Abstract
Pharmaceutical packaging materials play a crucial role in ensuring the safety and efficacy of medications. This mini-review examines the properties of common packaging materials (glass, plastics, metals, and rubber) and their implications for drug safety. By analyzing 127 research articles from PubMed, Web of Science, and CNKI databases (2000–2025), we also discuss recent regulatory updates in China and highlight emerging technologies, including nanomaterials, sustainable packaging solutions, and intelligent packaging systems that present new opportunities for the pharmaceutical industry. Key findings include the following: (1) The physicochemical properties of packaging materials and potential microbial contamination risks during production significantly impact drug quality and safety, underscoring the need for enhanced research and regulatory oversight. (2) Each material exhibits distinct advantages and limitations: glass demonstrates superior chemical stability but may leach ions; plastics offer versatility but risk plasticizer migration; metals provide exceptional strength yet have limited applications; rubber ensures effective sealing but may release additives compromising drug quality. (3) The pharmaceutical packaging sector is evolving toward intelligent systems and sustainable solutions to address contemporary healthcare challenges. This review can aid pharmaceutical companies in selecting drug packaging and guide manufacturers in developing innovative packaging solutions.
1. Introduction
Pharmaceutical packaging is an indispensable component of drugs, consisting of a collection of packaging components made from various materials [1]. It plays a crucial role in the entire process from drug production to patient use, ensuring safety, adaptability, stability, functionality, protection, and convenience of drugs [2]. The various types of packaging materials, including glass, plastics, metals, and rubber, each have unique properties. Glass has good chemical stability and is generally used for packaging injectable drugs; plastics have good processing properties and are cost-effective, widely used in various pharmaceutical packaging; metal containers have high strength and are suitable for packaging aerosols and other pressurized products; rubber plays a key role in sealing applications such as bottle caps and stoppers [3]. During drug packaging, numerous key factors require careful consideration, with patient safety serving as the paramount principle [4,5]. Ensuring consistent drug efficacy and maintaining drug quality are core requirements. Strict supervision of packaging material components and production processes is necessary to prevent drug contamination and deterioration, effectively limiting microbial contamination, which plays a key role in maintaining drug safety and effectiveness [6,7,8].
Pharmaceutical packaging materials may have a significant impact on medication safety. From a physical performance perspective, if the barrier properties are poor, oxygen, moisture, and light can enter the packaging, causing the drug quality to deteriorate. For example, vitamin C is highly susceptible to oxidation, aspirin is prone to hydrolysis, and chlorpromazine is susceptible to photochemical reactions. If the mechanical performance is poor, such as insufficient strength, toughness, and elasticity of the packaging material, it may cause damage, leakage, or deterioration of drugs during production, transportation, and storage. From a chemical performance perspective, migration phenomena can cause additives or components from the packaging material to migrate into the drug, affecting its quality and safety. For example, plasticizers in PVC infusion bags can migrate, and glass packaging materials can release alkaline substances. Adsorption can cause insufficient drug content and changes in properties, affecting efficacy and stability. Microbial contamination risk is particularly evident in the production stage of packaging materials. Production environment, equipment, and process conditions, as well as the hygiene habits of operators, can all cause microbial contamination, posing a threat to drug quality and user health. With the steady progress of science and technology, the application of new materials in pharmaceutical packaging has attracted increasing attention. Nanomaterials, with their unique properties such as high specific surface area, excellent UV-blocking effect, and antibacterial effect, have opened up new development opportunities for pharmaceutical packaging [9]. Intelligent packaging materials, with the help of modern science and technology, have practical functions such as self-inspection of drug quality, anti-counterfeiting, and measurement, optimizing the safety and convenience of drug circulation and use. Green packaging materials, with their characteristics of environmental protection, safety, and biodegradability, have become a key direction for the sustainable development of the pharmaceutical packaging industry. In-depth research on the relationship between pharmaceutical packaging materials and medication safety is of great significance in improving drug quality, ensuring patient medication safety, and promoting the development of the pharmaceutical packaging industry [10].
In this mini-review, the key aspects of pharmaceutical packaging materials and their impact on medication safety were summarized, the recent regulatory and research progress of pharmaceutical packaging materials in China were discussed, and emerging materials that may offer new opportunities for the pharmaceutical industry were explored. With the aim to provide references for material selection, risk management, and technical innovation, the intended readers of this mini-review include researchers, regulators, and industry practitioners.
2. Methods
2.1. Literature Search and Screening
This study employed a systematic literature review method to conduct literature retrieval and screening. The search scope encompassed commonly used English and Chinese literature databases such as “PubMed”, “Web of Science”, and “China National Knowledge Infrastructure (CNKI)” from 2000 to 2025, utilizing keywords such as “pharmaceutical packaging” and “pharmaceutical packaging materials”. The literature screening process involved the following steps: first, deduplication; second, preliminary screening based on titles and abstracts; third, full-text evaluation. Inclusion criteria were experimental studies or systematic reviews, articles published in either Chinese or English, and studies directly related to pharmaceutical packaging materials. Exclusion criteria included non-research articles (e.g., editorials, news reports) and literature unrelated to pharmaceutical packaging materials. A total of 127 relevant articles were ultimately included for analysis.
2.2. Data Analysis and Quality Assessment
We systematically collated relevant research data, focusing on the performance characteristics and safety parameters of packaging materials. During analysis, we implemented scientifically rigorous assessment methodologies to ensure all data underwent dual independent verification. Professional analytical approaches were employed to cross-compare property differentials among materials, with comprehensive evaluation of practical applicability across packaging variants. All referenced data originated from standard specifications issued by authoritative institutions, thereby guaranteeing research reliability.
3. Classification of Pharmaceutical Packaging
The primary purpose of pharmaceutical packaging is to protect the product from external factors. For instance, it safeguards medications from physical shocks such as vibration and friction to prevent impact damage, while also shielding them from environmental variations to avoid contamination by biological pollutants like mold, yeast, and bacteria. Pharmaceutical packaging also provides consumers with critical information about the medication, including usage instructions, side effects, warnings, manufacturer details, and pricing. Additionally, companies often use bold colors, logos, and symbols on pharmaceutical packaging to stand out from competitors and promote their products, aiming to influence purchasing decisions [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. To achieve these objectives, pharmaceutical packaging is generally divided into external packing and immediate packaging [45,46,47,48].
3.1. External Packing
Pharmaceutical external packaging plays a crucial role throughout the entire process from drug production to actual use. During transportation, medications usually face diverse and complex environments with potential risks: collisions during loading/unloading, vibrations from vehicle movement, and environmental humidity. For example, injectable medications in glass vials could easily shatter during transit without the protection of boxes, leading to spillage, waste, and delayed treatment [45], while as a kind of external packaging, sturdy corrugated boxes provide cushioning effects, reducing external forces and preventing breakage, deformation, or damage caused by vibrations. Humidity is also a key factor affecting drug quality: hygroscopic medications, such as granules or powders, may clump or develop mold when exposed to humidity, reducing efficacy or even generating harmful substances that endanger patient safety. Cartons and boxes with moisture-resistant properties effectively block dampness, preventing moisture-induced degradation [46].
Maintaining the integrity of external packaging is important during manufacturing, transportation, and storage. Manufacturers must use high-quality, standardized materials to ensure cartons and boxes possess robust moisture and shock resistance. Transport personnel should handle packages with care to avoid sharp impacts. Storage facilities must monitor environmental conditions, regularly inspect packaging for damage, and address issues promptly. By prioritizing these measures, the protective functions of external packaging are fortified, ensuring effective medication use and safeguarding patient safety [47].
3.2. Immediate Packing
Immediate packing, such as ampoule bottles, vials, and medicinal pouches, is critically linked to drug quality and safety. Immediate packing materials form the “first line of defense” for pharmaceutical integrity. Ampoule bottles, typically made of hard borosilicate glass, offer excellent chemical stability and hermetic sealing to block air, moisture, and microorganisms, ensuring sterile storage. Vials often utilize borosilicate glass for its thermal shock resistance and chemical inertness, making them ideal for lyophilized drugs and injectables. Medicinal pouches are commonly fabricated from polymers like polyethylene or polypropylene, whose physicochemical properties (e.g., gas permeability, moisture resistance, and corrosion resistance) directly determine drug shelf life and stability.
Even minor material defects of immediate packing can significantly compromise drug quality. For instance, inadequate microbial barrier properties in pouches may allow pathogen infiltration during storage, leading to contamination, reduced efficacy, and severe patient health risks. Consequently, such packaging materials must undergo rigorous quality testing and safety evaluations during production and application to ensure pharmaceutical safety [48].
4. Common Pharmaceutical Packaging Materials
Common pharmaceutical packaging materials include the following categories:
4.1. Glass
Glass is a non-crystalline amorphous fused solid that does not crystallize under rigid cooling conditions [12,13,14]. Glass pharmaceutical packaging materials, owing to their excellent physicochemical properties, have become the most important pharmaceutical packaging materials with the longest application history and the widest application range in China [14]. The United States Pharmacopeia (USP) and European Pharmacopoeia (EP) also specifically recognize two types of glass for safe parenteral drug packaging: Type I borosilicate glass and Type II soda–lime glass. Ampoule bottles are normally made of borosilicate glass or soda–lime glass, but vials are commonly made of borosilicate glass [12].
Chemical durability is a critical property of pharmaceutical glass, where borosilicate glass outperforms soda–lime glass. The typical chemical composition of borosilicate glass is as follows: silicon dioxide (SiO2) accounts for 70.00~80.00 wt%, boric anhydride (B2O3) accounts for 7.00~13.00 wt%, sodium oxide (Na2O) or potassium oxide (K2O) accounts for 4.00~8.00 wt%, and aluminum oxide (Al2O3) accounts for 2.00~8.00 wt%. Borosilicate glass, which can be sterilized before and after filling, exhibits exceptional properties: high hydrolytic resistance, superior chemical durability and inertness, excellent thermal shock resistance, and ultra-low thermal expansion coefficient. Therefore, this material has been widely used for sensitive drugs requiring stringent packaging, such as parenteral medications and blood products [13].
4.2. Metals
Metals for pharmaceutical packaging commonly refer to containers made from various metallic materials (including metallic coatings and alloys) used for packaging pharmaceuticals. In practical applications, they are primarily categorized into two types based on the base material: aluminum and its alloys, and stainless steel containers. Depending on whether internal surface treatment is applied, they can be classified as containers with inner coatings/platings or bare metal containers. These metal containers are suitable for packaging aerosols, sprays, ointments, and other formulations given the wide variety of involved dosage forms, the quality control of metals has received significant attention [15]. Elemental impurities introduced through raw materials or residual from manufacturing processes may leach into pharmaceuticals during storage, potentially affecting drug quality and safety. Aluminum is widely used in pharmaceutical packaging, especially in the form of aluminum foil. Aluminum foil is used for blister packaging of solid dosage forms [16]. It provides a good barrier against light, moisture, and oxygen, protecting the drugs from degradation. For example, many tablets and capsules are packaged in blister packs with an aluminum foil backing [17].
4.3. Plastics
As synthetic polymers, plastic materials have largely replaced inorganic materials such as metal and glass in pharmaceutical packaging due to their flexibility, low cost, and ease of production. Polyethylene (PE), polypropylene (PP) and polyvinyl chloride (PVC) are well-known plastic materials used in pharmaceutical packaging. PE exhibits good flexibility, allowing it to conform to various drug shapes, along with excellent chemical stability—rarely reacting chemically with pharmaceuticals. It also processes well, enabling the production of diverse packaging containers [21,22]. PP offers high transparency, facilitating visual inspection of medications, and outstanding heat resistance, making it suitable for sterilization processes. It is durable, chemically stable, and widely used in pharmaceutical packaging [23,24]. PVC is flexible, transparent, and excels in heat-sealing performance, ensuring rapid and secure packaging closure [25,26]. Despite their advantages, plastics also present significant drawbacks such as plasticizer migration. During production, plasticizers are often added to plastic materials like PVC, which may further migrate into medications, posing health risks, especially affecting children’s endocrine and reproductive development. Additionally, broken plastic packaging fragments could be ingested by children, causing harm. To ensure medication safety, rigorous screening of plastic materials is essential for pediatric drug packaging [27,28].
On the other hand, compared to glass and metal, most plastic materials exhibit inferior barrier properties, allowing gradual permeation of oxygen and moisture that can compromise drug stability. To address this, plastics with enhanced barrier performance have been developed. Commonly used barrier materials in pharmaceutical packaging include polyvinylidene chloride (PVDC), ethylene-vinyl alcohol copolymer (EVOH), and polyvinyl alcohol (PVA). The barrier properties of typical plastic packaging materials are summarized in Table 1.

Table 1.
Barrier performance of typical plastic packaging materials [27].
4.4. Rubbers
Rubbers are a kind of polymer that are renowned for their exceptional elasticity, making them an indispensable component of pharmaceutical packaging systems. Its quality and safety have always been focal points of industry attention. Rubber materials are commonly used in critical components such as bottle caps and stoppers, where they form tight seals with container openings. This creates a reliable barrier against external moisture, oxygen, and microorganisms, ensuring the stability of pharmaceuticals during storage and transportation [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
Elastomeric rubber stoppers play a unique role in pharmaceutical packaging as core primary packaging components for numerous sealed container systems. They serve not only as sealing closures for vials and syringes but also as plunger tips (pistons) in pre-filled syringes or cartridge-based systems. Elastomeric materials possess distinctive mechanical properties, enabling smooth penetration by hypodermic needles without bending or dulling them. They require minimal insertion force while rapidly regaining elasticity post-withdrawal to maintain a secure seal, preserving the integrity of the packaging system [42,43]. Currently, halogenated butyl rubber stoppers (primarily chlorinated and brominated butyl rubber) dominate applications for injectables, inhalants, and liquid formulations due to their low gas/moisture permeability, high chemical stability, heat/aging resistance, and superior self-sealing capabilities [30].
From a manufacturing perspective, most rubbers are produced by vulcanizing raw rubber to form crosslinked network structures. This vulcanization process imparts high strength and corrosion resistance, enabling vulcanized rubber to undergo significant deformation under stress while rapidly recovering its original shape post-stress removal. Sulfur, the earliest vulcanizing agent, faces limitations in butyl rubber applications. When exceeding its saturation solubility in butyl rubber stoppers, sulfur causes a “blooming” phenomenon that disrupts material homogeneity and compromises drug quality. Thus, stringent sulfur content monitoring has become a critical quality control parameter [36]. Nowadays, high-performance liquid chromatography (HPLC) is the standard method for detecting sulfur levels in halogenated butyl rubber stoppers, enabling precise quality risk mitigation [37,38,39,40].
Nevertheless, rubber materials present ongoing challenges. Issues such as permeability, adsorption tendencies, extractables, and release of subvisible particles require advanced solutions to ensure packaging quality and medication safety [43,44].
5. Impact on Medication Safety
5.1. Barrier Performance
Barrier properties of pharmaceutical packaging materials against oxygen, water vapor, and light play crucial roles in ensuring medication quality and safety [49]. Suboptimal barrier performance of packaging materials may lead to reduced efficacy and increased impurities in drugs and could pose significant threats to medication safety. Selecting appropriate packaging materials is therefore essential for maintaining drug quality. For example, vitamin C exhibits strong reducing properties and is prone to oxidation. If ordinary plastic bottles are used as packaging, oxygen can easily penetrate the plastic and oxidize vitamin C into dehydroascorbic acid, which further decomposes into products such as 2,3-diketogulonic acid. Prolonged consumption of deteriorated vitamin C tablets will not only fail to deliver the intended benefits of vitamin C supplementation but may also introduce harmful impurities, adversely affecting health. Therefore, good oxygen-resistant packaging is necessary to protect vitamin C tablets [50]. In humid environments, aspirin is susceptible to hydrolysis, generating salicylic acid and acetic acid. If the packaging material has weak water vapor barrier capabilities, moisture ingress can cause enteric-coated aspirin tablets to absorb humidity, accelerating hydrolysis reaction [51]. Light-blocking performance is equally important. For instance, chlorpromazine tablets are photosensitive [52]. If transparent packaging is used in a light-exposed environment, chlorpromazine will undergo a series of photochemical reactions [53,54], decomposing into toxic free radicals and other products. This not only renders the drug ineffective but may also cause serious adverse reactions, such as skin allergies [55], liver damage, and so on [55,56].
5.2. Mechanical Performance
The mechanical properties of pharmaceutical packaging materials also profoundly impact medication safety [57]. Firstly, packaging materials must possess sufficient compressive, tensile, and impact resistance capabilities [58,59,60,61]. During production, transportation, and storage, pharmaceuticals inevitably endure external forces such as stacking, collisions, and vibrations [62]. Excessively thin packaging boxes may collapse under stacking pressure, causing internal medications to break or deform, compromising drug efficacy. Plastic bottles with inadequate rigidity may crack upon minor impacts, leading to drug leakage, cross-contamination, and posing risks to patient health [63]. Secondly, pharmaceutical packaging materials should demonstrate appropriate toughness [64]. For example, pharmaceutical blister packaging requires plastic materials with excellent toughness to securely encapsulate medications while protecting tablets or capsules from damage through deformation when compressed [65]. Additionally, the elasticity of packaging materials is also related to medication safety [66]. For instance, flexible tubes for topical ointments must maintain elasticity to ensure smooth extrusion and preserve airtightness after repeated compression. This prevents air ingress that could oxidize and degrade the ointment, thereby ensuring safe usage and storage [67].
5.3. Migration Phenomenon
The migration phenomenon in pharmaceutical packaging materials poses significant risks to medication safety [68]. During the production of plastic, rubber, and other packaging materials, various additives such as plasticizers, stabilizers, and antioxidants are incorporated to enhance material performance. However, during the storage of pharmaceuticals, these additives may migrate from the packaging materials into the drugs [69]. Di(2-ethylhexyl)phthalate (DEHP), a plasticizer, may be added to PVC infusion bags. But if DEHP migrates into the human body, it may interfere with the endocrine system, hinder reproductive development, and pose greater risks to sensitive populations such as infants and pregnant women [70]. Beyond additives, the migration of intrinsic packaging components can also occur. Certain glass packaging materials, when in contact with aqueous solutions like injectables, may release alkaline substances or insoluble flakes. This not only alters the pH of medications but also introduces particulate contamination. If such particles enter the bloodstream, they increase the risk of thrombosis [71]. In addition, interactions between highly oxidizing drugs and reducing packaging materials may trigger redox reactions. This can degrade drug efficacy or generate toxic substances, severely compromising patient safety [72]. Therefore, rigorously controlling the chemical properties of pharmaceutical packaging materials and preventing migration-related hazards are critical to safeguarding medication safety.
To assess whether substances in packaging materials migrate into pharmaceutical products and ensure packaging safety, migration testing of packaging materials must be conducted [73]. Various analytical methods are employed during migration testing, such as gas chromatography–mass spectrometry (GC-MS), liquid chromatography mass spectrometry (LC-MS), ion chromatography (IC), inductively coupled plasma optical emission spectrometry (ICP-OES), and atomic absorption spectroscopy (AAS) [74]. These methods help identify and quantify potentially migratable compounds. To reduce costs and time, researchers increasingly employ mathematical models to predict migration behavior [75,76,77].
5.4. Adsorption Effect
Adsorption in pharmaceutical packaging poses critical safety risks. Many plastic and rubber packaging materials adsorb fat-soluble drugs, volatile compounds, or active ingredients. For example, antibiotics in plastic bottles may lose potency as packaging adsorbs active ingredients, leading to underdosing and delayed treatment [78]. Protein-based biologics stored in rubber-sealed vials risk structural changes if the rubber adsorbs the proteins, which reduces efficacy and may trigger allergic reactions due to immunogenic byproducts [79,80]. Plastic films adsorbing drug volatiles can become brittle, compromising seal integrity and accelerating drug degradation [81]. Since adsorption impacts drug potency, stability, and packaging performance, selecting low-adsorption materials is vital for medication safety.
5.5. Microbial Contamination Risk
During the production of pharmaceutical packaging, microbial contamination represents one of the key factors affecting pharmaceutical safety [82]. If the manufacturing environment fails to meet required cleanliness standards, microbial (such as bacteria, fungi and viruses) growth becomes highly likely [83]. For instance, in facilities producing paper-based packaging materials, improper humidity control or inadequate sanitation can lead to excessive proliferation of mold and other microorganisms. Even if contaminated paper is used for outer packaging without direct contact with medications, microbes may migrate into the drugs through gaps or pores during storage or transportation, jeopardizing product quality [84]. Production equipment and processes also contribute to microbial contamination. During plastic packaging manufacturing, residual microbes may adhere to surfaces if machinery like injection molding machines is not regularly cleaned and sterilized. For plastic bottles directly contacting medications, bacterial contamination during production can lead to microbial growth post-filling, causing drug degradation, loss of efficacy, or even user infections. The hygiene practices of operators are equally important. Inadequate handwashing by workers may introduce pathogenic microbes such as Escherichia coli or Staphylococcus aureus to packaging materials. This risk is especially acute for components like injection vial stoppers or eye-drop dispensers that directly contact medications. Even trace microbial contamination in such parts can render drugs unsafe, posing severe health risks to users [85]. Therefore, strict control across all production stages of pharmaceutical packaging materials, particularly manufacturing environments, is essential to eliminate microbial contamination and safeguard pharmaceutical safety.
Currently in China, pharmaceutical packaging material manufacturers are required to establish cleanrooms based on the intended use and characteristics of the materials, with cleanliness levels corresponding to those of the drug production environments they serve. Regulatory authorities are mandated to inspect and validate the performance parameters of these cleanrooms, with testing methods aligned to referenced standards such as those outlined in Table 2.

Table 2.
Detection items, methods, and standard for cleanrooms.
6. Standards for Pharmaceutical Packaging Materials
Owing to the significant impact of pharmaceutical packaging materials on medication safety, the production, processing, and use of all pharmaceutical packaging materials must strictly comply with the regulations and standards established by regulatory authorities [88,89]. USP serves as the comprehensive standard for drug quality control in the United States. Compiled by the United States Pharmacopeial Convention, it provides the legal basis for the production, use, and regulatory testing of pharmaceuticals in the country. As shown in Table A1, the USP-NF-2023 edition includes extensive and highly detailed requirements for pharmaceutical packaging materials. The EP, edited and published by the European Directorate for the Quality of Medicines & HealthCare (EDQM), saw its 11th edition (EP 11.0) come into effect in January 2023. EP 11.0 is currently the sole authoritative guideline for pharmaceutical quality testing in Europe. All manufacturers must comply with its quality standards when marketing and using pharmaceutical products within the European region. Table A2 illustrates that the sections on pharmaceutical packaging materials in EP 11.0 are divided into two main parts: “3.1 Materials for Containers for Manufacturing” and “3.2 Containers”. The Japanese Pharmacopoeia (JP) was developed by the Japanese Pharmacopoeia Editorial Committee and enforced by the Japanese Ministry of Health, Labour and Welfare. The latest edition, JP 18, became effective in 2021. As indicated in Table A3, JP 18 provides relatively concise guidelines for pharmaceutical packaging materials, with a specific focus on containers for injectable formulations.
In 2015, the Chinese Pharmacopoeia Commission published the 2015 Edition of the National Standard for Pharmaceutical Packaging Materials, which comprehensively covers packaging materials for all dosage forms used in China’s pharmaceutical industry. The Pharmacopoeia of the People’s Republic of China (ChP) 2020, also compiled and promulgated by the Chinese Pharmacopoeia Commission, dedicates 18 chapters to pharmaceutical packaging materials (Table 3). Unlike USP-NF-2023, which serves as a reference standard, both the National Standard for Pharmaceutical Packaging Materials and ChP 2020 are legally mandatory standards in China.

Table 3.
ChP 2020 pharmaceutical packaging material standard system.
7. Novel Materials for Pharmaceutical Packaging
7.1. Nanomaterials
Recent studies have shown that nanomaterials, leveraging their unique physicochemical properties—particularly their high specific surface area—have become critical elements for optimizing process efficiency and enhancing the comprehensive performance of materials. As next-generation UV-blocking composite materials, nanocomposite films demonstrate significant potential in pharmaceutical packaging and storage. By effectively blocking ultraviolet radiation, they prevent drug degradation and deterioration caused by light exposure, thereby ensuring drug quality and safety [90,91,92,93,94]. Rukmanikrishnan et al. incorporated zinc oxide nanoparticles (NPs) into gellan and xanthan gum nanocomposite films, significantly improving their glass transition temperature, thermal stability and tensile strength. These new nanocomposite films may be suitable for pharmaceutical packaging applications [91]. As shown in Figure 1, Patil et al. successfully fabricated a flexible, highly transparent, and re-emissive PVA/waste tea residue carbon dot (PVA@WTR-CD) composite thin films through solvent casting method. Their research confirmed that higher concentrations of WTR-CDs in PVA films enhance UV-blocking capabilities, achieving 100% UV-C (230–280 nm) and UV-B (280–315 nm) blocking, along with 20–60% blocking in the UV-A (315–400 nm) region. PVA@WTR-CDs can be used as a modern UV-blocking material in pharmaceutical storage, packaging, and coating [92]. Razali et al. developed bionanocomposite films based on gellan gum and TiO2 nanotube. These new bionanocomposite films not only exhibit high tensile strength and young modulus but also display strong antibacterial activity against pathogens such as Escherichia coli, Streptococcus, Pseudomonas aeruginosa, and Staphylococcus aureus, which may also make a beneficial contribution to pharmaceutical packaging [93].
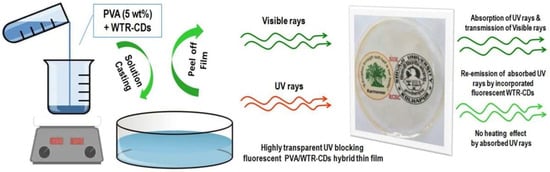
Figure 1.
Schematic for fabrication of PVA@WTR-CDs composite thin films and their UV-blocking applications [92].
7.2. Intelligent Packaging Materials
Intelligent pharmaceutical packaging materials and smart packaging encompass various categories such as information technology-based, active material-based, and structurally protective types. These innovations represent interdisciplinary achievements integrating computer science, materials science, artificial intelligence, industrial design, modern control theory, and microelectronics. The novel packaging materials and their derivative functions not only significantly enhance management efficiency and information collection capabilities in pharmaceutical logistics but also provide consumers with safe and convenient medication protection through comprehensive safety design and functional implementation. They have become frontier directions for global pharmaceutical packaging exploration and development [95,96,97,98,99,100,101,102,103,104,105].
Child Resistant Packaging (CRP), a typical example of structurally protective smart packaging, effectively prevents unauthorized opening by children through its special safety cap locking mechanism design. This design balances safety and convenience by avoiding accidental medication ingestion while maintaining accessibility for adults [104]. Furthermore, RFID technology offers efficient solutions for the wireless measurement of environmental parameters. By enabling real-time monitoring of temperature, humidity, and light intensity, it provides timely warnings for abnormal conditions, preventing drug deterioration caused by unsuitable environments and ensuring pharmaceutical quality at the source [95,96,97]. As shown in Figure 2, objective therapy compliance measurement based on intelligent packaging with conductive tracks and RFID tags are applied by the company “Qolpac” as self-adhesive labels to standard blister packs. When a pill is removed, near field communications will transmit data to the health records wirelessly [98]. RFID technology enables unified identification, anti-counterfeiting, traceability, and tamper resistance for pharmaceuticals. This not only reduces production and distribution costs while minimizing waste and shortening delivery time but also enables precise drug tracking to prevent counterfeit products from entering the market [99]. In anti-counterfeiting innovation, intelligent pharmaceutical packaging materials demonstrate unique advantages. Some packaging utilizes special inks to print hidden patterns and texts that only reveal distinct colors under ultraviolet or infrared light, creating difficult-to-replicate anti-counterfeiting identifiers. This technology effectively combats counterfeit drug production and distribution, safeguarding consumer medication safety [100]. Leem et al. developed edible and scalable matrix codes using fluorescent silk fibroin, which can serve as labels for pharmaceutical information representation and data storage. These codes enable drug serialization, tracking, tracing, and authentication, enhancing pharmaceutical security and combating illegal drugs [101].
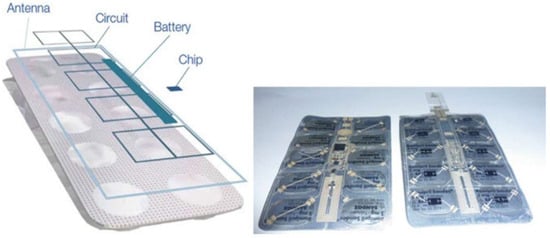
Figure 2.
Principles behind electronic packs of Qolpac and their application to Sandoz’s Ramipril tablets [98].
7.3. Green Packaging Materials
Microbial green packaging materials refer to environmentally friendly packaging solutions that pose no harm to human health and ecosystems throughout their entire lifecycle—from production and use to disposal. Primarily composed of natural bio-based polymers, these materials ensure pharmaceutical safety during packaging, transportation, and storage due to their excellent mechanical properties and biocompatibility. Their non-toxic nature eliminates contamination risks from harmful substances in packaging materials, while their biodegradability significantly reduces long-term environmental pollution caused by traditional packaging waste [106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121]. With rising global environmental awareness and sustainability priorities, the development and application of green packaging materials have gained international momentum. Worldwide governments are implementing supportive policies, including financial subsidies and tax incentives, to promote industry growth. Concurrent advancements in production technologies have enabled green packaging materials to match or surpass conventional materials in critical properties such as strength, toughness, and barrier performance [107].
As shown in Figure 3, Mohammadi et al. developed biodegradable PVA/CS/GO-PEG films by incorporating graphene oxide-polyethylene glycol nanohybrids (GO-PEG) into a polyvinyl alcohol/chitosan-carbohydrate (PVA/CS) blend. This material exhibits enhanced glass transition temperature (Tg), Young’s modulus, tensile strength, elongation at break, and antimicrobial properties, positioning it as a promising candidate for drug packaging [108]. Nanocellulose materials derived from plant cellulose (e.g., cotton, bamboo, wheat, rice, sugarcane) not only improve mechanical and barrier performance of packaging—effectively preventing moisture damage and drug degradation during storage—but also offer cost-effectiveness, making them ideal for balancing safety and affordability in pharmaceutical applications. This innovation has attracted significant academic and industrial interest [109]. Yuan et al. systematically analyzed the structural properties and applications of biodegradable plastics, highlighting polybutylene succinate (PBS) as a safe, eco-friendly option for pharmaceutical outer packaging. Although PBS is not bio-based, its biodegradability avoids the persistent environmental hazards associated with traditional plastic waste [110]. In green composite research, polylactic acid-based composites have gained prominence for their high performance. Incorporating natural fibers as reinforcements further enhances packaging strength and stability, better protecting pharmaceuticals during transit [111,112]. Plant-based natural fibers such as sisal, jute, cotton, coir, and bamboo are now widely used in biocomposite packaging across industries. These materials combine robust physical properties with inherent non-toxicity, providing multi-layered safety assurance for pharmaceutical packaging [113,114,115].
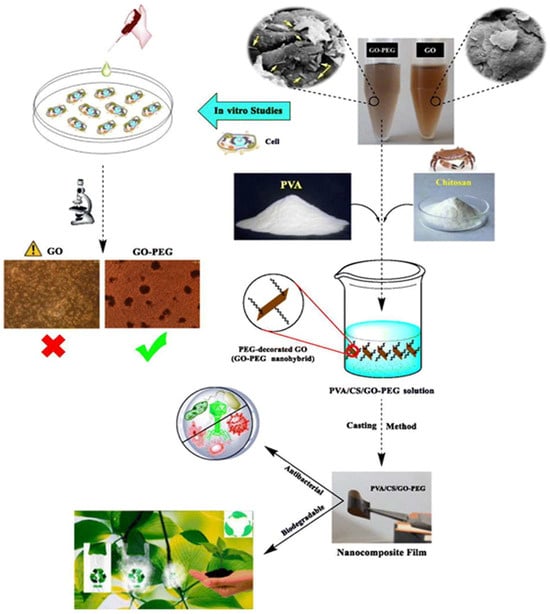
Figure 3.
Synthesis strategy of biodegradable PVA/CS/GO-PEG films [108].
As listed in Table 4, several bio-derived plastic materials demonstrate potential to replace traditional synthetic polymers as next-generation pharmaceutical packaging materials. Despite their immense potential, most bio-based materials have yet to achieve large-scale production for pharmaceutical applications. As packaging materials directly contact drugs, their safety critically impacts drug quality. Rigorous experimental analysis is required to comprehensively evaluate material–drug compatibility and potential effects on pharmaceutical stability under varied storage conditions [116].

Table 4.
Characteristics of different biodegradable plastics.
8. Summary and Outlook
In conclusion, the physical and chemical properties of pharmaceutical packaging materials, as well as their risks of microbial contamination during production processes, are critically linked to drug quality and safety. It is imperative to strengthen research and regulation on these materials. Common materials, such as glass, plastic, metal, and rubber, each have advantages and drawbacks: glass materials offer excellent chemical stability but may release ions; plastics are widely used but poses risks like plasticizer migration; metals provide high strength and are typically reserved for specific drug packaging; and rubbers are essential for sealing but may leach additives that compromise drug quality.
Recent advancements in pharmaceutical sciences, materials science, and electronic information technology, coupled with rapid innovation in drug development, have created new demands for packaging materials. Beyond meeting basic safety and protection requirements, packaging materials must now address higher-level needs such as intelligence and sustainability: nanomaterials enhance barrier properties and mechanical strength; smart packaging enables real-time quality monitoring; eco-friendly green packaging materials reduce environmental impact while ensuring safety; and innovations in production processes further drive industry growth. These advancements will accelerate the development of pharmaceutical packaging materials, align with the evolving needs of the healthcare sector, and ultimately safeguard patient safety. With the global warming situation becoming increasingly severe, we predict that new low-carbon, environmentally friendly, and energy-efficient packaging materials and related technologies will be increasingly applied in the design, production, usage, and recycling processes of pharmaceutical packaging at an accelerated pace.
In this review, the literature search was limited to Chinese and English databases, which may have overlooked significant research findings in other languages. In future studies, we will strengthen international collaboration and expand our investigations to include the literature in other languages (e.g., French, Spanish, Arabic, Hindi, Russian, etc.). However, we found that current short-term evaluations (<12 months) of novel packaging materials are insufficient to reflect their long-term safety. Additionally, the laboratory-accelerated testing data also diverges from that in actual storage conditions. Therefore, we recommend conducting the long-term observation of new packaging materials under real-world environmental conditions as an essential step forward.
Author Contributions
Conceptualization, Y.L. and N.L.; methodology, Y.L. and N.L.; formal analysis and investigation, Y.L., N.L., C.C. and Z.C.; data collection, Y.L., N.L., C.C. and Z.C.; writing—original draft preparation, Y.L. and N.L.; writing—review and editing, Y.L., N.L., C.C., Z.C. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Project of Zhejiang Province (No. 2022C01182) and the Zhejiang Provincial Natural Science Foundation of China (No. LY19B010003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank for the finacial supports from Public Service Platform for Testing, Evaluation, and Industrialization of Innovative Achievements of Drug Excipients and Packaging Materials, Ministry of Industry and Information Technology, P. R. China.
Conflicts of Interest
Author Jianhang Li was employed by the company Hangzhou Plastics Industry Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A

Table A1.
Pharmaceutical packaging material standard system table in USP NF—2023.
Table A1.
Pharmaceutical packaging material standard system table in USP NF—2023.
| Number | Name of Standard |
|---|---|
| <87> | Biological Reactivity Tests, In Vitro [122] |
| <88> | Biological Reactivity Tests, In Vivo |
| <381> | Elastomeric Closures For Injections |
| <659> | Packaging And Storage Requirements |
| <660> | Containers—Glass |
| <661> | Plastic Packaging Systems And Their Materials Of Construction |
| <661.1> | Plastic Materials Of Construction |
| <661.2> | Plastic Packaging Systems For Pharmaceutical Use |
| <661.3> | Pharmaceutical Components And Systems Used In The Manufacturing |
| <665> | Polymeric Components Used In The Manufacturing Of Pharmaceutical And Biopharmaceutical Drug Products |
| <670> | Auxiliary Packaging Components [123] |
| <671> | Containers—Performance Testing |
| <1031> | The Biocompatibility Of Materials Used In Drug Containers, Medical Devices, And Implants |
| <1136> | Packaging Repackaging-Single-Unitcontainers |
| <1177> | Good Repackaging Practices |
| <1178> | Good Packaging Practices |
| <1207> | Package Integrity Evaluation-Sterile Products |
| <1207.1> | Package Integrity Testing In The Product Life Cycle- Test Method Selection And Validation |
| <1207.2> | Package Integrity Leak Test Technologies |
| <1207.3> | Package Seal Quality Test Technologies |
| <1660> | Evaluation Of The Inner Surface Durability Of Glass Containers |
| <1661> | Evaluation Of Plastic Packaging Systems And Their Materials Of Construction With Respect To Their User Safety Impact [124] |
| <1663> | Assessment Of Extractables Associated With Pharmaceutical Packaging/Delivery Systems |
| <1664> | Assessment Of Drug Product Leachables Associated With Pharmaceutical Packaging/Delivery Systems |
| <1665> | Plastic Components And Systems Used To Manufacture Pharmaceutical Drug Products |

Table A2.
Pharmaceutical packaging material standard system table in EP 11.0.
Table A2.
Pharmaceutical packaging material standard system table in EP 11.0.
| Number | Name of Standard |
|---|---|
| 3.1 | Materials Used For The Manufacture of Containers |
| 3.1.3 | Polyolefines [125] |
| 3.1.4 | Polyethylenes Without Additives For Containers For Parenteral Preparations And For Ophthalmic Preparations |
| 3.1.5 | Polyethylenes With Additives For Containers For Parenteral Preparations And For Ophthalmic Preparations |
| 3.1.6 | Polypropylene For Containers And Closures For Parenteral Preparations And Ophthalmic Preparations |
| 3.1.7 | Polyethylene-Vinyl Acetate For Containers And Tubing For Total Parenteral Nutrition Preparations |
| 3.1.8 | Silicone Oil Used As A Lubricant |
| 3.1.9 | Silicone Elastomer For Closures And Tubing |
| 3.1.10 | Materials Based On Non-Plasticised Poly (Vinyl Chloride) For Containers For Non- Injectable, Aqueous Solutions |
| 3.1.11 | Materials Based On Non-Plasticised Poly (Vinyl Chloride) For Containers For Dry Dosage Forms For Oral Administration |
| 3.1.13 | Plastic Additives [126] |
| 3.1.14 | Materials Based On Plasticised Poly (Vinyl Chloride) For Containers For Aqueous Solutions For Intravenous Infusion |
| 3.1.15 | Polyethylene Terephthalate For Containers For Preparations Not For Parenteral Use |
| 3.2 | Containers |
| 3.2.1 | Glass Containers For Pharmaceutical Use |
| 3.2.2 | Plastic Containers And Closures For Pharmaceutical Use |
| 3.2.2.1 | Plastic Containers For Aqueous Solutions For Infusion |
| 3.2.9 | Rubber Closures For Containers For Aqueous Parenteral Preparations, For Powders And For Freeze-Dried Powders |

Table A3.
Pharmaceutical packaging material standard system table in JP 18.
Table A3.
Pharmaceutical packaging material standard system table in JP 18.
| Number | Name of Standard |
|---|---|
| 7 | Test methods for container packaging materials |
| 7.01 | Test methods for glass containers for injection |
| 7.02 | Test methods for plastic containers for pharmaceutical products [127] |
| 7.03 | Test methods for rubber plugs for infusion |
References
- Ibrahim, I.D.; Hamam, Y.; Sadiku, E.R.; Ndambuki, J.M.; Kupolati, W.K.; Jamiru, T.; Eze, A.A.; Snyman, J. Need for Sustainable Packaging: An Overview. Polymers 2022, 14, 4430. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Linh, T.T.T.; Vo, T.-K.; Nguyen, Q.H.; Van, T.-K. Analytical Techniques for Determination of Heavy Metal Migration from Different Types of Locally Made Plastic Food Packaging Materials Using ICP-MS. Food Sci. Nutr. 2023, 11, 4030–4037. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Q.; Wei, Y.; Qiao, Y.; Duan, X.; Li, L.; Chen, Y.; Qian, Z. Vitrification of Cerium(III) Fluoride in Iron Phosphoborate Glasses: A Study on Redox, Structure, and Stability. Ceram. Int. 2024, 50, 38435–38444. [Google Scholar] [CrossRef]
- Olawore, O.; Ogunmola, M.; Desai, S. Engineered Nanomaterial Coatings for Food Packaging: Design, Manufacturing, Regulatory, and Sustainability Implications. Micromachines 2024, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T. Patient Safety as a Global Health Priority. Cardiovasc. Ther. Prev. 2022, 21, 3427. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.L.G.; da Silva, R.E.; da Silva Junior, J.B.; Bosio, C.G.P.; Novaes, M.R.C.G. Post-Marketing Authorisation Safety and Efficacy Surveillance of Advanced Therapy Medicinal Products in Brazil, the European Union, the United States and Japan. Cytotherapy 2023, 25, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Gil-Pérez, I.; Rebollar, R.; Lidón, I.; Martín, J.; van Trijp, H.C.M.; Piqueras-Fiszman, B. Hot or Not? Conveying Sensory Information on Food Packaging Through the Spiciness-Shape Correspondence. Food Qual. Prefer. 2019, 71, 197–208. [Google Scholar] [CrossRef]
- Pattnaik, R.; Panda, S.K.; Biswas, S.; De, S.; Satahrada, S.; Kumar, S. Prospects and Challenges of Nanomaterials in Sustainable Food Preservation and Packaging: A Review. Discov. Nano 2024, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Qian, S.; Lan, T.; Wu, Y.; Liu, J.; Zhang, H. Recent Advances in Bio-Based Smart Active Packaging Materials. Foods 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Sustainability Team. Back to Sustainable Value Creation. How Sustainable Is Packaging? Sustain. Team Discuss. Pap. 2009, 2–3. [Google Scholar]
- Yang, R.; Liu, H.; Ding, Z.; Zheng, J.; Mauro, J.C.; Kim, S.H.; Zheng, Q. Chemical Durability of Borosilicate Pharmaceutical Glasses: Mixed Alkaline Earth Effect with Varying [MgO]/[CaO] Ratio. J. Am. Ceram. Soc. 2021, 104, 3973–3981. [Google Scholar] [CrossRef]
- Mathaes, R.; Streubel, A. Parenteral Container Closure Systems. In Challenges in Protein Product Development; Warne, N., Mahler, H.C., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: Cham, Switzerland, 2018; pp. 191–202. [Google Scholar]
- Tian, Y.L. Pharmaceutical Glass; Chemical Industry Press: Beijing, China, 2015; pp. 8–10. [Google Scholar]
- YBB00142002-2015; Guidelines for Compatibility Testing of Pharmaceutical Packaging Materials and Drugs. National Medical Products Administration: Beijing, China, 2015; pp. 446–448.
- de Oliveira, D.P.; Costa, J.S.R.; Oliveira-Nascimento, L. Sustainability of Blisters for Medicines in Tablet Form. Sustain. Chem. Pharm. 2021, 21, 100423. [Google Scholar] [CrossRef]
- Müller, S.; Weygand, S.M. Finite Element Simulation of Stretch Forming of Aluminium-Polymer Laminate Foils Used for Pharmaceutical Packaging. MATEC Web Conf. 2016, 80, 16001. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future Scenarios of Global Plastic Waste Generation and Disposal. Palgrave Commun. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bhoi, P.R. An Overview of Non-Biodegradable Bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-Sourced Polymers as Alternatives to Conventional Food Packaging Materials: A Review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Yu, D.; Basumatary, I.B.; Liu, Y.; Zhang, X.; Kumar, S.; Ye, F.; Dutta, J. Chitosan-Photocatalyst Nanocomposite on Polyethylene Films as Antimicrobial Coating for Food Packaging. Prog. Org. Coat. 2024, 186, 108069. [Google Scholar] [CrossRef]
- Falk, Y.Z.; Runnsjö, A.; Pettigrew, A.; Scherer, D.; Engblom, J.; Kocherbitov, V. Interactions of Perfluorohexyloctane with Polyethylene and Polypropylene Pharmaceutical Packaging Materials. J. Pharm. Sci. 2020, 109, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Ansari, J.R.; Sadeghi, K.; Seo, J. Applicability of Polypropylene/Polyethylene Glycol/Molecular Sieve Composites as Desiccant Pharmaceutical Packaging Materials. Food Packag. Shelf Life 2024, 42, 101266. [Google Scholar] [CrossRef]
- Hazer, B.; Ashby, R.D. Synthesis of Polyvinyl Chloride/Chlorinated Polypropylene-Active Natural Substance Derivatives for Potential Packaging Materials Application. Tannic Acid, Menthol and Lipoic Acid. Food Chem. 2023, 403, 134475. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.; Morsy, M.; Ateia, M.A.; Abdel-Haleem, F.M. Ultrafast Response Humidity Sensors Based on Polyvinyl Chloride/Graphene Oxide Nanocomposites for Intelligent Food Packaging. Sens. Actuators A Phys. 2021, 331, 112918. [Google Scholar] [CrossRef]
- Veronica, N.; Heng, P.W.S.; Liew, C.V. Relative Humidity Cycling: Implications on the Stability of Moisture-Sensitive Drugs in Solid Pharmaceutical Products. Mol. Pharm. 2023, 20, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, J. Package Technology for Pharmaceutical Packing Blister Film; Tsinghua University Press: Beijing, China, 2024; pp. 25–39. [Google Scholar]
- Nakayama, T.; Osako, M. Plastic Trade-Off: Impact of Export and Import of Waste Plastic on Plastic Dynamics in Asian Region. Ecol. Model. 2024, 489, 110624. [Google Scholar] [CrossRef]
- Meng, W.J.; Qiu, Y.X.; Yu, P.F.; Qi, Y.H.; Deng, Z.W. Research Progress on Regulations Related to Pharmaceutical Packaging Materials in China and Analytical Methods for Compatibility of Halogenated Butyl Rubber Stoppers with Drugs. Chin. J. Pharm. Anal. 2021, 41, 1860–1867. [Google Scholar]
- Jia, F.F.; Zhao, X.; Yang, H.Y.; Xiao, X.Y. Research Progress on Common Vulcanization Systems and Compatibility of Halogenated Butyl Rubber Stoppers for Pharmaceutical Use. Chin. Pharm. Aff. 2022, 36, 913–920. [Google Scholar]
- Sharma, R.K.; Mohanty, S.; Gupta, V. Advances in Butyl Rubber Synthesis via Cationic Polymerization: An Overview. Polym. Int. 2021, 70, 1165–1175. [Google Scholar] [CrossRef]
- McDonald, M.F.; Shaffer, T.D.; Tsou, A.H. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Aoshima, S.; Kanaoka, S. A Renaissance in Living Cationic Polymerization. Chem. Rev. 2009, 109, 5245–5287. [Google Scholar] [CrossRef] [PubMed]
- Kostjuk, S.V.; Ganachaud, F. Cationic Polymerization of Vinyl Ethers: A Critical Reappraisal. Acc. Chem. Res. 2010, 43, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Kostjuk, S.V. Recent Progress in the Cationic Polymerization of (Meth)Allyl Monomers and Macromolecular Engineering. RSC Adv. 2015, 5, 13125–13144. [Google Scholar] [CrossRef]
- Fortuna, L.; Rizzo, A.; Sinatra, M.; Xibilia, M.G. Soft analyzers for a sulfur recovery unit. Control Eng. Pract. 2003, 11, 1491–1500. [Google Scholar] [CrossRef]
- Li, C.H.; Xue, W.L.; Xing, S.; Hang, B.J.; You, P.F. Determination of Leaching and Migration Amounts of 11 Antioxidants and Free Sulfur in Brominated Butyl Rubber Stoppers by HPLC. Chin. J. Anal. Lab. 2020, 39, 91–96. [Google Scholar]
- Feng, J.; Cai, X.Y.; Liu, Y. Determination of Antioxidant-BHT and Vulcanizing Agent-Extractable Sulphur in Medicinal Butyl Rubber. Chin. J. Pharm. Anal. 2017, 37, 702–706. [Google Scholar]
- Li, Y.; Liang, T.T.; Sun, H.M. HPLC Determination of Antioxidant 1010 and Vulcanizing Agent Migration from Halogenated Butyl Rubber Stoppers for Freeze-Dried Injections into Drug Solutions. Chin. Pharm. Aff. 2019, 33, 283–289. [Google Scholar]
- Li, Y.; Yang, H.Y.; Wang, F.; Sun, H.M. Study on the Detection of Extractable Sulfur in Halogenated Butyl Rubber Stoppers for Pharmaceutical Use. Chin. Pharm. Aff. 2014, 28, 1257–1260. [Google Scholar]
- Zhu, B.J.; Li, T.T.; Sun, H.M.; Hu, X.Y.; He, J.Y.; Luo, Y.H. Simultaneous Determination of Five Antioxidants and Free Sulfur in Halogenated Butyl Rubber Stoppers for Pharmaceutical Use by HPLC. China Pharm. 2018, 29, 2475–2479. [Google Scholar]
- Gera, A.K.; Burra, R.K. Design of hollow tapered PMMA polymeric microneedles for enhanced structural stability and drug delivery efficiency. AIP Adv. 2025, 15, 015034. [Google Scholar] [CrossRef]
- Hopkins, G.H. Elastomeric Closures for Pharmaceutical Packaging. J. Pharm. Sci. 1965, 54, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.; Fradkin, A.; Krueger, A.; Kraft, C.; Paskiet, D. Evaluation of Particle Techniques for the Characterization of Subvisible Particles from Elastomeric Closure Components. J. Pharm. Sci. 2020, 109, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.B.; Xu, H.; Xie, X.Y.; Fang, M. Research Progress on Compatibility of Chemical Drug Injections with Pharmaceutical Glass. Guangzhou Chem. Ind. 2016, 44, 50–52. [Google Scholar]
- Almeter, P.J.; Isaacs, J.T.; Hunter, A.N.; Henderson, B.S.; Platt, T.; Mitchell, B.J.; Do, D.; Brainard, A.B.; Brown, J.E.; Stone, R.M.; et al. FDA Approaches in Monitoring Drug Quality, Forces Impacting the Drug Quality, and Recent Alternative Strategies to Assess Quality in the US Drug Supply. J. Pharm. Innov. 2022, 17, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Seidling, H.M.; Woltersdorf, R. Improving Drug Therapy Safety with and for the Patient. Bundesgesundheitsblatt 2018, 61, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P. Silanization Treatment of Glass Containers for Pharmaceutical Packaging. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2023. [Google Scholar]
- Bucci, M.; Deane, C.; Miura, G.; Song, Y. Research Highlights. Nat. Chem. Biol. 2019, 15, 651. [Google Scholar]
- Dewhirst, R.A.; Fry, S.C. The Oxidation of Dehydroascorbic Acid and 2,3-Diketogulonate by Distinct Reactive Oxygen Species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef] [PubMed]
- Eichie, F.E.; Okor, R.S.; Groning, R. Structure and Barrier Property of Acrylate-Methacrylate Film Coating in Aspirin Microcapsules. J. Appl. Polym. Sci. 2006, 99, 725–727. [Google Scholar] [CrossRef]
- Mostafa, I.M.; Mohamed, A.A.; Alahmadi, Y.; Shehata, A.M.; Almikhlafi, M.A.; Omar, M.A. Facile, Eco-Friendly and Sensitive Fluorimetric Approach for Detection of Chlorpromazine: Application in Biological Fluids and Tablet Formulations as Well as Greenness Evaluation of the Analytical Method. Luminescence 2024, 39, e4897. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Bilal, M.; Rasool, N.; Imran, M. Photochemical Reactions as Synthetic Tool for Pharmaceutical Industries. Chin. Chem. Lett. 2024, 35, 109498. [Google Scholar] [CrossRef]
- Bochet, C.G. On the Sustainability of Photochemical Reactions. Chimia 2019, 73, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Barrier Dysfunction in the Skin Allergy. Allergol. Int. 2018, 67, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortes, M.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug Induced Liver Injury: An Update. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef] [PubMed]
- Antón, N.; González-Fernández, Á.; Villarino, A. Reliability and Mechanical Properties of Materials Recycled from Multilayer Flexible Packages. Materials 2020, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Vrabić-Brodnjak, U.; Yavorov, N.; Lasheva, V.; Todorova, D. Chitosan-Coated Packaging Papers—Strength and Thermal Stability. Coatings 2023, 13, 828. [Google Scholar] [CrossRef]
- Gajewski, T.; Grabski, J.K.; Cornaggia, A.; Garbowski, T. On the Use of Artificial Intelligence in Predicting the Compressive Strength of Various Cardboard Packaging. Packag. Technol. Sci. 2024, 37, 97–105. [Google Scholar] [CrossRef]
- Rahmadiawan, D.; Abral, H.; Railis, R.M.; Iby, I.C.; Mahardika, M.; Handayani, D.; Natrana, K.D.; Juliadmi, D.; Akbar, F. The Enhanced Moisture Absorption and Tensile Strength of PVA/Uncaria gambir Extract by Boric Acid as a Highly Moisture-Resistant, Anti-UV, and Strong Film for Food Packaging Applications. J. Compos. Sci. 2022, 6, 337. [Google Scholar] [CrossRef]
- Maurya, D.K.; Upadhyay, C.S.; Kumari, P. A Green Light-Weight Material for Packaging and Impact Resistant Structures. Ind. Crops Prod. 2024, 216, 118711. [Google Scholar] [CrossRef]
- Pathak, A.; Rao, N.R.; Grover, P.; Sharma, V.; Malik, A.; Rawat, A.P.; Singh, S.; Maurya, A. Ecofriendly Pharmaceutical Packaging Material: A Review. Mater. Today Proc. 2024, 103, 423–431. [Google Scholar]
- Matos, A.M.; Bonin, E.; Carvalho, V.M.; Duarte, V.; Tagiariolli, M.A.; Costa e Silva, L.F.; do Prado, R.M.; Vital, A.C.P.; do Prado, I.N. Effect of Film Packaging or Vacuum Packaging on Characteristics Before and After Maturation of Beef from Feedlot-Finished Cattle. PUBVET 2020, 14, 1–11. [Google Scholar]
- Li, H.; Pei, Q.-X.; Sha, Z.-D.; Branicio, P.S. Intrinsic and Extrinsic Effects on the Fracture Toughness of Ductile Metallic Glasses. Mech. Mater. 2021, 162, 104066. [Google Scholar] [CrossRef]
- Shukla, S.; Halli, P.; Khalid, M.K.; Lundström, M. Waste Pharmaceutical Blister Packages as a Source of Secondary Aluminum. Jom 2022, 74, 612–621. [Google Scholar] [CrossRef]
- Ciavarella, M.; Papangelo, A.; McMeeking, R. Crack Propagation at the Interface Between Viscoelastic and Elastic Materials. Eng. Fract. Mech. 2021, 257, 108009. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Namjoshi, S.N.; Telaprolu, K.C.; Jung, N.; Shewan, H.M.; Stokes, J.R.; Benson, H.A.E.; Grice, J.E.; Raney, S.G.; Rantou, E.; et al. Impact of Different Packaging Configurations on a Topical Cream Product. Pharm. Res. 2024, 41, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Pipliya, S.; Karunanithi, S.; Eswaran U, G.M.; Kumar, S.; Mandliya, S.; Srivastav, P.P.; Suthar, T.; Shaikh, A.M.; Harsányi, E.; et al. Migration of Chemical Compounds from Packaging Materials into Packaged Foods: Interaction, Mechanism, Assessment, and Regulations. Foods 2024, 13, 3125. [Google Scholar] [CrossRef] [PubMed]
- Galmán Graíño, S.; Sendón, R.; López Hernández, J.; Rodríguez-Bernaldo de Quirós, A. GC-MS Screening Analysis for the Identification of Potential Migrants in Plastic and Paper-Based Candy Wrappers. Polymers 2018, 10, 802. [Google Scholar] [CrossRef] [PubMed]
- Kwansa, A.L.; Pani, R.C.; DeLoach, J.A.; Tieppo, A.; Moskala, E.J.; Perri, S.T.; Yingling, Y.G. Molecular Mechanism of Plasticizer Exudation from Polyvinyl Chloride. Macromolecules 2023, 56, 4775–4786. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Lee, C.-F.; Chen, Y.-J.; Chang, G.-J.; Chong, K.-Y. Risks in Induction of Platelet Aggregation and Enhanced Blood Clot Formation in Platelet Lysate Therapy: A Pilot Study. J. Clin. Med. 2022, 11, 3972. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C. Evaluation of the Compatibility between Pharmaceutical Packaging Materials and Drugs Based on National Standards of Pharmaceutical Packaging Materials. Eng. Manag. 2025, 6, 267–269. [Google Scholar]
- Clotea, D.; Ungureanu, E.; Mustatea, G.; Popa, M. Chemical Contamination in Packaging Material of Pharmaceutical Use. In Bulletin of the Transilvania University of Brasov. Series I-Engineering Sciences; Transilvania University Press: Braşov, Romania, 2021; pp. 1–8. [Google Scholar]
- Gotardo, M.A.; Monteiro, M. Migration of diethylhexyl phthalate from PVC bags into intravenous cyclosporine solutions. J. Pharm. Biomed. Anal. 2005, 38, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, P.; Brunsteiner, M.; Khinast, J. Investigation of Migrant–Polymer Interaction in Pharmaceutical Packaging Material Using the Linear Interaction Energy Algorithm. J. Pharm. Sci. 2014, 103, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Cueff, R.; Chagnon, M.C.; Abdoulouhab, F.; Décaudin, B.; Breysse, C.; Kauffmann, S.; Cosserant, B.; Souweine, B.; Sautou, V.; et al. Migration of plasticizers from PVC medical devices: Development of an infusion model. Int. J. Pharm. 2015, 494, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Perou, A.L.; Laoubi, S.; Vergnaud, J.M. Effect of the thickness of food packages made of recycled and virgin polymer layers coextruded in sandwich form on the time of food protection. Comput. Theor. Polym. Sci. 1998, 8, 331–338. [Google Scholar] [CrossRef]
- Bai, F.; Chen, G.; Hu, Y.; Liu, Y.; Yang, R.; Liu, J.; Cai, H. Understanding the Effect of Plastic Food Packaging Materials on Food Flavor: A Critical Review. Trends Food Sci. Technol. 2024, 148, 104502. [Google Scholar] [CrossRef]
- Wei, B.; Zhao, Y.; Xu, M.S. Research Progress on the Relationship between Protein Structure Changes and in vitro Digestibility under Different Treatment Methods. Food Sci. 2019, 40, 327–333. [Google Scholar]
- Chen, S.; Quan, D.H.; Sam, G.; Ozberk, V.; Wang, X.T.; Halfmann, P.; Rehm, B.H.A. Assembly of Immunogenic Protein Particles toward Advanced Synthetic Vaccines. Small 2023, 19, 2205819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, D.; Wang, C.; Liu, Z.; Yang, M.; Cui, Z.; Chen, X. Shape Morphing of Plastic Films. Nat. Commun. 2022, 13, 7294. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhatt, S.; Shukla, P.; Kumar, A. Microbial Contamination in Pharmaceutical Manufacturing. J. Drug Discov. Health Sci. 2024, 1, 21–27. [Google Scholar]
- Saeed, M.; Ilyas, N.; Arshad, M.; Sheeraz, M.; Ahmed, I.; Bhattacharya, A. Development of a Plant Microbiome Bioremediation System for Crude Oil Contamination. J. Environ. Chem. Eng. 2021, 9, 105401. [Google Scholar] [CrossRef]
- Wada, S.; Satoh, Y.; Hama, T. Massive Loss and Microbial Decomposition in Reproductive Biomass of Zostera marina. Estuar. Coast. Shelf Sci. 2022, 275, 107986. [Google Scholar] [CrossRef]
- Luoreng, Z.-M.; Wang, X.-P.; Mei, C.-G.; Zan, L.-S. Comparison of microRNA Profiles between Bovine Mammary Glands Infected with Staphylococcus aureus and Escherichia coli. Int. J. Biol. Sci. 2018, 14, 87–99. [Google Scholar] [CrossRef] [PubMed]
- GB 50591-2010; Code for Construction and Acceptance of Cleanrooms. China Architecture & Building Press: Beijing, China, 2010.
- GB/T 16293-16294-2010; Test Method for Airborne Microbe in Clean Room (Zone) of the Pharmaceutical Industry. General Administration of Quality Supervision, Inspection and Quarantine of China & Standardization Administration of China: Beijing, China, 2010.
- Herber, O.R.; Gies, V.; Schwappach, D.; Thürmann, P.; Wilm, S. Patient Information Leaflets: Informing or Frightening? A Focus Group Study Exploring Patients’ Emotional Reactions and Subsequent Behavior towards Package Leaflets of Commonly Prescribed Medications in Family Practices. BMC Fam. Pract. 2014, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- White, N.D.; Kibalama, W. Prevention of Pediatric Pharmaceutical Poisonings. Am. J. Lifestyle Med. 2018, 12, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Nooshkam, M.; Zargar, M. Green Synthesis of Nanomaterials for Smart Biopolymer Packaging: Challenges and Outlooks. J. Nanostruct. Chem. 2024, 14, 113–136. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Ismail, F.R.M.; Manoharan, R.K.; Kim, S.S.; Lee, J. Blends of Gellan Gum/Xanthan Gum/Zinc Oxide Based Nanocomposites for Packaging Application: Rheological and Anti-Microbial Properties. Int. J. Biol. Macromol. 2020, 148, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.S.; Waghmare, R.D.; Pawar, S.P. Photophysical Insights of Highly Transparent, Flexible and Re-emissive PVA@WTR-CDs Composite Thin Films: A Next Generation Food Packaging Material for UV Blocking Applications. J. Photochem. Photobiol. A 2020, 400, 112647. [Google Scholar] [CrossRef]
- Razali, M.H.; Ismail, N.A.; Amin, K.A.M. Titanium Dioxide Nanotubes Incorporated Gellan Gum Bio-nanocomposite Film for Wound Healing: Effect of TiO2 Nanotubes Concentration. Int. J. Biol. Macromol. 2020, 153, 1117–1135. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiong, H.; Tang, S.; Zou, P. A Starch-Based Biodegradable Film Modified by Nano Silicon Dioxide. J. Appl. Polym. Sci. 2009, 113, 34–40. [Google Scholar] [CrossRef]
- Ramakanth, D.; Singh, S.; Maji, P.K. Advanced Packaging for Distribution and Storage of COVID-19 Vaccines: A Review. Environ. Chem. Lett. 2021, 19, 3597–3608. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Gupta, P. Internet of Things: The New Rx for Pharmaceutical Manufacturing and Supply Chains. In An Industrial IoT Approach for Pharmaceutical Industry Growth; Academic Press: London, UK, 2020; pp. 257–288. [Google Scholar]
- Saha, E.; Rathore, P.; Parida, R.; Rana, N.P. The Interplay of Emerging Technologies in Pharmaceutical Supply Chain Performance: An Empirical Investigation for the Rise of Pharma 4.0. Technol. Forecast. Soc. Change 2022, 181, 121799. [Google Scholar] [CrossRef]
- Woudhuysen, J.; Rivers, P. Rethinking packaging: How electronic packs for pharmaceuticals work with mobile IT to improve patient adherence to medication regimens. In Smart Design: First International Conference Proceedings; Springer: London, UK, 2012; pp. 1–11. [Google Scholar]
- Botcha, K.M.; Chakravarthy, V.V. Enhancing Traceability in Pharmaceutical Supply Chain Using Internet of Things (IoT) and Blockchain. In Proceedings of the 2019 IEEE International Conference on Intelligent Systems and Green Technology (ICISGT), Visakhapatnam, India, 29–30 June 2019; pp. 445–453. [Google Scholar]
- Kumarka, A.; Guptanv, B.; Lalasap, C. A Review on Packaging Materials with Anti-Counterfeit, Tamper-Evident Features for Pharmaceuticals. Int. J. Drug Dev. Res. 2013, 5, 26–34. [Google Scholar]
- Leem, J.W.; Jeon, H.; Ji, Y.; Park, S.M.; Kwak, Y.; Park, J.; Kim, K.; Kim, S.; Kim, Y.L. Edible Matrix Code with Photogenic Silk Proteins. ACS Cent. Sci. 2022, 8, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Lotoshynska, N.; Izonin, I.; Nazarkevych, M. Consumer-Centered Design of the Secondary Packaging for Industrial Pharmaceuticals. CIRP J. Manuf. Sci. Technol. 2021, 32, 257–265. [Google Scholar] [CrossRef]
- Wang, S.; Yu, P.; Fan, G. Application Research of Smart Pharmaceutical Packaging Materials in the Multisensory Dimension. Packag. Eng. 2022, 43, 76–87. [Google Scholar]
- Zadbuken, N.; Shahi, S.; Gulecha, B. Recent Trends and Future of Pharmaceutical Packaging Technology. J. Pharm. Bioallied Sci. 2013, 5, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xiong, L. Intelligent Design of Pharmaceutical Packaging under the Background of Healthy China Initiative. Packag. Eng. 2022, 43, 235–239. [Google Scholar]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B. Biopolymers and Composites: Properties, Characterization and Their Applications in Food, Medical and Pharmaceutical Industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Lin, H.; Tian, S.; Mei, L. Research Progress of Pharmaceutical Packaging Materials. Chin. J. Pharm. 2023, 54, 339–346. [Google Scholar]
- Mohammadi, S.; Babaei, A. Poly(vinyl alcohol)/Chitosan/Polyethylene Glycol-Assembled Graphene Oxide Bionanocomposites as a Prosperous Candidate for Biomedical Applications and Drug/Food Packaging Industry. Int. J. Biol. Macromol. 2022, 201, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.P.S.A.; Davoudpour, Y.; Saurabh, C.K. A Review on Nanocellulosic Fibres as New Material for Sustainable Packaging: Process and Applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Yuan, C.; Hou, H. Application and Prospect of Bioplastics in Food and Pharmaceutical Packaging. Chin. J. Pharm. 2020, 51, 1356–1363. [Google Scholar]
- Ren, Y.; Ren, J. Polylactic Acid Pharmaceutical Packaging Material, Its Preparation Method and Application. CN Patent 101914273A, 16 August 2010. [Google Scholar]
- Dejene, B.K.; Gudayu, A.D. Eco-Friendly Packaging Innovations: Integrating Natural Fibers and ZnO Nanofillers in Polylactic Acid (PLA) Based Green Composites—A Review. Polym.-Plast. Technol. Mater. 2024, 63, 1645–1681. [Google Scholar] [CrossRef]
- Zhu, X. Discussion on the Optimal Application of Pharmaceutical Packaging Materials and the Development Trend of New Materials. China Packag. 2022, 42, 30–34. [Google Scholar]
- Thyavihalli Girijappa, Y.G.; Mavinkere Rangappa, S.; Parameswaranpillai, J.; Siengchin, S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019, 6, 226. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Naik, L.L. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Nat. Resour. J. 2016, 7, 108–114. [Google Scholar]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites Reinforced with Natural Fibers: 2000–2010. Prog. Polym. Sci. 2014, 39, 9–26. [Google Scholar] [CrossRef]
- Xie, B.; Bai, R.R.; Sun, H.S. Research Progress on the Synthesis, Biodegradation and Waste Disposal of Polylactic Acid Plastics. Chin. J. Biotechnol. 2023, 39, 1912–1929. [Google Scholar]
- Cheng, Y.Z.; Ding, X.; Wu, Y. Research Progress on the Preparation of Bioplastics from Crop Wastes. Eng. Plast. Appl. 2022, 50, 172–176. [Google Scholar]
- Zhang, Z.C.; Sun, Z.Y.; Wang, X. Research Progress and Prospect of Cellulose-Based Biodegradable Plastics. Eng. Plast. Appl. 2022, 50, 168–172. [Google Scholar]
- Zhang, J.X.; Wang, Y.Z.; Chen, H. Research Progress of Bio-Based Plastics Based on Animal and Plant Proteins. Leather Sci. Eng. 2021, 31, 9–15. [Google Scholar]
- Lei, Y.J. Reading the “Script” of Plastic Reduction from the Source of Production and Use. Environ. Econ. 2021, 19, 18–23. [Google Scholar]
- Valant, J.; Drobne, D. Biological reactivity of TiO2 nanoparticles assessed by ex vivo testing. Protoplasma 2012, 249, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Hera, D.; Berndt, A.; Gunther, T.; Schmiel, S.; Harendt, C.; Zimmermann, A. Flexible packaging by film-assisted molding for microintegration of inertia sensors. Sensors 2017, 17, 1511. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.R.; de Azevedo, A.R.G.; Cecchin, D.; Marvila, M.T.; Amran, M.; Fediuk, R.; Vatin, N.; Karelina, M.; Klyuev, S.; Szelag, M. Application of Plastic Wastes in Construction Materials: A Review Using the Concept of Life-Cycle Assessment in the Context of Recent Research for Future Perspectives. Materials 2021, 14, 3549. [Google Scholar] [CrossRef] [PubMed]
- Kurbanova, R.V. Research of Structural Features and Properties of Dressed Hybrid Nanocomposites Based on Polyolefines. Inorg. Mater. 2020, 21, 258–267. [Google Scholar]
- Lahimer, M.C.; Ayed, N.; Horriche, J.; Belgaied, S. Characterization of plastic packaging additives: Food contact, stability and toxicity. Arab. J. Chem. 2017, 10 (Suppl. S2), S1938–S1954. [Google Scholar] [CrossRef]
- Perkola, N.; Äystö, L.; Hagström, M.; Kauppi, S.; Fjäder, P. Pharmaceutical residues in plastic tablet containers: Impacts on recycling and the environment. Waste Manag. 2024, 189, 159–165. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).