, B.; Gebauer, B. CT fluoroscopy-guided percutaneous gastrostomy (CT-PG)—A single center experience in 233 patients. Eur. J. Radiol. 2022, 152, 110333. [Google Scholar] [CrossRef] [PubMed]
- Canaz, E.; Sehouli, J.; Gebauer, B.; Segger, L.; Collettini, F.; Auer, T.A. CT Fluoroscopy-Guided Percutaneous Gastrostomy in the Palliative Management of Advanced and Relapsed Ovarian Cancer: The Charite Experiences and a Review of the Literature. Cancers 2023, 15, 4540. [Google Scholar] [CrossRef]
- Hu, H.-T.; Yuan, H.; Guo, C.-Y.; Yao, Q.-J.; Geng, X.; Cheng, H.-T.; Ma, J.-L.; Zhao, Y.; Jiang, L.; Zhao, Y.-Q. Radiological gastrostomy: A comparative analysis of different image-guided methods. Int. J. Gastrointest. Interv. 2021, 10, 67–71. [Google Scholar] [CrossRef]
- Carlson, S.K.; Felmlee, J.P.; Bender, C.E.; Ehman, R.L.; Classic, K.L.; Hu, H.H.; Hoskin, T.L. Intermittent-Mode CT Fluoroscopy–guided Biopsy of the Lung or Upper Abdomen with Breath-hold Monitoring and Feedback: System Development and Feasibility. Radiology 2003, 229, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Brönnimann, M.P.; Christe, A.; Heverhagen, J.T.; Gebauer, B.; Auer, T.A.; Schnapauff, D.; Collettini, F.; Schroeder, C.; Dorn, P.; Ebner, L. Pneumothorax risk reduction during CT-guided lung biopsy–Effect of fluid application to the pleura before lung puncture and the gravitational effect of pleural pressure. Eur. J. Radiol. 2024, 176, 111529. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.; Dixon, R.G.; Collins, J.D.; Walser, E.M.; Nikolic, B. Best practice guidelines for CT-guided interventional procedures. J. Vasc. Interv. Radiol. 2018, 29, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Song, H.-Y.; Kim, K.R.; Shin, J.H.; Choi, E.K. The one-anchor technique of gastropexy for percutaneous radiologic gastrostomy: Results of 248 consecutive procedures. J. Vasc. Interv. Radiol. 2008, 19, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Thornton, F.; Fotheringham, T.; Haslam, P.; McGrath, F.; Keeling, F.; Lee, M. Percutaneous radiologic gastrostomy with and without T-fastener gastropexy: A randomized comparison study. Cardiovasc. Interv. Radiol. 2002, 25, 467–471. [Google Scholar] [CrossRef]
- de Baere, T.; Chapot, R.; Kuoch, V.; Chevallier, P.; Delille, J.P.; Domenge, C.; Schwaab, G.; Roche, A. Percutaneous gastrostomy with fluoroscopic guidance: Single-center experience in 500 consecutive cancer patients. Radiology 1999, 210, 651–654. [Google Scholar] [CrossRef]
- Gang, M.H.; Kim, J.Y. Short-term complications of percutaneous endoscopic gastrostomy according to the type of technique. Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 214–222. [Google Scholar] [CrossRef]
- Halyard. Halyard Gastrointestinal Anchor Set Instructions for Use. Available online: https://online.pubhtml5.com/ymcs/fudu/#p=3 (accessed on 1 October 2024).
- Kabi, F. Indications of the Freka ® Pexact, ENFIT. Available online: https://www.fresubin.com/sites/default/files/2021-10/Freka%20Pexact%20II%20Brochure.pdf (accessed on 1 October 2024).
- Kandarpa, K.; Machan, L. Handbook of Interventional Radiologic Procedures; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Mousavi-Gazafroudi, S.S.; Sajjadieh-Khajouei, A.; Moradi, M.; Mousavi-Gazafroudi, S.S.; Yadegarfar, G.; Tavakoli, M.B. Evaluation of image quality and radiation dose in low tube voltage coronary computed tomography angiography. Arya Atheroscler. 2019, 15, 205–210. [Google Scholar]
- Brönnimann, M.P.; Kulagowska, J.; Gebauer, B.; Auer, T.A.; Collettini, F.; Schnapauff, D.; Magyar, C.T.; Komarek, A.; Krokidis, M.; Heverhagen, J.T. Fluoroscopic-Guided vs. Multislice Computed Tomography (CT) Biopsy Mode-Guided Percutaneous Radiologic Gastrostomy (PRG)—Comparison of Interventional Parameters and Billing. Diagnostics 2024, 14, 1662. [Google Scholar] [CrossRef]
- Nattenmüller, J.; Filsinger, M.; Bryant, M.; Stiller, W.; Radeleff, B.; Grenacher, L.; Kauczor, H.-U.; Hosch, W. Complications in CT-guided procedures: Do we really need postinterventional CT control scans? Cardiovasc. Interv. Radiol. 2014, 37, 241–246. [Google Scholar] [CrossRef]
- Prosch, H.; Stadler, A.; Schilling, M.; Bürklin, S.; Eisenhuber, E.; Schober, E.; Mostbeck, G. CT fluoroscopy-guided vs. multislice CT biopsy mode-guided lung biopsies: Accuracy, complications and radiation dose. Eur. J. Radiol. 2012, 81, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Nutricia. Applikationstechnik für Kinder und Erwachsene. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.nutricia.de/content/dam/sn/local/dach/hc/none-brand/download-hcp/flocare_katalog_applikationstechnik_d.pdf&ved=2ahUKEwiskrnt6fuFAxXjBdsEHYluD40QFnoECBUQAQ&usg=AOvVaw2sUweKd8z3z1DRu4GDVWyj (accessed on 1 October 2024).
| Center 1 | Center 2 | |
|---|---|---|
| Staff present | 1 × interventionalist, radiographer, interventional radiology nurse | |
| Preparation | ||
| Required lab values | INR ≤ 1.5, Quick ≥ 60%, Hb ≥ 80g/L, platelate value ≥ 50 × 109/L | INR ≤ 1.5, Quick ≥ 50%, aPTT < 50 s, platelate value ≥ 50 × 109/L |
| Drugs during intervention | Lidocaine 1% (max. 20 mL), 20 mg Buscopan | Lidocaine 1%, 5 mg Midazolam, 8 mg Ondansetron and 15 mg Piritramid, if necessary (Buscopan 20 mg) |
| Stomach insufflated volume | 600 mL (room air) | 800 mL (room air) |
| Scanner | ||
| Type | Toshiba Asteion 4SL/Somatom X.cite | Somatom Definition AS |
| Mode | MS-CT BM | RT-CTF |
| Name | i-Sequence | i-Fluoro |
| CTF | intermittent | continuous |
| Acquired slices | 3 × 4 mm | 1 × 5 mm |
| Tube settings | 120 kV, 25 mAs, rotation time 0.5 s | 100 kV, 40 mAs, rotation time 0.36 s |
| Operator experience | board-certified radiologist and at least one year of experience in this procedure | |
| Material | ||
| Gastric tube | Flocare® Gastro Tube CH 14 | Freka® Pexact CH/FR 15 |
| Fixation material | 4 × single anchors | gastropexy device |
| Fixation technique | Free choice of anchor number, min 1. | 4 point with 2 sutures, distance to each of 2 cm |
| Dilatation | Enteral Access Dilation System 12–16 F | several, single dilatators 10, 12 and 16 F |
| Additional material | metalline® aluminum vaporized coating compress | Slit compress |
| Start of nutrition | early feeding 6 h after intervention | |
| Survey of PRG | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | All (n = 100) | C1, MS-CT BM with Single Anchor (n = 50) | C2, RT-CTF with Gastropexy Device (n = 50) | p Value | |||
| Female | 27 | 27% | 14 | 28% | 13 | 26% | 1.000 |
| Age (y) | 62.52 | ±12.36 | 62.52 | ±12.36 | 64.18 | ±12.44 | 0.288 |
| Indication | 0.011 * | ||||||
| HN-cancer | 80 | 80% | 35 | 70% | 45 | 90% | |
| Neurological disorders | 14 | 14% | 12 | 24% | 2 | 4% | |
| Other indications | 6 | 6% | 3 | 6% | 3 | 6% | |
| DLP of CTF (mGy×cm) | 43.59 | ±53.10 | 56.28 | ±67.897 | 30.91 | ±27.539 | <0.001 * |
| Exposure Time (s) | 11.63 | ±12.86 | 5.88 | ±5.618 | 16.69 | ±15.183 | <0.001 * |
| Proportion of CTF DLP to total DLP | 0.13 | ±0.12 | 0.12 | ±0.134 | 0.14 | ±0.111 | 0.011 * |
| No. of CT | 2.31 | ±1.71 | 3.42 | ±1.797 | 1.20 | ±0.404 | <0.001 * |
| DLP of CTs (mGy×cm) | 390.43 | ±545.82 | 582.37 | ±714.3 | 198.50 | 128.54 | <0.001 * |
| Total DLP (mGy×cm) | 462.90 | ±582.92 | 681.00 | ±751.49 | 244.62 | ±156.74 | <0.001 * |
| GT (min) | 11.14 | ±5.725 | 11.50 | ±5.239 | 11.17 | ±6.015 | 0.463 |
| Procedure time (min) | 35.74 | ±21.27 | 41.08 | ±16.97 | 30.40 | ±23.83 | <0.001 * |
| Complications | 0.458 | ||||||
| Minor | 19 | 19% | 7 | 14% | 12 | 24% | |
| Major | 2 | 2% | 1 | 2% | 1 | 2% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brönnimann, M.P.; Tarca, M.; Segger, L.; Kulagowska, J.; Fleckenstein, F.N.; Gebauer, B.; Fehrenbach, U.; Collettini, F.; Heverhagen, J.T.; Auer, T.A. Comparative Analysis of CT Fluoroscopy Modes and Gastropexy Techniques in CT-Guided Percutaneous Radiologic Gastrostomy. Tomography 2024, 10, 1754-1766. https://doi.org/10.3390/tomography10110129
Brönnimann MP, Tarca M, Segger L, Kulagowska J, Fleckenstein FN, Gebauer B, Fehrenbach U, Collettini F, Heverhagen JT, Auer TA. Comparative Analysis of CT Fluoroscopy Modes and Gastropexy Techniques in CT-Guided Percutaneous Radiologic Gastrostomy. Tomography. 2024; 10(11):1754-1766. https://doi.org/10.3390/tomography10110129
Chicago/Turabian StyleBrönnimann, Michael P., Mauro Tarca, Laura Segger, Jagoda Kulagowska, Florian N. Fleckenstein, Bernhard Gebauer, Uli Fehrenbach, Federico Collettini, Johannes T. Heverhagen, and Timo A. Auer. 2024. "Comparative Analysis of CT Fluoroscopy Modes and Gastropexy Techniques in CT-Guided Percutaneous Radiologic Gastrostomy" Tomography 10, no. 11: 1754-1766. https://doi.org/10.3390/tomography10110129
APA StyleBrönnimann, M. P., Tarca, M., Segger, L., Kulagowska, J., Fleckenstein, F. N., Gebauer, B., Fehrenbach, U., Collettini, F., Heverhagen, J. T., & Auer, T. A. (2024). Comparative Analysis of CT Fluoroscopy Modes and Gastropexy Techniques in CT-Guided Percutaneous Radiologic Gastrostomy. Tomography, 10(11), 1754-1766. https://doi.org/10.3390/tomography10110129






