Biomimetic Antibacterial Pro-Osteogenic Cu-Sericin MOFs for Osteomyelitis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silk Sericin
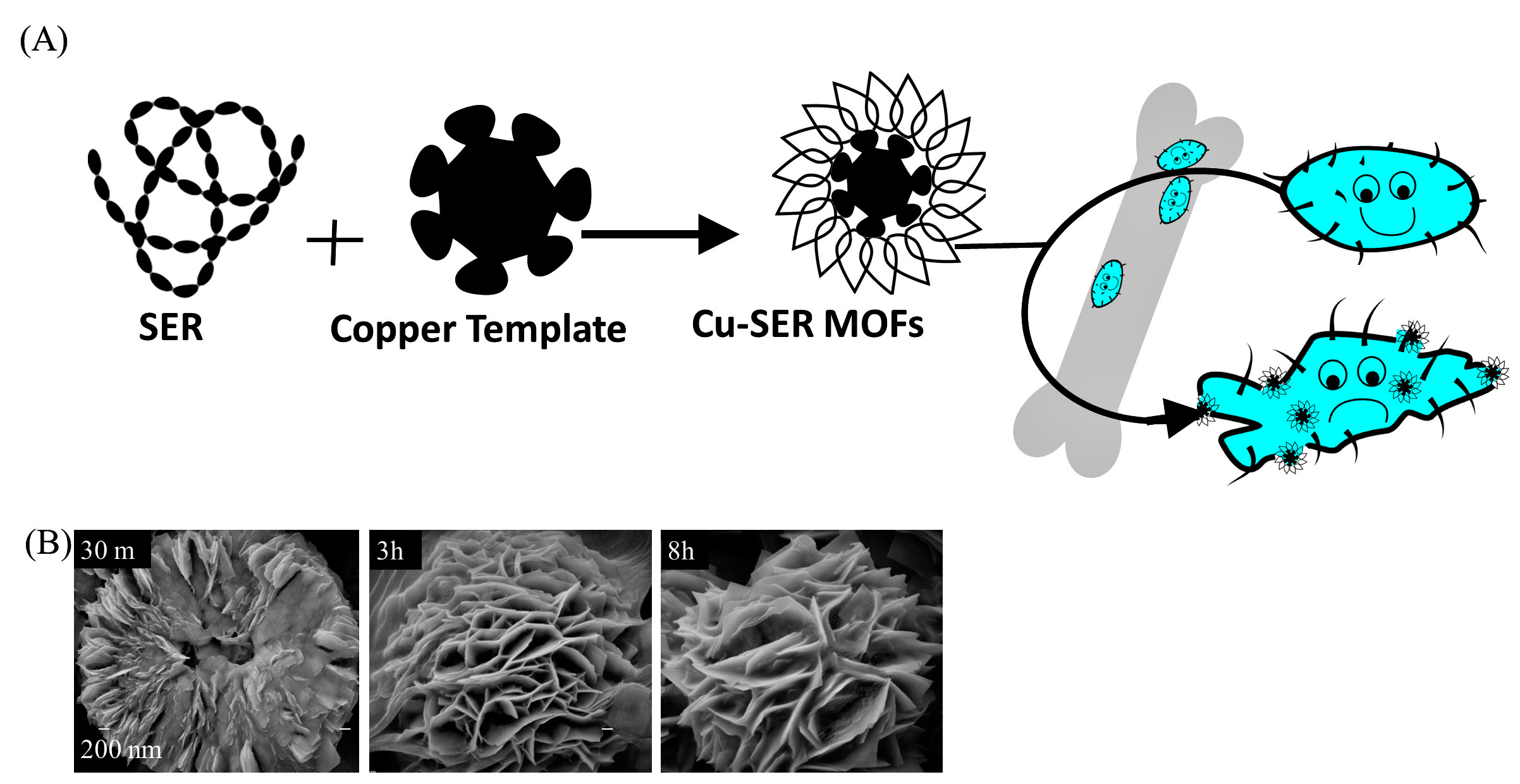
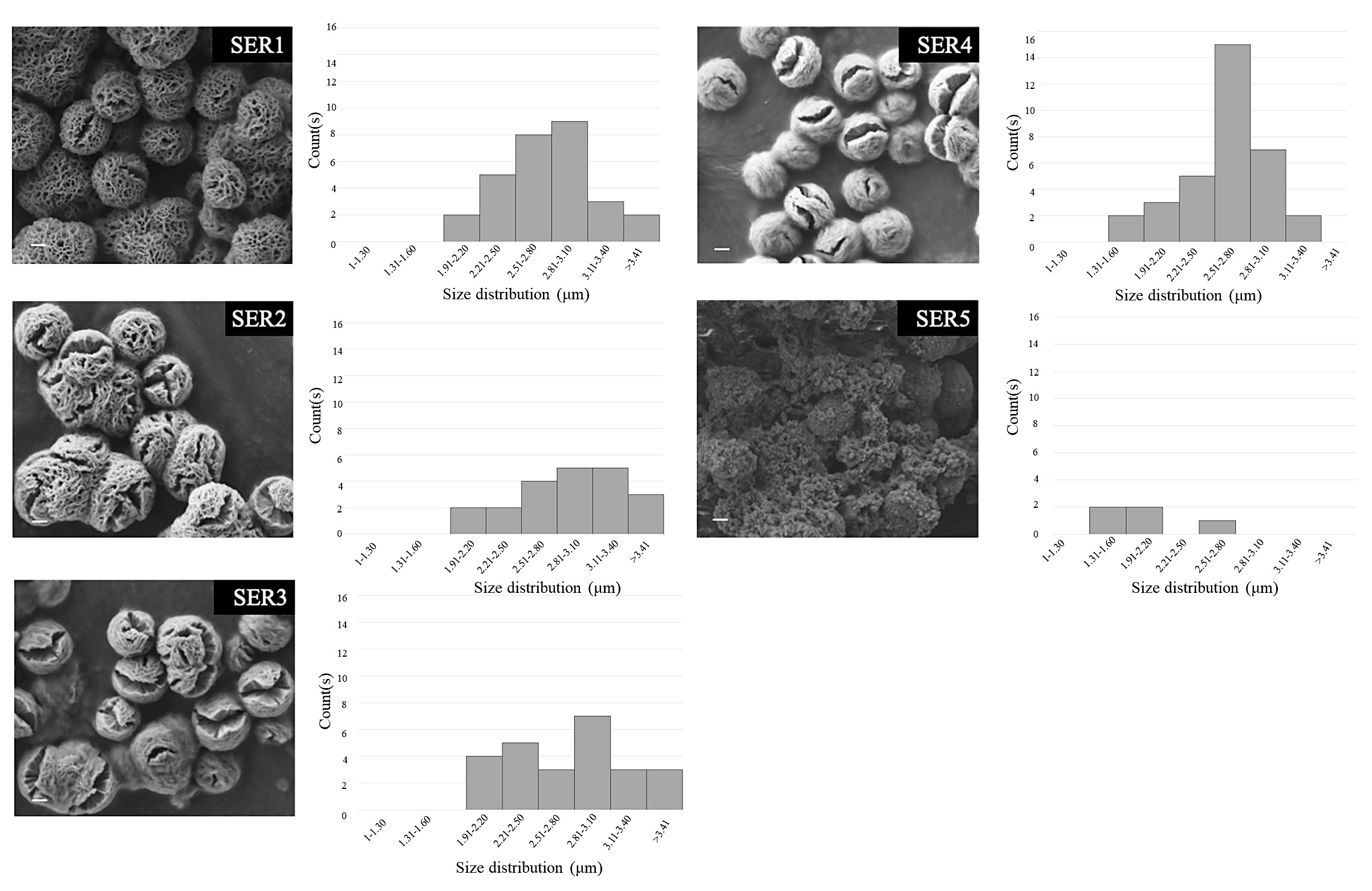
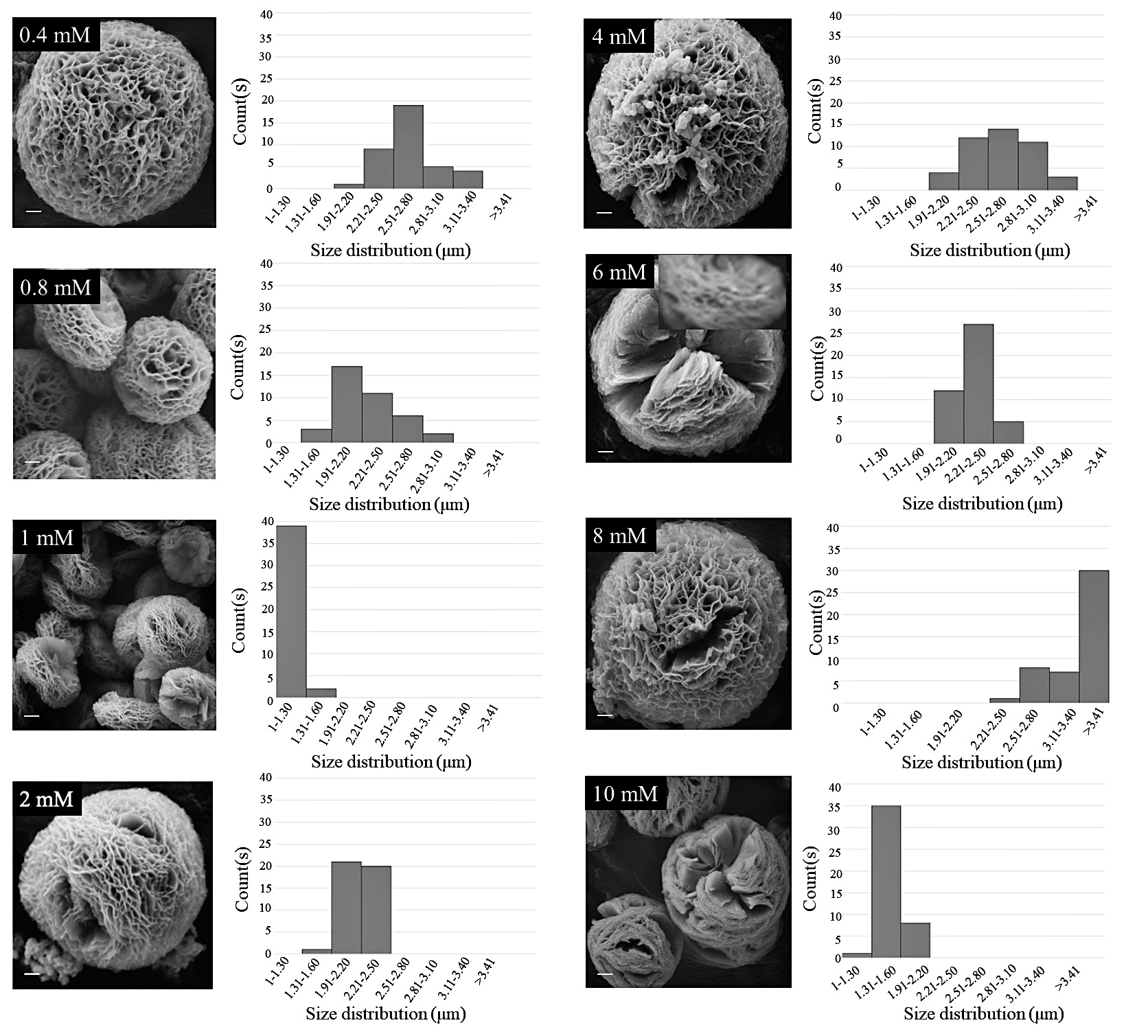
2.2. Synthesis of Copper-Silk Sericin MOFs
2.3. Biophysical Characterization
2.4. Isolation and Culture of Cells
2.5. alamarBlue Assay
2.6. Fluorescence Microscopy
2.7. Histology Analysis
2.8. Antibacterial Activity
2.9. Statistical Analysis
3. Results
3.1. Formation of Cu-SER MOFs
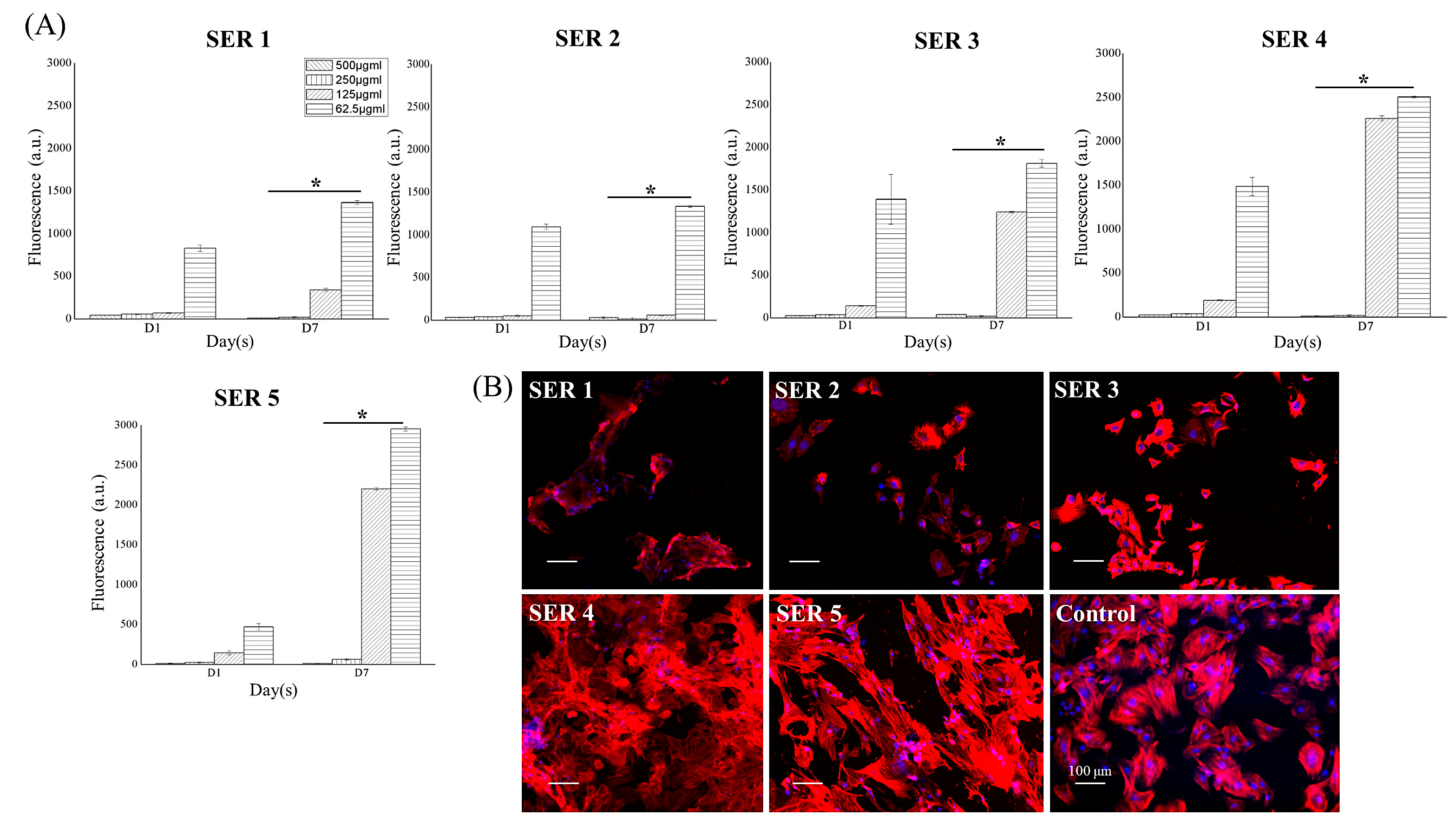
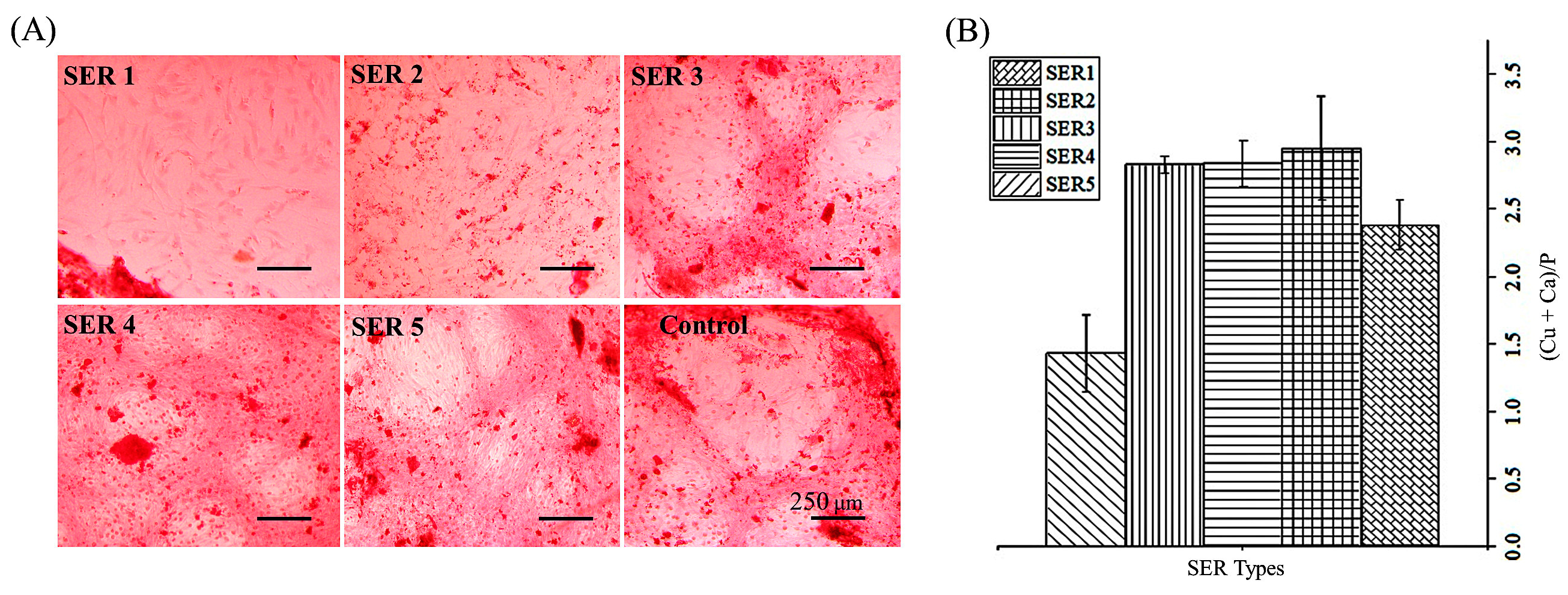
3.2. Pro-Osteogenic Activity of Cu-SER MOFs
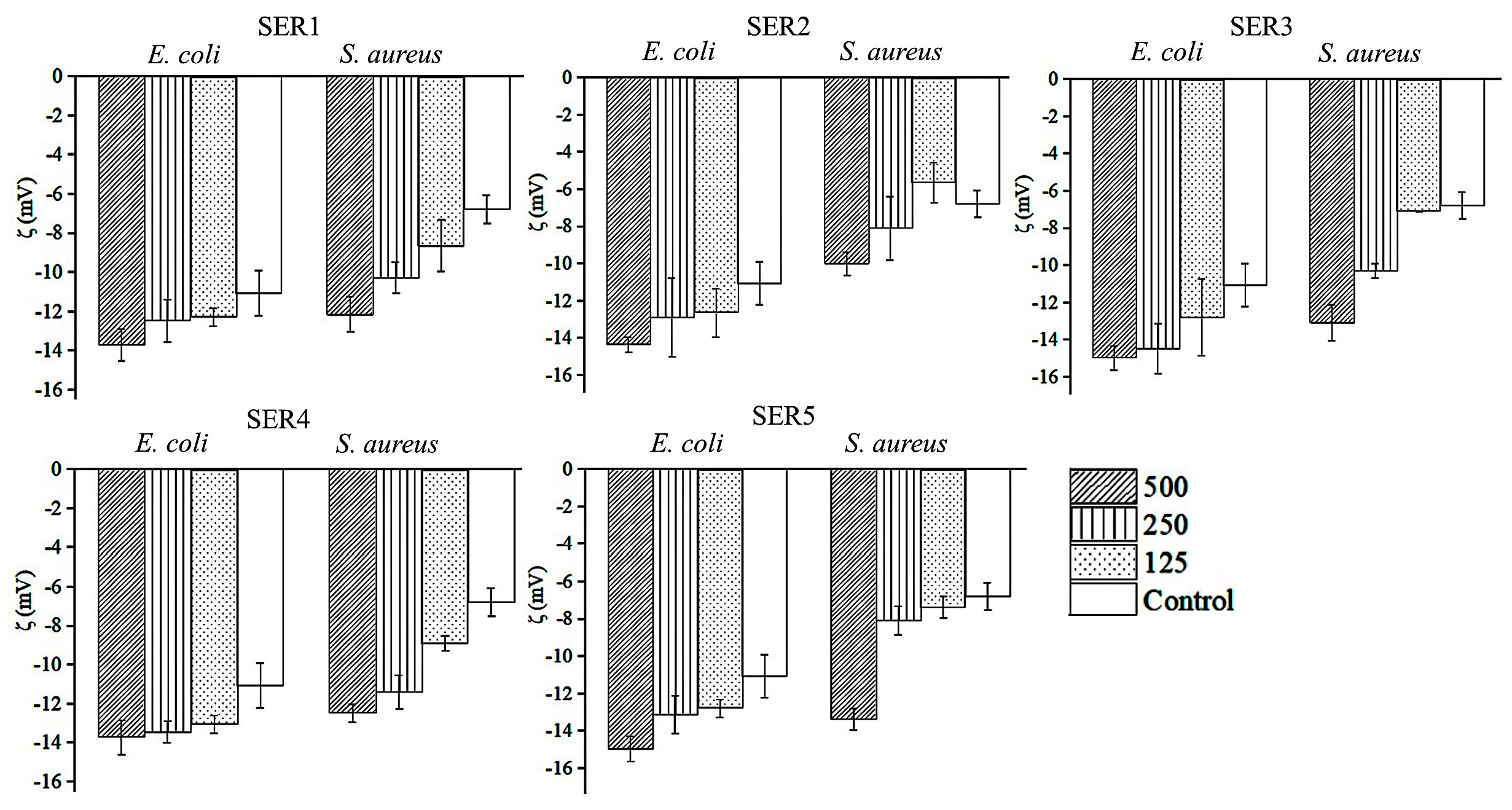
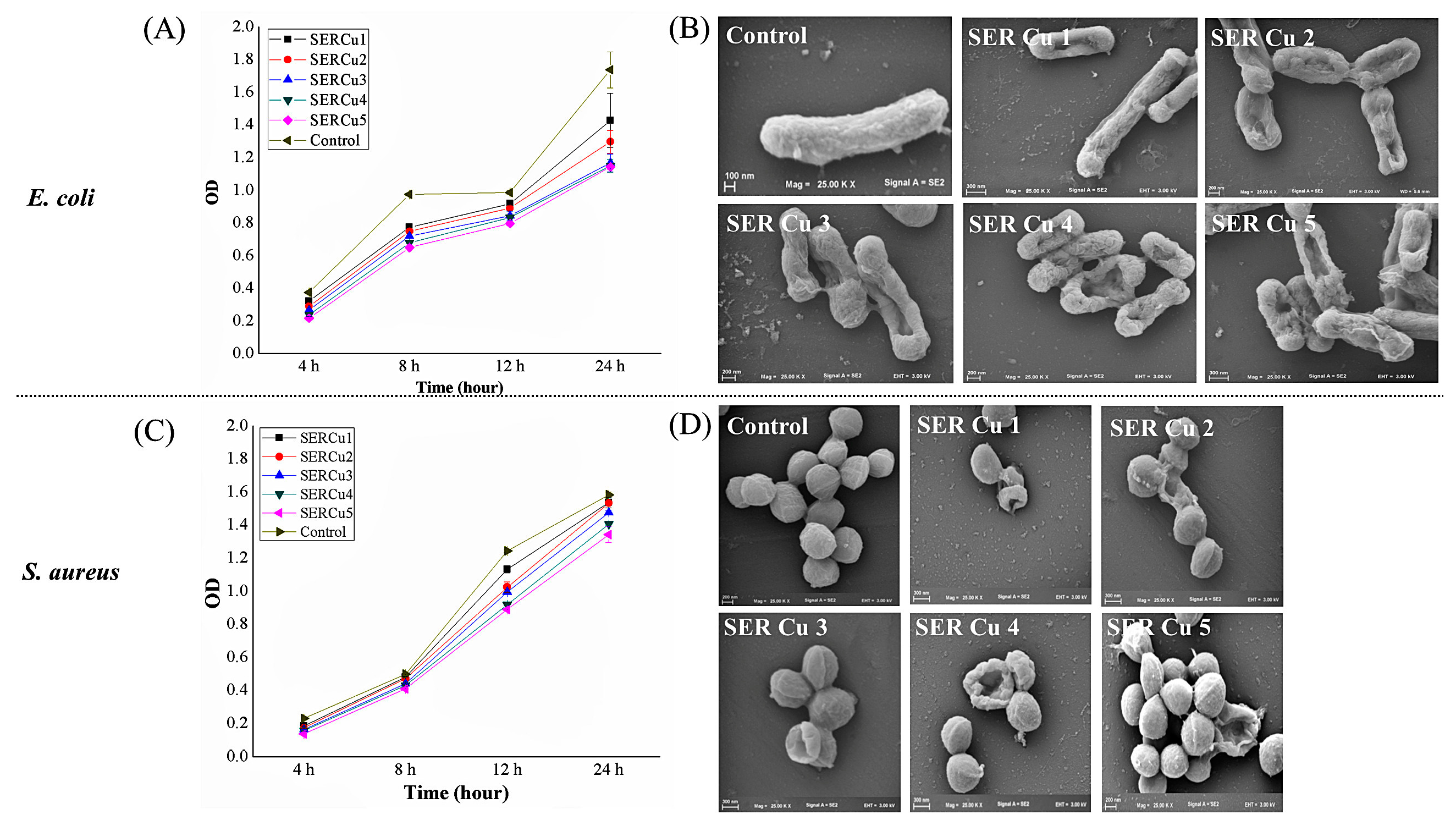
3.3. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osteomyelitis Market: Global Industry Forecast (2022–2027) by Type, Treatment, End-User, and Region. Available online: https://www.maximizemarketresearch.com/market-report/global-osteomyelitis-market/71688/ (accessed on 10 April 2022).
- Murray, C.J.L.; Ezzati, M.; Flaxman, A.D.; Lim, S.; Lozano, R.; Michaud, C.; Naghavi, M.; Salomon, J.A.; Kenji Shibuya, K.; Vos, T.; et al. GBD 2010: Design, definitions, and metrics. Lancet 2012, 380, 2063–2066. [Google Scholar] [CrossRef]
- “No Time To Wait”—AMR Could Cause 10 Million Deaths Annually by 2050, Warns UN Report. Available online: https://healthpolicy-watch.news/no-time-to-wait-amr-could-cause-10-million-deaths-annually-by-2050-warns-un-report/#:~:text=Deaths%20from%20infections%20resistant%20to,United%20Nations%20report%20released%20today (accessed on 10 April 2022).
- Securing new Drugs for Future Generations: The Pipeline of Antibiotics/the Review on Antimicrobial Resistance Chaired by Jim O’Neill. Available online: https://wellcomecollection.org/works/zqv86kgr (accessed on 10 April 2022).
- Koley, P.; Sakurai, M.; Aono, M. Controlled fabrication of silk protein sericin mediated hierarchical hybrid flowers and their excellent adsorption capability of heavy metal ions of Pb(II), Cd(II) and Hg(II). ACS Appl. Mater. Interfaces 2016, 8, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. An ancient remedy returning to fight microbial, fungal and viral infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef]
- Sankar, R.; Maheswari, R.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 234–239. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, Y.; Cho, H.; Lee, S.; Yang, S.-H.; Kim, S.-J.; Lee, D.N. Robust copper metal–organic framework-embedded polysiloxanes for biomedical applications: Its antibacterial effects on MRSA and in vitro cytotoxicity. Nanomaterials 2021, 11, 719. [Google Scholar] [CrossRef]
- Noosak, C.; Jantorn, P.; Meesane, J.; Voravuthikunchai, S.; Saeloh, D. Dual-functional bioactive silk sericin for osteoblast responses and osteomyelitis treatment. PLoS ONE 2022, 17, e0264795. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired design of sericin/chitosan/Ag@MOF/GO hydrogels for efficiently combating resistant bacteria, rapid hemostasis, and wound healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef]
- Chitichotpanya, P.; Pisitsak, P.; Chitichotpanya, C. Sericin–copper-functionalized silk fabrics for enhanced ultraviolet protection and antibacterial properties using response surface methodology. Text. Res. J. 2019, 89, 1166–1179. [Google Scholar] [CrossRef]
- Kundu, B.; Kundu, S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 2012, 33, 7456–7467. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Zhang, C.; Chen, Y.; Zhu, L.; Mao, C.; OuYang, H. Biomimetic nucleation of hydroxyapatite crystals mediated by Antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biomacromolecules 2014, 15, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-Y.; Kweon, H.-Y.; Kim, D.-W.; Baek, K.; Chae, W.-S.; Kang, Y.-J.; Oh, J.-H.; Kim, S.-G.; Garagiola, U. Silk sericin application increases bone morphogenic protein-2/4 expression via a toll-like receptor-mediated pathway. Int. J. Biol. Macromol. 2021, 190, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.P.; Mandal, B.B. Silk sericin induced pro-oxidative stress leads to apoptosis in human cancer cells. Food Chem. Toxicol. 2019, 123, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Bastos, A.R.F.; Brancato, V.; Cerqueira, M.T.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Mechanical property of hydrogels and the presence of adipose stem cells in tumor stroma affect spheroid formation in the 3D osteosarcoma model. ACS Appl. Mater. Interfaces 2019, 11, 14548–14559. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. adipoSIGHT in therapeutic response: Consequences in osteo-sarcoma treatment. Bioengineering 2021, 8, 83. [Google Scholar] [CrossRef]
- Kundu, B.; Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Silk fibroin promotes mineralization of gellan gum hydrogels. Int. J. Biol. Macromol. 2020, 153, 1328–1334. [Google Scholar] [CrossRef]
- Eltohamy, M.; Kundu, B.; Moon, J.; Lee, H.Y.; Kim, H.W. Anti-bacterial zinc-doped calcium silicate cements: Bone filler. Ceram. Int. 2018, 44, 13031–13038. [Google Scholar] [CrossRef]
- Shen, Q.; Qi, Y.; Kong, Y.; Bao, H.; Wang, Y.; Dong, A.; Wu, H.; Xu, Y. Advances in copper-based biomaterials with antibacterial and osteogenic properties for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 9, 795425. [Google Scholar] [CrossRef]
- Kokubo, T.; Miyaji, F.; Kim, H.M.; Nakamura, T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Hanib, N.H.; Hamzah, F.; Omar, Z.; Subuki, I. Biomimetic apatite deposition in modified dulbecco’s phosphate-buffered saline solution on the alkali-heat treated TI6AL4V. J. Eng. Appl. Sci. 2019, 14, 3083–3093. [Google Scholar] [CrossRef]
- Xiaolin, D.; Zhouqi, Q.; Jinjing, P.; Xing, C.; Lin, L.; Qingqing, N.; Juming, Y. Silk sericin-assisted synthesis of architectured porous copper@cuprous oxide hybrid microspheres with enhanced visible light photocatalytic activity. Mater. Sci. Semicond. Process. 2018, 86, 157–163. [Google Scholar] [CrossRef]
- Ewald, A.; Käppel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. A 2012, 100, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wu, X.; Luo, J.; Yang, G.; Lu, Y.; Lin, S.; Jiang, F.; Zhang, W.; Jiang, X. Copper peptide-incorporated 3D-printed silk-based scaffolds promote vascularized bone regeneration. Chem. Eng. J. 2021, 422, 130147. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ergun, C.; Webster, T.J.; Bahadir, A.; Ueda, K.; Narushima, T.; Nakano, T. Effect of precursor deficiency induced Ca/P Ratio on antibacterial and osteoblast adhesion properties of Ag-incorporated hydroxyapatite: Reducing Ag toxicity. Materials 2021, 14, 3158. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Edirisinghe, M. Nanocomposites: Suitable alternatives as antimicrobial agents. Nanotechnology 2018, 29, 282001. [Google Scholar] [CrossRef] [Green Version]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram- positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [Green Version]
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Alves, C.S.; Melo, M.N.; Franquelim, H.G.; Ferre, R.; Planas, M.; Feliu, L.; Bardají, E.; Kowalczyk, W.; Andreu, D.; Santos, N.C.; et al. Escherichia coli cell surface perturbation and disruption induced by antimicrobial peptides BP100 and pepR. J. Biol. Chem. 2010, 285, 27536–27544. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, J.; Barwinska-Sendra, A.; Tarrant, E.; Waldron, K.J. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2013; pp. 468–479. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundu, B.; Reis, R.L.; Kundu, S.C. Biomimetic Antibacterial Pro-Osteogenic Cu-Sericin MOFs for Osteomyelitis Treatment. Biomimetics 2022, 7, 64. https://doi.org/10.3390/biomimetics7020064
Kundu B, Reis RL, Kundu SC. Biomimetic Antibacterial Pro-Osteogenic Cu-Sericin MOFs for Osteomyelitis Treatment. Biomimetics. 2022; 7(2):64. https://doi.org/10.3390/biomimetics7020064
Chicago/Turabian StyleKundu, Banani, Rui L. Reis, and Subhas C. Kundu. 2022. "Biomimetic Antibacterial Pro-Osteogenic Cu-Sericin MOFs for Osteomyelitis Treatment" Biomimetics 7, no. 2: 64. https://doi.org/10.3390/biomimetics7020064
APA StyleKundu, B., Reis, R. L., & Kundu, S. C. (2022). Biomimetic Antibacterial Pro-Osteogenic Cu-Sericin MOFs for Osteomyelitis Treatment. Biomimetics, 7(2), 64. https://doi.org/10.3390/biomimetics7020064