Abstract
Diabetic wounds remain a major clinical challenge due to impaired angiogenesis, chronic inflammation, oxidative stress, and persistent infection, all of which delay tissue repair. Conventional dressings provide only passive protection and fail to modulate the wound microenvironment effectively. Chitosan (CS) is a naturally derived polysaccharide inspired by biological structures in crustaceans and fungi. It has emerged as a multifunctional biomimetic polymer with excellent biocompatibility, antimicrobial activity, and hemostatic properties. Recent advances in biomimetic materials science have enabled the development of stimuli-responsive CS hydrogels. These systems can sense physiological cues such as pH, temperature, glucose level, light, and reactive oxygen species (ROS). These smart systems emulate natural wound healing mechanisms and adapt to environmental changes. They release bioactive agents on demand and promote tissue homeostasis through controlled angiogenesis and collagen remodeling. This review discusses the biomimetic design rationale, crosslinking mechanism, and emerging strategies underlying single and dual-responsive hydrogel systems. It further emphasizes how nature-inspired structural and functional designs accelerate diabetic wound repair and outlines the current challenges and future prospects for translating these bioinspired intelligent hydrogels into clinical wound care applications.
1. Introduction
The increasing prevalence of diabetes worldwide has led to a rise in complications, particularly chronic wounds, which are often difficult to heal and highly susceptible to infection [1,2]. These non-healing wounds such as diabetic foot ulcers are a major cause of morbidity and may result in lower-limb amputation if not effectively treated [3]. The World Health Organization (WHO) and recent epidemiological studies project that diabetes could affect more than 700 million people globally by 2045. This rapid increase underscores the urgent need for innovative wound-care technologies [4]. Under healthy conditions, wound healing proceeds through a regulated sequence of biological events including clot formation, inflammation, tissue regeneration, and remodeling [5]. In diabetic patients, the healing process becomes impaired. This is due to metabolic abnormalities that interfere with normal tissue repair. Elevated blood glucose levels trigger oxidative stress, impair immune responses, inhibit angiogenesis, and promote bacterial colonization, all of which hinder tissue repair and prolong inflammation. This pathological state results in a wound environment that is difficult to resolve and often transitions into chronicity [6].
Conventional treatments such as gauze dressings provide only basic coverage and limited influence over the biochemical environment of the wound [7]. As a result, the medical field has turned its attention to functional materials like hydrogels that not only protect but also actively participate in the healing process. The term “gel” originates from the Latin word meaning snow or ice. Fundamentally, a gel is an elastic, semisolid system that exhibits both solid and liquid characteristics. It can retain its shape like a solid yet behaves as a viscous liquid under stress [8]. According to the United States Pharmacopeia (USP), gels are semisolid colloidal systems in which inorganic particles or organic macromolecules are uniformly dispersed within a liquid medium [9]. Hydrogels are particularly suited for wound management due to their high-water content, biocompatibility, and ability to support tissue regeneration by mimicking the extracellular matrix (ECM) [10]. Among the various biomaterials explored for hydrogel synthesis, chitosan (CS) a naturally derived polysaccharide has emerged as a leading candidate [11]. It possesses multiple properties such as antibacterial activity, biodegradability, and hemostasis, which are beneficial to wound healing [12]. CS can adhere to wound tissue, control microbial growth, and facilitate moisture retention, thereby creating a more favorable environment for repair [13]. Nonetheless, its native form has limitations such as poor mechanical resilience and pH-dependent solubility, which restrict its application in complex wound settings [14]. An illustration of the functional characteristic of CS is shown in Figure 1A.
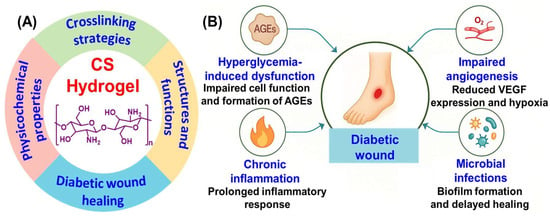
Figure 1.
(A) Functional roles of CS hydrogel and (B) key complications in diabetic wound healing.
The development of CS-based hydrogels is rooted in biomimetic principles, inspired by the adaptive and regenerative abilities of natural systems [15]. In nature, organisms exhibit self-healing, structural flexibility, and responsiveness to environmental cues-features that are now being replicated in synthetic hydrogel design. CS derived from chitin found in crustacean shells, embodies nature’s blueprint for strength, flexibility and biocompatibility [16]. Modern biomimetic material science applies these concepts to create hydrogels that mimic the ECM allowing cell attachment, nutrient transport, and biological signaling [17]. Stimuli-responsive CS hydrogel mimics the body’s natural feedback mechanisms, adjusting their structure or releasing therapeutic molecules in response to environmental signals such as pH, glucose or oxidative stress at the wound site.
Recent advances have addressed these issues through the development of modified CS hydrogels. Techniques such as nanoparticle incorporation, chemical crosslinking, and the design of injectable or stimuli-responsive systems have significantly improved their performance [18]. These innovations aim to enhance wound site delivery, promote tissue regeneration, and respond dynamically to the local wound environment [19]. This review provides a detailed analysis of the role of CS-based hydrogels in diabetic wound healing. It outlines the pathophysiological challenges posed by diabetic wounds, summarizes the key properties and functions of CS and evaluates the progress made in designing advanced hydrogel systems. Furthermore, it also discusses the translational potential and current limitations, paving the way for future research and clinical adoption of these next-generation wound care materials.
2. Pathophysiology of Diabetic Wounds
Chronic wounds in diabetic individuals arise from a complex interaction of systemic metabolic imbalances and local tissue impairments. These wounds often fail to progress through the normal stages of healing, coagulation, inflammation, proliferation, and tissue remodeling. As a result, they exhibit delayed closure and a higher risk of complications (Figure 1B) [5]. Elevated blood glucose levels significantly interfere with cellular activities essential for wound repair, as fibroblasts and keratinocytes show reduced proliferation and migration, while collagen synthesis is compromised [20]. Furthermore, chronic hyperglycemia promotes the accumulation of advanced glycation end products (AGEs), which disrupt ECM organization and trigger inflammatory responses via receptors for advanced glycation end products (RAGE)-mediated pathways. A hallmark feature of diabetic wounds is prolonged inflammation driven by pro-inflammatory macrophages (M1 phenotype) and elevated cytokines such as tumor necrosis factor (TNF)-α, Interleukin (IL)-1β, and IL-6. These factors inhibit tissue regeneration and promote ECM degradation through the overexpression of matrix metalloproteinases (MMPs). In addition, oxidative stress is exacerbated in diabetic wounds due to excess reactive oxygen species (ROS), which impair cell viability by damaging membranes, proteins, and DNA, while antioxidant defenses like glutathione are often depleted. Impaired vascularization further complicates wound healing in diabetics. Disrupted angiogenic signaling including reduced vascular endothelial growth factor (VEGF) and hypoxia inducible factor (HIF)-1α, resulting in localized hypoxia that hinders tissue repair and immune defense [20,21,22]. The compromised immune function and poor perfusion characteristic of diabetes also increase susceptibility to infection with pathogens like Staphylococcus aureus and Pseudomonas aeruginosa commonly forming biofilms that resist antibiotics and immune clearance [23,24]. Finally, matrix remodeling becomes dysregulated with MMPs upregulated and their inhibitors downregulated. This imbalance causes excessive ECM degradation and prevents scaffold formation for proper cell attachment and tissue regeneration. These challenges highlight the need for targeted therapies such as CS-based hydrogels [25].
3. Chemistry, Structure, and Sources of CS
CS is a naturally derived linear polysaccharide that consists of repeating units of D-glucosamine and N-acetyl-D-glucosamine joined by β-(1→4) linkages [26], as illustrated in Figure 2A. Its chemical nature is largely determined by the abundance of reactive amino and hydroxyl groups along the backbone, which allow for hydrogen bonding, ion chelation, and a wide range of structural modifications [27]. Two important parameters that influence its properties are the degree of deacetylation (DD) and molecular weight [28]. Typically, the DD falls between 60 and 100%, and this strongly affects solubility, charge density, and bioactivity [29]. CS with a low molecular weight (below 100 kDa) is more soluble and readily bioavailable, making it especially useful for applications in wound repair, tissue regeneration, and drug delivery. Medium molecular weight material (approximately 190–310 kDa) offers a balance between solubility and viscosity, which is advantageous for controlled release systems, whereas high molecular weight CS (above 1000 kDa) forms stable gels and films with strong adhesive capacity, particularly suited for wound dressings and scaffolds that require extended performance [30,31,32].
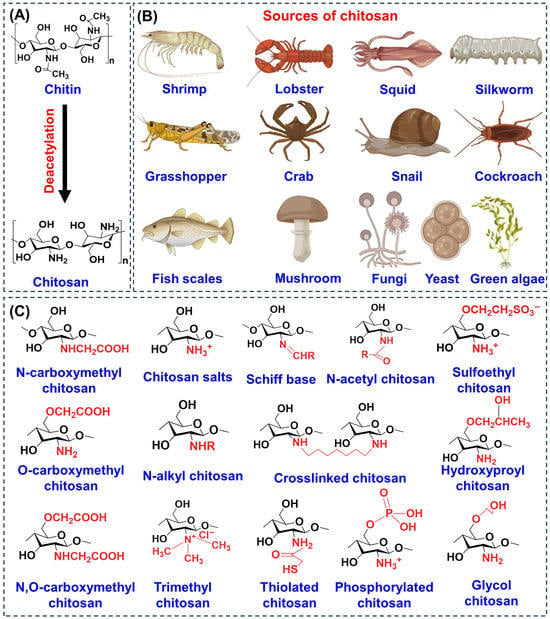
Figure 2.
Overview of CS chemistry and sources. (A) Basic structure and key derivative of CS. (B) Natural sources including crustaceans, insects, mollusks, fungi, algae, and mushrooms. (C) Functionalized derivatives illustrating chemical modifications that enhance solubility, stability, and biomedical performance.
The functional groups of CS, primarily the amino group at the C-2 position and hydroxyl groups at C-3 and C-6 are central to its biological functions [33]. They enable electrostatic interactions with negatively charged cell membranes, promote chemical derivatization, and contribute to its mucoadhesive behavior [34,35]. Beyond its native form, CS can be chemically modified to yield a wide range of derivatives, as shown in Figure 2C, including carboxymethyl, thiolated, phosphorylated, glycol, and trimethyl CS [36]. These modifications improve solubility at physiological pH, enhance stability, strengthen mucoadhesion, and expand antimicrobial or tissue-regenerative properties, thereby overcoming the limitations of unmodified CS. The natural sources of CS are diverse ranging from crustacean shells such as shrimp, crab, lobster to insects (silkworm, grasshopper, cockroach), mollusks (squid, snail), fungi, yeast, green algae, mushrooms, and even fish scales (Figure 2B). This wide availability highlights its renewable and abundant nature [37].
4. Advantages and Biomimetic Perspective of CS Hydrogels
Several natural and synthetic hydrogels including alginate, hyaluronic acid, gelatin, collagen, and synthetic polymers such as polyethylene glycol (PEG) and polyvinyl alcohol (PVA) have been widely applied in diabetic wound management due to their moisture-retention and biocompatibility [38,39,40,41,42,43]. However, these materials often lack intrinsic antimicrobial activity, hemostatic capability, or the ability to modulate inflammation, requiring additional modification or incorporation of active agents. In contrast, CS offers several inherent advantages including broad-spectrum antimicrobial activity, strong hemostatic action, mucoadhesive behavior. Its cationic structure also supports cell adhesion and promote re-epithelization [44,45,46]. Its reactive amino and hydroxyl groups also allow efficient chemical modification, enabling the development of stimuli-responsive, self-healing and multifunctional hydrogels. Beyond these material benefit, CS is biodegradable, sustainably source, and exhibits low toxicity, making it more versatile and multifunctional than many other hydrogel matrices [47].
These structural characteristics and derivatization strategies translate into important bioactivities relevant to promoting platelet aggregation for hemostasis, disrupting microbial membranes for antibacterial action, and enhancing granulation tissue formation, angiogenesis, and epithelialization for regeneration [48]. Clinical studies further confirmed these benefits, demonstrating that CS-based hydrogels accelerate wound closure and improve overall healing outcomes [49,50]. From a biomimetic perspective, CS and its derivatives exemplify a natural model for material design. Their cationic nature and hydrated network mimic the ECM, supporting cell adhesion, migration and nutrient diffusion, where biological environments respond dynamically to pH, or oxidative stress. These properties collectively position CS based hydrogels as bioinspired and adaptive materials that emulate the body’s intrinsic healing, aligning closely with the principles of biomimetics.
5. Mechanism of CS Based Responsive Formation
CS-based responsive hydrogels are typically fabricated through physical or chemical crosslinking approaches. The choice of method largely determines their structural stability, responsiveness, and biomedical applicability [51,52]. These strategies are designed to impart stimuli-responsiveness to the hydrogel. As a result, the material can undergo reversible swelling, degradation, or sol–gel transitions in response to triggers such as pH, temperature, redox potential, enzymatic activity, or even multiple combined stimuli [53]. These crosslinking strategies draw inspiration from natural self-assembly and adhesion processes. They reflect the core biomimetic principle of replicating nature’s hierarchical and adaptive material organization. The following sections provide detailed approaches.
5.1. Physical Crosslinking Methods
Physical crosslinking relies on non-covalent interactions such as ionic bonds, hydrogen bonding, hydrophobic associations, or on physical processing techniques like freeze–thaw cycles and host-guest inclusion [54]. These methods do not require potentially toxic chemical reagents, making them particularly attractive for wound healing applications. Hydrogels formed by physical crosslinking are typically biocompatible, reversible, and often injectable, though they usually exhibit lower mechanical strength compared with chemically crosslinked counterparts [55,56].
5.1.1. Ionic/Electrostatic Interaction
In acidic media, CS possesses protonated amino groups that readily interact with multivalent anions such as sodium tripolyphosphate (STP), citrate, sulfate or phosphate [35]. These electrostatic interactions facilitate rapid hydrogel formation under mild conditions. The method is straightforward, non-toxic, and compatible with sensitive bioactive molecules. Cao et al. [57] developed polyelectrolyte complex (PEC) hydrogels through the in situ polymerization of acrylic acid monomers within a CS solution (Figure 3B). Subsequently, various cations (Na+, Mg2+, and Al3+) and anions were incorporated into the PEC network to establish strong electrostatic interactions with the polymer chains, leading to the formation of double polyelectrolyte complex (DPC) hydrogels. The incorporation of these ions played a critical role in determining the mechanical performance of the resulting hydrogels. The tensile strengths of the ion-modified DPC hydrogels (2.36, 12.59, 65.1, and 2.80 MPa, respectively) were markedly higher than that of the blank control (0.29 MPa). Notably, the DPC–Ca hydrogels exhibited distinct ionic conductivity and exceptional anti-freezing behavior, remaining unfrozen even at −20 °C. This DPC–Ca strategy provides a promising route for the design of CD-based hydrogels with enhanced mechanical strength, conductivity, and temperature tolerance. Wang et al. [58] designed an injectable CS-based hydrogel by utilizing CS as the primary polymeric matrix. The amino groups on the CS backbone enabled the conjugation of catechol moieties, mimicking mussel-inspired adhesive proteins, through electrostatic interactions and cooperative reactions with the sulfonic acid groups of the thermosensitive monomer N-isopropylacrylamide. This innovative formulation produced a biodegradable, biocompatible, and temperature-responsive hydrogel with remarkable tissue adhesion and stability, making it highly promising for biomedical and wound-healing applications. Their fast swelling and controlled release properties make them particularly useful for drug delivery applications. However, such hydrogels often suffer from limited mechanical integrity and tend to disintegrate quickly under physiological conditions.
5.1.2. Hydrogen Bonding
Hydrogen bonding plays a significant role in stabilizing CS hydrogels, either through interactions between the hydroxyl and amino groups of CS itself or in blends with other polymers such as polyvinyl alcohol (PVA) and poly (ethylene glycol) (PEG) [59]. These reversible interactions contribute to pH- or temperature-responsive behavior. For instance, hydrogen bonding in CS–PVA hydrogels enhances elasticity and swelling control. In a study, Yan et al. [60] employed agar and CS to demonstrate the fabrication of a double network hydrogel via strong H-bonding between the -OH group of agars and the NH3+ group CS. Another study by Han and coworkers reported the fabrication of hydrogel reinforced cellulose nanocrystals through electrostatic and hydrogen bonding interaction between xanthan solution and CS [61]. These studies revealed that hydrogels can be fabricated via simple hydrogen bond formation and can be made stimuli-responsive. Nonetheless, hydrogen-bonded networks are often sensitive to environmental fluctuations and may lack long-term stability [62].
5.1.3. Hydrophobic Interaction
Hydrophobic modification of CS involving grafting with alkyl chains, cholesterol, or aromatic groups, induces self-association in aqueous environments. These hydrophobic domains aggregate, forming a 3D-network. In general, hydrogels can be synthesized by utilizing both techniques and the resultant gels can be tailored to achieve sol-to-gel transition under specific conditions. Hydrogels can also be structured by regulating hydrophobic interactions, which are achieved mainly through two fabrication routes. In the first route, micellar structures act as dynamic crosslinkers, where the hydrophobic cores not only entrap poorly water-soluble drugs but also connect polymer chains to form a stable network. This technique effectively enhances the drug-loading efficiency, distribution uniformity, and overall stability of the hydrogel [63]. The second route involves introducing hydrophobic segments such as long alkyl, steroidal, or aromatic groups onto otherwise hydrophilic polymer backbones. This modification promotes spontaneous self-association in aqueous media, leading to a three-dimensional network held together by hydrophobic domains [64]. In addition, hydrophobic interactions can be triggered or strengthened through two external signals: thermal modulation, utilizing phase transition behavior such as lower critical solution temperature (LCST) or upper critical solution temperature (UCST), and ultrasonic treatment, which facilitates molecular aggregation and network formation [52]. Together, these approaches allow the development of robust, stimuli-responsive hydrogels with improved capacity to incorporate and deliver hydrophobic bioactive molecules. Hydrogels prepared through this mechanism frequently display temperature- or pH-dependent responsiveness, since hydrophobic interactions are strengthened under physiological conditions. A major advantage is their capacity to encapsulate and protect hydrophobic drugs and proteins, although achieving reproducible and structurally uniform gels remains a challenge.
5.1.4. Metal-Chelating
The metal-chelating method utilizes the abundant amino and hydroxyl groups of CS to coordinate with multivalent metal ions, forming stable crosslinked hydrogel networks. Transition metals such as Fe3+, Cu2+, Zn2+ or Ca2+ interact electrostatically or through coordination bonds with the functional groups of CS, resulting in improved mechanical strength, structural integrity and antibacterial activity. The reversible nature of metal-ligand coordination also imparts self-healing and stimuli-responsive properties as the crosslinkers can dissociate and reform under changes in pH or ionic strength. For instance, Yang et al. [65] prepared gallic acid-modified carboxymethyl chitosan/ferric ion (GA-CMCS/Fe III) composite hydrogels using non-covalent interaction and metal coordination (Figure 3A). GA-CMCS/Fe3+ composite hydrogels showed good compressive strength and reasonable mechanical strength, providing a new opportunity to construct physical multi-crosslinking systems with excellent mechanical strength. Metal-chelated CS hydrogel offers simple fabrication, improved mechanical strength, and pH responsive behavior. However, excessive metal-ion release during degradation may reduce long-term stability under fluctuating pH or ionic environments.
5.1.5. Freeze–Thaw Method
Freeze–thaw is one of the physical cross-linking techniques, which is primarily accomplished by single or repeated cycles. When CS is blended with PVA and subjected to repeated freeze–thaw cycles, crystallite regions form that act as physical junction points, stabilizing the hydrogel network [66]. This approach eliminates the need for chemical crosslinkers and produces non-toxic hydrogels with enhanced elasticity and toughness [67]. Such hydrogels have shown promise in wound dressings and biomedical scaffolds [68]. However, multiple cycles are usually required to obtain adequate stability and their swelling properties may still be limited compared to chemically crosslinked systems.
5.1.6. Host-Guest Interaction
This host-guest mechanism generates supramolecular hydrogels with reversible gelation and tunable responsiveness [69]. Cyclodextrins (CDs) are widely employed as host molecules to form inclusion complexes with hydrophobic guest moieties attached to CS [70]. For example, CS modified with adamantane (guest) can interact with β-cyclodextrin (host) to yield self-assembled networks [71]. These hydrogels are particularly valuable in drug delivery and tissue engineering, where controlled responsiveness and reversible crosslinking are advantageous. Host–guest interaction hydrogels can also be engineered to respond to external stimuli such as temperature, pH, or light, making them highly suitable for controlled and targeted drug delivery applications. Their main limitation lies in the relatively weak binding affinities of host–guest pairs under physiological conditions, which may compromise stability.
5.2. Chemical Crosslinking Methods
Chemical crosslinking produces covalent bonds between or within polymer chains, resulting in robust, permanent hydrogel networks. These networks offer superior mechanical strength, tunable degradation, and highly controlled swelling behavior [72]. Unlike physically crosslinked hydrogels, these systems are more resistant to dissolution under physiological conditions, making them particularly attractive for load-bearing wound healing applications. However, the cytotoxicity of some crosslinkers and the need for careful purification remain important considerations.
5.2.1. Free Radical Polymerization
Free radical polymerization is widely used to graft monomers such as acrylic acid, acrylamide, or N-isopropylacrylamide onto the backbone [68]. This approach generates hydrogels with enhanced stimuli-responsiveness, including pH-sensitivity (via acrylic acid) and thermo-responsiveness (via poly (N-isopropylacrylamide) PNIPAM) [73]. In a typical free-radical polymerization process, four fundamental stages occur: initiation, propagation, chain transfer, and termination. During initiation, reactive radical species are generated by external triggers such as ultraviolet or visible light, heat, or redox reactions [74]. These radicals attack monomer units, creating active growing centers. In the propagation stage, these centers continuously add monomer molecules, allowing the polymer chains to extend. Eventually, the reaction is terminated when two radical chains combine or a chain transfer reaction takes place, producing a stable crosslinked polymeric network that forms the hydrogel structure [75]. These grafted networks provide high versatility and tunability of mechanical and physicochemical properties. Nevertheless, residual initiators or unreacted monomers must be carefully removed to ensure safety for biomedical use.
5.2.2. Enzymatic Crosslinking
Enzymatic methods offer a greener alternative, where enzymes such as horseradish peroxidase (HRP), tyrosinases, peroxidases, phosphopantetheinyl transferase, transglutaminase catalyze specific reactions in derivatives [76,77]. For example, HRP can crosslink gallic acid onto the CS backbone [78], while transglutaminase are the members of the thiol group of enzyme that catalyzes amide bonds between carboxylic and amide/amino groups in the presence of calcium ions as cofactors [79]. These processes yield biocompatible hydrogels under physiological conditions with excellent cytocompatibility, spatiotemporal control, and suitability for cell encapsulation. Limitations include higher cost and sensitivity to enzyme activity and storage conditions.
5.2.3. Photo-Crosslinking
Photo-crosslinking has emerged as an effective approach for developing CS-based hydrogels suitable for a wide range of biomedical uses, including drug delivery, tissue repair, and wound healing. The process involves incorporating light-responsive groups or photoinitiators that can absorb energy from ultraviolet or visible light and produce reactive species capable of linking polymer chains into a stable three-dimensional network [76]. To ensure biological safety, non-toxic and biocompatible initiators such as riboflavin, eosin Y, or camphorquinone are typically employed [62,80,81]. Hydrogels produced by photo-crosslinking generally exhibit good cytocompatibility, improved mechanical behavior, and efficient cell encapsulation, making them promising candidates for tissue-engineered scaffolds [82]. Additionally, this technique provides spatiotemporal control over gelation, allowing hydrogel formation to occur in situ and without direct physical manipulation, which is particularly beneficial for irregular or deep wound sites.
5.2.4. Click Chemistry
Click chemistry represents a group of highly efficient and selective reactions designed to covalently attach functional molecules to specific reactive sites on another compound [83]. These reactions generally occur under mild, catalyst-friendly, and high-yielding conditions, and they are largely unaffected by the presence of other species in the reaction mixture [84,85]. Because of these advantages, click chemistry has become a preferred approach for constructing hydrogel networks with precise structural control. In hydrogel crosslinking, click-based strategies are commonly categorized into five principal reaction types: the Huisgen cycloaddition, thiol-ene coupling, Diels-Alder cycloaddition, Michael addition, and Schiff-base condensation [86,87,88]. These reactions are highly efficient, selective, and bio-orthogonal, proceeding under mild conditions with minimal byproducts. For example, in the modified C6 O-allyl (OAL)-CS system, the thiol-containing crosslinkers generates thiol radicals under UV irradiation, which undergo a thiol-ene reaction with allyl groups on OAL-CS to rapidly form hydrogel, as shown in Figure 3C [89]. The solubility of the UV-triggered thiol-ene hydrogels varies with the pH of the surrounding medium, enabling their use as a pH-responsive slow drug release system. The resulting networks offer excellent reproducibility and structural control, making them well suited for drug delivery, biosensing, and regenerative medicine. The main challenge lies in the need to pre-functionalize CS with reactive moieties, which adds complexity to the preparation process.
5.2.5. Graft Copolymerization Techniques
Graft copolymerization is an effective strategy to modify CS and enhance its physiochemical and biological properties for hydrogel fabrication. In this approach, synthetic or natural monomers are covalently grafted onto the CS backbone through reactive amino or hydroxyl groups, which improve solubility, mechanical strength, stability and stimuli-responsiveness. Li et al. [90] successfully developed CS-polyethylene glycol-hydro caffeic acid composite hydrogels, in which catechol groups were grafted onto CS chains via 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-mediated coupling chemistry, as illustrated in Figure 3D. The resulting CS-PEG-HA hydrogels exhibited excellent self-healing ability and effectively promoted angiogenesis and epidermal regeneration. Such self-healing, chemically crosslinked hydrogels broaden the potential of CS-based materials for advanced biomedical and regenerative applications.
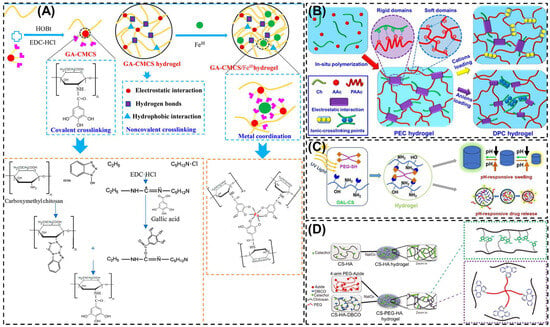

Figure 3.
Schematic representation of the various formation mechanism of CS based hydrogels. (A) Schematic diagram for the preparation of GA-CMCS hydrogel and GA-CMCS/Fe III hydrogel. (B) Illustration of the preparation of PEC and DPC hydrogels (C) Formation of C6 O-allyl-CS hydrogel by UV-triggered thiol-ene click chemistry. (D) Formation of CS-HA and CS-PEG-HA hydrogel. Reprinted with permission from Yang et al. [65] (Copyright, 2019, American Chemical Society (ACS)), Cao et al. [57] (Copyright, 2020, Elsevier), Ding et al. [89] (Copyright, 2021, Elsevier) and Li et al. [90] (Copyright, 2022, Elsevier).
5.3. Self-Assembly Methods
Self-assembly represents a unique strategy for the preparation of CS hydrogels, relying on the intrinsic amphiphilicity and charge of CS or its derivatives to spontaneously organize into 3D networks under aqueous conditions [91]. These methods do not require chemical crosslinkers, making them attractive for injectable and in situ gelling systems. However, the resulting hydrogels often show relatively weaker mechanical properties compared with covalently crosslinked systems.
One common approach is polyelectrolyte complexation, where positively charged interacts with negatively charged biopolymers such as alginate, carrageenan, hyaluronic acid, or DNA [92]. The electrostatic attraction between these oppositely charged chains leads to hydrogel formation with high biocompatibility and biodegradability. Such systems are particularly well suited for wound dressings, gene delivery, and tissue regeneration. Another important approach involves amphiphilic derivatives, where hydrophobic chains are grafted onto CS backbones. In aqueous environments, these derivatives undergo micellization and self-aggregation, forming hydrogel networks that can respond to pH, temperature, and ionic strength. These hydrogels are especially useful for the encapsulation and controlled release of hydrophobic drugs or proteins [93]. Table 1 represent the classification of CS-based hydrogel-based on crosslinking mechanisms.

Table 1.
Classification of CS-based hydrogels according to crosslinking mechanism, representative crosslinkers, advantage, limitation and major biomedical applications.
Table 1.
Classification of CS-based hydrogels according to crosslinking mechanism, representative crosslinkers, advantage, limitation and major biomedical applications.
| Category | Method | Mechanism/Crosslinker | Advantages | Limitations | Applications | References |
|---|---|---|---|---|---|---|
| Physical crosslinking | Ionic/electrostatic | Electrostatic interaction between protonated and multivalent anions (TPP, citrate, sulfate, phosphate) | Simple, non-toxic, mild conditions and biocompatible | Poor mechanical strength and unstable in physiological media. | Drug delivery and controlled release | [94,95,96] |
| Hydrogen bonding | Intermolecular hydrogen bonds (–OH, –NH2 groups) or blending with PVA and PEG. | Reversible, responsive to pH/temperature and enhances elasticity | Sensitive to environment and weak long-term stability | Swelling control and wound dressing | [97,98,99] | |
| Hydrophobic interaction | Grafted alkyl, cholesterol and aromatic moieties induce aggregation | Encapsulation of hydrophobic drugs and pH/temperature responsiveness | Poor reproducibility and structural heterogeneity | Protein/drug delivery and responsive carriers | [100,101] | |
| Freeze–thaw | Repeated freeze–thaw cycles with PVA form crystallite junctions | Non-toxic, reinforced elasticity and toughness | Multiple cycles required and moderate swelling | Wound dressings and biomedical scaffolds | [102,103,104] | |
| Host-guest inclusion | Cyclodextrin (host) + hydrophobic CS moiety (guest) | Reversible, tunable and injectable | Weak host–guest binding under physiological conditions | Drug delivery and tissue engineering | [105,106,107] | |
| Chemical crosslinking | Free radical polymerization | Grafting monomers (acrylic acid, N-isopropylacrylamide (NIPAM)) onto CS | Strong, versatile and stimuli-responsive networks | Toxic residual initiators and complex purification | Smart drug release and responsive gels | [108,109] |
| Enzymatic | HRP or transglutaminase catalyzed crosslinking. | Biocompatible, mild and spatiotemporal control | High cost and enzyme sensitivity | Injectable gels and tissue regeneration | [78,110] | |
| Photo-crosslinking | UV/visible light crosslinking of methacrylated/cinnamate CS | Precise spatial/temporal control and rapid gelation. | UV cytotoxicity and photo initiator concerns | 3D bioprinting and localized delivery. | [111,112,113] | |
| Click chemistry | Azide-alkyne or thiol-ene reactions | High specificity, reproducibility and minimal byproducts | Requires functionalization, and added complexity | Drug delivery, biosensing, and regenerative medicine | [114,115] | |
| Self-assembly | Polyelectrolyte complexation | Electrostatic interaction with alginate, carrageenan, HA, and DNA | Mild, biocompatible, and biodegradable | Weak stability, less mechanical strength | Gene delivery, wound healing | [116,117] |
| Amphiphilic derivatives | Hydrophobic modification (alkyl, cholesterol, PNIPAM)-micellization and aggregation | Multi-stimuli responsive and encapsulates hydrophobic drugs | Structural inconsistency with weak strength | Drug/protein delivery and smart carriers | [118,119,120] |
6. Stimuli-Responsiveness in Diabetic Wound Healing
Stimuli-responsive hydrogels are a class of smart polymeric materials that can sense and react to environmental changes by altering their physical or chemical properties [121]. These hydrogels respond to specific stimuli such as pH, temperature, light, glucose concentration, ROS or enzymatic activity leading to controlled swelling, degradation or therapeutic release [122,123]. Depending on the nature of the stimulus, they are categorized into several types such as pH, thermosensitive, photo, glucose and ROS-responsive hydrogel [124,125] (Figure 4). Among various polymers used to fabricate such systems, CS has gained significant attention due to its unique chemical functionality, physiological compatibility, enzymatic degradability, and inherent bioactivity. The presence of –OH and –NH2 groups in its molecular structure allows facile chemical modification, enabling the incorporation of responsive moieties that make the hydrogel sensitive to physiological stimuli [126].
In the context of diabetic wound healing, where the wound environment is often acidic, infected, and rich in glucose or ROS, these smart hydrogels offer multiple therapeutic benefits [18]. They can deliver drugs, growth factors, or signaling molecules in response to local stimuli while maintaining a moist and antimicrobial microenvironment. Additionally, they promote angiogenesis, collagen deposition, and overall tissue regeneration [127]. A comparative analysis of single and dual-stimuli-responsive CS-based hydrogel including their triggering mechanisms, major therapeutic advantages and current limitation are summarized in Table 2. The following sections provide a detailed overview of each type of stimuli-responsive CS hydrogel and their specific mechanism contributing to diabetic wound healing.
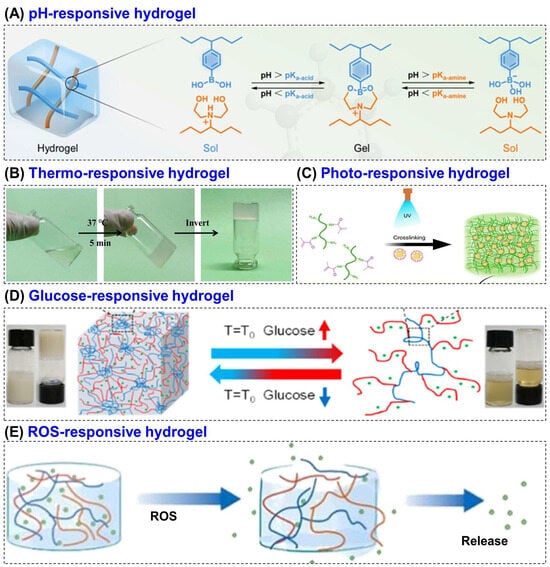
Figure 4.
Schematic representation of various stimuli-responsive hydrogels, including (A) pH, (B) thermo, (C) photo, (D) glucose, and (E) ROS-responsive systems. Reprinted with permission from Kang et al. [128] (Copyright, 2023, ACS), Liu et al. [129] (copyright, 2022, MDPI), Kurian et al. [130] (copyright, 2023, Elsevier), Hu et al. [131] (Copyright, 2021, ACS) and Zhao et al. [132] (copyright, 2024, Elsevier).

Table 2.
Comparative analysis of single and dual-stimuli responsive CS-based hydrogel for diabetic wound healing.
Table 2.
Comparative analysis of single and dual-stimuli responsive CS-based hydrogel for diabetic wound healing.
| Hydrogel Type | Stimulus/Trigger | Example Representative Composition | Key Advantage | Limitations | References |
|---|---|---|---|---|---|
| pH- responsive | Acidic microenvironment in infected wounds | Quaternary ammonium CS + oxidized dextran-dopamine (OD-DA) + silver nanoparticles (AgNPs)/deferoxamine (DFO) | Enables acid-triggered antibacterial and angiogenic release, good adhesion and self-healing | Limited mechanical strength, and possible instability under neutral pH | [133] |
| Thermo- responsive | Body temperature | CS + beta-glycerophosphate + Cu/Mg-MOF nano enzyme | Injectable, in situ gelation, modulates inflammation and promotes angiogenesis | Slow gelation and temperature-sensitive stability | [134] |
| Photo- responsive | Near-infrared (NIR) light | Carboxymethyl CS + gelatin + polydopamine-coated ZIF-8 NPs | On demand antibacterial activity via mild photothermal effect and enhances angiogenesis | Require external light sources, limited source and limited tissue penetration | [135] |
| Glucose- responsive | Elevated glucose concentration | CS + hyaluronic acid + L-arginine + glucose oxidase (GOx) | Self-regulated NO generation, improved angiogenesis and collagen synthesis | Complex fabrication and limited long term stability | [136] |
| ROS- responsive | High oxidative stress | Quaternized CS + metal–organic framework (MOF) enzymes | Scavenges ROS, restores redox balance, promotes oxygen generation and tissue repair | Costly nanozyme preparation and unclear biodegradation profile | [137] |
| Dual- responsive | pH/ROS or ROS/glucose | Quaternized CS + phenylboronic acid + catechol derivatives | Synergistic response to multiple stimuli and enhanced control over drug release | Complicated synthesis and reproducibility issues | [138,139] |
6.1. pH-Responsive CS Hydrogel
During the wound healing process, the pH level of the wound site serves as an important indicator of healing status. Normal wounds generally exhibit a neutral or slightly alkaline pH (around 7–8), which promotes cellular activity and tissue regeneration. In contrast, diabetic wounds often shift toward an acidic environment (pH-6.5) as a result of bacterial infection, inflammation, and limited oxygen supply, which delays the healing process [140,141,142]. pH-responsive CS hydrogels are designed to detect this acidic condition and release therapeutic agents such as antibacterial compounds or growth-promoting molecules on demand [143]. Their sensitivity originates from the ionizable amino groups (–NH2) in the CS structure, which can gain or lose protons depending on pH fluctuations. These reversible interactions enable the hydrogel to swell, contract, or degrade as needed. This allows precise drug release, localized antibacterial action, and restoration of a favorable wound environment that supports tissue repair [144,145].
Several studies have been carried out to investigate the therapeutic potential of pH-responsive CS based hydrogels for diabetic wound healing applications. For example, Hu et al. [133] designed a dual-crosslinked, mussel-inspired pH-responsive CS hydrogel by coupling quaternary ammonium CS (HTCC) with oxidized dextran–dopamine (OD–DA) through Schiff base linkages and catechol–catechol interactions. This approach resulted in a self-healing, injectable, and adhesive hydrogel matrix (Figure 5A). This dual-crosslinking strategy provided the hydrogel with enhanced mechanical strength, structural stability, and dynamic responsiveness. To achieve multifunctionality, AgNPs and the angiogenic agent DFO were incorporated as active therapeutic components. Under the acidic conditions typical of infected diabetic wounds, the imine bonds within the hydrogel were cleaved, resulting in pH-triggered release of AgNPs for potent antibacterial action and DFO for angiogenic stimulation via HIF-1α and VEGF pathway activation (Figure 5B). Furthermore, in vivo studies in diabetic rat models demonstrated significantly accelerated wound closure (~79% within 7 days), enhanced collagen deposition, reduced macrophage infiltration, and robust neovascularization compared with control groups. This hydrogel’s acid-responsive degradation, self-repairing behavior, and moist, adhesive surface synergistically contributed to effective bacterial elimination and rapid tissue regeneration, making it a promising therapeutic platform for treating chronic and infected diabetic wounds.
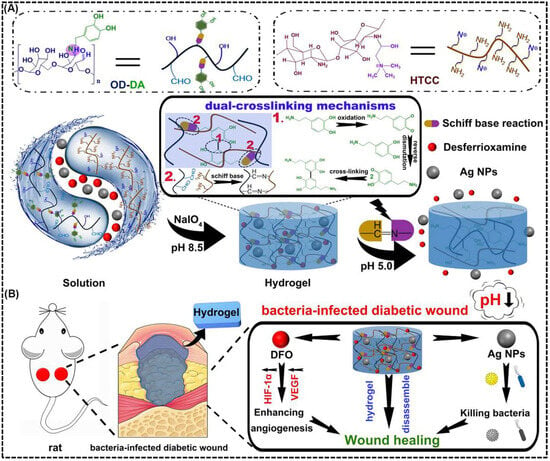
Figure 5.
pH-responsive antibacterial and angiogenic CS-based hydrogel for diabetic wound healing. (A) Schematic illustration of the preparation and dual-crosslinking mechanism of the mussel-inspired hydrogel composed of OD-DA and HTCC. The hydrogel is formed through dynamic Schiff base reactions and coordination with AgNPs. This structure enables pH-triggered degradation and drug release under acidic wound conditions. (B) Illustration of the CS-based hydrogel applied to a bacteria-infected diabetic wound, showing pH-triggered disassembly that releases AgNPs and DFO. The release AgNPs provide antibacterial action, while DFO promote angiogenesis through HIF-1α/VEGF signaling to enhance wound healing. Reprinted with permission from Hu et al. [133] (copyright, 2021, Elsevier).
Another study by Yang et al. [146] developed a pH-responsive hydrogel composed of tannic acid, quaternized carboxymethyl CS, oxidized sodium alginate, and carbon quantum dots for diabetic wound healing and real-time pH monitoring. The dynamic Schiff-base bonds between quaternized carboxymethyl CS (QCMCS) and oxidized sodium alginate (OSA) endowed the system with clear pH sensitivity, enabling controlled drug release and structural self-healing under acidic wound conditions. In diabetic mice, this pH-responsive CS-based hydrogel achieved about 97% wound closure within 12 days, enhanced angiogenesis, and offered simultaneous therapeutic and diagnostic benefits for advanced diabetic wound management. An injectable, self-healing, pH-responsive CS hydrogel for sustained insulin delivery in diabetic wound healing was developed by Li et al. [147] from N-carboxyethyl CS, hyaluronic acid–aldehyde, and adipic acid dihydrazide. The developed hydrogel exhibited acid-triggered degradation and controlled insulin release under wound-like pH. It also maintained insulin bioactivity, promoted cell migration, and achieved accelerated wound closure, angiogenesis, and collagen deposition in diabetic rats. These results suggest that pH-responsive CS hydrogels are simple, biocompatible systems that effectively release therapeutic agents in acidic wound environments. They show strong antibacterial and angiogenic activity but depend heavily on wound pH. Their main limitation are weak mechanical stability and short-term responsiveness, which restrict sustained use.
6.2. Thermo-Responsive CS Hydrogel
Temperature is critical in human physiology and can be conveniently controlled in both in vitro and in vivo conditions [148]. Thus, temperature-responsive hydrogel has attracted much attention in the past few decades. Thermo-responsive hydrogels can reversibly switch between sol and gel states with temperature, typically forming gels at body temperature (~37 °C) [149]. This property enables injectable and in situ-forming wound dressings that conform to irregular diabetic wounds and provide sustained drug release [150]. The phase transition of thermo-responsive hydrogels depends on their critical solution temperature, which can be classified as either lower critical solution temperature (LCST) or upper critical solution temperature (UCST). Hydrogels with a LCST undergo gelation when the temperature exceeds this threshold, whereas those with a UCST form gels when the temperature falls below it [151]. The temperature sensitivity is often achieved by blending CS with β-glycerophosphate (β-GP), poly (N-isopropylacrylamide) (PNIPAM), or Pluronic F127, allowing rapid gelation under physiological conditions [152,153].
For examples, a thermosensitive CS hydrogel was prepared by grafting NIPAM onto carboxymethyl CS and reinforcing it with graphene [154]. The hydrogel showed gelation near body temperature (~34 °C), strong antibacterial activity, and excellent biocompatibility, making it a promising temperature-responsive system for drug delivery and wound healing. Zhang et al. [134] developed a thermosensitive CS-based hydrogel incorporating copper–magnesium bimetallic MOF (Cu/Mg-MOF) nano-enzymes to promote diabetic wound healing by regulating the wound microenvironment. The hydrogel, composed of CS and ε-polyline (PL) with β-glycerophosphate as the thermal gelling agent, that exhibited excellent injectability, temperature sensitivity, and biocompatibility, forming a stable gel at physiological temperature. They also demonstrated in vitro studies, which showed that the hydrogel effectively protected fibroblasts from oxidative stress, stimulated their migration, and modulated macrophage polarization by suppressing pro-inflammatory (M1) activity and enhancing anti-inflammatory (M2) behavior. In vivo diabetic mouse experiments revealed accelerated wound closure (~90.6% within 14 days) along with enhanced collagen formation, angiogenesis, and re-epithelialization (Figure 6A). These thermos-responsive hydrogels maintain a moist, antibacterial environment, while incorporated bioactive agents such as VEGF or insulin enhance angiogenesis, collagen synthesis, and tissue repair [155]. Thermos-responsive hydrogel offers good injectability and form stable gels at body temperature, enabling localized therapy. They promote angiogenesis and tissue repair but have slow gelation kinetics and limited temperature stability. Compared to other stimuli, they are less specific to diabetic biochemical cues.
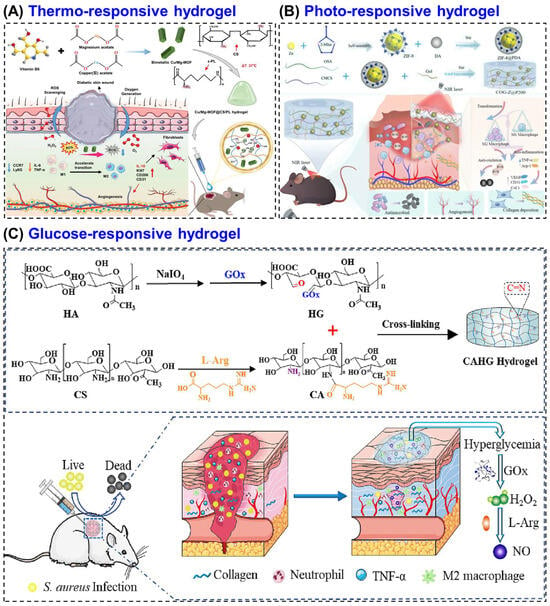
Figure 6.
CS-based hydrogel for diabetic wound healing. (A) Schematic illustration of thermosensitive CS-based hydrogel incorporating Cu/Mg-MOF nano-enzymes to promote diabetic wound healing. (B) An illustration of the photo-responsive multifunctional hydrogel (COG-Z@P200) for methicillin resistant Staphylococcus aureus (MRSA)-infected diabetic wound healing. (C) A glucose responsive hydrogel prepared from CS, hyaluronic acid, and L-arginine (L-Arg) to promotes angiogenesis, collagen synthesis, and antibacterial defense in diabetic wounds. Reprinted with permission from Zhang et al. [134] (copyright, 2025, Wiley-VCH GmbH), Gao et al. [135] (copyright, 2025, BMC), and Zhou et al. [136] (copyright, 2023, Elsevier).
6.3. Photo-Responsive CS Hydrogel
Photo-responsive CS hydrogels are a class of light-activated biomaterials that can transform light energy into therapeutic effects such as localized heating, drug release, or ROS generation [156]. These hydrogels are typically constructed by embedding photoactive agents for example, gold nanoparticles (AuNPs) [157], carbon quantum dots (CQDs) [158], or photosensitizers into a CS-based matrix. Upon exposure to visible or near-infrared (NIR) light, these photoactive components trigger controlled physicochemical changes within the hydrogel, allowing on-demand antibacterial, anti-inflammatory, and regenerative actions [159,160].
In normal healing, the tissue environment naturally supports inflammation resolution, collagen remodeling, and angiogenesis [161]. Here, photo-responsive CS hydrogels primarily act as antibacterial and regenerative enhancers, accelerating closure by sterilizing the wound surface, stimulating fibroblast proliferation, and maintaining a moist healing environment [162]. The mild photothermal or photodynamic effects produced under light exposure can promote microcirculation, thereby speeding up re-epithelialization and granulation tissue formation without damaging healthy cells. For example, Wang et al. [163] compounded that the dopamine-assisted exfoliated molybdenum disulfide into lipoic acid modified CS (LAMC-MoS2@PDA) hydrogel promotes wound healing through a photothermal antibacterial and tissue-regenerative mechanism. Under 808 nm NIR irradiation, the MoS2 nanosheets convert light into mild localized heat, effectively eliminating bacteria and reducing inflammation. Simultaneously, the CS matrix supports cell proliferation, collagen synthesis, and angiogenesis, creating a favorable microenvironment that accelerates normal wound closure and tissue regeneration.
In contrast, diabetic wounds are characterized by persistent infection, excessive oxidative stress, reduced oxygen supply, and impaired angiogenesis [164]. In such a pathological microenvironment, photo-responsive CS hydrogels provide a more complex and targeted therapeutic approach. The CS backbone contributes inherent antibacterial and hemostatic properties, while light activation enhances ROS regulation, inflammation control, and vascular regeneration. For instance, CS–AuNP hydrogels under NIR irradiation exert localized photothermal antibacterial action while simultaneously stimulating angiogenesis and collagen deposition in diabetic wounds [165]. Similarly, CS methacryloyl (CSMA)-based hydrogels integrated with soy isoflavones and Au NPs demonstrated photo-triggered drug release, which leading to reduced inflammation and enhanced VEGF-mediated neovascularization in diabetic models [166].
An advanced CS-based multifunctional hydrogel was developed by Gao et al. [135] that combines carboxymethyl CS (CMCS), oxidized sodium alginate and gelatin with polydopamine-coated ZIF-8 nanoparticles (COG-Z@P200), forming a mild photothermal system for MRSA-infected diabetic wounds (Figure 6B). Upon NIR irradiation (808 nm), the composite generated localized heat (≈40–45 °C) and released Zn2+ ions, which synergistically disrupted bacterial membranes and metabolism achieving >99.5% MRSA elimination. Multi-omics analysis confirmed that the hydrogel suppressed glycolysis, the TCA cycle, and arginine biosynthesis while down-regulating quorum-sensing and virulence genes. In vivo, the system accelerated wound closure by 48%, promoted M2 macrophage polarization, enhanced VEGF-mediated angiogenesis, and increased collagen deposition without damaging healthy tissue. Further, the developed CS network provided biocompatibility, ROS scavenging, and pH-responsive degradation, making this photo-responsive CS hydrogel a potent, antibiotic-free platform for diabetic wound therapy through metabolic interference and microenvironment regulation. Overall, these demonstration highlight that photo-responsive CS hydrogels system allow precise, on-demand antibacterial and regenerative effects under light irradiation. They provide high control and strong antimicrobial activity but require external irradiation, limiting deep wound application.
6.4. Glucose-Responsive CS Hydrogel
Glucose-responsive CS hydrogels are smart biomaterials designed to sense elevated glucose concentrations in diabetic wounds and respond by triggering a therapeutic action [167]. Chronic hyperglycemia in diabetic wounds delays healing and contributes to inflammation, infection, and oxidative stress [168]. These hydrogels use the high-glucose microenvironment as a biological cue to regulate drug delivery, nitric oxide (NO) generation, or hydrogel degradation in a controlled way. Their responsiveness is typically achieved through three main mechanisms [169]: (i) the glucose oxidase reaction system, (ii) phenylboronic acid (PBA) modification, where PBA groups reversibly bind with glucose, altering the hydrogel structure and triggering swelling or release. (iii) the Concanavalin A (ConA)-mediated competitive binding system.
The glucose oxidase (GOx) catalyzes glucose to produce gluconic acid and hydrogen peroxide (H2O2). The products induce local chemical changes that promote drug release or hydrogel breakdown [170,171]. PBA and its derivatives are among the most widely used components for developing glucose-responsive hydrogels. Their responsiveness is based on the reversible covalent interaction between PBA and cis-diol groups. In a hyperglycemic environment, glucose molecules compete with the existing diol-containing components to form boronate ester bonds with PBA. This competitive binding weakens or disrupts the pre-formed PBA–diol crosslinks within the hydrogel network, leading to a decrease in crosslinking density. As a result, the hydrogel undergoes swelling, partial degradation, or a sol–gel transition, which can be strategically used to control the release rate of encapsulated therapeutic molecules and thus allowing on-demand glucose-responsive drug delivery [172,173,174]. ConA is a lectin protein capable of binding selectively and reversibly to carbohydrate molecules. Its glucose sensitivity arises from a competitive binding process, in which elevated glucose concentrations cause free glucose molecules to displace immobilized carbohydrate ligands attached to the hydrogel matrix. This displacement alters the noncovalent interactions between ConA and the bound ligands, leading to a reorganization of the hydrogel’s crosslinked structure. As a result, the network undergoes swelling or structural relaxation, which facilitates glucose-dependent and controlled release of therapeutic agents [19].
A representative system is the CAHG hydrogel, prepared by in situ crosslinking of L-arginine-coupled CS and GOx-modified hyaluronic acid based on Schiff-base reaction. In this system, GOx converts glucose into H2O2, which subsequently reacts with L-Arg to generate NO, a signaling molecule that promotes angiogenesis, collagen synthesis, and antibacterial defense (Figure 6C) [136]. This ensures therapeutic action only under high-glucose conditions, reducing unnecessary release in normal tissue. Glucose-responsive CS hydrogels offer self-regulated drug or NO release, closely mimicking biological feedback. They effectively address hyperglycemia-related stress but involve complex synthesis and enzyme instability.
6.5. ROS-Responsive CS Hydrogel
ROS-responsive CS hydrogels are designed to sense the abnormally high levels of ROS in diabetic wounds and convert that signal into therapy [169]. Two core strategies are used: (i) ROS-cleavable linkers such as thioketals and aryl boronic/boronate esters are grafted into CS networks. When H2O2 level rise, these linkers undergo bond scission, causing network loosening and enabling on-demand release of antibiotics, growth factors, or NO donors. For instance, Kulkarni et al. [175] designed a ROS-responsive nucleobase-conjugated CS hydrogel using thioketal linkers for oxidative stress-triggered drug release. The guanine-functionalized variant (P4) showed strong adhesion, ROS sensitivity, and antibacterial activity. Under H2O2 exposure, the hydrogel degraded to release drugs while scavenging ROS, reducing inflammation, and promoting tissue repair. As shown in Figure 7A, this system combines CS’s biocompatibility and antibacterial properties with ROS-cleavable bonds and nucleobase functionalization, enabling on-demand drug delivery and accelerated healing in diabetic wounds. (ii) ROS-scavenging components such as tannic/gallic acid, polydopamine, ceria/manganese dioxide (MnO2) nanozymes, Prussian blue, selenium and melanin-like nanoparticles are embedded in CS to catalytically quench ROS, and restoring redox balance. For example, Wang et al. [176] developed a ROS-responsive quaternized CS hydrogel incorporating thioctic acid-functionalized PEGs and polydopamine-coated MnO2 nanozymes to treat diabetic wounds (Figure 7B). The MnO2 nanozymes mimicked superoxide dismutase (SOD) and catalase activity, scavenging excess ROS and releasing oxygen to relieve hypoxia, while CS provided antibacterial and adhesive properties. The hydrogel promoted M2 macrophage polarization, angiogenesis, and collagen deposition, resulting in ~95% wound closure within 14 days. These combined effect make it an effective antioxidant and antimicrobial dressing for diabetic wound healing.
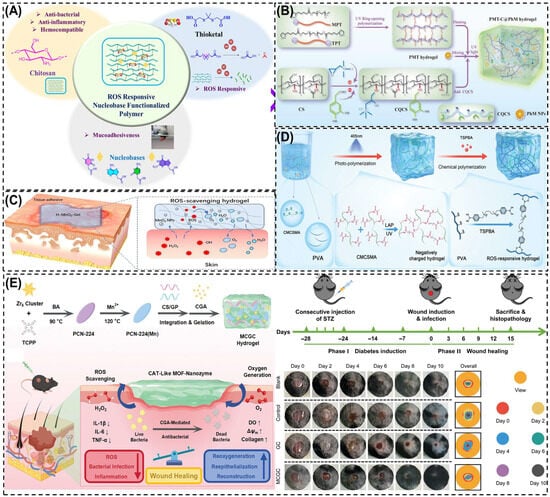
Figure 7.
Schematic illustration of different ROS-responsive CS hydrogels for diabetic wound healing. (A) Nucleobase-functionalized CS hydrogel with thioketal linkers for ROS-triggered degradation and drug release. (B) Quaternized CS hydrogel (PMT-C@PhM) with MnO2 nanozymes mimicking SOD/CAT to remove ROS and enhance angiogenesis. (C) H-MnO2–CS hydrogel decomposing H2O2 into O2/H2O, reducing oxidative stress and inflammation. (D) Immunomodulatory CS hydrogel (NCRH) adsorbing cytokines, scavenging ROS, and promoting M2 macrophage polarization. (E) Dual-metal MOF-decorated CS hydrogel (MCGC) combining Mn/Zr nanozymes and CGA for ROS scavenging, oxygen generation, and accelerated wound repair. Reprinted with permission from Kulkarni et al. [175] (copyright, 2025, Elsevier), Wang et al. [176] (copyright, 2025, Elsevier), Wu et al. [177], (copyright, 2023, BMC), Wang et al. [178] (copyright, 2024, Wiley-VCH GmbH), and Wei et al. [137] (copyright, 2024, Wiley-VCH GmbH).
These hydrogels directly address diabetic-wound pathologies-persistent oxidative stress, infection, and delayed angiogenesis. By lowering excessive ROS, they limit tissue damage and macrophage M1 polarization, while ROS-triggered release supplies antimicrobials or pro-angiogenic signals exactly where needed. A ROS-responsive CS-based hydrogel (H-MnO2-Gel) was developed by incorporating hollow MnO2 nanoparticles (H-MnO2 NPs) into a quaternary CS–tannic acid matrix to alleviate oxidative stress and inflammation in chronic wounds [177]. The MnO2 nanozymes catalytically converted excess H2O2 into O2 and H2O, effectively scavenging ROS and restoring redox balance, while the CS network offered antibacterial activity, tissue adhesion, and biocompatibility (Figure 7C). The hydrogel protected fibroblasts from oxidative injury and reduced inflammatory cytokine expression, demonstrating strong potential for oxidative microenvironment regulation in diabetic wound healing. Another study by Wang et al. [178] developed an immunomodulatory CS-based negatively charged and ROS-responsive hydrogel (NCRH) capable of capturing pro-inflammatory cytokines and scavenging ROS to promote chronic diabetic wound remodeling. The hydrogel was synthesized from methacrylamide carboxymethyl chitosan (CMCSMA) and TSPBA-modified polyvinyl alcohol (PVA) via dual polymerization (Figure 7D). The negatively charged CMCSMA electrostatically adsorbed positively charged cytokines (IL-1β, TNF-α), while the ROS-responsive PVA network eliminated excess ROS. In diabetic models, NCRH systems consistently show reduced inflammatory cytokines, increased M2 macrophages, enhanced HIF-1α/VEGF signaling, faster re-epithelialization, and denser collagen I/III, culminating in accelerated closure. For intense, Wei et al. [137] developed a MOF nanozymes/chlorogenic acid/genipin crosslinked CS-based hydrogel (MCGC) with potent ROS-scavenging, oxygen-generating, and antibacterial properties for the treatment of infected diabetic wounds. As shown in Figure 7E, the hydrogel was synthesized by integrating Mn/Zr-based PCN-224(Mn) MOF nanozymes, chlorogenic acid (CGA), and a CS-gelatin (CS/GP) matrix via genipin crosslinking. Mechanistically, the Mn–Zr MOF nanozymes acted as catalase-like (CAT) mimics, catalytically decomposing excess H2O2 into oxygen, which reduced oxidative stress and relieved tissue hypoxia. In vivo diabetic wound studies revealed that the MCGC-treated group achieved the fastest wound closure, surpassing control and blank groups by Day 8, with evident re-epithelialization and collagen remodeling. The hydrogel not only eliminated ROS but also promoted cell proliferation (TNF-α/IL-6/ IL-1β), collagen synthesis, and angiogenesis, leading to accelerated wound healing. These results demonstrate that ROS-responsive CS hydrogels convert excessive oxidative stress, restore redox balance, and promote angiogenesis. They show superior antioxidative and healing performance but they rely on uncertain biodegradability. Developing natural antioxidant alternatives may improve clinical viability.
6.6. Dual-Responsive CS Hydrogel
Dual-responsive CS hydrogels are multifunctional materials designed to react to two biological stimuli simultaneously, such as pH/ROS, pH/glucose, or ROS/glucose. This dual action enables control over drug delivery and wound repair [179]. A common design involves pH/ROS-responsive CS-based hydrogels, which contain dynamic bonds like Schiff base and boronate ester linkages. When exposed to acidic and oxidative conditions of diabetic wounds, these linkages break, leading to controlled hydrogel degradation and site-specific release of therapeutic molecules such as antibacterial or angiogenic agents. Another approach combines ROS and glucose sensitivity, where thioketal or boronate groups are integrated with GOx to trigger drug or NO release only under both oxidative and hyperglycemic conditions. This dual response helps restore redox balance, enhances vascular growth, and reduces inflammation [180,181]. For instance, Li et al. [138] created an ROS/glucose-responsive quaternized CS hydrogel containing salvianolic acid B, which regulated oxidative stress and glucose metabolism to promote collagen formation and rapid wound closure.
Similarly, Dai et al. [182] designed a multi-stimuli CS-based hydrogel that responded to pH, ROS, and mild heat, improving angiogenesis and immune modulation in diabetic wounds. Overall, dual-responsive CS hydrogels can adapt to the changing wound microenvironment and deliver drugs when needed. They also maintain moisture balance, control inflammation, and stabilize oxidative conditions, making them a powerful platform for treating chronic diabetic wounds. However, their complex fabrication and batch-to-batch variability remain significant challenges. In addition, mechanical and stability limitations associated with added responsive components may affect long-term performance and clinical translation. Examples of some single and dual-stimuli-responsive CS-based hydrogel and wound healing mechanisms are summarized in Table 3.

Table 3.
Examples of single and dual-stimuli responsive CS-based hydrogels and their therapeutic applications in diabetic wound healing.
7. Clinical and Commercial Translation of CS-Based Hydrogels
Although many studies on CS hydrogel have been conducted in animal models, several CS-based wound dressings have advanced to human clinical trials and commercial use. These developments demonstrate their strong translation potential. Products such as HemConTM, ChitoHeal, Celox®, and Kytocel® have shown significant improvements in wound closure, infection control, and hemostasis in both hospital and field settings. These dressings highlight CS’s clinical versatility, combining biocompatibility, antimicrobial action, and moisture retention capacity. Table 4 summarizes the representative CS-based wound dressing materials evaluated at the human level and their therapeutic outcomes.

Table 4.
Commercially available CS-based hydrogel dressings for wound healing applications [183].
8. Research Gaps and Future Outlook
This review has identifies several significant gaps and future directions in bio-inspired CS-based hydrogel research. These findings can assist researchers in recognizing underdeveloped areas and determine, which aspects should be prioritized to overcome existing limitations. The main considerations are summarized below.
8.1. Safety Considerations Related to Hydrogel Degradation
One important aspect in the clinical use of CS-based hydrogels is the fate of incorporated metallic nanoparticle, small molecules, and synthetic crosslinkers after hydrogel degradation. As the polymer network gradually breaks down, these components may be released into the wound environment. If their concentration exceeds physiological tolerance, they can potentially cause cytotoxicity, oxidative stress or inflammatory response. For example, excessive release of silver or copper nanoparticles can impair fibroblast proliferation, while residual aldehyde or epoxide based chemical crosslinkers may delay epithelialization.
Recent studies address these concerns by using biocompatible or bioresorbable metals, natural crosslinkers such as Genipin or tannic acid and surface-modified nanoparticles with controlled-release coatings to minimize burst release. Future hydrogel designs should include careful evaluation of degradation products, long-term cytotoxicity, and in vivo clearance pathways to ensure both therapeutic efficacy and clinical safety.
8.2. Development of Clinically Reliable and Multifunctional Hydrogels
Achieving consistent clinical outcomes with CS hydrogels requires addressing variability in physicochemical properties arising from differences in polymer source, DD, and molecular weight. Such inconsistencies often result in variable performance during large-scale production. Establishing standardized fabrication protocols and robust quality control measures are essential to ensure reproducibility, scalability, and regulatory acceptance.
Future directions should focus on developing standardized fabrication protocols, implementing quantifiable control metrics (e.g., fixed DD/molecular weight ranges, impurity thresholds, gel strength standards), and validating this procedure under Good Manufacturing Practice (GMP)-compatible conditions to enable reproducible, scalable, and regulatory-ready CS hydrogel production.
8.3. Incorporation of Bioactive Molecules and Nanostructures
Integrating biologically active agents into CS-based matrices can further amplify their regenerative capacity. Growth factors, antimicrobial peptides, nitric oxide donors, and angiogenic proteins (such as VEGF or FGF) can be encapsulated within the polymeric network to accelerate vascularization and tissue remodeling. However, these compounds often suffer from instability or denaturation in hostile wound environments. To overcome this limitation, nanocarrier-assisted encapsulation using liposomes, polymeric nanoparticles, or metal–organic frameworks can provide sustained release and molecular protection.
Nanocomposite CS hydrogels embedded with metallic or oxide nanoparticles have shown notable antibacterial, antioxidant, and pro-angiogenic performance. Advancing, hybrid nano-bio systems that combine catalytic nanomaterials with CS scaffolds could be designed to regulate intracellular pathways such as macrophage polarization, collagen synthesis, and fibroblast activation. In addition, gene-responsive and immunomodulatory hydrogels that deliver nucleic acids (e.g., siRNA, miRNA, or CRISPR components) hold potential for directly influencing cell behavior and restoring normal wound physiology.
8.4. Advanced Manufacturing and Smart Design
Emerging fabrication approaches such as 3D bioprinting, electrospinning, and microfluidic templating offer precise control over material architecture, enabling the fabrication of wound dressings tailored to specific wound geometries. Three-dimensional bioprinting can produce patient-specific scaffolds with spatial gradients of drugs, growth factors, or responsive domains. Incorporating biosensors within these printed matrices could transform CS hydrogels into theragnostic systems, capable of monitoring wound conditions such as pH, oxygen, and glucose while simultaneously delivering therapeutics.
Injectable and self-healing hydrogels also represent an important focus for future development. Hydrogels with dynamic covalent networks, such as those containing Schiff-base or boronate ester linkages can repair structural damage autonomously and extend dressing lifespan. Thermosensitive injectable hydrogels, which solidify at body temperature are particularly advantageous for treating deep or irregular wounds. Additionally, coupling conductive or magnetically responsive materials with CS networks may allow electro stimulated or remotely triggered wound repair, enhancing healing precision and comfort.
8.5. Computational and Artificial Intelligence Approaches
Artificial intelligence (AI) and computational modeling can accelerate the optimization of CS hydrogel formulations. Machine learning can predict key relationships among polymer composition, crosslinking density, degradation kinetics, and biological performance, guiding rational material design while reducing experimental workload. Furthermore, AI-assisted wound monitoring through digital imaging and pattern recognition could provide real-time insights into healing progression and hydrogel functionality. Integrating these data-driven strategies with experimental validation will support a new generation of predictive biomaterial engineering.
8.6. Translational Pathways and Preclinical Assessment
Although laboratory studies demonstrate significant potential, clinical implementation remains limited by insufficient long-term and large-animal evaluations. Current research predominantly relies on rodent models, which cannot fully reproduce the complexity of chronic diabetic wounds in humans. Future investigations should adopt clinically relevant preclinical models, standardized evaluation parameters, and extended follow-up durations to assess safety, immunogenicity, and degradation behavior comprehensively.
Regulatory approval also presents a significant hurdle, particularly for composite materials incorporating biological or nanostructured components. Close collaboration between researchers, clinicians, industry partners, and policymakers is needed to establish uniform testing standards and GMP–compliant protocols. Ensuring scalability while maintaining bioactivity and mechanical integrity will be crucial for industrial translation.
8.7. Future Vision for Next-Generation CS Hydrogels
Over the next decade, stimuli-responsive CS hydrogels are expected to advance significantly. They will likely evolve into bio-intelligent and multifunctional wound dressings capable of autonomous therapeutic action. These systems will combine multiple functionalities-antimicrobial, anti-inflammatory, antioxidative, and pro-angiogenic within a single, environmentally adaptive framework. The concept of living hydrogels-incorporating engineered or probiotic cells capable of secreting healing-promoting molecules offers an exciting new direction in regenerative therapy.
Sustainability will also become a key focus with emphasis on eco-friendly synthesis routes, renewable CS sources, and biomimetic crosslinking methods that reduce environmental impact and cost. Beyond diabetic wound treatment, such adaptive CS-based materials have potential applications in surgical healing, soft tissue engineering, and targeted drug delivery. Ultimately, success in this field will depend on synergistic collaboration among multidisciplinary scientists to translate laboratory innovations into practical and patient-centered therapies.
9. Conclusions
Stimuli-responsive CS hydrogels have emerged as advanced biomaterials for diabetic wound management. They combine the intrinsic biocompatibility, biodegradability, and antimicrobial nature of CS with responsiveness to physiological signals such as pH, glucose, temperature, light, and ROS. By dynamically interacting with the wound microenvironment, these hydrogels can modulate inflammation, suppress microbial proliferation, promote angiogenesis, and accelerate tissue regeneration. Their capability to provide localized and controlled therapeutic release allows precise regulation of the healing process, establishing them as a promising alternative to conventional wound dressings. However, challenges related to mechanical durability, batch-to-batch reproducibility, regulatory translation, and sterilization compatibility still limit large-scale clinical adoption. The progressive evolution of CS hydrogels toward intelligent, multi-stimuli-responsive systems marks a significant advancement in wound-healing technology. Continued innovation in material design, functional modification, and regulatory alignment is expected to facilitate their transition from laboratory studies to practical medical use.
Author Contributions
Conceptualization, D.B. and S.S.; validation, E.S. and D.B.; resources, D.B.; data curation, P.K.; writing—original draft preparation, S.S., E.S., P.K. and D.B.; writing—review and editing, D.B.; supervision, P.K. and D.B.; project administration, D.B. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cerolsaletti, K.; Hao, W.; Greenbaum, C.J. Genetics Coming of Age in Type 1 Diabetes. Diabetes Care 2019, 42, 189–191. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global Epidemiology of Diabetic Foot Ulceration: A Systematic Review and Meta-Analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Falanga, V. Wound Healing and Its Impairment in the Diabetic Foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Nayak, B.A.; Shubham; Prusty, R.K.; Ray, B.C. Effect of Nanosilica and Nanoclay Reinforcement on Flexural and Thermal Properties of Glass Fiber/Epoxy Composites. Mater. Today Proc. 2020, 33, 5098–5102. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical Applications of Hydrogels in Drug Delivery System: An Update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Nandana, C.N.; Christeena, M.; Bharathi, D. Synthesis and Characterization of Chitosan/Silver Nanocomposite Using Rutin for Antibacterial, Antioxidant and Photocatalytic Applications. J. Clust. Sci. 2022, 33, 269–279. [Google Scholar] [CrossRef]
- Shah, J.; Patel, D.; Rananavare, D.; Hudson, D.; Tran, M.; Schloss, R.; Langrana, N.; Berthiaume, F.; Kumar, S. Recent Advancements in Chitosan-Based Biomaterials for Wound Healing. J. Funct. Biomater. 2025, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef]
- Yi, B.; Wang, X.; Yu, J.; Diao, J.; Wang, G.; Li, S.; Bo, J.; Zhang, X.; Zhang, C.; Guimarães, C.F.; et al. Biomimetic Hydrogel Micro-/Nanofibers for in Situ Soft Tissue Repair and Regeneration. Bioact. Mater. 2026, 55, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, A.; Ashraf, A.; Ahmad, K.; Hou, H. Recent advances in chitosan-based smart hydrogel for drug delivery systems. Int. J. Biol. Macromol. 2024, 280, 135803. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Advancements in Biopolymer and Metal Nanoparticle-Based Materials in Diabetic Wound Healing Management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Mohsin, F.; Javaid, S.; Tariq, M.; Mustafa, M. Molecular Immunological Mechanisms of Impaired Wound Healing in Diabetic Foot Ulcers (DFU), Current Therapeutic Strategies and Future Directions. Int. Immunopharmacol. 2024, 139, 112713. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The Role of Extracellular Matrix in the Pathophysiology of Diabetic Wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of Advanced Glycation End Products (AGEs) and Oxidative Stress in Vascular Complications in Diabetes. Biochim. Biophys. Acta—Gen. Subj. 2012, 1820, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, C.; An, J.; Liu, J.; Wang, Y.; Cai, Y.; Krishna Janapati, Y.; Agwu, E. Mechanisms of Microbial Infection and Wound Healing in Diabetic Foot Ulcer: Pathogenicity in the Inflammatory-Proliferative Phase, Chronicity, and Treatment Strategies. Front. Endocrinol. 2025, 16, 1657928. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.S.; Singh, M.; Vinayagam, R.; Shukla, P. Therapies and Delivery Systems for Diabetic Wound Care: Current Insights and Future Directions. Front. Pharmacol. 2025, 16, 1628252. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Bio-Inspired Synthesis of Chitosan/Copper Oxide Nanocomposite Using Rutin and Their Anti-Proliferative Activity in Human Lung Cancer Cells. Int. J. Biol. Macromol. 2019, 141, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Preethi, S.; Abarna, K.; Nithyasri, M.; Kishore, P.; Deepika, K.; Ranjithkumar, R.; Bhuvaneshwari, V.; Bharathi, D. Synthesis and Characterization of Chitosan/Zinc Oxide Nanocomposite for Antibacterial Activity onto Cotton Fabrics and Dye Degradation Applications. Int. J. Biol. Macromol. 2020, 164, 2779–2787. [Google Scholar] [CrossRef]
- Kamaci, M.; Kaya, I. Chitosan Based Hybrid Hydrogels for Drug Delivery: Preparation, Biodegradation, Thermal, and Mechanical Properties. Polym. Adv. Technol. 2023, 34, 779–788. [Google Scholar] [CrossRef]
- Gaweł, M.; Domalik-Pyzik, P.; Douglas, T.E.L.; Reczyńska-Kolman, K.; Pamuła, E.; Pielichowska, K. The Effect of Chitosan on Physicochemical Properties of Whey Protein Isolate Scaffolds for Tissue Engineering Applications. Polymers 2023, 15, 3867. [Google Scholar] [CrossRef]
- Saputra, H.A. Andreas Chitosan and Its Biomedical Applications: A Review. Next Mater. 2025, 9, 101270. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A. A Review of Chitosan-Based Materials for Biomedical, Food, and Water Treatment Applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef]
- Santoso, J.; Adiputra, K.C.; Soerdirga, L.C.; Tarman, K. Effect of Acetic Acid Hydrolysis on the Characteristics of Water Soluble Chitosan. IOP Conf. Ser. Earth Environ. Sci. 2020, 414, 012021. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and Challenges in the Use of Chitosan and Its Derivatives in Biomedical Fields: A Review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, W.; Zhang, J.; Li, Q.; Guo, Z. Water-Soluble Amino Functionalized Chitosan: Preparation, Characterization, Antioxidant and Antibacterial Activities. Int. J. Biol. Macromol. 2022, 217, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiao, D.; Zhao, S.; Liu, P.; Xie, F.; Zhang, B. Biofunctional Chitosan–Biopolymer Composites for Biomedical Applications. Mater. Sci. Eng. R Rep. 2024, 159, 100775. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Grenha, A.; Remuñán-López, C. Chitosan and Its Derivatives as Nanocarriers for SiRNA Delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 29–42. [Google Scholar] [CrossRef]
- Bharathi, D.; Thiruvengadam Nandagopal, J.G.; Lee, J.; Ranjithkumar, R. Facile Synthesis and Characterization of Chitosan Functionalized Silver Nanoparticles for Antibacterial and Anti-Lung Cancer Applications. Polymers 2023, 15, 2700. [Google Scholar] [CrossRef]
- Da Silva, J.; Leal, E.C.; Gomes, A.; Gomes, P.; Calheiros, D.; Gonçalves, T.; Carvalho, E.; Silva, E.A. Alginate-Based Hydrogels for Sustained Antimicrobial Peptide Delivery to Enhance Wound Healing in Diabetes. Biomater. Adv. 2025, 175, 214337. [Google Scholar] [CrossRef]
- He, C.; Bi, S.; Zhang, R.; Chen, C.; Liu, R.; Zhao, X.; Gu, J.; Yan, B. A Hyaluronic Acid Hydrogel as a Mild Photothermal Antibacterial, Antioxidant, and Nitric Oxide Release Platform for Diabetic Wound Healing. J. Control. Release 2024, 370, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Q.; Wang, Q.; Zhang, S.; Liu, J.; Yang, L.; Ma, W.; Li, W.; Tan, P.; Yang, G. Gelatin-Based Adaptive Injectable Nanocomposite Hydrogel for Closure of Irregular Wounds and Immunoregulation in Diabetic Wound Healing. Int. J. Biol. Macromol. 2025, 315, 144313. [Google Scholar] [CrossRef]
- Suliman, M.; Alissa, M.; Alghamdi, A. Collagen-Based Hydrogel Encapsulated with SDF-1α Microspheres Accelerate Diabetic Wound Healing in Rats. Tissue Cell 2025, 95, 102877. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, S.; Wu, Z.; Li, Q.; Ren, S.; Chen, J.; Xu, X.; Wang, C.; Lu, C.; Yang, X.; et al. ADSC-Exo@MMP-PEG Smart Hydrogel Promotes Diabetic Wound Healing by Optimizing Cellular Functions and Relieving Oxidative Stress. Mater. Today Bio 2022, 16, 100365. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.; Mei, J.; Shao, M.; Zhang, J.; Hu, H.; Wu, H. A Glucose-Responsive Hydrogel Platform Based on Poly(Vinyl Alcohol) for Enhanced Diabetic Wound Healing. ACS Appl. Polym. Mater. 2024, 6, 6852–6863. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Gu, M.; Zhou, J.; He, L.; Wan, J.; Liu, Y.; Wang, S.; Xu, J. Carboxymethyl Chitosan Hydrogel Loaded with the Antimicrobial Peptide FK-13 Promotes Healing of Diabetic Skin Wounds by Inhibiting Infection and Biofilm Formation. ACS Appl. Polym. Mater. 2025, 7, 11514–11526. [Google Scholar] [CrossRef]
- Li, Z.; Fan, X.; Luo, Z.; Loh, X.J.; Ma, Y.; Ye, E.; Wu, Y.L.; He, C.; Li, Z. Nanoenzyme-Chitosan Hydrogel Complex with Cascade Catalytic and Self-Reinforced Antibacterial Performance for Accelerated Healing of Diabetic Wounds. Nanoscale 2022, 14, 14970–14983. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Y.; Huang, C.; Javeed, A.; Wang, Y.; Han, B.; Jiang, G. A Chitosan-Based Hydrogel Loaded with Fenofibrate for Diabetic Wound Healing. Biomater. Sci. 2024, 12, 4682–4694. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.S.; Hu, X.H.; Chu, L.Q. Recent Advances in Antimicrobial Hydrogels Containing Metal Ions and Metals/Metal Oxide Nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Naveedunissa, S.; Meenalotchani, R.; Manisha, M.; Ankul Singh, S.; Nirenjen, S.; Anitha, K.; Harikrishnan, N.; Prajapati, B.G. Advances in Chitosan Based Nanocarriers for Targetted Wound Healing Therapies: A Review. Carbohydr. Polym. Technol. Appl. 2025, 11, 100891. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Wang, Y.; Chen, W.; Zhu, Z.; Wang, S. Hydrogels and Hydrogel-Based Drug Delivery Systems for Promoting Refractory Wound Healing: Applications and Prospects. Int. J. Biol. Macromol. 2025, 285, 138098. [Google Scholar] [CrossRef]
- Falbo, F.; Spizzirri, U.G.; Restuccia, D.; Aiello, F. Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing. Pharmaceutics 2023, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, X.; Hu, H.; Xu, F.-J. Stimulus-Responsive Polysaccharide-Based Hydrogels: From Design to Biomedical Applications. Precis. Med. Eng. 2024, 1, 100001. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Q.; Qin, L. Innovative Applications of Multidimensional Engineered Hydrogels in Wound Healing. J. Adv. Res. 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, J.; Guo, Z.; Liu, W. Hydrogels: A Review on Their Versatile Applications for Efficient and Stable Oil-Water Separation. J. Mater. Chem. A 2025, 13, 6919–6953. [Google Scholar] [CrossRef]
- León-Campos, M.I.; Mendoza, J.J.; Aguayo-Morales, H.; Cobos-Puc, L.E.; Cabrera-Munguía, D.A.; Claudio-Rizo, J.A. The Biological Applications of IPN Hydrogels. ADMET DMPK 2024, 12, 581–621. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering Multifunctional Dynamic Hydrogel for Biomedical and Tissue Regenerative Applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; He, C.; Kang, Y.; Zhou, J. Ionically Crosslinked Chitosan/Poly(Acrylic Acid) Hydrogels with High Strength, Toughness and Antifreezing Capability. Carbohydr. Polym. 2020, 242, 116420. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Dong, J.; Li, D.; Dong, W.; Li, H.; Zhou, Y.; Liu, Q.; Deng, B. Mussel-Inspired Multifunctional Hydrogels with Adhesive, Self-Healing, Antioxidative, and Antibacterial Activity for Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 16515–16525. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials Diversity of Hydrogel: Synthesis, Polymerization Process and Soil Conditioning Properties in Agricultural Field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Yan, K.; Wan, Y.; Yang, C.; Chen, Y.; Wei, W.; Li, X.; Wang, D. Rational Programming of Polysaccharide-Based Double Network Hydrogel with Heterogeneous Architecture and Multifunction via Electrical Signal/Temperature Triggered Sequential Self-Assembly. Compos. Part B Eng. 2021, 226, 109343. [Google Scholar] [CrossRef]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Lee, D.H.; Han, S.S. Effect of Crosslinking Functionality on Microstructure, Mechanical Properties, and in Vitro Cytocompatibility of Cellulose Nanocrystals Reinforced Poly (Vinyl Alcohol)/Sodium Alginate Hybrid Scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef]
- Kolipaka, T.; Pandey, G.; Abraham, N.; Srinivasarao, D.A.; Raghuvanshi, R.S.; Rajinikanth, P.S.; Tickoo, V.; Srivastava, S. Stimuli-Responsive Polysaccharide-Based Smart Hydrogels for Diabetic Wound Healing: Design Aspects, Preparation Methods and Regulatory Perspectives. Carbohydr. Polym. 2024, 324, 121537. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Staropoli, M.; Appavou, M.S.; Wyss, H.M.; Pyckhout-Hintzen, W.; Sijbesma, R.P. Tough Supramolecular Hydrogel Based on Strong Hydrophobic Interactions in a Multiblock Segmented Copolymer. Macromolecules 2017, 50, 3333–3346. [Google Scholar] [CrossRef] [PubMed]
- Fredrick, R.; Podder, A.; Viswanathan, A.; Bhuniya, S. Synthesis and Characterization of Polysaccharide Hydrogel Based on Hydrophobic Interactions. J. Appl. Polym. Sci. 2019, 136, 47665. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Wang, Y.; Wu, H.; Ji, N.; Dai, L.; Li, Y.; Xiong, L.; Shi, R.; Sun, Q. High-Strength Physically Multi-Cross-Linked Chitosan Hydrogels and Aerogels for Removing Heavy-Metal Ions. J. Agric. Food Chem. 2019, 67, 13648–13657. [Google Scholar] [CrossRef]
- Bercea, M. Recent Advances in Poly(Vinyl Alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, L.; Yan, L.; Tang, L. Recent Advances in Polysaccharide-Based Physical Hydrogels and Their Potential Applications for Biomedical and Wastewater Treatment. Macromol. Biosci. 2022, 22, e2200153. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef] [PubMed]
- Mantooth, S.M.; Munoz-Robles, B.G.; Webber, M.J. Dynamic Hydrogels from Host–Guest Supramolecular Interactions. Macromol. Biosci. 2019, 19, e1800281. [Google Scholar] [CrossRef]
- Sehgal, V.; Pandey, S.P.; Singh, P.K. Prospects of Charged Cyclodextrins in Biomedical Applications. Carbohydr. Polym. 2024, 323, 121348. [Google Scholar] [CrossRef]
- Xue, W.; Zhao, R.; Liu, T.; Ran, X.; Liu, R.; Lu, T.; Wei, R.; Du, G.; Li, J.; Yang, L. Innovative Bio-Based Bamboo Adhesive: Performance Breakthrough Through the Host-Guest Interaction of Cyclodextrin and Adamantane. Colloids Surf. A Physicochem. Eng. Asp. 2025, 716, 136765. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, L.Y.; Li, Y.K.; Lee, Y.M. Dual Thermo- and pH-Sensitive Poly(N-Isopropylacrylamide-Co-Acrylic Acid) Hydrogels with Rapid Response Behaviors. Polymer 2007, 48, 1718–1728. [Google Scholar] [CrossRef]
- Elkhoury, K.; Zuazola, J.; Vijayavenkataraman, S. Bioprinting the Future Using Light: A Review on Photocrosslinking Reactions, Photoreactive Groups, and Photoinitiators. SLAS Technol. 2023, 28, 142–151. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural Polymers and the Hydrogels Prepared from Them; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128164211. [Google Scholar]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.L.; Le Thi, P.; Hoang Thi, T.T.; Park, K.D. Novel Enzymatically Crosslinked Chitosan Hydrogels with Free-Radical-Scavenging Property and Promoted Cellular Behaviors under Hyperglycemia. Prog. Nat. Sci. Mater. Int. 2020, 30, 661–668. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bode, F.; Drake, A.F.; Goldoni, S.; Stevens, M.M.; Dreiss, C.A. Enzymatically Cross-Linked Gelatin/Chitosan Hydrogels: Tuning Gel Properties and Cellular Response. Macromol. Biosci. 2014, 14, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Karimi, A.R.; Hadizadeh, M. Multifunctional Riboflavin/Chitosan-Based Dianhydride Crosslinked Hydrogels: Photodynamic Therapy, Antioxidant, and Antibacterial Properties. Next Res. 2025, 2, 100139. [Google Scholar] [CrossRef]
- Hu, J.; Hou, Y.; Park, H.; Choi, B.; Hou, S.; Chung, A.; Lee, M. Visible Light Crosslinkable Chitosan Hydrogels for Tissue Engineering. Acta Biomater. 2012, 8, 1730–1738. [Google Scholar] [CrossRef]
- Ma, H.; Peng, Y.; Zhang, S.; Zhang, Y.; Min, P. Effects and Progress of Photo-Crosslinking Hydrogels in Wound Healing Improvement. Gels 2022, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bratlie, K.M. Click Chemistry and Material Selection for in Situ Fabrication of Hydrogels in Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2018, 4, 2276–2291. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” Chemistry in Polymeric Scaffolds: Bioactive Materials for Tissue Engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Yigit, S.; Sanyal, R.; Sanyal, A. Fabrication and Functionalization of Hydrogels through “Click” Chemistry. Chem.—Asian J. 2011, 6, 2648–2659. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, T.; Gravesande, J.; Baysah, C.; Song, X.; Xing, J. Injectable and Self-Healing Polysaccharide-Based Hydrogel for PH-Responsive Drug Release. Int. J. Biol. Macromol. 2019, 123, 140–148. [Google Scholar] [CrossRef]
- McKay, C.S.; Finn, M.G. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef]
- Srivastava, N.; Choudhury, A.R. Stimuli-Responsive Polysaccharide-Based Smart Hydrogels and Their Emerging Applications. Ind. Eng. Chem. Res. 2023, 62, 841–866. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Jiang, Y.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. PH-Responsive UV Crosslinkable Chitosan Hydrogel via “Thiol-Ene” Click Chemistry for Active Modulating Opposite Drug Release Behaviors. Carbohydr. Polym. 2021, 251, 117101. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Luo, L.; Ding, Q.; Tang, S. Bio-Orthogonally Crosslinked Catechol–Chitosan Hydrogel for Effective Hemostasis and Wound Healing. Carbohydr. Polym. 2022, 281, 119039. [Google Scholar] [CrossRef]
- Webber, M.J.; Pashuck, E.T. (Macro)Molecular Self-Assembly for Hydrogel Drug Delivery. Adv. Drug Deliv. Rev. 2021, 172, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.; Guzmán, L.; Valdés, O.; Muñoz-Vera, M.; Morales-Quintana, L.; Castro, R.I. Development and Evaluation of Cross-Linked Alginate–Chitosan–Abscisic Acid Blend Gel. Polymers 2023, 15, 3217. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.J.; Faneca, H.; Lima, M.P.; Gil, M.H.; Figueiredo, M.M. In Situ Forming Chitosan Hydrogels Prepared via Ionic/Covalent Co-Cross-Linking. Biomacromolecules 2011, 12, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Bédouet, L.; Denys, E.; Courtois, B.; Courtois, J. Changes in Esterified Pectins during Development in the Flax Stems and Leaves. Carbohydr. Polym. 2006, 65, 165–173. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Z.; Wu, D.; Gan, W.; Zhu, S.; Li, W.; Tian, J.; Li, L.; Zhou, C.; Lu, L. Antibacterial Poly (Ethylene Glycol) Diacrylate/Chitosan Hydrogels Enhance Mechanical Adhesiveness and Promote Skin Regeneration. Carbohydr. Polym. 2019, 225, 115110. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and Chitosan in Selected Biomedical Applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Chen, X.G.; Park, H.J. Chemical Characteristics of O-Carboxymethyl Chitosans Related to the Preparation Conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Poly(Vinyl Alcohol) Hydrogels: Reinforcement of Radiation-crosslinked Networks by Crystallization. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 441–457. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly(Vinyl Alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2000; Volume 153, pp. 37–65. [Google Scholar]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-Based Host-Guest Supramolecular Hydrogel and Its Application in Biomedical Fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Zou, H.; Guo, W.; Yuan, W. Supramolecular Hydrogels from Inclusion Complexation of α-Cyclodextrin with Densely Grafted Chains in Micelles for Controlled Drug and Protein Release. J. Mater. Chem. B 2013, 1, 6235–6244. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Wang, X. Cyclodextrin-Based Supramolecular Hydrogels in Tissue Engineering and Regenerative Medicine. Molecules 2025, 30, 3225. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Guo, L.; Chang, X.; Yang, M. Thermo-Sensitive Chitosan Based Semi-IPN Hydrogels for High Loading and Sustained Release of Anionic Drugs. Int. J. Biol. Macromol. 2012, 50, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, Z.P.; Ping, C.S.; Bing, L.H.; Xiao, X.Z. Synthesis and Characterization of Thermo- and pH- Sensitive Hydrogels Based on Chitosan-Grafted N-Isopropylacrylamide via γ-Radiation. Radiat. Phys. Chem. 2005, 74, 26–30. [Google Scholar] [CrossRef]
- Salamat, Q.; Moradi, R.; Nadizadeh, Z.; Kavehpour, P.; Soylak, M.; Asimov, A.; Zillur Rahman, M.; Kovářík, T.; Babuška, V.; Deshmukh, K. Chitosan Based Smart Injectable Hydrogels for Biomedical Applications: A Comprehensive Review. Bioact. Mater. 2026, 55, 703–753. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Wu, Z.; Cao, L. Photocrosslinked Methacrylated Natural Macromolecular Hydrogels for Tissue Engineering: A Review. Int. J. Biol. Macromol. 2023, 246, 125570. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Lee, M.Y.; Cha, J.M.; Min, K.H.; Lee, S.C.; Shin, S.Y.; Bae, H. Injectable Hydrogel Derived from Chitosan with Tunable Mechanical Properties via Hybrid-Crosslinking System. Carbohydr. Polym. 2021, 251, 117036. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Mao, J.; Xu, W.; Zhou, Y.; Xiao, P. Photocrosslinkable Chitosan Hydrogels and Their Biomedical Applications. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1862–1871. [Google Scholar] [CrossRef]
- Sojdeh, S.; Panjipour, A.; Yaghmour, A.; Arabpour, Z.; Djalilian, A.R. Click Chemistry-Based Hydrogels for Tissue Engineering. Gels 2025, 11, 724. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and Interactions in Chitosan Hydrogels Formed by Complexation or Aggregation for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Barroso, N.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L. Self-Healable Hyaluronic Acid/Chitosan Polyelectrolyte Complex Hydrogels and Multilayers. Eur. Polym. J. 2019, 120, 109268. [Google Scholar] [CrossRef]
- Nichifor, M. Role of Hydrophobic Associations in Self-Healing Hydrogels Based on Amphiphilic Polysaccharides. Polymers 2023, 15, 1065. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-Grafted Chitosan Micelles as a Nanocarrier System for Drug-SiRNA Co-Delivery to the Lung Cancer Cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Don, T.M.; Chiu, W.Y. Preparation of Environmental-Responsive Chitosan-Based Nanoparticles by Self-Assembly Method. Carbohydr. Polym. 2011, 84, 765–769. [Google Scholar] [CrossRef]
- Khattak, S.; Ullah, I.; Sohail, M.; Akbar, M.U.; Rauf, M.A.; Ullah, S.; Shen, J.; Xu, H. Endogenous/Exogenous Stimuli-responsive Smart Hydrogels for Diabetic Wound Healing. Aggregate 2025, 6, e688. [Google Scholar] [CrossRef]
- Ding, K.; Liao, M.; Wang, Y.; Lu, J.R. Advances in Composite Stimuli-Responsive Hydrogels for Wound Healing: Mechanisms and Applications. Gels 2025, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wen, Y.; Zhang, Z.; Zhu, J.; Song, X.; Phan, T.T.; Li, J. Recent Advances in Smart Hydrogels Derived from Polysaccharides and Their Applications for Wound Dressing and Healing. Biomaterials 2025, 318, 123134. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Applications of Chitosan-Based Hydrogels in Diabetic Wound Healing: A Review. Int. J. Biol. Macromol. 2025, 324, 147264. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Malviya, R.; Kaushik, N. Chitosan in Biomedicine: A Comprehensive Review of Recent Developments. Carbohydr. Polym. Technol. Appl. 2024, 8, 100551. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Wei, W.; Sun, L.; Yu, R.; Yang, E.; Wu, X.; Dai, H. Modular Design and Bonding Mechanism of Internal Boron-Nitrogen Coordinated Boronic Ester Hydrogels with Alkaline PH Responsiveness and Tunable Gelation PH. Chem. Mater. 2023, 35, 2408–2420. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Wang, C.; Zhang, Q.; Qu, X.; Liang, C.; Si, C.; Wang, L. Treatment of Periodontal Inflammation in Diabetic Rats with IL-1ra Thermosensitive Hydrogel. Int. J. Mol. Sci. 2022, 23, 13939. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.G.; Mandakhbayar, N.; Singh, R.K.; Lee, J.H.; Jin, G.; Kim, H.W. Multifunctional Dendrimer@nanoceria Engineered GelMA Hydrogel Accelerates Bone Regeneration through Orchestrated Cellular Responses. Mater. Today Bio 2023, 20, 100664. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.N.; Ju, X.J.; Pu, X.Q.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.Y. Injectable Temperature/Glucose Dual-Responsive Hydrogels for Controlled Release of Insulin. Ind. Eng. Chem. Res. 2021, 60, 8147–8158. [Google Scholar] [CrossRef]
- Zhao, Q.; Gu, M.; Ni, M.; Li, J.; Wu, T.; Zhu, S.; Zhou, Y.; Lu, Y.; Li, X.; Xu, H.; et al. ROS Responsive Hydrogel for Inhibition of MUC5AC against Allergic Rhinitis: A New Delivery Strategy for Ipratropium Bromide. Colloids Surf. B Biointerfaces 2024, 242, 114112. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-Crosslinked Mussel-Inspired Smart Hydrogels with Enhanced Antibacterial and Angiogenic Properties for Chronic Infected Diabetic Wound Treatment via PH-Responsive Quick Cargo Release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Li, F.; Li, B.; Zhang, M.; Li, W.; Zhuge, P.; Yao, J.; Zhang, Y.; Chen, S.; et al. Thermosensitive Hydrogel Integrated with Bimetallic Nano-Enzymes for Modulating the Microenvironment in Diabetic Wound Beds. Adv. Sci. 2025, 12, e2411575. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hu, F.; Chai, Z.; Zheng, C.; Zhang, W.; Pu, K.; Yang, Z.; Zhang, Y.; Ramrkrishna, S.; Wu, X.; et al. Multifunctional Hydrogel with Mild Photothermal Properties Enhances Diabetic Wound Repair by Targeting MRSA Energy Metabolism. J. Nanobiotechnol. 2025, 23, 380. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, B.B.; Wang, L.; Yang, L.; Chen, H.; Chen, W.; Qiao, H.; Qian, H. A Glucose-Responsive Nitric Oxide Release Hydrogel for Infected Diabetic Wounds Treatment. J. Control. Release 2023, 359, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, H.; Zhou, Z.; Liu, C.; Cai, C.; Li, J.; Yu, X.; Zhang, J.; Liu, Y.; Wang, N. Kill Two Birds with One Stone: Dual-Metal MOF-Nanozyme-Decorated Hydrogels with ROS-Scavenging, Oxygen-Generating, and Antibacterial Abilities for Accelerating Infected Diabetic Wound Healing. Small 2024, 20, e2403679. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Miao, C.; Song, M.; Cao, Z. Quaternized Chitosan/Salvianolic Acid B Multifunctional Hydrogel with ROS/Glucose Dual Responsive Properties for Diabetic Wound Healing. Carbohydr. Polym. 2025, 367, 123995. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, X.; Chen, Y.; Chang, H.; Lu, D.; Pei, D.; Geng, Z.; Zeng, Z.; Guo, C.; Huang, J.; et al. Dual Glucose/ROS-Sensitive Injectable Adhesive Self-Healing Hydrogel with Photothermal Antibacterial Activity and Modulation of Macrophage Polarization for Infected Diabetic Wound Healing. ACS Mater. Lett. 2023, 5, 3142–3155. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of PH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Guo, J.; Cao, Y.; Wu, Q.Y.; Zhou, Y.M.; Cao, Y.H.; Cen, L.S. Implications of PH and Ionic Environment in Chronic Diabetic Wounds: An Overlooked Perspective. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2669–2686. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Remesh, R.; Kalathil, K.K.; Anie, Y. Responsive to Adaptive Supramolecular Hydrogels for Diabetic Wound Treatment. Supramol. Mater. 2025, 4, 100081. [Google Scholar] [CrossRef]
- Patroklou, G.; Triantafyllopoulou, E.; Goula, P.E.; Karali, V.; Chountoulesi, M.; Valsami, G.; Pispas, S.; Pippa, N. PH-Responsive Hydrogels: Recent Advances in Pharmaceutical Applications. Polymers 2025, 17, 1451. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ma, D.; Wu, D.; Qiu, X.; Yang, S.; Wang, Y.; Xiao, L.; Ji, X.; Zhang, W.; Han, S.; et al. A PH-Responsive, Injectable and Self-Healing Chitosan-Coumarin Hydrogel Based on Schiff Base and Hydrogen Bonds. Int. J. Biol. Macromol. 2024, 255, 128122. [Google Scholar] [CrossRef]
- Gao, K.; Xu, K. Advancements and Prospects of PH-Responsive Hydrogels in Biomedicine. Gels 2025, 11, 293. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, C.; Zhang, Z.; Yu, F.; Wang, Y.; Ding, J.; Zhao, Z.; Liu, Y. A PH Responsive Tannic Acid/Quaternized Carboxymethyl Chitosan/Oxidized Sodium Alginate Hydrogels for Accelerated Diabetic Wound Healing and Real-Time Monitoring. Int. J. Biol. Macromol. 2024, 264, 130741. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. PH-Responsive Hydrogel Loaded with Insulin as a Bioactive Dressing for Enhancing Diabetic Wound Healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Wendering, P.; Nikoloski, Z. Model-Driven Insights into the Effects of Temperature on Metabolism. Biotechnol. Adv. 2023, 67, 108203. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide-PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Chen, R.; Wang, P.; Xie, J.; Tang, Z.; Fu, J.; Ning, Y.; Zhong, Q.; Wang, D.; Lei, M.; Mai, H.; et al. A Multifunctional Injectable, Self-Healing, and Adhesive Hydrogel-Based Wound Dressing Stimulated Diabetic Wound Healing with Combined Reactive Oxygen Species Scavenging, Hyperglycemia Reducing, and Bacteria-Killing Abilities. J. Nanobiotechnol. 2024, 22, 444. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Park, S.J.; Lee, Y.W.; Yang, Y.J. LCST/UCST Behavior of Polysaccharides for Hydrogel Fabrication. RSC Adv. 2024, 14, 35754–35768. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, J.; Sun, J.; Jiang, Y.; Zheng, W.; Hu, W.; Qian, H. Recent Advances on Thermosensitive Hydrogels-Mediated Precision Therapy. Asian J. Pharm. Sci. 2024, 19, 100911. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Chen, Y.; Li, N.; Liao, X.; Heng, Y.; Guo, Y.; Hu, K. A Comprehensive Review of Thermosensitive Hydrogels: Mechanism, Optimization Strategies, and Applications. Gels 2025, 11, 544. [Google Scholar] [CrossRef]
- Kang, W.; Liang, J.; Liu, T.; Long, H.; Huang, L.; Shi, Q.; Zhang, J.; Deng, S.; Tan, S. Preparation of Silane-Dispersed Graphene Crosslinked Vinyl Carboxymethyl Chitosan Temperature-Responsive Hydrogel with Antibacterial Properties. Int. J. Biol. Macromol. 2022, 200, 99–109. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Huang, S.; Guo, B. Chitosan-Based Self-Healing Hydrogel Dressing for Wound Healing. Adv. Colloid Interface Sci. 2024, 332, 103267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, H.; Ma, Y.; Lu, T.J.; Xu, F.; Genin, G.M.; Lin, M. Spatiotemporally Controlled Photoresponsive Hydrogels: Design and Predictive Modeling from Processing through Application. Adv. Funct. Mater. 2020, 30, 2000639. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.N.; Kumar, P.; Faísca, P.; Ferreira, H.A.; Coelho, J.M.P.; Gaspar, M.M.; Reis, C.P. Gold Nanoparticle-Mediated Photothermal Therapy: Expanding the Frontiers of Cancer Treatment and Theragnostics. Biomed. Pharmacother. 2025, 190, 118399. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, Y.; Peng, H.; Zhou, J.; Zhang, Q.; Yan, J.; Liu, Y.; Guo, S.; Wu, X.; Li, B. Carbon Dots as a Novel Photosensitizer for Photodynamic Therapy of Cancer and Bacterial Infectious Diseases: Recent Advances. J. Nanobiotechnol. 2024, 22, 210. [Google Scholar] [CrossRef]
- Yang, Y.; Long, K.; Chu, Y.; Lu, H.; Wang, W.; Zhan, C. Photoresponsive Drug Delivery Systems: Challenges and Progress. Adv. Funct. Mater. 2024, 34, 2402975. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, G.; Li, L.; Piao, Z.; Wang, J.; Chen, R.; Hao, Z.; Zhang, Z.; Li, Z.; Huang, Y.; et al. Recent Advances in Smart Responsive Hydrogel Microspheres for Tissue Regeneration: Preparation, Characteristics and Applications. Mater. Horiz. 2025, 12, 8943–8988. [Google Scholar] [CrossRef] [PubMed]
- Jonidi Shariatzadeh, F.; Currie, S.; Logsetty, S.; Spiwak, R.; Liu, S. Enhancing Wound Healing and Minimizing Scarring: A Comprehensive Review of Nanofiber Technology in Wound Dressings; Elsevier Ltd.: Amsterdam, The Netherlands, 2025; Volume 147, ISBN 0000000303019. [Google Scholar]
- Xu, C.; Chen, Y.; Lin, C.; Xiao, J.A.; Li, P.; Su, W. NIR Photo-Responsive Injectable Chitosan/Hyaluronic Acid Hydrogels with Controlled NO Release for the Treatment of MRSA Infections. Int. J. Biol. Macromol. 2025, 300, 140304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, K.; Huang, K.; Wei, W.; Huang, Y.; Dai, H. Photothermal Antibacterial MoS2 Composited Chitosan Hydrogel for Infectious Wound Healing. Biomater. Adv. 2024, 156, 213701. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, Y.; Cai, H.; You, D.; Wang, Y.; Wu, S.; Wang, Y.; Guo, W.; Qu, W. Hydrogels Containing Chitosan-Modified Gold Nanoparticles Show Significant Efficacy in Healing Diabetic Wounds Infected with Antibiotic-Resistant Bacteria. Int. J. Nanomed. 2024, 19, 1539–1556. [Google Scholar] [CrossRef]
- Ma, P.; Da, J.; Zhao, G.; Suo, F.; Li, Y.; Zhou, X.; Li, Y.; Han, Y.; Zou, M.; Dou, X. Injectable Light-Responsive Hydrogel Dressing Promotes Diabetic Wound Healing by Enhancing Wound Angiogenesis and Inhibiting Inflammation. Polymers 2025, 17, 607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, X.; Shen, Z.; Zhang, R.; Zhang, G.; Yu, B.; Li, Y.; Xu, F.J. Glucose-Responsive Hydrogel with Adaptive Insulin Release to Modulate Hyperglycemic Microenvironment and Promote Wound Healing. Biomaterials 2026, 326, 123641. [Google Scholar] [CrossRef] [PubMed]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, Y.; Xu, K. Hydrogel-Based Treatment of Diabetic Wounds: From Smart Responsive to Smart Monitoring. Gels 2025, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, Z.; Guan, L.; Liu, A.; Li, L.; Nešić, M.D.; Yang, B.; Qu, W.; Lin, Q. Glucose-Responsive Hydrogel Optimizing Fenton Reaction to Eradicate Multidrug-Resistant Bacteria for Infected Diabetic Wound Healing. Chem. Eng. J. 2024, 487, 150545. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, K.; Xiao, C.; Chen, X. Guanosine-Driven Hyaluronic Acid-Based Supramolecular Hydrogels with Peroxidase-like Activity for Chronic Diabetic Wound Treatment. Acta Biomater. 2023, 172, 206–217. [Google Scholar] [CrossRef]
- Morariu, S. Advances in the Design of Phenylboronic Acid-Based Glucose-Sensitive Hydrogels. Polymers 2023, 15, 582. [Google Scholar] [CrossRef]
- Shen, D.; Yu, H.; Wang, L.; Chen, X.; Feng, J.; Li, C.; Xiong, W.; Zhang, Q. Glucose-Responsive Hydrogel-Based Microneedles Containing Phenylborate Ester Bonds and N-Isopropylacrylamide Moieties and Their Transdermal Drug Delivery Properties. Eur. Polym. J. 2021, 148, 110348. [Google Scholar] [CrossRef]
- Maity, B.; Moorthy, H.; Govindaraju, T. Glucose-Responsive Self-Regulated Injectable Silk Fibroin Hydrogel for Controlled Insulin Delivery. ACS Appl. Mater. Interfaces 2023, 15, 49953–49963. [Google Scholar] [CrossRef]
- Kulkarni, N.; Jadhav, G.S.; Kombe, P.R.; Dewangan, B.; Apparao, C.V.; Patra, S.; Sakla, A.P.; Borah, S.; Sahu, B. ROS-Responsive Nucleobase Conjugated Chitosan: Synthesis and Evaluations for Biomedical Applications. Carbohydr. Polym. 2025, 356, 123353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; He, C.; Dong, W.; Yang, X.; Kong, Q.; Yan, B.; He, J. Quaternized Chitosan-Based Biomimetic Nanozyme Hydrogels with ROS Scavenging, Oxygen Generating, and Antibacterial Capabilities for Diabetic Wound Repair. Carbohydr. Polym. 2025, 348, 122865. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Z.; Zhang, M.; Li, S.; Sun, M.; Song, Z. Hollow Manganese Dioxide-Chitosan Hydrogel for the Treatment of Atopic Dermatitis Through Inflammation-Suppression and ROS Scavenging. J. Nanobiotechnol. 2023, 21, 432. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Chen, J.; Zhang, S.; Wang, B.; Wang, J. Immunomodulatory Hydrogel for Electrostatically Capturing Pro-Inflammatory Factors and Chemically Scavenging Reactive Oxygen Species in Chronic Diabetic Wound Remodeling. Adv. Healthc. Mater. 2024, 13, e2402080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Wu, P.; Wu, S.; Qin, J.; Zhang, H.; Sun, G. pH- and Glucose-Responsive Antioxidant Hydrogel Promotes Diabetic Wound Healing. Biomater. Adv. 2025, 169, 214177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent Advances in Responsive Hydrogels for Diabetic Wound Healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef]
- Yu, C.; Jiang, Z.; Li, G.; Cao, Y.; Zhang, Z.; Zhou, C.; Zhang, P.; Gu, X. Hydrogel Complex of Natural Actives for Diabetic Wound Treatment. Mater. Des. 2025, 257, 114515. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, J.; Chen, F.; Chen, X.; Lee, C.J.; Liang, H.; Zhao, L.; Tan, H. A Multi-Responsive Hydrogel Combined with Mild Heat Stimulation Promotes Diabetic Wound Healing by Regulating Inflammatory and Enhancing Angiogenesis. Adv. Sci. 2024, 11, e2408783. [Google Scholar] [CrossRef]
- Xu, J.; Chang, L.; Xiong, Y.; Peng, Q. Chitosan-Based Hydrogels as Antibacterial/Antioxidant/Anti-Inflammation Multifunctional Dressings for Chronic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2401490. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).