Abstract
Chitin, the second most abundant biopolymer after cellulose, is an important resource for biosourced materials. The global demand for chitin is rapidly increasing, however, the majority of industrial chitin is sourced from crustacean shells, which may be less accessible in regions without seafood waste. Therefore, it is crucial to explore alternative chitin sources, such as those derived from beetles and other arthropods. This study investigated chitin extraction from nine species of Curculionidae (true weevils), which are recognized as crop pests. The extraction process and yields were described, and the isolated chitin was characterized by SEM, IR spectroscopy, elemental analysis, XRD, and ash and water content measurements. This work highlights the potential of Curculionidae as an alternative chitin source.
1. Introduction
In a world where climate change is expected to have a considerable impact on the ability of humans to produce food, invasive insects and pests that can significantly harm or destroy vegetable production are of particular concern. Invasive insects are often responsible for the complete destruction of an agricultural culture, and thus it is very important to prevent these events with different treatments. Prevention may include different control strategies including: physical, chemical, biological, or cultural methods [1,2]. But if prevention fails, an important part of the production is lost. Considering these challenges, it is of interest to identify resources that can be produced or extracted from invasive species, and the mechanisms by which this extraction may be feasible. Ultimately, identifying strategies to create value from invasive species that cannot be eliminated easily would have a significant impact.
One interesting method to obtain a valuable product from invasive insect species would be through chitin extraction. Insects are arthropods, and as a consequence, they have an exoskeleton rich in chitin. Chitin is a chain polymer of N-acetylglucosamine and is the second most abundant polysaccharide polymer after cellulose [3]. In Arthropoda’s exoskeleton and as consequence in insect’s exoskeleton, the most abundant form of chitin is the α form. In this form, chitin presents a fully anti-parallel organization. This organization leads to many hydrogen bonds and, as consequence, high stability and low solubility [4].
Unfortunately, due to this poor solubility in most common solvents, α-chitin is difficult to use in materials applications in its pristine form. This lack of solubility is a consequence of strong intermolecular interactions and a compact macromolecular structure. Typically, to avoid this solubility limitation, chitin is modified prior to industrial use. For example, chitin is the main raw component in chitosan production once it is partially deacetylated. Chitosan is an important material that can be used for many application domains including food, medicine, and textiles [5,6,7,8,9,10,11,12]. Chitin-sourced chitosan is already produced on an industrial scale, mainly from seafood waste as the exoskeletons of shrimp, lobster and crabs are rich in chitin [13,14,15]. Due to the human consumption of crustaceans, this is a relatively large resource. Despite this, and due to the important industrial interest, new chitin sources are investigated. Recent works highlight that mushroom, corals, sponges and terrestrial Arthropoda are promising sources of chitin [16,17,18,19,20,21,22,23]. Our prior work investigated beetles (giant flower beetles or dung beetles) as a source of chitin and therefore chitosan [24,25,26]. Building upon this foundation, here we focus on the possibility to use Curculionidae as a potential source of chitin (Figure 1). This includes extensive SEM observation of the specimens prior to and following chitin extraction, and the extracted chitins are fully characterized. Curculionidae include a great diversity of species including some species that are considered pest insects. For example, true weevils cause havoc in cocoa, palm, sugar beet and banana productions [27,28,29,30,31,32]. Of course, not all Curculionidae are classified as pest insects, and some are beneficial as pollinators. For this study we chose various species from different genus (including pest and beneficial species) to determine the best candidates for chitin extraction, which can inform future strategies for materials development.
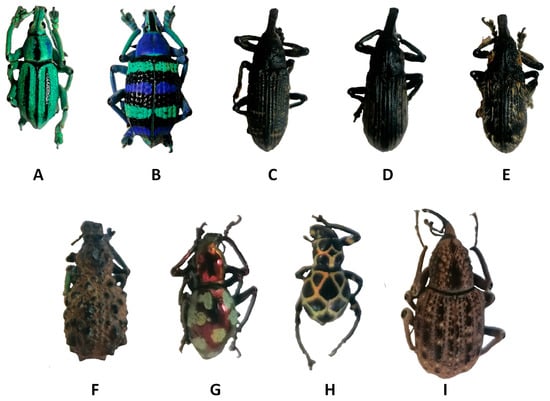
Figure 1.
Examples of Curculionidae specimens. (A) Eupholus cuvieri Guérin-Méneville, 1830, (B) Eupholus magnificus Kirsch, 1877, (C) Lixus sturmii Boheman, 1836, (D) Lixus gigas Fairmaire, 1904, (E) Lixus albicornis Fairmaire, 1904, (F) Holonychus saxosus Coquerel, 1859, (G) Pachyrhynchus gemmatus purpureus Kraatz, 1888, (H) Pachyrhynchus reticulatus Waterhouse, 1841 and (I) Sipalinus gigas Fabricius, 1775.
2. Materials and Methods
2.1. Materials
The specimens used in this work came from the private collection of G. Godeau. All observations were performed on dried samples of dead specimens. No specimens were sacrificed for this work. Due to the small size of some species studied, such as animals of the genus Pachyrhynchus, it is difficult to separate the internal elements and exoskeleton of the specimens. Therefore, all specimens were used whole to have the same treatment for all species. The internal elements are hydrolyzed, degraded and eliminated during chemical treatment. To consider the diversity of the Curculionidae family, 9 species belonging to 5 genera (including Lixus and Sipalinus) were investigated in this work. With this design, both beneficial and pest insects are considered. The complete list of the studied species is reported in Table 1.

Table 1.
Studied species data.
2.2. Chitin Extraction [24]
Demineralization: Dry samples were immersed in 1 M HCl aqueous solution. The solution was then heated for 2 h (95 °C) using a dry bath. The liquid phase was filtered off, and the resulting exoskeleton was rinsed with deionized water until reaching a neutral pH. The exoskeleton was then dried in an oven overnight at 90 °C to estimate the demineralization yield:
The exoskeleton was used for deproteination without any further purification.
Deproteination: After demineralization, the exoskeletons were placed in an aqueous 2 M NaOH solution. The solution was then warmed (95 °C) over a period of 36 h using a dry bath. During this treatment, the solution rapidly turned black. Therefore, the NaOH solution was refreshed hourly during the first 6 h of the treatment. The liquid phase was then removed, and the exoskeletons were rinsed with deionized water until achieving a neutral pH. The exoskeletons were then dried in an oven overnight at 90 °C to determine the deproteination yield:
The resulting material is directly used for bleaching without further purification.
Bleaching: The deproteinated exoskeletons were bleached using an aqueous solution of sodium hypochlorite (3.6 wt. %) at room temperature for 1 h. The bleached exoskeletons were then washed multiple time with deionized water and then with ethanol. The samples were finally dried in an oven (90 °C). The overall yields are determined as:
All yields are presented in Table 2.

Table 2.
Chitin extraction data.
2.3. Chitin Characterization
2.3.1. FT-IR Characterization
Fourier Transform Infrared spectroscopy (FT-IR) measurements were carried out using a Spectrum Two FT-IR spectrometer from Perkin Elmer with universal ATR accessory. The measurements were performed between 4000 cm−1 and 500 cm−1.
2.3.2. Thermal Analysis (TGA)
Thermogravimetric (TGA) measurements were performed on a TGA/DSC 1 from Mettler Toledo. The samples were heated from 25 °C to 850 °C with a heating rate of 10 K.min−1 under nitrogen flow of 50 mL.min−1, the gas was then switched to air to oxidize carbon and determine the ash content.
2.3.3. X-ray Diffraction (XRD) Analysis
X-ray diffraction of powdered chitin samples were examined by a Panalytical X’Pert Pro with an Xcelerator fast detector operating at 45 kV and 30 mA. The radiation was generated from a Cu Kα (k = 0.15418 nm) source. The diffraction data were collected at 2θ values from 5° to 75°.
The crystallinity indices of isolated chitosan samples (CrI) were calculated from XRD data using the following equation [19,33]:
where Icr is the maximum intensity for crystalline lattices at 2θ = 19.6° and Iam is the maximum intensity at 2θ = 16°, corresponding to the amorphous region.
2.3.4. Elemental Analysis
Elemental analyses were carried out on an elemental analyzer Flash EA 1112 series (Thermo Finnigan, Waltham, MA, USA), equipped with Eager 300 Xperience software.
2.3.5. Scanning Electron Microscopy
SEM observations were carried out using Phenom ProX scanning electron microscope. Samples were observed with a gold coating at an accelerating voltage of 5 and 10 kV. The samples were coated using Q150R S Sp.
3. Results
Curculionoidae, also known as true weevils, are one of the most diverse groups of phytophagous Coleoptera. More than 51,000 species belonging to approximately 4600 genera of Curculionidae have been described [34]. Among this wide diversity, many weevils can be described as pest insects that harm various crops or ornamental plants including banana, cocoa, and palm [27,28,29]. For example, the Lixus genus is reported as major pests of sugar beet in Iran and leafy vegetables in Nigeria. Another example, Sipalinus gigas, are considered as one of the most significant wood pests in Japan [31,35,36]. All the specimens were treated for chitin extraction. The selected chitin extraction strategy is the classical chemical approach described for many decades for chitin extraction from shrimp [24,37]. It consists of a three step treatment that can be summarized as follows. The first step entails demineralization in an HCl solution (1 M in water) for 1 h at 95 °C. The second step consists of immersion in a sodium hydroxide solution (2 M in water) at 95° over 36 h. The third and final step is bleaching. For this bleaching step, the material is immersed in a sodium hypochlorite solution (3.6 wt. % in water) for 1 h at room temperature. Between each step, the material is washed with water. The final isolated material can be reported as Curculionidae’s chitin. The extracted material should theoretically present a chemical structure similar to the one presented in Figure 2.
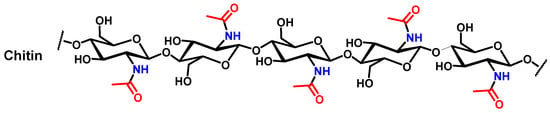
Figure 2.
Theoretical chemical structure of chitin.
Of course, the selected species went through the chitin extraction process separately to obtain species-specific data. All the data collected at each stage of the extraction are reported in Table 2, including yields for demineralization, deproteination and the overall yield of both steps.
The chitin extraction data mostly show uniform results. The demineralization step has an associated yield between 80 and 90%. For deproteination, the yield is roughly 15–25%, and the overall yield is between 12 and 19%. These values remain similar for all species. Compared with chitin extraction yields reported for other Coleoptera in the literature, the values are consistent but remain in the low range of reported values [22]. These low yields may be a consequence of the use of whole specimens that lead to underestimation of the overall yield. However, even if the extraction yields are in a low range compared with other beetles, these yields remain significant compared to the yield for chitin extraction from shrimps.
The extracted samples of chitin were then characterized. All treated surfaces were first investigated for their morphologies, using scanning electron microscopy (SEM) and compared with the corresponding virgin surfaces. Not surprisingly, depending on the species, the surface morphologies were very different. For example, some Curculionidae genera are known to exhibit structural coloration like Eupholus (Figure S1) and Pachyrhynchus (Figure S2).
For both genera, the surface morphology varies across the specimens. For example, the colored parts (blue or green) of E. cuvieri (Figure S1A) and E. magnificus (Figure S1C) show nanostructured (wrinkled) microscales which contribute to the structural color, as has been previously described in the literature [38]. Not surprisingly, the black part of both species, which lack any structural color, is smooth when observed via microscopy (Figure S1B for E. cuvieri and Figure S1D for E. magnificus). Similar microscales are observed for P. reticulatus and P. gemmatus purpureus. Here, these microscale structures correspond to the golden network present on P. reticulatus (Figure S2A) and the large green spots on P. gemmatus purpureus (Figure S2C).
On these species, other types of surface are observed, both the black part of P. reticulatus (Figure S2B) and the metallic red surface of P. gemmatus purpureus (Figure S2D) are smooth compared with the previous one. Of course, the red metallic surface of P. gemmatus purpureus is structurally colored but the structuration remains under the surface. For the Lixus genus, all species present similar surface morphologies. These surfaces are highlighted in the supporting information in Figure S3. For Lixus species, most of the observed surfaces are smooth, with or without hairs depending on the position imaged (Figure S3). Observations of H. saxosus, which lacks any structural coloration, reveals dense microscale organizations (Figure S4A,B). The surface of S. gigas has vertically aligned pins (Figure S4C,D). This structuration appears to be quite uniform along the darkest surface.
After the treatment used to extract chitin, surfaces reveal an inner-connected chitin network (Figure 3, Figure 4, Figure 5 and Figure 6). SEM images presented in Figure 3A–D show treated surfaces from Eupholus species. The surface morphology can provide valuable insights into the effectiveness of the extraction process. Differences in surface structure between species can affect the efficiency of deproteinization and demineralization, and SEM helps to visualize these effects. Additionally, the observation of different surface morphologies corresponding to various insect surface colors provides insights that could contribute to bionics research. These differences in morphology may have implications for bioinspired design, offering potential applications beyond chitin extraction.
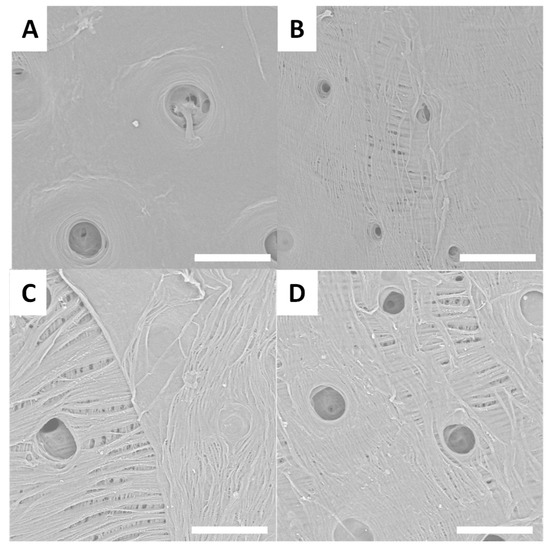
Figure 3.
Examples of SEM images (scale bar = 30 µm) observed for treated surfaces of E. cuvieri (A,B), E. magnificus (C,D).
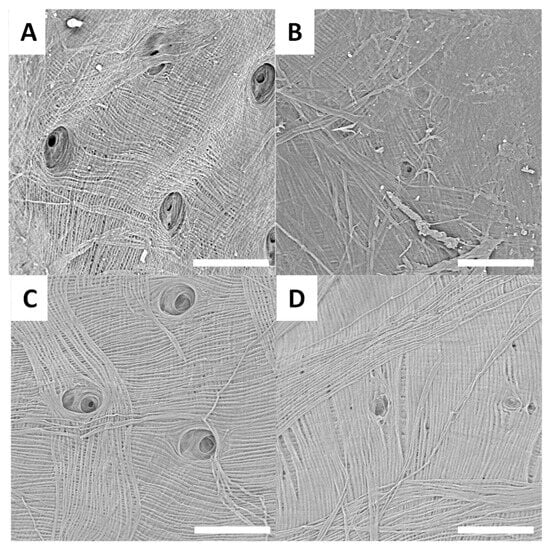
Figure 4.
Examples of SEM images (scale bar = 30 µm) observed for treated surfaces of P. gemmatus purpureus (A,B) and P. reticulatus (C,D).
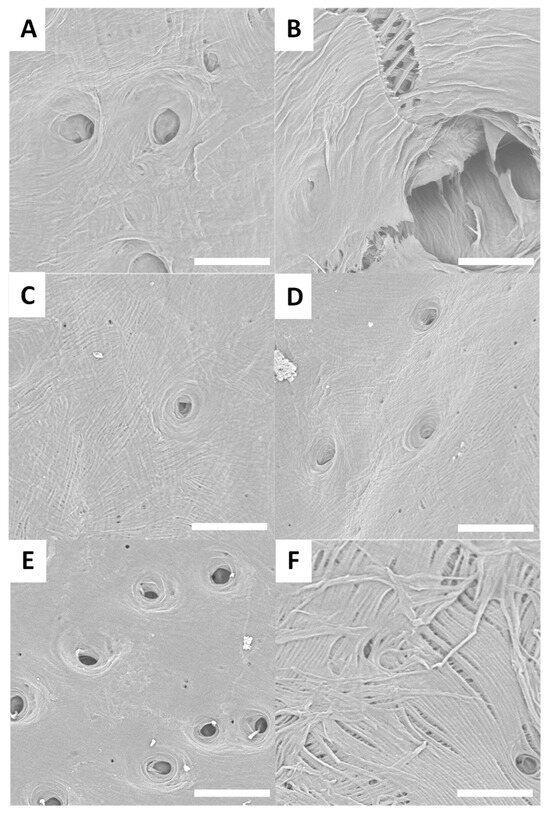
Figure 5.
Examples of SEM images (scale bar = 30 µm) observed for treated surfaces of L. sturmii (A,B), L. gigas (C,D) and L. albicornis (E,F).
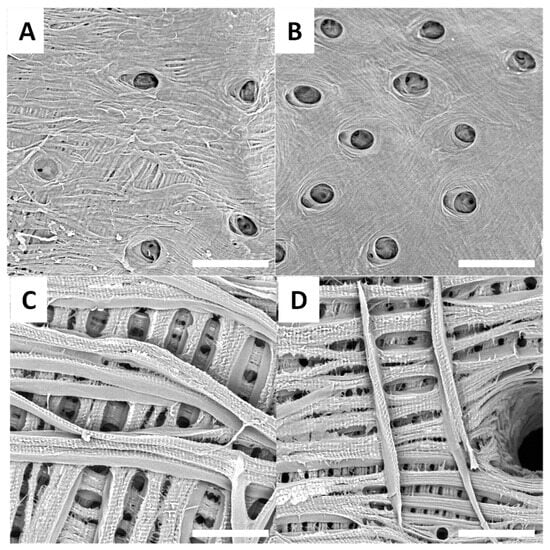
Figure 6.
Examples of SEM images (scale bar = 30 µm) observed for raw surfaces of H. saxosus (A,B) and S. gigas (C,D).
SEM images of Pachyrhynchus species’ treated surfaces are presented in Figure 4A–D.
Different Lixus species surfaces after treatment are presented in Figure 5A–F.
Treated surfaces from H. saxosus are presented in Figure 6A,B, and treated surfaces from S. gigas are presented in Figure 6C,D.
SEM observations of the treated surfaces reveal holes on most of them. These holes can be explained by the loss or degradation of insects’ surfaces microstructures during the chemical treatment. In the case of treated surfaces from Eupholus, Pachyrhynchus and Holonychus species, the holes may be linked to the loss of the scales from raw surfaces. These observations are consistent with the progressive loss of structural coloration seen during treatment, especially for Eupholus species. In the case of treated surfaces from Lixus species, the holes maybe linked to the degradation (during treatment) of the air shown by the raw surfaces. As the Sipalinus does not present such kind of microstructures, no holes were observed on the corresponding treated surfaces.
For most of the treated surfaces, a fiber network is observed even if the network has qualitative differences from one specie to the other. If such kind of network are consistent with expected structures for chitin materials, it remains only superficial observations. To investigate these variations in surface characterization further, additional experiments are needed.
FT-IR analyses were performed on all extracted chitin samples. Examples of FT-IR spectra for the select species are shown in Figure 7, and all other FT-IR spectra collected are provided in the Supplementary Data (Figures S5–S12).
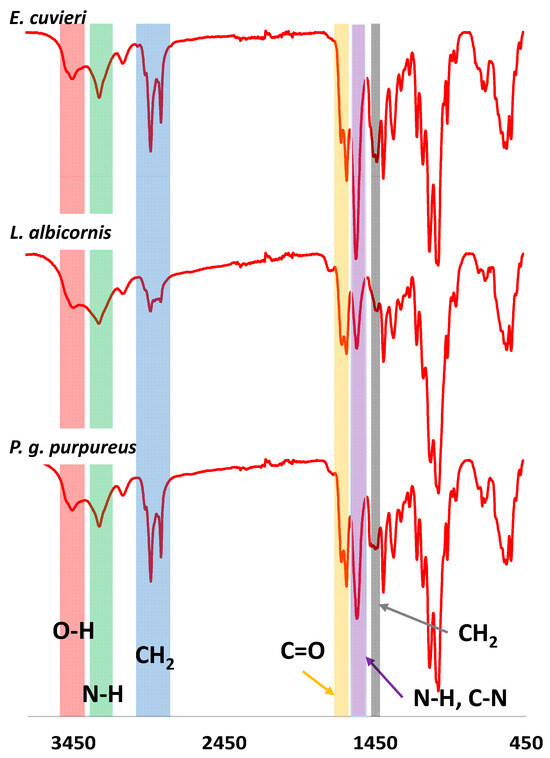
Figure 7.
Examples of FTIR spectra observed for Curculionidae specimens.
The FT-IR spectra for each extract have results consistent with chitin material. For example, strong bands at 3380–3450 cm−1 and 3250–3300 cm−1 are observed, consistent with the stretching of O-H (red) and N-H bonds (green), respectively. Additionally, a band corresponding to sp3 CH2 vibration is observed at 2850–2950 cm−1 (blue), and the C=O band from amide group is observed at 1635–1660 cm−1 (orange). The bending and vibration bands from N-H and stretching band from C-N are observed at 1560–1580 cm−1 (purple). Finally, a deformation band of CH2 is reported at 1410–1425 cm−1 (grey). This first observation is consistent with bands expected for chitin. However, to conclude on the chitin form isolated from Curculionidae, IR observation should be more detailed. All observed IR bands are presented in Table 3.

Table 3.
IR data observed for Curculionides, α and β-chitin [39].
Comparison of IR observed for extracted chitin, α -chitin (anti-parallel organization) and β-chitin (parallel organization) allows the identification of the chitin form from Curculionidae’s species. Some bands are of particular interest. In particular, the split C=O bands observed between 1635 and 1660 cm−1 are characteristic of the α-form compared to the β-form that shows only one band. Additionally, the displacement observed for the O-H, N-H, CH2, N-H and C-N bands are closer from the α-chitin instead of the β-chitin. For bands from 850 cm−1 to 1400 cm−1 differences are more difficult to distinguish. However, these differences are enough to suggest that the extracted chitin form is the α-chitin [39]. This observation is consistent with the literature that describes the chitin from most coleoptera as α-chitin.
The extracted materials were also characterized using thermal gravimetric analysis (TGA). Examples of TGA curves are presented in Figure 8 (data for other species are presented in the Supplementary Materials, Figures S13–S21).
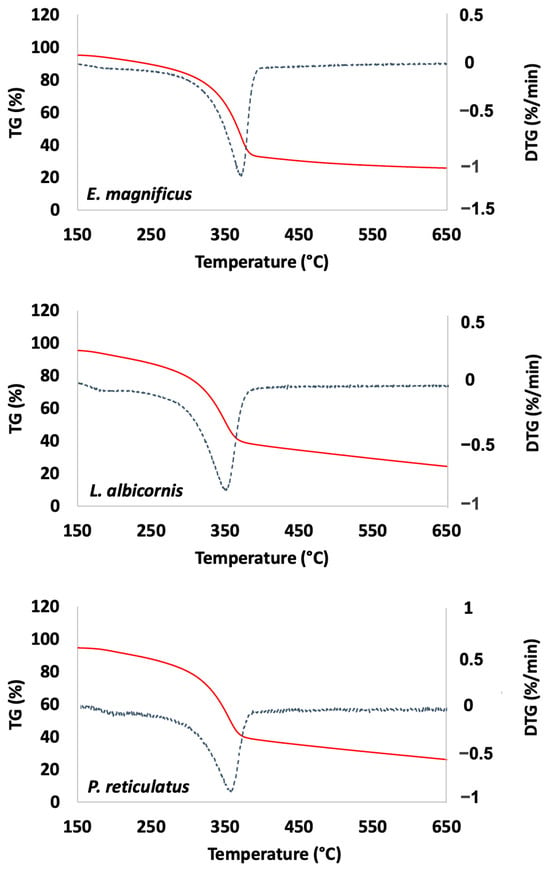
Figure 8.
Examples of thermal analysis observed for Curculionidae specimens (TG: red and DTG: blue).
TGA is used here to determine thermal degradation temperature, then the ash and water content. The ash content is related to the remaining mineral fraction of the extracted chitin and the water content corresponds to the amount of water spontaneously trapped in the material (Table 4).

Table 4.
Thermal analysis data.
The TGA data reveal a degradation temperature greater than 350 °C for all extracted chitins. This is consistent with temperatures reported in the literature for α-chitin [40]. For most species, the ash content is reported near or below 5% except for P. gemmatus purpureus, which is higher (9.7%). All these values remain consistent with values previously reported for beetles in the literature [22]. Regarding the measured water content (moisture content), the reported data are from 2 to 5%.
The extracted chitin was also characterized using elemental analysis. All elemental analysis data are reported in Table 5 and are consistent with theoretical values considered for chitin.

Table 5.
Elemental analysis data.
Finally, the extracted chitins were evaluated using X-ray diffraction. Examples of the X-ray observations are shown in Figure 9, and data for the remaining species are provided in the Supplementary Data.
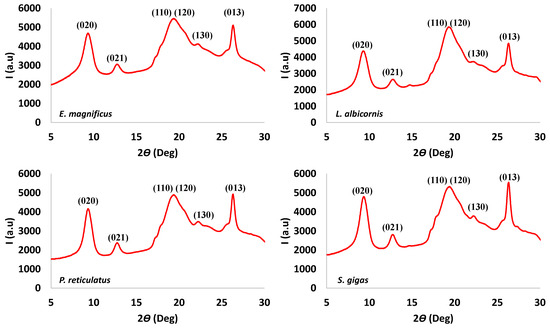
Figure 9.
Examples of X-ray spectra observed for Curculionidae specimens.
All chitin samples have similar XRD patterns, which are at 2θ values of 9.4°, 12.9°, 19.6° shouldering with 20.6, 22.3° and 26.4°. These data were compared with XRD patterns reported for α and β chitin (Table 6).

Table 6.
XRD values observed for extracted, α and β-chitin [39].
Most of the values reported for extracted chitins are consistent with the α-form. Those results confirm the observation made with the IR and thermogravimetric data. Considering the extracted chitin as α-chitin, it is possible to hypothesize that the observed rays may be respectively attributed to the plane (020), (021), (110) shouldering with (120), (130) and (013) [39]. The crystallinity results for the extracted materials are presented in Table 7.

Table 7.
Calculated crystallinity index.
For all species, the crystallinity index is between 45 and 60%. This index can be described as low compared with crystallinity index reported in the literature for other coleopters [19,22]. With all the performed characterizations, it is concluded that the extracted material has similar characteristics to classical shrimp chitin which is also α-chitin. The average overall yield near 20% for Curculionidae is comparable (even if in the low range) to other beetle chitin yields [22]. However, compared with shrimp, the yield is greater. As consequence, it is reasonable to consider Curculionidae as a potential source of chitin for future industrial exploitation.
4. Conclusions
As a conclusion of this work, we report here for the first-time chitin extraction from nine Curculionidae species from five different genera. Chitin was obtained using simple, straight-forward chemical treatments including demineralization, deproteination and bleaching. After the three-step process, the treated cuticles produced chitin with yield comprised between 12 and 20%. The observed yield can be reported as low compared to other beetles, but remains high if compared to chitin extraction yields for shrimp. The extracted chitins were characterized for all species using various techniques including SEM, FTIR, TGA, X-Ray and elemental analysis.
SEM observations show important modification of the materials after treatment. All surfaces present a fibrous network with different shapes. As the extracted chitin needs to be processed to reach chitosan, the morphology differences should not have an impact for further material application. FTIR and X-Ray observations reveal indeed that the extracted material can be described as chitin but also that the extracted chitin is α-chitin. As for FTIR and X-Ray, the elemental analysis gives values very similar for all extracted chitins. Those values are also consistent with expected values for chitin. Thermogravimetric analysis revealed that extracted materials present ash and water contents around 3 to 10% and 2 to 5%, respectively, but also that the extracted materials present high degradation temperature (greater than 350°). In summary, all the characterizations are consistent with chitin and, more precisely, with α-chitin. Except for the morphological observations, the presented data show no significant difference, depending on the studied species. As a consequence, it is possible to assume that all studied Curculionidae species are suitable for chitin extraction. These preliminary data are of particular interest and demonstrate that ravaging animals may have an application as a potential source of chitin for industrial uses and that pest insect invasion may be considered as an underestimated resource of biosourced material elaboration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomimetics9100608/s1, Figure S1. Examples of SEM images (scale bar = 30 µm) observed for raw surfaces of E. cuvieri (A and B), E. magnificus (C and D).; Figure S2. Examples of SEM images (scale bar = 30 µm) observed for raw surfaces of P. gemmatus purpureus (A and B) and P. reticulatus (C and D); Figure S3. Examples of SEM images (scale bar = 30 µm) observed for raw surfaces of L. sturmii (A and B), L. gigas (C and D) and L. albicornis (E and F); Figure S4. Examples of SEM images (scale bar = 30 µm) observed for raw surfaces of H. saxosus (A and B) and S. gigas (C and D); Figure S5. FT-IR spectrum for chitin extracted from E. cuvieri; Figure S6. FT-IR spectrum for chitin extracted from E. magnificus; Figure S7. FT-IR spectrum for chitin extracted from L. albicornis; Figure S8. FT-IR spectrum for chitin extracted from L. gigas; Figure S9. FT-IR spectrum for chitin extracted from L. sturmii; Figure S10. FT-IR spectrum for chitin extracted from H. saxosus; Figure S11. FT-IR spectrum for chitin extracted from P. reticulatus; Figure S12. FT-IR spectrum for chitin extracted from P. purpureus; Figure S13. FT-IR spectrum for chitin extracted from S. gigas; Figure S14. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from E. cuvieri; Figure S15. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from E. magnificus; Figure S16. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from L. albicornis; Figure S17. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from L. gigas; Figure S18. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from L. sturmii; Figure S19. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from H. saxosus; Figure S20. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from P. reticulatus; Figure S21. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from P. purpureus; Figure S22. Thermal analysis (TGA in red and DTG in blue) of chitin extracted from S. gigas.
Author Contributions
Z.M.: investigation, formal analysis. L.V.: investigation, formal analysis, writing—original draft. C.R.S.: writing—original draft, formal analysis. R.-P.G.: writing—original draft, conceptualization, formal analysis. P.K.: funding acquisition, writing—original draft, conceptualization, formal analysis. G.G.: investigation, conceptualization, funding acquisition, writing—original draft, project administration, resources, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article and Supplementary File.
Acknowledgments
The authors thank the China Scholar Council for funding Zhenying Mei’s Ph.D. The group also thanks Olivier Montreuil from UMR 7179 MNHN/CNRS, MECADEV, (Muséum National d’Histoire Naturelle, Entomologie) for advice and constructive comments. This work was supported by the French National Research Agency (ANR, Agence Nationale de la Recherche) as a future investment project UCAJEDI with reference: n° ANR-15-IDEX-01 (Perception of Insects by Human and RheoGels). IR and SEM were performed at technological platform « Smart City Innovation Center » (Université Côte d’Azur-IMREDD). The “Smart City Innovation Center” is a project funded by the European union with the European fund for regional development and co-funded by Metropole Nice Côte d’Azur, the Département Alpes-Maritimes, the Région Sud Provence-Alpes-Côte d’Azur and France for the “initiative d’excellence” (Investissements d’avenir).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manosathiyadevan, M.; Bhuvaneshwari, V.; Latha, R. Impact of Insects and Pests in Loss of Crop Production: A Review. In Sustainable Agriculture towards Food Security; Dhanarajan, A., Ed.; Springer: Singapore, 2017; pp. 57–67. ISBN 978-981-10-6646-7. [Google Scholar]
- Oman, P. Prevention, Surveillance and Management of Invading Pest Insects. Bull. Entomol. Soc. Am. 1968, 14, 98–102. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Sikorski, P.; Hori, R.; Wada, M. Revisit of α-Chitin Crystal Structure Using High Resolution X-Ray Diffraction Data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Ravikumar, M.N.V.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 307–354. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and Its Derivatives for Tissue Engineering Applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Ali Khan, Z.; Jamil, S.; Akhtar, A.; Mustehsan Bashir, M.; Yar, M. Chitosan Based Hybrid Materials Used for Wound Healing Applications- A Short Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 419–436. [Google Scholar] [CrossRef]
- Rufato, K.B.; Galdino, J.P.; Ody, K.S.; Pereira, A.G.; Corradini, E.; Martins, A.F.; Paulino, A.T.; Fajardo, A.R.; Aouada, F.A.; La Porta, F.A.; et al. Hydrogels Based on Chitosan and Chitosan Derivatives for Biomedical Applications. In Hydrogels—Smart Materials for Biomedical Applications; Popa, L., Violeta Ghica, M., Dinu-Pîrvu, C.-E., Eds.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78985-875-4. [Google Scholar]
- Shahid, M.; Mohammad, F. Green Chemistry Approaches to Develop Antimicrobial Textiles Based on Sustainable Biopolymers—A Review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Oyatogun, G.M.; Esan, T.A.; Akpan, E.I.; Adeosun, S.O.; Popoola, A.P.I.; Imasogie, B.I.; Soboyejo, W.O.; Afonja, A.A.; Ibitoye, S.A.; Abere, V.D.; et al. Chitin, Chitosan, Marine to Market. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–381. ISBN 978-0-12-817966-6. [Google Scholar]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of Marine-Derived Biowaste to Develop Chitin/Fish Gelatin Products as Bioactive Carriers and Moisture Scavengers. Sci. Total Environ. 2020, 706, 135747. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of Chitin Nanofibers from Mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef]
- Vetter, J. Chitin Content of Cultivated Mushrooms Agaricus Bisporus, Pleurotus Ostreatus and Lentinula Edodes. Food Chem. 2007, 102, 6–9. [Google Scholar] [CrossRef]
- Juárez-de la Rosa, B.A.; Quintana, P.; Ardisson, P.-L.; Yáñez-Limón, J.M.; Alvarado-Gil, J.J. Effects of Thermal Treatments on the Structure of Two Black Coral Species Chitinous Exoskeleton. J. Mater. Sci. 2012, 47, 990–998. [Google Scholar] [CrossRef]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and Physicochemical Characterization of Chitin and Chitosan from Zophobas Morio Larvae in Varying Sodium Hydroxide Concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and Identification of Chitin in Three-Dimensional Skeleton of Aplysina Fistularis Marine Sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Mentes, A.; Asaroglu, M.; Sezen, G.; Tozak, K.O. Extraction and Characterization of α-Chitin and Chitosan from Six Different Aquatic Invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Kabalak, M.; Aracagök, D.; Torun, M. Extraction, Characterization and Comparison of Chitins from Large Bodied Four Coleoptera and Orthoptera Species. Int. J. Biol. Macromol. 2020, 145, 402–409. [Google Scholar] [CrossRef]
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Mohamed Anuar, N.A.F.; Abu Bakar, M.F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. IJMS 2020, 21, 4978. [Google Scholar] [CrossRef]
- Godeau, X.Y.; Andrianandrasana, F.J.; Volkova, O.; Szczepanski, C.R.; Zenerino, A.; Montreuil, O.; Godeau, R.-P.; Kuzhir, P.; Godeau, G. Investigation on Dung Beetle’s (Heliocopris Hope, 1838) Chitosan Valorisation for Hydrogel 3D Printing. Int. J. Biol. Macromol. 2022, 199, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Marmier, T.; Szczepanski, C.R.; Candet, C.; Zenerino, A.; Godeau, R.-P.; Godeau, G. Investigation on Mecynorhina Torquata Drury, 1782 (Coleoptera, Cetoniidae, Goliathini) Cuticle: Surface Properties, Chitin and Chitosan Extraction. Int. J. Biol. Macromol. 2020, 164, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Szczepanski, C.R.; Godeau, R.-P.; Godeau, G. Chitosan Extraction from Goliathus Orientalis Moser, 1909: Characterization and Comparison with Commercially Available Chitosan. Biomimetics 2020, 5, 15. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A. Fruit Production. In Food Crop Production by Smallholder Farmers in Southern Africa; Elsevier: Amsterdam, The Netherlands, 2018; pp. 275–312. ISBN 978-0-12-814383-4. [Google Scholar]
- Van Huis, A. Cultural Significance of Beetles in Sub-Saharan Africa. Insects 2021, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Gressitt, J.L. Ecology and Biogeography of New Guinea Coleoptera (Beetles). In Biogeography and Ecology of New Guinea; Gressitt, J.L., Ed.; Monographiae Biologicae; Springer: Dordrecht, The Netherlands, 1982; Volume 42, pp. 709–734. ISBN 978-94-009-8634-3. [Google Scholar]
- Fernández, D.C.; VanLaerhoven, S.L.; McCreary, C.; Labbé, R.M. An Overview of the Pepper Weevil (Coleoptera: Curculionidae) as a Pest of Greenhouse Peppers. J. Integr. Pest Manag. 2020, 11, 26. [Google Scholar] [CrossRef]
- Fathi, S.A.A.; Abedi, A.A. Ovipositional Preference and Life History Parameters of Lixus incanescens (Coleoptera: Curculionidae) on Selected Sugar Beet Cultivars. Int. J. Pest Manag. 2014, 60, 293–299. [Google Scholar] [CrossRef]
- Sawicka, B.; Egbuna, C. Pests of Agricultural Crops and Control Measures. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–16. ISBN 978-0-12-819304-4. [Google Scholar]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H.M. Isolation and Characterization of Chitosan from Different Local Insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Shin, S.; Clarke, D.J.; Lemmon, A.R.; Moriarty Lemmon, E.; Aitken, A.L.; Haddad, S.; Farrell, B.D.; Marvaldi, A.E.; Oberprieler, R.G.; McKenna, D.D. Phylogenomic Data Yield New and Robust Insights into the Phylogeny and Evolution of Weevils. Mol. Biol. Evol. 2018, 35, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Okiwelu, S.N.; Ndome, C.B.; Ide, Y.F. Insect Pests of Leafy Vegetables in Rivers State, Nigeria: I. Feeding Habits and Infestation of the Bitterleaf Weevil Lixus Camerunus Kolbe (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 1988, 9, 557–561. [Google Scholar] [CrossRef]
- Nakamura, K.; Lang, X. Development and Survivorship of the Japanese Giant Weevil, Sipalinus Gigas(Fabricius)(Coleoptera: Rhynchophoridae), in Cut Pine Bolts. Appl. Entomol. Zool. 2002, 37, 111–115. [Google Scholar] [CrossRef][Green Version]
- Percot, A.; Viton, C.; Domard, A. Optimization of Chitin Extraction from Shrimp Shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Pouya, C.; Stavenga, D.G.; Vukusic, P. Discovery of Ordered and Quasi-Ordered Photonic Crystal Structures in the Scales of the Beetle Eupholus Magnificus. Opt. Express 2011, 19, 11355. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in Chitin Analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and Characterization of α-Chitin from Cicada Sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).