Concordance of Skin Prick Test, Intradermal Testing, Serum IgE Levels and Symptoms in Patients with Allergic Rhinitis
Abstract
:1. Introduction
Objective
2. Materials and Methods
Statistics
3. Results
3.1. Epidemiology
3.2. Spring Bloomer

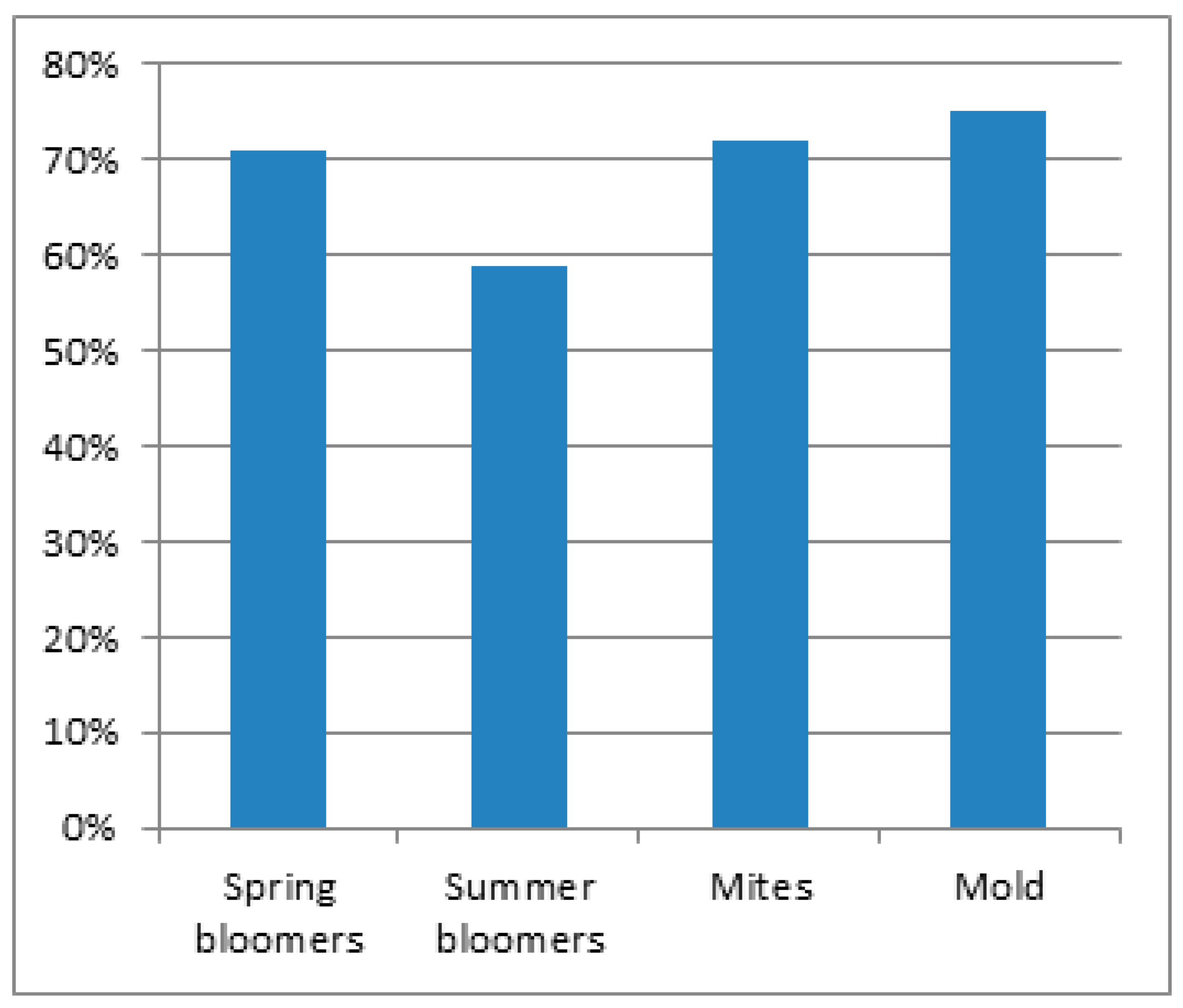


3.3. Summer Bloomer
3.4. Autumn Bloomer
3.5. Mites
3.6. Mold
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| AR | Allergic rhinitis |
| IgE | Immunoglobulin E |
| SPT | Skin prick test |
References
- Nathan, R.A.; Meltzer, E.O.; Derebery, J.; Campbell, U.B.; Stang, P.E.; Corrao, M.A.; Allen, G.; Stanford, R. The prevalence of nasal symptoms attributed to allergies in the United States: Findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008, 29, 600–608. [Google Scholar] [CrossRef]
- Juniper, E.F.; Guyatt, G.H.; Dolovich, J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: Development and testing of a questionnaire for clinical trials. J. Allergy Clin. Immunol. 1994, 93, 413–423. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. S86), 8–160. [Google Scholar] [CrossRef] [PubMed]
- Chinoy, B.; Yee, E.; Bahna, S.L. Skin testing versus radioallergosorbent testing for indoor allergens. Clin. Mol. Allergy 2005, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Stenius, B. Skin and provocation tests with Dermatophagoides pteronyssinus in allergic rhinitis. Comparison of prick and intracutaneous skin test methods and correlation with specific IgE. Acta Allergol. 1973, 28, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.B.; Wu, K.K. Total serum IgE, allergy skin testing, and the radioallergosorbent test for the diagnosis of allergy in asthmatic children. Ann. Allergy 1989, 62, 432–435. [Google Scholar] [PubMed]
- van der Zee, J.S.; de Groot, H.; van Swieten, P.; Jansen, H.M.; Aalberse, R.C. Discrepancies between the skin test and IgE antibody assays: Study of histamine release, complement activation in vitro, and occurrence of allergen-specific IgG. J. Allergy Clin. Immunol. 1988, 82, 270–281. [Google Scholar] [CrossRef]
- Agache, I.; Bilo, M.; Braunstahl, G.J.; Delgado, L.; Demoly, P.; Eigenmann, P.; Gevaert, P.; Gomes, E.; Hellings, P.; Horak, F.; et al. In vivo diagnosis of allergic diseases—Allergen provocation tests. Allergy 2015, 70, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Huss-Marp, J.; Darsow, U.; Brockow, K.; Pfab, F.; Weichenmeier, I.; Schöber, W.; Petersson, C.J.; Borres, M.P.; Ring, J.; Behrendt, H. Can immunoglobulin E-measurement replace challenge tests in allergic rhinoconjunctivits to grass pollen? Clin. Exp. Allergy 2011, 41, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Werninghaus, G. [A new questionaire in the diagnosis of allergic lung disease suitable for evaluation by computer (author’s transl)]. Prax Klin Pneumol. 1977, 31, 642–652. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Bjerg, A.; Ekerljung, L.; Eriksson, J.; Naslund, J.; Sjolander, S.; Ronmark, E.; Dahl, A.; Holmberg, K.; Wennergren, G.; Toren, K.; et al. Increase in pollen sensitization in Swedish adults and protective effect of keeping animals in childhood. Clin. Exp. Allergy 2016, 46, 1328–1336. [Google Scholar] [CrossRef]
- Arbes, S.J., Jr.; Gergen, P.J.; Elliott, L.; Zeldin, D.C. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2005, 116, 377–383. [Google Scholar] [CrossRef]
- Heinzerling, L.; Frew, A.J.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bresciani, M.; Carlsen, K.H.; van Cauwenberge, P.; Darsow, U.; Fokkens, W.J.; et al. Standard skin prick testing and sensitization to inhalant allergens across Europe—A survey from the GALEN network. Allergy 2005, 60, 1287–1300. [Google Scholar] [CrossRef]
- Craig, T.J.; King, T.S.; Lemanske, R.F.; Wechsler, M.E.; Icitovic, N.; Zimmerman, R.R.; Wasserman, S. Aeroallergen sensitization correlates with PC(20) and exhaled nitric oxide in subjects with mild-to-moderate asthma. J. Allergy Clin Immunol. 2008, 121, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Cirillo, I. Monosensitization and polysensitization in allergic rhinitis. Eur. J. Intern Med. 2011, 22, e75–e79. [Google Scholar] [CrossRef]
- Haftenberger, M.; Laussmann, D.; Ellert, U.; Kalcklosch, M.; Langen, U.; Schlaud, M.; Schmitz, R.; Thamm, M. [Prevalence of sensitisation to aeraoallergens and food allergens: Results of the German Health Interview and Examination Survey for Adults (DEGS1)]. Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Pastorello, E.A.; Incorvaia, C.; Pravettoni, V.; Marelli, A.; Farioli, L.; Ghezzi, M. Clinical evaluation of CAP System and RAST in the measurement of specific IgE. Allergy 1992, 47, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; Incorvaia, C.; Ortolani, C.; Bonini, S.; Canonica, G.W.; Romagnani, S.; Tursi, A.; Zanussi, C. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J. Allergy Clin. Immunol. 1995, 96 Pt 1, 580–587. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Nelson, H.S. Skin testing. Ann. Allergy Asthma Immunol. 2006, 96, S6–S12. [Google Scholar] [CrossRef]
- Molgaard, E.; Thomsen, S.F.; Lund, T.; Pedersen, L.; Nolte, H.; Backer, V. Differences between allergic and nonallergic rhinitis in a large sample of adolescents and adults. Allergy 2007, 62, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Zacharasiewicz, A.; Douwes, J.; Pearce, N. What proportion of rhinitis symptoms is attributable to atopy? J. Clin. Epidemiol. 2003, 56, 385–390. [Google Scholar] [CrossRef]
- Wuthrich, B.; Schindler, C.; Leuenberger, P.; Ackermann-Liebrich, U. Prevalence of atopy and pollinosis in the adult population of Switzerland (SAPALDIA study). Swiss Study on Air Pollution and Lung Diseases in Adults. Int. Arch. Allergy Immunol. 1995, 106, 149–156. [Google Scholar] [CrossRef]
- Cohn, J.R.; Padams, P.; Zwillenberg, J. Intradermal skin test results correlate with atopy. Ear Nose Throat J. 2011, 90, E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauchau, V.; Durham, S.R. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur. Respir J. 2004, 24, 758–764. [Google Scholar] [CrossRef]
- Ciprandi, G.; Klersy, C.; Cirillo, I.; Marseglia, G.L. Quality of life in allergic rhinitis: Relationship with clinical, immunological, and functional aspects. Clin. Exp. Allergy 2007, 37, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Devillier, P.; Bousquet, P.-J.; Grassin-Delyle, S.; Salvator, H.; Demoly, P.; Bousquet, J.; De Beaumont, O. Comparison of outcome measures in allergic rhinitis in children, adolescents and adults. Pediatr. Allergy Immunol. 2016, 27, 375–381. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Blaiss, M.; Naclerio, R.; Stoloff, S.; Derebery, M.; Nelson, H.; Boyle, J.; Wingertzahn, M. Burden of allergic rhinitis: Allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012, 33 (Suppl. S1), S113–S141. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Blaiss, M.; Derebery, M.; Mahr, T.; Gordon, B.; Sheth, K.; Simmons, A.; Wingertzahn, M.; Boyle, J. Burden of allergic rhinitis: Results from the Pediatric Allergies in America survey. J. Allergy Clin. Immunol. 2009, 124, S43–S70. [Google Scholar] [CrossRef]
- Roberts, G.; Ollert, M.; Aalberse, R.; Austin, M.; Custovic, A.; DunnGalvin, A.; A Eigenmann, P.; Fassio, F.; Grattan, C.; Hellings, P.; et al. A new framework for the interpretation of IgE sensitization tests. Allergy 2016, 71, 1540–1551. [Google Scholar] [CrossRef] [Green Version]
- Douladiris, N.; Savvatianos, S.; Roumpedaki, I.; Skevaki, C.; Mitsias, D.; Papadopoulos, N.G. A molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: Towards component-resolved management of allergic diseases. Int. Arch. Allergy Immunol. 2013, 162, 163–172. [Google Scholar] [CrossRef] [PubMed]
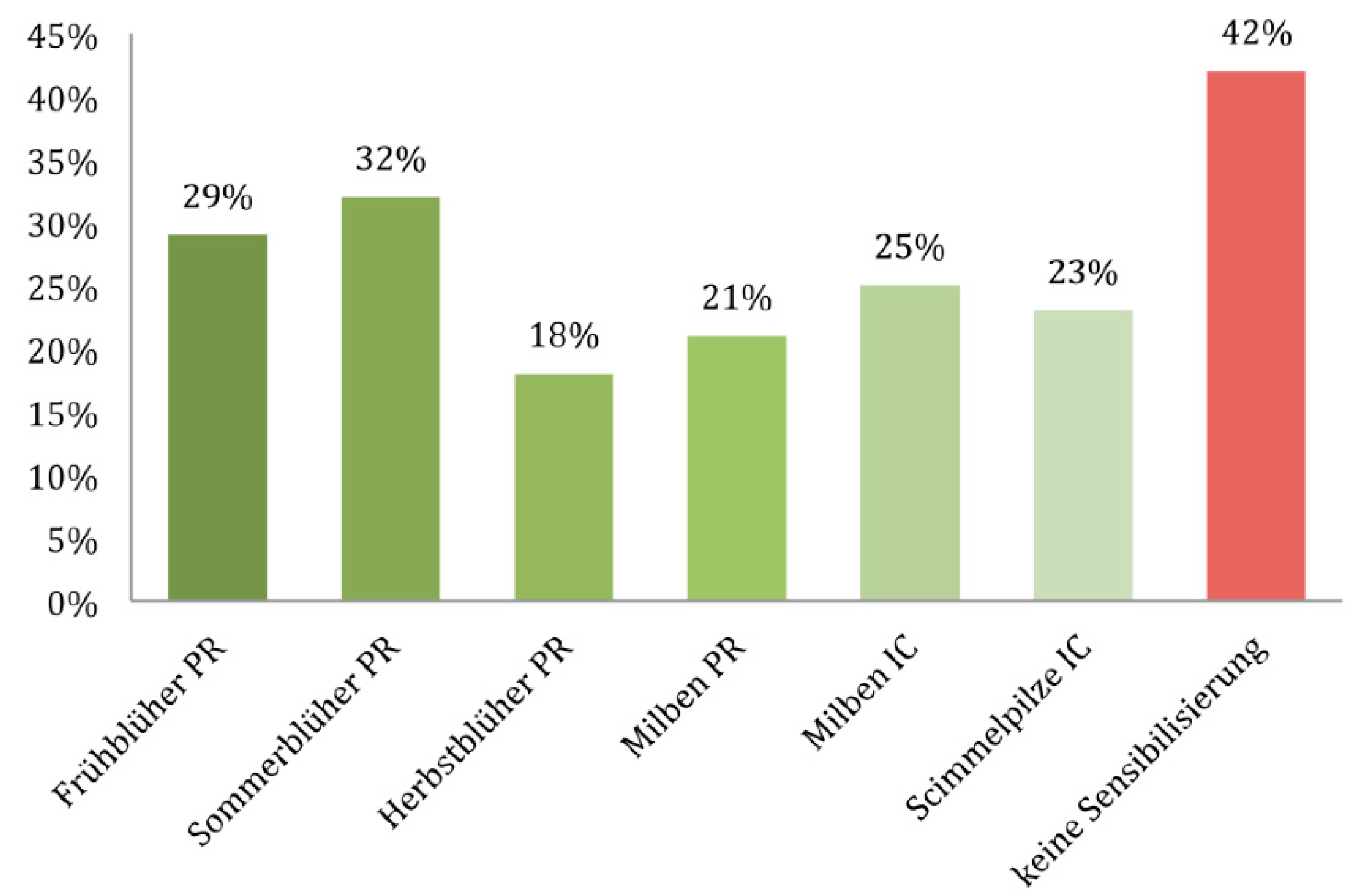

| Symptom | OR | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Value | Upper Value | |||
| Asthma | 1.635 | 1.370 | 1.952 | <0.001 |
| Cough | 1.106 | 0.937 | 1.306 | 0.234 |
| Rhinitis | 5.356 | 4.581 | 6.262 | <0.001 |
| Sneezing | 1.568 | 1.339 | 1.838 | <0.001 |
| Nasal obstruction | 0.877 | 0.761 | 1.012 | 0.072 |
| Eye symptoms | 2.410 | 2.070 | 2.806 | <0.001 |
| Urticaria | 1.718 | 1.317 | 2.240 | <0.001 |
| Itching | 1.944 | 1.644 | 2.298 | <0.001 |
| Atopic eczema | 1.922 | 1.407 | 2.624 | <0.001 |
| Gastrointestinal symptoms | 1.136 | 0.919 | 1.404 | 0.237 |
| Sense of smell | 1.626 | 1.408 | 1.877 | <0.001 |
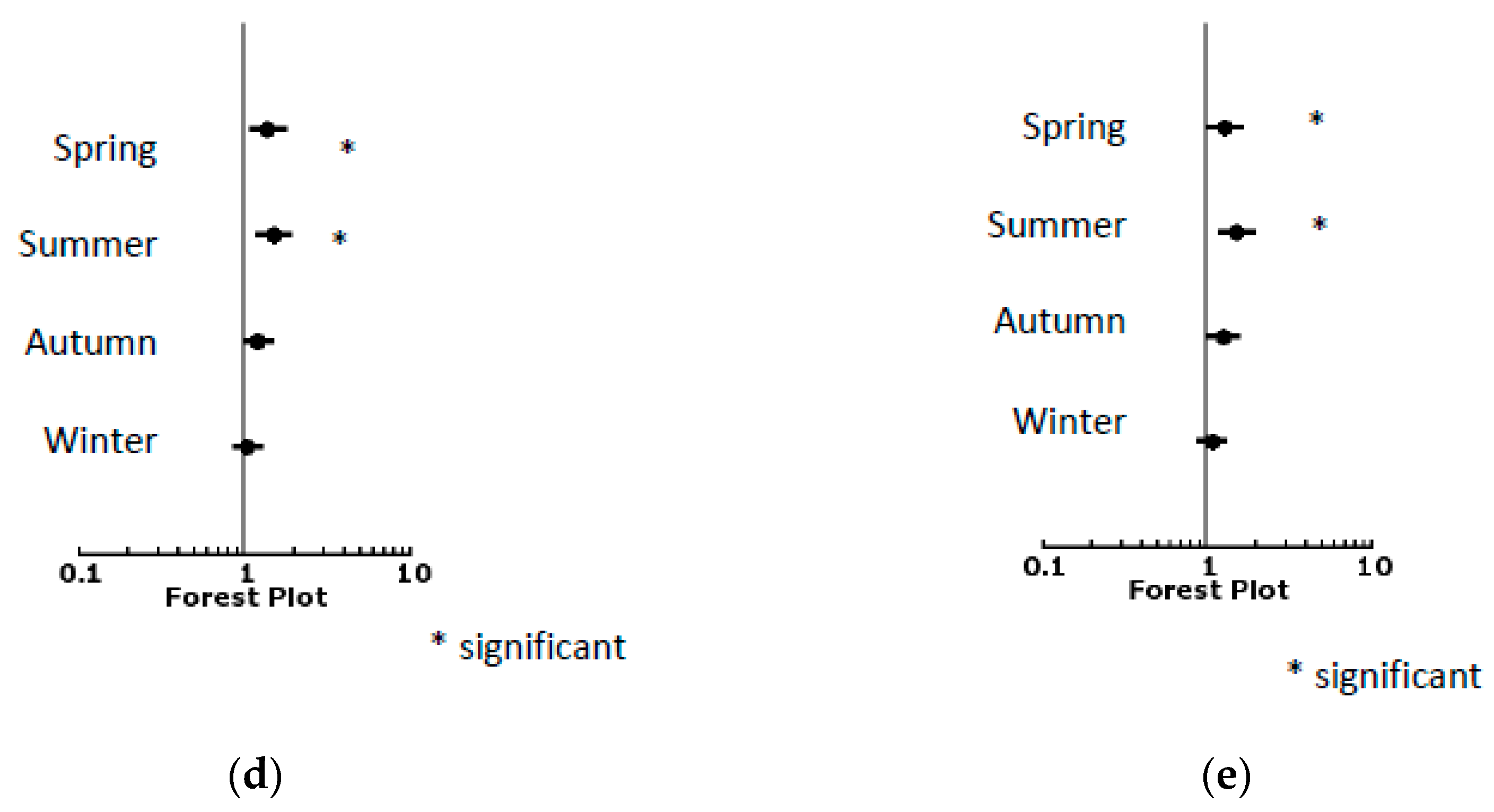
| Time of year | ||||
| Spring | 1.544 | 1.239 | 1.923 | <0.001 |
| Summer | 1.661 | 1.332 | 2.071 | <0.001 |
| Autumn | 0.792 | 0.656 | 0.956 | 0.015 |
| Winter | 0.659 | 0.549 | 0.791 | <0.001 |
| Symptom | OR | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Value | Upper Value | |||
| Asthma | 1.755 | 1.476 | 2.087 | <0.001 |
| Cough | 1.119 | 0.953 | 1.315 | 0.171 |
| Rhinitis | 5.053 | 4.330 | 5.897 | <0.001 |
| Sneezing | 1.545 | 1.323 | 1.804 | <0.001 |
| Nasal obstruction | 0.835 | 0.727 | 0.959 | 0.011 |
| Eye symptoms | 2.040 | 1.756 | 2.369 | <0.001 |
| Urticaria | 1.518 | 1.166 | 1.978 | 0.002 |
| Itching | 1.691 | 1.433 | 1.997 | <0.001 |
| Atopic eczema | 2.143 | 1.573 | 2.921 | <0.001 |
| Gastrointestinal symptoms | 0.986 | 0.799 | 1.217 | 0.895 |
| Sense of smell | 1.633 | 1.419 | 1.879 | <0.001 |
| Time of year | ||||
| Spring | 1.310 | 1.066 | 1.610 | 0.010 |
| Summer | 2.101 | 1.683 | 2.625 | <0.001 |
| Autumn | 1.058 | 0.876 | 1.278 | 0.557 |
| Winter | 0.746 | 0.624 | 0.893 | 0.001 |
| Symptom | OR | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Value | Upper Value | |||
| Asthma | 1.572 | 1.285 | 1.924 | <0.001 |
| Cough | 1.138 | 0.939 | 1.378 | 0.189 |
| Rhinitis | 3.583 | 3.018 | 4.253 | <0.001 |
| Sneezing | 1.455 | 1.212 | 1.747 | <0.001 |
| Nasal obstruction | 0.885 | 0.749 | 1.045 | 0.150 |
| Eye symptoms | 1.903 | 1.599 | 2.265 | <0.001 |
| Urticaria | 1.630 | 1.211 | 2.196 | 0.001 |
| Itching | 1.761 | 1.456 | 2.130 | <0.001 |
| Atopic eczema | 1.604 | 1.126 | 2.284 | 0.009 |
| Gastrointestinal symptoms | 0.910 | 0.703 | 1.179 | 0.476 |
| Sense of smell | 1.397 | 1.182 | 1.652 | <0.001 |
| Time of year | ||||
| Spring | 1.441 | 1.110 | 1.870 | 0.006 |
| Summer | 1.918 | 1.454 | 2.532 | <0.001 |
| Autumn | 0.977 | 0.780 | 1.225 | 0.843 |
| Winter | 0.794 | 0.640 | 0.983 | 0.034 |
| Symptom | OR | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Value | Upper Value | |||
| Asthma | 1.398 | 1.146 | 1.706 | 0.001 |
| Cough | 1.379 | 1.150 | 1.653 | 0.001 |
| Rhinitis | 2.170 | 1.833 | 2.570 | <0.001 |
| Sneezing | 1.328 | 1.110 | 1.590 | 0.002 |
| Nasal obstruction | 0.941 | 0.802 | 1.105 | 0.461 |
| Eye symptoms | 1.459 | 1.227 | 1.735 | <0.001 |
| Urticaria | 1.505 | 1.123 | 2.016 | 0.006 |
| Itching | 1.268 | 1.045 | 1.538 | 0.016 |
| Atopic eczema | 1.834 | 1.305 | 2.579 | <0.001 |
| Gastrointestinal symptoms | 0.899 | 0.700 | 1.155 | 0.405 |
| Sense of smell | 1.359 | 1.155 | 1.598 | <0.001 |
| Time of year | ||||
| Spring | 1.389 | 1.086 | 1.777 | 0.009 |
| Summer | 1.496 | 1.168 | 1.916 | 0.001 |
| Autumn | 1.211 | 0.968 | 1.515 | 0.094 |
| Winter | 1.039 | 0.840 | 1.286 | 0.724 |
| Symptom | OR | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Value | Upper Value | |||
| Asthma | 1.421 | 1.195 | 1.689 | <0.001 |
| Cough | 1.252 | 1.069 | 1.467 | 0.005 |
| Rhinitis | 1.568 | 1.351 | 1.821 | <0.001 |
| Sneezing | 0.939 | 0.801 | 1.099 | 0.432 |
| Nasal obstruction | 0.816 | 0.712 | 0.935 | 0.003 |
| Eye symptoms | 1.065 | 0.916 | 1.239 | 0.413 |
| Urticaria | 1.499 | 1.154 | 1.946 | 0.002 |
| Itching | 1.252 | 1.061 | 1.478 | 0.008 |
| Atopic eczema | 1.653 | 1.211 | 2.256 | 0.002 |
| Gastrointestinal symptoms | 1.099 | 0.895 | 1.350 | 0.367 |
| Sense of smell | 1.482 | 1.290 | 1.702 | <0.001 |
| Time of year | ||||
| Spring | 1.286 | 1.009 | 1.639 | 0.042 |
| Summer | 1.511 | 1.179 | 1.937 | 0.001 |
| Autumn | 1.248 | 0.996 | 1.563 | 0.054 |
| Winter | 1.070 | 0.864 | 1.325 | 0.536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huppertz, T.; Dahlem, M.; Weyer-Elberich, V.; Haxel, B.R. Concordance of Skin Prick Test, Intradermal Testing, Serum IgE Levels and Symptoms in Patients with Allergic Rhinitis. Allergies 2021, 1, 181-194. https://doi.org/10.3390/allergies1030017
Huppertz T, Dahlem M, Weyer-Elberich V, Haxel BR. Concordance of Skin Prick Test, Intradermal Testing, Serum IgE Levels and Symptoms in Patients with Allergic Rhinitis. Allergies. 2021; 1(3):181-194. https://doi.org/10.3390/allergies1030017
Chicago/Turabian StyleHuppertz, Tilman, Martha Dahlem, Veronika Weyer-Elberich, and Boris R. Haxel. 2021. "Concordance of Skin Prick Test, Intradermal Testing, Serum IgE Levels and Symptoms in Patients with Allergic Rhinitis" Allergies 1, no. 3: 181-194. https://doi.org/10.3390/allergies1030017
APA StyleHuppertz, T., Dahlem, M., Weyer-Elberich, V., & Haxel, B. R. (2021). Concordance of Skin Prick Test, Intradermal Testing, Serum IgE Levels and Symptoms in Patients with Allergic Rhinitis. Allergies, 1(3), 181-194. https://doi.org/10.3390/allergies1030017






