Abstract
Objective: Positron emission tomography with 2-deoxy-2-[fluorine-18] fluoro- D-glucose integrated with computed tomography (18F-FDG PET/CT) or magnetic resonance imaging (18F-FDG PET/MRI) has emerged as a promising tool for managing various types of cancer. This review study was conducted to investigate the role of 18F- FDG PET/CT and FDG PET/MRI in the management of gynecological malignancies. Search strategy: We searched for relevant articles in the three databases PubMed/MEDLINE, Scopus, and Web of Science. Selection criteria: All studies reporting data on the FDG PET/CT and FDG PET MRI in the management of gynecological cancer, performed anywhere in the world and published exclusively in the English language, were included in the present study. Data collection and analysis: We used the EndNote software (EndNote X8.1, Thomson Reuters) to list the studies and screen them on the basis of the inclusion criteria. Data, including first author, publication year, sample size, clinical application, imaging type, and main result, were extracted and tabulated in Excel. The sensitivity, specificity, and diagnostic accuracy of the modalities were extracted and summarized. Main results: After screening 988 records, 166 studies published between 2004 and 2022 were included, covering various methodologies. Studies were divided into the following five categories: the role of FDG PET/CT and FDG-PET/MRI in the management of: (a) endometrial cancer (n = 30); (b) ovarian cancer (n = 60); (c) cervical cancer (n = 50); (d) vulvar and vagina cancers (n = 12); and (e) gynecological cancers (n = 14). Conclusions: FDG PET/CT and FDG PET/MRI have demonstrated potential as non-invasive imaging tools for enhancing the management of gynecological malignancies. Nevertheless, certain associated challenges warrant attention.
1. Introduction
Gynecological malignancies, prevalent cancers affecting women globally, result in substantial morbidity and mortality [1,2,3]. Effective management and improved survival rates necessitate early diagnosis, accurate staging, and the prompt recurrence detection [4].
Various imaging modalities, including ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), functional MRI, and combined imaging with positron emission tomography (PET/MRI) and positron-emission-tomography-computed tomography (PET-CT), are employed for the management of common gynecologic malignancies, specifically cervical, endometrial, and ovarian malignancies [4]. Recent advancements in imaging, such as 18F-fluorodeoxyglucose positron-emission-computed tomography (18F-FDG PET/CT) and 18F-FDG-PET/MRI, have significantly contributed to the characterization and management of gynecological malignancies [5]. While the use of FDG PET/CT for gynecological cancer management is not mandatory, it has been recommended in the Danish Gynecological Cancer Ovarian Guidelines since 2009 for evaluating women with suspected ovarian cancer. FDG PET/CT aids in cancer diagnosis, metastatic lymph node detection, and identifying distant metastases [6].
Upon reviewing the literature, we identified conflicting data regarding the role of FDG PET/CT and FDG PET/MRI in gynecological cancers. These hybrid imaging techniques, in conjunction with robotic-assisted surgical tools and artificial intelligence (AI) applications for personalized treatment strategies, hold promise for achieving improved outcomes with fewer adverse effects. Thus, believing that emerging imaging technologies will play a pivotal role in gynecological cancer diagnosis and treatment, this study was designed to assess the proposed roles of FDG PET/CT and FDG PET/MRI in managing various types of gynecological cancers.
2. Materials and Methods
2.1. Search Strategy and Information Sources
We conducted a thorough search for pertinent articles in three databases: PubMed/MEDLINE, Scopus, and Web of Science. The following keywords were used for the search in March 2023: “gynecologic malignancies”, “gynecological neoplasms”, “gynecological tumors”, “management”, “hybrid imaging using 18F-FDG PET CT” and “18F- FDG PET/MRI”. MeSH keywords and Boolean (AND, OR) operators were employed to enhance the selection of entries.
2.2. Study Selection
We utilized EndNote software (EndNote X8.1 BLD 11010, Thomson Reuters) to compile and screen studies based on inclusion criteria. The study selection process consisted of three phases: screening, selection, and data extraction. During the screening phase, two trained authors (LA and AMM) evaluated titles and abstracts of all retrieved articles. Of these, 204 articles were selected for full-text review. Two authors (LA and HS) independently completed a checklist-style form and included articles meeting the inclusion criteria. Any discrepancies during the full-text review were resolved by a third expert (IA). We included all studies reporting data on FDG PET/CT and FDG PET/MRI in gynecological cancer management, published exclusively in English, without imposing a time limit on the search. Exclusions comprised review studies, in vitro or animal studies, studies related to cancer during pregnancy, and studies employing mathematical, deep learning, or radiomics models to assess the two imaging methods or quantitative parameters, like maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG).
2.3. Data Extraction and Synthesis
We extracted data from the included studies using a standardized form and recorded it in Excel. Extracted information included the first author, publication year, sample size, clinical application, imaging modality, and main result. Any disagreements were resolved through discussion, involving a third external colleague when necessary. Given the diversity in reporting methods, we conducted a narrative synthesis of the studies and summarized sensitivity, specificity, and diagnostic accuracy data.
3. Results
3.1. Search Results
In total, 988 publications were identified across various databases, of which 581 were duplicates. After evaluating the titles and abstracts of the remaining papers, 203 were excluded. Of the remaining articles, 38 were omitted due to a lack of alignment with the study objectives. Ultimately, the present review encompassed 166 studies (Figure 1) employing various methodological approaches.
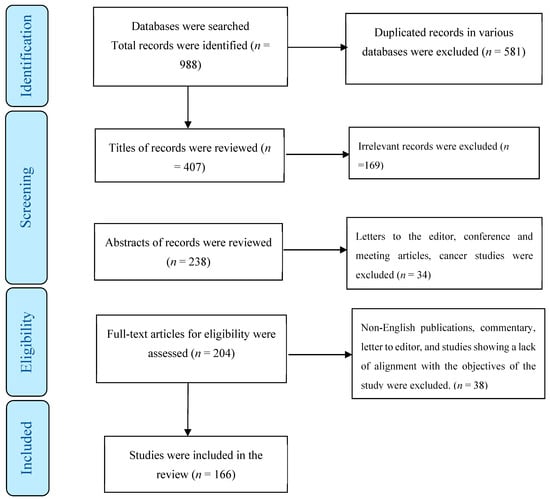
Figure 1.
The process of screening and selecting relevant studies.
3.2. Study Characteristics
One hundred and sixty-six studies met the criteria for inclusion in this current review. These studies were categorized into five groups as follows: (a) FDG PET/MRI and FDG PET/CT for the management of endometrial cancer (EC) (n = 30), (b) ovarian cancer (OC) (n = 60), (c) cervical cancer (CC) (n = 50), (d) vulvar and vagina cancers (n = 12), and (e) gynecological cancers (n = 14). Studies that did not report separate results for gynecological cancers were included in the fifth group.
3.2.1. FDG PET/CT and FDG PET/MRI in the Management of the EC
We identified 30 studies that examined the role of FDG PET/CT and FDG PET/MRI in managing EC. These studies were further categorized into the following subgroups: (a) the staging or diagnosis of primary EC (n = 9), (b) the diagnosis or prediction of recurrence of EC (n = 5), and (c) the detection or prediction of metastases of EC (n = 16).
Staging or Diagnosis of Primary EC
Five studies, involving a total of 506 patients, focused on staging EC using FDG PET/CT or FDG PET/MRI [7,8,9,10,11]. The sensitivity and specificity of FDG PET/CT for EC staging ranged from 90% to 93% and 49% to 96.3%, respectively [7,9]. In contrast, FDG PET/MRI exhibited a sensitivity and specificity of 77% and 84%, respectively [8]. The overall accuracy for staging was 86.0% for PET/MRI and 77.2% for PET/CT [11]. Additionally, four eligible FDG PET/CT studies, involving 158 patients, addressed the diagnosis of primary EC [12,13,14,15]. The sensitivity and specificity of FDG PET/CT for EC diagnosis ranged from 90.6% to 98.1% and 33.3% to 99%, respectively [12,14,15]. The overall accuracy of EC diagnosis with FDG PET/CT was 94.6% [12]. Bezzi and colleagues conducted a systematic review and meta-analysis on the use of hybrid FDG PET/MR imaging for EC staging/re-staging, analyzing eleven articles. Their results indicated that FDG PET/MRI is a valid imaging technique for EC patients, both for staging and re-staging, while also potentially reducing radiation exposure [16]. However, the limited availability in the literature of studies on FDG PET/MRI underscores the need for more prospective trials with larger and more homogeneous cohorts.
Diagnosis or Prediction of Recurrence of EC
In this study, we evaluated the diagnostic accuracy of FDG PET/CT in cases of recurrent EC. Five eligible FDG PET/CT studies, involving 345 patients, were included in the assessment of recurrent EC diagnosis [17,18,19,20,21]. The sensitivity and specificity of FDG PET/CT in studies were reported as being greater than 88.9% and 93.6%, respectively. The overall accuracy for EC diagnosis with FDG PET/CT ranged from 91% to 97% [18,19,20,21]. In a systematic review by Bollineni et al. in 2016, F-FDG PET/CT exhibited excellent diagnostic performance for detecting postoperative recurrence in EC patients [22].
Detection or Prediction of Metastases of EC
The diagnostic value of FDG PET/CT and FDG PET/MRI in detecting EC metastases was assessed through 15 studies involving 1904 patients [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. The sensitivity and specificity for FDG PET/CT ranged from 45.4% to 100% and 66.67% to 99.8%, respectively [23,24,25,26,27,28,29,30,31,32,33]. In contrast, FDG PET/MRI demonstrated a sensitivity of 100% and specificity of 96.9% for detecting regional nodal metastasis [38]. The range of overall accuracy of diagnosis of EC for FDG PET/MRI was 81.8–97% [36,38].
A 2016 systematic review and meta-analysis by Bollineni et al. aimed to investigate the diagnostic performance of (18)F-FDG PET/CT in the preoperative evaluation of endometrial cancer recurrence (ECR) and lymph node metastases (LNM) in EC patients. Their analysis included 21 studies. The pooled sensitivity, specificity, positive likelihood ratio (LH+), negative likelihood ratio (LH-), diagnostic odds ratio (DOR), and area under the ROC curve (AUC) for F-FDG PET/CT in detecting ECR were 0.95, 0.91, 8.8, 0.08, 171.7, and 0.97, respectively. For the diagnosis of LNM, these values were 0.72, 0.94, 10.9, 0.36, 39.7, and 0.94, respectively [22]. In a 2021 study by Bezzi et al., PET/MRI was found to offer an opportunity to distinguish post-treatment changes from local recurrence and to detect small and indeterminate lymph node metastases in patients with recurrent EC, showing superior performance compared to other imaging modalities [16]. Based on the literature review, it can be concluded that FDG PET/CT exhibits appropriate diagnostic performance for the preoperative detection of LNM in EC patients [22].
Metastasis contributes to the vast majority of cancer-related mortality and the regulatory mechanisms of the multistep invasion–metastasis cascade are gradually being uncovered. The tumor suppressor p53 is the most frequently mutated gene in human cancers. Accumulating evidence suggests that TP53 mutations not only result in loss-of-function or dominant negative effects but also promote gain-of-function characteristics. Specifically, gain-of-function mutant p53 has been implicated in enhancing cancer cell motility, invasion, and metastasis [39]. The molecular classification of EC has independent prognostic value, particularly among women with high-grade EC. P53-aberrant EC is associated with poor clinical outcomes [40]. However, it remains unclear whether PET/CT or PET/MRI can further predict distant metastasis in patients with p53 mutations, and this issue warrants further investigation in future studies.
The roles of PET/CT and PET/MRI in the management of the EC are summarized in Table 1.

Table 1.
FDG PET/CT and FDG-PET/MRI in management of endometrial cancer (EC).
3.2.2. FDG PET/CT and FDG PET/MRI in the Management of OC
We identified 60 studies that examined the utilization of FDG PET/MRI and FDG PET/CT in the management of ovarian cancer (OC). These studies were categorized into the following subgroups: (a) the staging and diagnosis performance of OC (n = 12), (b) the prediction of the optimal primary treatment/treatment prognosis and response of OC (n = 6), (c) the diagnosis or prediction of recurrence of OC (n = 31), (d) the detection or prediction of metastases of OC (n = 9), and (e) the prediction of OC survival (n = 2).
Staging and Diagnosis Performance of OC
We reviewed three FDG PET/CT studies involving a total of 102 patients aimed at staging OC [41,42,43]. The sensitivity and specificity of the pre-surgical staging of OC was 78% and 68%, respectively [43]. Additionally, FDG PET/CT and FDG PET/MRI demonstrated accuracy rates of 71% and 92.5%, respectively, in the peritoneal staging and characterization of suspected OC [41]. FDG PET/MRI exhibited an accuracy of 92.5% for characterizing suspected OC [44]. Moreover, eight eligible FDG PET/CT studies, with a total of 766 patients, addressed the diagnosis of OC. The sensitivity and specificity of FDG PET/CT for OC diagnosis ranged from 82.4% to 94.7% and 76.9% to 100%, respectively [45,46,47,48,49,50,51,52]. FDG PET/CT showed an accuracy of 81.1% to 92.9% for detecting primary OC [46,48,49,51]. In a systematic review by Suppiah and et al., 23 studies regarding the role of PET/CT in the diagnosis (10 articles, 734 patients) and staging (13 studies, 604 patients) were reviewed and the range of sensitivity and specificity of PET/CT in the diagnosis of OC was 82.4–100%% and 33.0–78.9%, respectively [53]. Although CT remains the preferred imaging modality for assessing ovarian cancer, PET/CT appears advantageous for staging and follow-up in OC [54].
Prediction Optimal Primary Treatment/Treatment Prognosis and Response of OC
We assessed two FDG PET/CT studies involving a total of 75 patients that aimed to predict optimal primary treatment in OC patients [55,56]. These studies reported sensitivity, specificity, and accuracy values of 91%, 67%, and 86%, respectively, for characterizing ovarian masses with PET/CT [55]. Additionally, results from four FDG PET/CT studies, including 134 patients, focused on assessing treatment prognosis and response in OC [57,58,59,60]. These studies demonstrated that FDG PET/CT is valuable for evaluating neoadjuvant chemotherapy response [57,59,60].
Diagnosis or Prediction of Recurrence of OC
In the present study, we evaluated the diagnostic accuracy of FDG PET/CT for recurrent OC. We reviewed 30 FDG PET/CT studies, which reported sensitivity and specificity values ranging from 40.7% to 100% and 55.5% to 100%, respectively. FDG PET/CT exhibited an accuracy of 72% to 97% for detecting recurrent OC [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. A meta-analysis conducted in 2013 estimated the diagnostic accuracy of PET/CT for suspected recurrent OC by reviewing 29 studies (1651 patients with OC). The pooled sensitivity, specificity, positive likelihood ratio (LH+), negative likelihood ratio (LH-), and diagnostic odds ratio (DOR) for recurrent OC were 88.6%, 90.3%, 6.104, 0.122, and 57.032, respectively [92]. These findings suggest that FDG PET/CT is a valuable tool for predicting the diagnosis and restaging of suspected recurrent OC.
Detection or Prediction of Metastases of OC
Nine FDG PET/CT studies involving 746 patients focused on diagnosing metastases in OC [42,93,94,95,96,97,98,99,100]. These studies reported sensitivity and specificity values of 64% to 96.2% and 90% to 98.2%, respectively, for FDG PET/CT in diagnosing peritoneal carcinomatosis, distant metastases, and detecting nodal metastases [42,94,95,96,98]. FDG PET/CT showed an accuracy of 88.6% to 95.6% for diagnosing OC metastasis [94,96]. A systematic review conducted in 2012 compared the diagnostic performance of PET/CT, CT, and MR for detecting metastatic lymph nodes in OC patients. This review, encompassing 18 relevant studies with a total of 882 patients, revealed that PET/CT was more accurate, with a sensitivity, specificity, and odds ratio (OR) of 73.2%, 96.7%, and 90.32, respectively [101].
A meta-analysis in 2022 reported that the sensitivity, specificity, and area under the ROC curve (AUC) of FDG PET/CT for diagnosing epithelial OC recurrence were 0.88, 0.89, and 0.94, respectively [102]. Collectively, these results suggest that FDG-PET or FDG PET/CT is more accurate than CT and MR imaging for detecting lymph node metastasis in OC patients, although its superiority for intra-abdominal disease spread remains debatable, while, in a study by Hynninen et al., PET/CT was not superior to CT for the detection of intra-abdominal disease spread but was more effective for the detection of extra-abdominal disease than CT [42].
Prediction of OC Survival
The role of FDG PET/CT in the prediction of OC survival was evaluated in the present study. We found that a negative FDG PET/CT has a high negative predictive value for disease presence and is notably associated with a very good disease-specific survival outcome [103,104]. The roles of FDG PET/CT and FDG-PET/MRI in the management of the OC are summarized in Table 2.

Table 2.
FDG PET/CT and FDG-PET/MRI in management of ovarian cancer (OC).
3.2.3. FDG PET/CT and FDG PET/MRI in the Management of CC
We encountered 50 studies that have delved into the utilization of FDG PET/CT and FDG PET/MRI for managing cervical cancer (CC). These studies were categorized into the following groups: (a) the staging or diagnosis of primary CC (n = 12), (b) the treatment prognosis and prediction of response to treatment of CC (n = 8), (c) the diagnosis or prediction of recurrence of CC (n = 3), (d) the detection or prediction of CC metastasis (n = 21), and (e) the prognostic value and prediction of survival of CC (n = 6).
Staging or Diagnosis of Primary CC
In the present study, we evaluated the diagnostic accuracy of FDG PET/CT versus FDG PET/MRI in staging and diagnosing CC. A total of eight FDG PET/CT or PET/MRI studies focusing on CC staging were reviewed [106,107,108,109,110,111,112,113]. The study by Anner et al. in 2018 found that PET/MRI exhibited slightly superior results compared to PET/CT in terms of specificity (77% vs. 69%), positive predictive value (PPV) (75% vs. 69%), and negative predictive value (NPV) (67% vs. 64%) [106]. Conversely, Lazzari et al.’s 2014 study favored FDG PET/CT for CC staging and disease definition [109]. The sensitivity and specificity of FDG PET/MRI in CC staging across studies ranged from 77% to 91% and 90% to 94% [106,110,111].
For diagnosing CC, five eligible studies utilizing FDG PET/CT and FDG PET/MRI with a total of 188 patients were included [114,115,116,117,118]. The sensitivity and specificity of FDG PET/CT in CC diagnosis ranged from 75% to 86.66% and 44.4% to 96%, respectively [115,118]. The diagnostic accuracy of PET/CT for early-stage CC was reported as 95.45% [117]. In contrast, the accuracy, sensitivity, and NPV of FDG PET/MRI in CC diagnosis were 78.5%, 64.9%, and 74.5%, respectively [116]. Given that the present study is the first to address the diagnostic accuracy of FDG PET/CT versus FDG PET/MRI in CC staging and diagnosis, direct data comparison is not feasible. MRI has higher imaging sensitivity in detecting the area in which the cervical cancer involved, while PET imaging can provide the favorable detection of cervical cancer [119]. It is unknown whether MRI, with its superior soft-tissue contrast, can improve the combined accuracy of a PET/MRI scanner as compared to PET/CT in detecting malignant lymph nodes [120] or whether it is useful in image-guided adaptive brachytherapy [121]. Further in-depth studies are recommended to explore this issue comprehensively.
Treatment Prognosis and Prediction of Response to Treatment of CC
Eight eligible FDG PET/CT studies involving 728 patients were analyzed to assess the role of FDG PET/CT in treatment prognosis and in predicting the response to CC treatment [122,123,124,125,126,127,128,129]. Draghini et al.’s 2019 study found that FDG PET/CT significantly influenced stage assessment and radiotherapy treatment planning due to its high specificity in detecting distant metastases and nodal involvement [128]. The sensitivity and specificity of PET/CT in detecting or predicting residual local and regional disease ranged from 20% to 94.8% and 62% to 100% [122,129,130]. The accuracy of PET/CT for detecting residual local and regional disease was reported as 89% [130]. In a 2012 study by Chang et al., post-treatment PET/CT scans were deemed sensitive and accurate for monitoring and providing prognostic information in CC [129]. A systematic review in 2021 assessed the diagnostic accuracy of FDG PET/CT in predicting tumor response in chemotherapy-treated advanced cervical cancer (LACC). This review, covering 15 articles with 1132 patients published from 2010 to 2020, reported sensitivities and specificities of PET/CT at 83.5% and 77.8%, respectively. The diagnostic sensitivity and specificity of PET/CT for detecting residual tumor were 86% and 95%, respectively [131]. While FDG PET/CT shows promise in assessing treatment response, further studies are needed to establish it as a standard option for evaluating treatment response.
Diagnosis or Prediction of Recurrence of CC
In our study, we included three eligible FDG PET/CT studies with a total of 74 patients focused on diagnosing recurrence [132,133,134]. The sensitivity, specificity, and accuracy of PET/CT for detecting recurrent CC were 90.3%, 81.0%, and 86.5%, respectively [134]. A systematic review and meta-analysis conducted by Chu et al. in 2014 assessed the diagnostic value of PET or FDG PET-CT in recurrent CC. Twenty studies were included, and the meta-analysis indicated pooled sensitivity and specificity values of 0.82 and 0.98, respectively, for local-regional recurrence [135]. In summary, FDG-PET has demonstrated its value in assessing recurrent CC.
Detection or Prediction of CC Metastasis
This study examined the diagnostic value of FDG PET/CT in detecting pelvic lymph nodal and para-aortic nodal metastasis of CC through 21 eligible FDG PET/CT studies comprising 1729 CC patients [136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156]. The sensitivity and specificity of FDG PET/CT in detecting CC metastasis ranged from 28.6% to 92.8% and 58.33% to 98.8%, respectively [136,137,138,139,140,141,142,143,144,145,146,147,148,150,152,153,154,155,156]. The accuracy of PET/CT in detecting CC metastasis was reported to range from 65.1% to 99.3% [136,142,143,144,148,150]. Adam and co-workers conducted a systematic review evaluating FDG-PET/CT’s performance for lymph node metastases in LACC, reviewing twelve studies with 778 patients. For pelvic nodes, summary estimates of sensitivity, specificity, LR+, and LR- were 0.88, 0.93, 11.90, and 0.13, respectively [157]. It is important to note that PET/CT plays a valuable role in guiding radiation field planning for locally advanced cervical cancer. PET/CT can effectively detect pelvic nodes and guide clinicians in making decisions regarding radiation fields to eliminate potential microscopic para-aortic metastases [158,159].
Prognostic Value and Prediction of Survival of CC
We explored the prognostic value of FDG PET/CT in CC through six studies involving 571 patients [160,161,162,163,164,165]. The estimated 5-year overall survival (OS), disease-free survival (DFS), and local control (LC) rates ranged from 81% to 82%, 70% to 74%, and 84.4% to 86%, respectively [162,165]. The extent of lymph node involvement in para-aortic, retrocrural, and supraclavicular areas was identified as a significant prognostic factor for CC progression [160,161,163]. A systematic review by Han et al. in 2018 analyzed twelve studies comprising 660 patients and indicated that volume-based FDG PET/CT parameters were significant prognostic factors in CC patients [166]. In summary, FDG PET/CT stands out as the preferred method for staging advanced CC. In addition, in locally advanced CC, FDG PET/CT may be used for staging, in planning for radiotherapy, and in evaluating treatment response, disease recurrence, and long-term survival [167]. The role of FDG PET/CT and FDG-PET/MRI for the management of CC is summarized in Table 3.

Table 3.
FDG PET/CT and FDG-PET/MRI in management of cervical cancer (CC).
3.2.4. FDG PET/CT in the Management of Vulvar and Vagina Cancers
We identified 12 studies that focused on the use of FDG PET/CT in the management of vulvar and vaginal cancers. These studies were categorized into the following groups: (a) the staging or diagnosis performance of vulvar and vaginal cancer (n = 8), (b) the recurrence of vulvar (n = 1), and (c) the detection and prediction of metastases of the vulvar and vaginal cancer (n = 3).
Staging or Diagnosis Performance of Vulvar and Vaginal Cancer
In our current investigation, we evaluated the diagnostic accuracy of FDG PET/CT for staging and diagnosing vulvar and vaginal cancers [168,169,170,171,172,173,174,175]. A total of four FDG PET/CT studies involving 243 patients, which aimed to stage vulvar cancer, were examined. The range of sensitivity, specificity, and accuracy of FDG PET/CT for vulvar staging was found to be 50–100%, 65.5–89%, and 59–82.5%, respectively [168,169,170,171]. Additionally, four eligible FDG PET/CT studies with a total of 79 patients were included in the diagnosis of vulvar and vaginal cancers [172,173,174,175]. FDG PET/CT exhibited a sensitivity of 95% and a PPV of 100% in identifying the primary tumor [173]. In the study by Peiro et al., PET/CT demonstrated a sensitivity of 100% for detecting vulvar lesions in squamous cell carcinomas and 60% in non-squamous cell carcinomas [174]. A systematic review conducted by Triumbari et al. in 2021 assessed the role of FDG PET/CT in vulvar cancer patients and provided summary estimates of its diagnostic performance for preoperative lymph node staging. The review of ten articles revealed sensitivity, specificity, PPV, NPV, and DOR values of 0.70, 0.90, 0.86, 0.77, and 10.49, respectively [176].
Recurrence of Vulvar Cancer
In our study, we also investigated the role of FDG PET/CT in detecting recurrences of vulvar cancer. In a retrospective multicentric study, PET/CT showed sensitivity, specificity, PPV, NPV, and accuracy values of 100%, 92%, 98%, 100%, and 98%, respectively. FDG PET/CT proved to be a precise tool for assessing recurrent vulvar cancer with high sensitivity and specificity, significantly impacting clinical decision-making [177]. However, due to the limited number of additional studies available, a direct data comparison was not feasible.
Detection and Prediction of Metastases Vulvar and Vaginal Cancer
The diagnostic value of FDG PET/CT in detecting and predicting metastases in vulvar and vaginal cancer was another aspect examined in our study. A total of three FDG PET/CT studies involving 180 patients, aimed at detecting and predicting metastases in vulvar cancer, were reviewed [178,179,180]. The range of sensitivity, specificity, and accuracy of FDG PET/CT in detecting metastases in vulvar and vaginal cancer was found to be 56–89%, 67–88%, and 74–84%, respectively [178,179]. In summary, our study delved into the various aspects of FDG PET/CT’s role in managing vulvar and vaginal cancers, including the staging, diagnosing, assessing of recurrences, and detecting of metastases. These findings shed light on the potential of FDG PET/CT as a valuable tool in the management of these cancers, although further research is needed to expand our understanding of its full capabilities in these domains.
Three domains of FDG PET/CT and FDG-PET/MRI in the management of vulvar and vaginal cancer are summarized in Table 4.

Table 4.
FDG PET/CT in management of vulvar and vagina cancers.
3.2.5. FDG PET/MRI and FDG PET/CT in the Management of Gynecological Cancer
We identified 15 studies that investigated the use of FDG PET/MRI and FDG PET/CT in the management of gynecological cancers. These studies were categorized into the following groups: (a) the diagnosis of primary or recurrent gynecological cancers (n = 10) and (b) the detection and prediction of metastases of gynecological cancers (n = 4).
Diagnosis of Primary or Recurrent Gynecological Cancers
We reviewed a total of nine FDG PET/CT and FDG-PET/MRI studies focused on diagnosing gynecological cancers [181,182,183,184,185,186,187,188,189,190]. The sensitivity and specificity of FDG PET/CT for detecting malignant or borderline malignant pelvic tumors ranged from 50% to 71.4% and 81.3% to 93.9%, respectively [183,184]. Notably, Sawicki et al. found that FDG-PET/MRI correctly identified cancer recurrence in 100% of patients with a diagnostic accuracy of 99.2% [186]. Additionally, Schwartz et al. (2018) suggested that FDG-PET/MRI may be superior to FDG-PET/CT for the early radiographic evaluation of cervical cancers, with at least comparable diagnostic ability for detecting primary cervical and endometrial tumors and regional metastases [187]. Kirchner et al. (2017) reported sensitivity and specificity values of 50% and 58% for FDG-PET/MRI and 98% and 83% for FDG-PET/CT in diagnosing gynecologic cancers [188]. FDG PET/MRI appears to be a promising diagnostic modality for primary tumors, nodal staging, and recurrence in patients with pelvic gynecologic malignancies.
A systematic literature review by Virarkar et al. in 2020 evaluated the diagnostic performance of FDG PET/MRI for gynecological cancers, reporting sensitivity, specificity, DOR, and AUC values of 74.2%, 89.8%, 26, and 0.834, respectively [191]. Another meta-analysis by Virarkar et al. in 2020 compared the diagnostic performance of FDG PET/CT versus FDG PET/MRI for gynecological pelvic malignancies. This review of nine studies published up to 2019 found sensitivity and specificity values of 62.6% and 91.6% for PET/CT and 73.3% and 91.2% for PET/MRI, respectively, with no significant difference in DOR between the two modalities [192]. Compared with PET/CT, PET/MRI seems to have slightly better diagnostic performance than FDG PET/CT in gynecological malignancies. It is essential to note that the evaluation of the female pelvic region using FDG-PET can be affected by various factors, including the menstrual cycle and menopausal status. Incidental findings in the female genital region can also pose diagnostic challenges due to potential false positives and false negatives, particularly in cases of infections and post-therapy changes [193].
Detection and Prediction of Metastases of Gynecological Cancers
We also assessed the diagnostic value of FDG PET/CT in detecting and predicting metastases in gynecological cancers through four studies [190,194,195,196,197]. Jónsdóttir et al. reported sensitivity and specificity values of 43.5% to 100% and 50% to 100%, respectively, for PET/MRI in detecting and predicting metastases in the small bowel regions [197]. Gee et al. found that PET/CT demonstrated sensitivity and specificity values of 54.8% and 97.7% for CC metastasis and 64.6% and 98.6% for EC metastasis, respectively [194]. Kitajima et al. reported sensitivity, specificity, and accuracy values of 91.3%, 100%, and 93.3%, respectively, for PET/CT in detecting intra-pelvic recurrence/metastasis [190]. However, Bentivegna et al. observed a low accuracy of FDG PET/CT in predicting pelvic nodal status for patients with early-stage cervical and vaginal cancer [196]. A systematic review by Liu et al. in 2023, which included 13 studies with 473 patients and 2775 lesions, calculated detection rates for FDG PET/CT in the primary staging and recurrence assessment of pelvic cancers. The detection rate for early-stage assessment was 0.91, while it was 0.56 for the recurrence assessment of pelvic cancer. The sensitivity and specificity for detecting distant or lymph node metastases were 0.525 and 0.821, respectively [198]. In summary, FDG PET/CT and FDG-PET/MRI show promise in diagnosing primary and recurrent gynecological cancers, with some variations in sensitivity and specificity. These modalities also have potential in detecting metastases in pelvic cancers, although diagnostic challenges should be considered. Further research is needed to fully understand the diagnostic capabilities and nuances of these imaging techniques in the management of gynecological cancers. The domains of FDG PET/CT and FDG-PET/MRI in the management of gynecological cancers are summarized in Table 5.

Table 5.
FDG PET/CT and FDG-PET/MRI in management of gynecological cancers.
Limitation and Strengths
The primary limitation of this study arises from the diverse methods and patient characteristics reported in the included studies, resulting in substantial heterogeneity in sensitivity, specificity, and diagnostic accuracy estimates.
Nonetheless, despite this limitation, the comprehensive review of published studies involving FDG PET/CT and FDG-PET/MRI methods still provides valuable insights into their roles in the management of gynecological cancers.
4. Conclusions
The early detection of gynecological malignancies plays a pivotal role in patient management and can significantly improve survival rates. This article offers an updated review of all the relevant studies on the application of FDG PET/CT and FDG PET/MRI in gynecological malignancies. Since 2004, FDG PET/CT has been increasingly employed for various purposes, including tumor staging, primary tumor diagnosis, recurrence prediction, metastasis detection, treatment prognosis assessment, and prognostic value prediction in gynecological malignancies. Furthermore, PET/MRI, as a promising “one-stop shop” approach, has been explored in patients with different gynecologic malignancies since 2008. These studies have evaluated the role of PET/MRI in staging, primary tumor diagnosis, and recurrence and metastasis detection in gynecologic malignancies. According to the available literature, FDG PET/MRI shows promise in enhancing cancer staging. However, its potential impact on prognosis, clinical decision-making, and treatment strategies for gynecological cancers remains to be fully determined. The development of rapid acquisition protocols and optimized attenuation correction methods is essential in this regard.
Despite the numerous advantages of PET/MRI and PET/CT in managing gynecological cancers, they come with challenges. These include limited capability to detect early-stage or small tumors, a risk of high false-negative results, and relatively low specificity [199]. Additionally, distinguishing tumors from benign lesions, such as corpus luteum cysts, dermoid cysts, and serous cysts, remains a challenge for these imaging modalities [199,200,201].
Another hurdle to consider is the cost-effectiveness of these imaging methods. Given the higher equipment cost, longer examination time, and expertise required, the incremental cost of PET/MRI is currently estimated to be as high as USD 4000 per examination. Nonetheless, the use of these modalities should be justified by their potential to improve the survival rates of patients with gynecological cancers, outweighing the additional costs and the exposure to ionizing radiation associated with their use. Consequently, numerous prospective studies employing standardized methods are needed before hybrid molecular imaging can be established as the primary imaging approach for gynecological cancers.
Author Contributions
Conceptualization, L.A.; methodology, L.A., S.H., A.S.L., A.M., H.S., M.M.G. and I.A., data curation, L.A., A.M., Z.M., A.R., A.F., M.M.G. and I.A., writing-original draft preparation, L.A., A.M., H.S., Z.M. and I.A., writing—review and editing: L.A., A.S.L., A.R., Z.M. and I.A., supervision. L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Momenimovahed, Z.; Mazidimoradi, A.; Banakar, N.; Allahqoli, L.; Salehiniya, H. Temporal Trends of Ovarian Cancer Between 1990 and 2019, in Asian Countries by Geographical Region and SDI, Comparison with Global Data. Indian J. Gynecol. Oncol. 2023, 21, 38. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Mazidimoradi, A.; Maroofi, P.; Allahqoli, L.; Salehiniya, H.; Alkatout, I. Global, regional and national burden, incidence, and mortality of cervical cancer. Cancer Rep. 2023, 6, e1756. [Google Scholar] [CrossRef] [PubMed]
- Mazidimoradi, A.; Momenimovahed, Z.; Allahqoli, L.; Tiznobaik, A.; Hajinasab, N.; Salehiniya, H.; Alkatout, I. The global, regional and national epidemiology, incidence, mortality, and burden of ovarian cancer. Health Sci. Rep. 2022, 5, e936. [Google Scholar] [CrossRef] [PubMed]
- Daoud, T.; Sardana, S.; Stanietzky, N.; Klekers, A.R.; Bhosale, P.; Morani, A.C. Recent Imaging Updates and Advances in Gynecologic Malignancies. Cancers 2022, 14, 5528. [Google Scholar] [CrossRef]
- Virarkar, M.; Vulasala, S.S.; Calimano-Ramirez, L.; Singh, A.; Lall, C.; Bhosale, P. Current Update on PET/MRI in Gynecological Malignancies—A Review of the Literature. Curr. Oncol. 2023, 30, 1077–1105. [Google Scholar] [CrossRef]
- Viswanathan, C.; Bhosale, P.R.; Shah, S.N.; Vikram, R. Positron emission tomography-computed tomography imaging for malignancies in women. Radiol. Clin. N. Am. 2013, 51, 1111–1125. [Google Scholar] [CrossRef]
- Vural Topuz, Ö.; Aksu, A.; Erinç, S.R.; Tokgözoğlu, N.; Tamam, M. The Evaluation of Preoperative (18)F-FDG PET/CT in Patients with Endometrial Cancer and the Correlation between PET Parameters and Postoperative Pathology Results. Mol. Imaging Radionucl. Ther. 2022, 31, 16–22. [Google Scholar] [CrossRef]
- Ironi, G.; Mapelli, P.; Bergamini, A.; Fallanca, F.; Candotti, G.; Gnasso, C.; Taccagni, G.L.; Sant’Angelo, M.; Scifo, P.; Bezzi, C.; et al. Hybrid PET/MRI in Staging Endometrial Cancer: Diagnostic and Predictive Value in a Prospective Cohort. Clin. Nucl. Med. 2022, 47, e221–e229. [Google Scholar] [CrossRef]
- Antonsen, S.L.; Jensen, L.N.; Loft, A.; Berthelsen, A.K.; Costa, J.; Tabor, A.; Qvist, I.; Hansen, M.R.; Fisker, R.; Andersen, E.S.; et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer—A multicenter prospective comparative study. Gynecol. Oncol. 2013, 128, 300–308. [Google Scholar] [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Takahashi, S.; Ebina, Y.; Miyahara, Y.; Yamada, H.; Sugimura, K. Value of fusion of PET and MRI for staging of endometrial cancer: Comparison with ¹⁸F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur. J. Radiol. 2013, 82, 1672–1676. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Sultana, B.; Wang, B.; Sun, H. Diagnostic value of integrated 18F-FDG PET/MRI for staging of endometrial carcinoma: Comparison with PET/CT. BMC Cancer 2022, 22, 947. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.-Y.; Lee, J.J.; Kim, M.H.; Kim, D.-Y.; Suh, D.-S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Comparison of MRI and 18F-FDG PET/CT in the preoperative evaluation of uterine carcinosarcoma. Gynecol. Oncol. 2016, 140, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Sudo, S.; Hattori, N.; Manabe, O.; Kato, F.; Mimura, R.; Magota, K.; Sugimori, H.; Hirata, K.; Sakuragi, N.; Tamaki, N. FDG PET/CT diagnostic criteria may need adjustment based on MRI to estimate the presurgical risk of extrapelvic infiltration in patients with uterine endometrial cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 676–684. [Google Scholar] [CrossRef]
- Boonya-ussadorn, T.; Choi, W.H.; Hyun, J.; Kim, S.H.; Chung, S.K.; Yoo, I. 18F-FDG PET/CT findings in endometrial cancer patients: The correlation between SUVmax and clinicopathologic features. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2014, 97, S115–S122. [Google Scholar]
- Picchio, M.; Mangili, G.; Samanes Gajate, A.M.; De Marzi, P.; Spinapolice, E.G.; Mapelli, P.; Giovacchini, G.; Sigismondi, C.; Viganò, R.; Sironi, S.; et al. High-grade endometrial cancer: Value of [(18)F]FDG PET/CT in preoperative staging. Nucl. Med. Commun. 2010, 31, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, C.; Zambella, E.; Ghezzo, S.; Fallanca, F.; Samanes Gajate, A.M.; Franchini, A.; Ironi, G.; Bergamini, A.; Monaco, L.; Evangelista, L.; et al. 18 F-FDG PET/MRI in endometrial cancer: Systematic review and meta-analysis. Clin. Transl. Imaging 2021, 10, 45–58. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, R.; Singh, H.; Jeph, S.; Sharma, D.N.; Bal, C.; Malhotra, A. Carcinoma endometrium: Role of 18-FDG PET/CT for detection of suspected recurrence. Clin. Nucl. Med. 2012, 37, 649–655. [Google Scholar] [CrossRef]
- Ozcan Kara, P.; Kara, T.; Kaya, B.; Kara Gedik, G.; Sari, O. The value of FDG-PET/CT in the post-treatment evaluation of endometrial carcinoma: A comparison of PET/CT findings with conventional imaging and CA 125 as a tumour marker. Rev. Esp. Med. Nucl. Imagen Mol. 2012, 31, 257–260. [Google Scholar]
- Chung, H.H.; Kang, W.J.; Kim, J.W.; Park, N.-H.; Song, Y.-S.; Chung, J.-K.; Kang, S.B. The clinical impact of [18 F] FDG PET/CT for the management of recurrent endometrial cancer: Correlation with clinical and histological findings. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1081–1088. [Google Scholar] [CrossRef]
- Sironi, S.; Picchio, M.; Landoni, C.; Galimberti, S.; Signorelli, M.; Bettinardi, V.; Perego, P.; Mangioni, C.; Messa, C.; Fazio, F. Post-therapy surveillance of patients with uterine cancers: Value of integrated FDG PET/CT in the detection of recurrence. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 472–479. [Google Scholar] [CrossRef]
- Albano, D.; Zizioli, V.; Odicino, F.; Giubbini, R.; Bertagna, F. Clinical and prognostic value of (18)F-FDG PET/CT in recurrent endometrial carcinoma. Rev. Esp. Med. Nucl. Imagen Mol. 2019, 38, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bollineni, V.R.; Ytre-Hauge, S.; Bollineni-Balabay, O.; Salvesen, H.B.; Haldorsen, I.S. High Diagnostic Value of 18F-FDG PET/CT in Endometrial Cancer: Systematic Review and Meta-Analysis of the Literature. J. Nucl. Med. 2016, 57, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Taşkin, S.; Varli, B.; Ersöz, C.C.; Altin, D.; Soydal, Ç.; Ortaç, F. Complementary role of 18F-FDG PET/CT for sentinel lymph node algorithm in endometrial cancer with high-risk factors for lymphatic metastasis. Nucl. Med. Commun. 2020, 41, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Konuralp Atakul, B.; Taşkın, S.; Soydal, Ç.; Sükür, Y.E.; Kahraman, A.; Koyuncu, K.; İbiş, E.; Ortac, F. Preoperative 18F-Fluorodeoxyglucose Positron Emission Tomography/CT in Prediction of Uterine Risk Factors and Lymph Node Metastasis: An Analysis of 111 Endometrioid Endometrial Cancer Patients. Gynecol. Obstet. Investig. 2017, 82, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Atri, M.; Zhang, Z.; Dehdashti, F.; Lee, S.I.; Marques, H.; Ali, S.; Koh, W.J.; Mannel, R.S.; DiSilvestro, P.; King, S.A.; et al. Utility of PET/CT to Evaluate Retroperitoneal Lymph Node Metastasis in High-Risk Endometrial Cancer: Results of ACRIN 6671/GOG 0233 Trial. Radiology 2017, 283, 450–459. [Google Scholar] [CrossRef]
- Liu, D.D.; Li, J.; Li, X.; Xie, L.; Qin, L.; Peng, F.; Cheng, M.H. Prognostic value of metabolic tumor volume and total lesion glycolysis from ¹⁸F-FDG PET/CT in lymph node metastases and risk stratification of endometrial carcinoma. J. Gynecol. Oncol. 2019, 30, e89. [Google Scholar] [CrossRef]
- Gholkar, N.S.; Saha, S.C.; Prasad, G.; Bhattacharya, A.; Srinivasan, R.; Suri, V. The Accuracy of Integrated [18F] Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography in Detection of Pelvic and Para-aortic Nodal Metastasis in Patients with High Risk Endometrial Cancer. World J. Nucl. Med. 2014, 13, 170–177. [Google Scholar]
- Husby, J.A.; Reitan, B.C.; Biermann, M.; Trovik, J.; Bjørge, L.; Magnussen, I.J.; Salvesen, Ø.O.; Salvesen, H.B.; Haldorsen, I.S. Metabolic Tumor Volume on 18F-FDG PET/CT Improves Preoperative Identification of High-Risk Endometrial Carcinoma Patients. J. Nucl. Med. 2015, 56, 1191–1198. [Google Scholar] [CrossRef]
- Crivellaro, C.; Signorelli, M.; Guerra, L.; De Ponti, E.; Pirovano, C.; Fruscio, R.; Elisei, F.; Montanelli, L.; Buda, A.; Messa, C. Tailoring systematic lymphadenectomy in high-risk clinical early stage endometrial cancer: The role of 18F-FDG PET/CT. Gynecol. Oncol. 2013, 130, 306–311. [Google Scholar] [CrossRef]
- Signorelli, M.; Guerra, L.; Buda, A.; Picchio, M.; Mangili, G.; Dell’Anna, T.; Sironi, S.; Messa, C. Role of the integrated FDG PET/CT in the surgical management of patients with high risk clinical early stage endometrial cancer: Detection of pelvic nodal metastases. Gynecol. Oncol. 2009, 115, 231–235. [Google Scholar] [CrossRef]
- Kitajima, K.; Murakami, K.; Yamasaki, E.; Kaji, Y.; Sugimura, K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur. Radiol. 2009, 19, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Nayot, D.; Kwon, J.S.; Carey, M.S.; Driedger, A. Does preoperative positron emission tomography with computed tomography predict nodal status in endometrial cancer? A pilot study. Curr. Oncol. 2008, 15, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, E.N.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol. Oncol. 2008, 108, 486–492. [Google Scholar] [CrossRef]
- Soydal, Ç.; Varlı, B.; Araz, M.; Bakırarar, B.; Taşkın, S.; Ortaç, U.F. Radiomics analysis of uterine tumors in 18F-fluorodeoxyglucose positron emission tomography for prediction of lymph node metastases in endometrial carcinoma. Turk. J. Med. Sci. 2022, 52, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Laurent, J.D.S.; Davis, M.R.; Feltmate, C.M.; Goodman, A.; Del Carmen, M.G.; Horowitz, N.E.; Lee, S.I.; Growdon, W.B. Prognostic Value of Preoperative Imaging: Comparing 18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography to Computed Tomography Alone for Preoperative Planning in High-risk Histology Endometrial Carcinoma. Am. J. Clin. Oncol. 2020, 43, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.H.; Wang, M.; Gong, J.; Liu, H.H.; Wang, N.; Wen, N.; Fan, W.S.; Xu, B.X.; Wang, M.Y.; Ye, M.X.; et al. Comparison of integrated PET/MRI with PET/CT in evaluation of endometrial cancer: A retrospective analysis of 81 cases. PeerJ 2019, 7, e7081. [Google Scholar] [CrossRef]
- De Bernardi, E.; Buda, A.; Guerra, L.; Vicini, D.; Elisei, F.; Landoni, C.; Fruscio, R.; Messa, C.; Crivellaro, C. Radiomics of the primary tumour as a tool to improve 18F-FDG-PET sensitivity in detecting nodal metastases in endometrial cancer. EJNMMI Res. 2018, 8, 86. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging 2020, 20, 75. [Google Scholar] [CrossRef]
- Tang, Q.; Su, Z.; Gu, W.; Rustgi, A.K. Mutant p53 on the Path to Metastasis. Trends Cancer 2020, 6, 62–73. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Horeweg, N.; Peters, E.E.M.; Rutten, T.; ter Haar, N.; Smit, V.T.; Kroon, C.D.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol. Oncol. 2022, 164, 577–586. [Google Scholar] [CrossRef]
- Michielsen, K.; Vergote, I.; Op de Beeck, K.; Amant, F.; Leunen, K.; Moerman, P.; Deroose, C.; Souverijns, G.; Dymarkowski, S.; De Keyzer, F.; et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: A clinical feasibility study in comparison to CT and FDG-PET/CT. Eur. Radiol. 2014, 24, 889–901. [Google Scholar] [CrossRef]
- Hynninen, J.; Kemppainen, J.; Lavonius, M.; Virtanen, J.; Matomäki, J.; Oksa, S.; Carpén, O.; Grénman, S.; Seppänen, M.; Auranen, A. A prospective comparison of integrated FDG-PET/contrast-enhanced CT and contrast-enhanced CT for pretreatment imaging of advanced epithelial ovarian cancer. Gynecol. Oncol. 2013, 131, 389–394. [Google Scholar] [CrossRef]
- De Iaco, P.; Musto, A.; Orazi, L.; Zamagni, C.; Rosati, M.; Allegri, V.; Cacciari, N.; Al-Nahhas, A.; Rubello, D.; Venturoli, S.; et al. FDG-PET/CT in advanced ovarian cancer staging: Value and pitfalls in detecting lesions in different abdominal and pelvic quadrants compared with laparoscopy. Eur. J. Radiol. 2011, 80, e98–e103. [Google Scholar] [CrossRef] [PubMed]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic value of [18F]FDG PET/MRI for staging in patients with ovarian cancer. EJNMMI Res. 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, J.-H.; Cho, A.; Yun, M.; Lee, J.D.; Kim, Y.T.; Kang, W.J. The Performance of Contrast-Enhanced FDG PET/CT for the Differential Diagnosis of Unexpected Ovarian Mass Lesions in Patients with Nongynecologic Cancer. Clin. Nucl. Med. 2015, 40, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.A.; Atta, H.; Diab, W.A.; Eloteify, L.M.; Imam, H.; Gabr, A.; Mekkawy, M.A.; Omar, W.M. The predominant role of 18F-FDG PET/CT over MDCT in assessment of ovarian cancer patients. Egypt. J. Radiol. Nucl. Med. 2015, 46, 1313–1322. [Google Scholar] [CrossRef][Green Version]
- Kim, C.; Chung, H.H.; Oh, S.W.; Kang, K.W.; Chung, J.-K.; Lee, D.S. Differential Diagnosis of Borderline Ovarian Tumors from Stage I Malignant Ovarian Tumors using FDG PET/CT. Nucl. Med. Mol. Imaging 2013, 47, 81–88. [Google Scholar] [CrossRef]
- Zytoon, A.A.; Murakami, K.; Eid, H.; El-Gammal, M. High impact of FDG-PET/CT in diagnostic strategies for ovarian cancer. Acta Radiol. 2013, 54, 340–348. [Google Scholar] [CrossRef]
- Castellucci, P.; Perrone, A.M.; Picchio, M.; Ghi, T.; Farsad, M.; Nanni, C.; Messa, C.; Meriggiola, M.C.; Pelusi, G.; Al-Nahhas, A.; et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: Correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl. Med. Commun. 2007, 28, 589–595. [Google Scholar] [CrossRef]
- Dauwen, H.; Van Calster, B.; Deroose, C.M.; Op de Beeck, K.; Amant, F.; Neven, P.; Berteloot, P.; Leunen, K.; Timmerman, D.; Vergote, I. PET/CT in the staging of patients with a pelvic mass suspicious for ovarian cancer. Gynecol. Oncol. 2013, 131, 694–700. [Google Scholar] [CrossRef]
- Kitajima, K.; Suzuki, K.; Senda, M.; Kita, M.; Nakamoto, Y.; Onishi, Y.; Maeda, T.; Yoshikawa, T.; Ohno, Y.; Sugimura, K. FDG-PET/CT for diagnosis of primary ovarian cancer. Nucl. Med. Commun. 2011, 32, 549–553. [Google Scholar] [CrossRef]
- Nam, E.J.; Yun, M.J.; Oh, Y.T.; Kim, J.W.; Kim, J.H.; Kim, S.; Jung, Y.W.; Kim, S.W.; Kim, Y.T. Diagnosis and staging of primary ovarian cancer: Correlation between PET/CT, Doppler US, and CT or MRI. Gynecol. Oncol. 2010, 116, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, S.; Chang, W.; Hassan, H.; Kaewput, C.; Asri, A.; Saad, F.; Nordin, A.; Vinjamuri, S. Systematic review on the accuracy of positron emission tomography/computed tomography and positron emission tomography/magnetic resonance imaging in the management of ovarian cancer: Is functional information really needed? World J. Nucl. Med. 2017, 16, 176–185. [Google Scholar] [CrossRef]
- Engbersen, M.P.; Van Driel, W.; Lambregts, D.; Lahaye, M. The role of CT, PET-CT, and MRI in ovarian cancer. Br. J. Radiol. 2021, 94, 20210117. [Google Scholar] [CrossRef] [PubMed]
- Alessi, A.; Martinelli, F.; Padovano, B.; Serafini, G.; Lorusso, D.; Lorenzoni, A.; Ditto, A.; Lecce, F.; Mira, M.; Donfrancesco, C.; et al. FDG-PET/CT to predict optimal primary cytoreductive surgery in patients with advanced ovarian cancer: Preliminary results. Tumori 2016, 102, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ebina, Y.; Watari, H.; Kaneuchi, M.; Takeda, M.; Hosaka, M.; Kudo, M.; Yamada, H.; Sakuragi, N. Impact of FDG PET in optimizing patient selection for cytoreductive surgery in recurrent ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 446–451. [Google Scholar] [CrossRef]
- Du, X.L.; Jiang, T.; Sheng, X.G.; Li, Q.S.; Wang, C.; Yu, H. PET/CT scanning guided intensity-modulated radiotherapy in treatment of recurrent ovarian cancer. Eur. J. Radiol. 2012, 81, 3551–3556. [Google Scholar] [CrossRef]
- Sironi, S.; Messa, C.; Mangili, G.; Zangheri, B.; Aletti, G.; Garavaglia, E.; Vigano, R.; Picchio, M.; Taccagni, G.; Maschio, A.D.; et al. Integrated FDG PET/CT in patients with persistent ovarian cancer: Correlation with histologic findings. Radiology 2004, 233, 433–440. [Google Scholar] [CrossRef]
- Vallius, T.; Hynninen, J.; Kemppainen, J.; Alves, V.; Auranen, K.; Matomäki, J.; Oksa, S.; Virtanen, J.; Grénman, S.; Auranen, A.; et al. 18F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1224–1232. [Google Scholar] [CrossRef]
- Vallius, T.; Peter, A.; Auranen, A.; Carpén, O.; Kemppainen, J.; Matomäki, J.; Oksa, S.; Roering, P.; Seppänen, M.; Grénman, S.; et al. 18F-FDG-PET/CT can identify histopathological non-responders to platinum-based neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol. Oncol. 2016, 140, 29–35. [Google Scholar] [CrossRef]
- Bristow, R.E.; Giuntoli, I.I.R.L.; Pannu, H.K.; Schulick, R.D.; Fishman, E.K.; Wahl, R.L. Combined PET/CT for detecting recurrent ovarian cancer limited to retroperitoneal lymph nodes. Gynecol. Oncol. 2005, 99, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Rubello, D.; Farsad, M.; De Iaco, P.; Sansovini, M.; Erba, P.; Rampin, L.; Mariani, G.; Fanti, S. 18F-FDG PET/CT in the evaluation of recurrent ovarian cancer: A prospective study on forty-one patients. Eur. J. Surg. Oncol. 2005, 31, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Simcock, B.; Neesham, D.; Quinn, M.; Drummond, E.; Milner, A.; Hicks, R.J. The impact of PET/CT in the management of recurrent ovarian cancer. Gynecol. Oncol. 2006, 103, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Thrall, M.M.; DeLoia, J.A.; Gallion, H.; Avril, N. Clinical use of combined positron emission tomography and computed tomography (FDG-PET/CT) in recurrent ovarian cancer. Gynecol. Oncol. 2007, 105, 17–22. [Google Scholar] [CrossRef]
- Fulham, M.J.; Carter, J.; Baldey, A.; Hicks, R.J.; Ramshaw, J.E.; Gibson, M. The impact of PET-CT in suspected recurrent ovarian cancer: A prospective multi-centre study as part of the Australian PET Data Collection Project. Gynecol. Oncol. 2009, 112, 462–468. [Google Scholar] [CrossRef]
- Risum, S.; Høgdall, C.; Markova, E.; Berthelsen, A.K.; Loft, A.; Jensen, F.; Høgdall, E.; Roed, H.; Engelholm, S.A. Influence of 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography on recurrent ovarian cancer diagnosis and on selection of patients for secondary cytoreductive surgery. Int. J. Gynecol. Cancer 2009, 19, 600–604. [Google Scholar] [CrossRef]
- Bhosale, P.; Peungjesada, S.; Wei, W.; Levenback, C.F.; Schmeler, K.; Rohren, E.; Macapinlac, H.A.; Iyer, R.B. Clinical utility of positron emission tomography/computed tomography in the evaluation of suspected recurrent ovarian cancer in the setting of normal CA-125 levels. Int. J. Gynecol. Cancer 2010, 20, 936–944. [Google Scholar] [CrossRef]
- Bilici, A.; Ustaalioglu, B.B.O.; Seker, M.; Canpolat, N.; Tekinsoy, B.; Salepci, T.; Gumus, M. Clinical value of FDG PET/CT in the diagnosis of suspected recurrent ovarian cancer: Is there an impact of FDG PET/CT on patient management? Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1259–1269. [Google Scholar] [CrossRef]
- Sala, E.; Kataoka, M.; Pandit-Taskar, N.; Ishill, N.; Mironov, S.; Moskowitz, C.S.; Mironov, O.; Collins, M.A.; Chi, D.S.; Larson, S.; et al. Recurrent ovarian cancer: Use of contrast-enhanced CT and PET/CT to accurately localize tumor recurrence and to predict patients’ survival. Radiology 2010, 257, 125–134. [Google Scholar] [CrossRef]
- Nasu, K.; Abe, W.; Takai, N.; Tomonari, K.; Narahara, H. Impact of positron emission tomography/computed tomography in the management of patients with epithelial ovarian carcinoma after treatment. Arch. Gynecol. Obstet. 2011, 283, 1121–1126. [Google Scholar] [CrossRef]
- Pan, H.-S.; Lee, S.-L.; Huang, L.-W.; Chen, Y.-K. Combined positron emission tomography—Computed tomography and tumor markers for detecting recurrent ovarian cancer. Arch. Gynecol. Obstet. 2011, 283, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.-J.; Liou, W.-S.; Liu, R.-S.; Hu, C.; Tsay, D.-G.; Liu, C.-B. Early detection of recurrent ovarian cancer in patients with low-level increases in serum CA-125 levels by 2-[F-18] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography. Cancer Biother. Radiopharm. 2011, 26, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Antunovic, L.; Cimitan, M.; Borsatti, E.; Baresic, T.; Sorio, R.; Giorda, G.; Steffan, A.; Balestreri, L.; Tatta, R.; Pepe, G.; et al. Revisiting the clinical value of 18F-FDG PET/CT in detection of recurrent epithelial ovarian carcinomas: Correlation with histology, serum CA-125 assay, and conventional radiological modalities. Clin. Nucl. Med. 2012, 37, e184–e188. [Google Scholar] [CrossRef] [PubMed]
- Sanli, Y.; Turkmen, C.; Bakir, B.; Iyibozkurt, C.; Ozel, S.; Has, D.; Yilmaz, E.; Topuz, S.; Yavuz, E.; Unal, S.N.; et al. Diagnostic value of PET/CT is similar to that of conventional MRI and even better for detecting small peritoneal implants in patients with recurrent ovarian cancer. Nucl. Med. Commun. 2012, 33, 509–515. [Google Scholar] [CrossRef]
- Sari, O.; Kaya, B.; Ozcan Kara, P.; Kara Gedik, G.; Celik, C.; Ozbek, O.; Serdengecti, M. The Role of FDG-PET/CT in Ovarian Cancer Patients with High Tumor Markers or Suspicious Lesion on Contrast-Enhanced CT in Evaluation of Recurrence and/or in Determination of Intraabdominal Metastases. Rev. Española Med. Nucl. Imagen Mol. 2012, 31, 3–8. [Google Scholar] [CrossRef]
- Dragosavac, S.; Derchain, S.; Caserta, N.M.; De Souza, G. Staging recurrent ovarian cancer with 18FDG PET/CT. Oncol. Lett. 2013, 5, 593–597. [Google Scholar] [CrossRef]
- Ghosh, J.; Thulkar, S.; Kumar, R.; Malhotra, A.; Kumar, A.; Kumar, L. Role of FDG PET-CT in asymptomatic epithelial ovarian cancer with rising serum CA-125: A pilot study. Natl. Med. J. India 2013, 26, 327–331. [Google Scholar]
- Gouhar, G.K.; Siam, S.; Sadek, S.M.; Ahmed, R.A. Prospective assessment of 18F-FDG PET/CT in detection of recurrent ovarian cancer. Egypt. J. Radiol. Nucl. Med. 2013, 44, 913–922. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.M.; Chen, T.; Zee, C.S.; Shi, Y.P.; Wan, L.R.; Tong, L.J. Is there an impact of 18F-FDG PET/CT on the surveillance and clinical management of recurrent ovarian cancer? Research based on a large sample in a single PET/CT center. Nucl. Med. Commun. 2014, 35, 347–352. [Google Scholar] [CrossRef]
- Takeuchi, S.; Lucchini, M.; Schmeler, K.M.; Coleman, R.L.; Gershenson, D.M.; Munsell, M.F.; Macapinlac, H.A.; Ramirez, P.T. Utility of 18F-FDG PET/CT in follow-up of patients with low-grade serous carcinoma of the ovary. Gynecol. Oncol. 2014, 133, 100–104. [Google Scholar] [CrossRef]
- Evangelista, L.; Palma, M.D.; Gregianin, M.; Nardin, M.; Roma, A.; Nicoletto, M.O.; Nardelli, G.B.; Zagonel, V. Diagnostic and prognostic evaluation of fluorodeoxyglucose positron emission tomography/computed tomography and its correlation with serum cancer antigen-125 (CA125) in a large cohort of ovarian cancer patients. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Fularz, M.; Adamiak, P.; Czepczyński, R.; Jarząbek-Bielecka, G.; Rewers, A.; Kędzia, W.; Ruchała, M. Utility of PET/CT in the diagnosis of recurrent ovarian cancer depending on CA 125 serum level. Nukl. Nucl. Med. 2015, 54, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Carlier, T.; Colombié, M.; Goulon, D.; Fleury, V.; Rousseau, N.; Berton-Rigaud, D.; Jaffre, I.; Kraeber-Bodéré, F.; Campion, L.; et al. Clinical and Survival Impact of FDG PET in Patients with Suspicion of Recurrent Ovarian Cancer: A 6-Year Follow-Up. Front. Med. 2015, 2, 46. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, Y.; Tawakol, A.; Osama, A.; Hamada, E.; El-Refaei, S. Role of 18F-FDG PET/CT in the detection of ovarian cancer recurrence in the setting of normal tumor markers. Egypt. J. Radiol. Nucl. Med. 2016, 47, 1787–1794. [Google Scholar] [CrossRef][Green Version]
- Caobelli, F.; Alongi, P.; Evangelista, L.; Picchio, M.; Saladini, G.; Rensi, M.; Geatti, O.; Castello, A.; Laghai, I.; Popescu, C.E.; et al. Predictive value of 18F-FDG PET/CT in restaging patients affected by ovarian carcinoma: A multicentre study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 404–413. [Google Scholar] [CrossRef]
- Tawakol, A.; Abdelhafez, Y.G.; Osama, A.; Hamada, E.; El Refaei, S. Diagnostic performance of 18F-FDG PET/contrast-enhanced CT versus contrast-enhanced CT alone for post-treatment detection of ovarian malignancy. Nucl. Med. Commun. 2016, 37, 453–460. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, Y.-M.; Jung, P.-S.; Lee, J.-J.; Kim, J.-K.; Kim, Y.-T.; Nam, J.H. Diagnostic value of integrated 18F-fluoro-2-deoxyglucose positron emission tomography/computed tomography in recurrent epithelial ovarian cancer: Accuracy of patient selection for secondary cytoreduction in 134 patients. J. Gynecol. Oncol. 2018, 29, e36. [Google Scholar] [CrossRef]
- Cengiz, A.; Koç, Z.P.; Özcan Kara, P.; Yürekli, Y. The Role of 18F-FDG PET/CT in Detecting Ovarian Cancer Recurrence in Patients with Elevated CA-125 Levels. Mol. Imaging Radionucl. Ther. 2019, 28, 8–14. [Google Scholar] [CrossRef]
- Dolci, C.; Ceppi, L.; Guerra, L.; Crivellaro, C.; Lamanna, M.; Adorni, M.; Elisei, F.; Bonazzi, C.M.; Sina, F.; Fruscio, R.; et al. Role of 18F-fluoro-2-deoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in malignant ovarian germ cell tumors: A single-center experience with long term follow-up. Int. J. Gynecol. Cancer 2019, 29, 1298–1303. [Google Scholar] [CrossRef]
- García-Talavera, P.; Alejo, E.; Arias, P.; Verdú, A.; Tamayo, P.; Gómez-Caminero, F. 18F-FDG PET/CT in ovarian cancer recurrence: Clinical impact, correlation with ceCT and CA-125, and prognostic value. Rev. Española Med. Nucl. Imagen Mol. 2021, 40, 207–213. [Google Scholar] [CrossRef]
- Rusu, G.; Achimaș-Cadariu, P.; Piciu, A.; Căinap, S.S.; Căinap, C.; Piciu, D. A comparative study between 18F-FDG PET/CT and conventional imaging in the evaluation of progressive disease and recurrence in ovarian carcinoma. Healthcare 2021, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Limei, Z.; Yong, C.; Yan, X.; Shuai, T.; Jiangyan, X.; Zhiqing, L. Accuracy of positron emission tomography/computed tomography in the diagnosis and restaging for recurrent ovarian cancer: A meta-analysis. Int. J. Gynecol. Cancer 2013, 23. [Google Scholar] [CrossRef]
- Fruscio, R.; Sina, F.; Dolci, C.; Signorelli, M.; Crivellaro, C.; Dell’Anna, T.; Cuzzocrea, M.; Guerra, L.; Milani, R.; Messa, C. Preoperative 18F-FDG PET/CT in the management of advanced epithelial ovarian cancer. Gynecol. Oncol. 2013, 131, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, M.; Guerra, L.; Pirovano, C.; Crivellaro, C.; Fruscio, R.; Buda, A.; Cuzzucrea, M.; Elisei, F.; Ceppi, L.; Messa, C. Detection of nodal metastases by 18F-FDG PET/CT in apparent early stage ovarian cancer: A prospective study. Gynecol. Oncol. 2013, 131, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Won, K.S.; Zeon, S.K.; Ahn, B.C.; Gayed, I.W. Peritoneal carcinomatosis in patients with ovarian cancer: Enhanced CT versus 18F-FDG PET/CT. Clin. Nucl. Med. 2013, 38, 93–97. [Google Scholar] [CrossRef]
- Rubini, G.; Altini, C.; Notaristefano, A.; Merenda, N.; Rubini, D.; Ianora, A.A.; Asabella, A.N. Role of 18F-FDG PET/CT in diagnosing peritoneal carcinomatosis in the restaging of patient with ovarian cancer as compared to contrast enhanced CT and tumor marker Ca-125. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 22–27. [Google Scholar]
- Funicelli, L.; Travaini, L.; Landoni, F.; Trifirò, G.; Bonello, L.; Bellomi, M. Peritoneal carcinomatosis from ovarian cancer: The role of CT and [18 F] FDG-PET/CT. Abdom. Imaging 2010, 35, 701–707. [Google Scholar] [CrossRef]
- Schmidt, S.; Meuli, R.A.; Achtari, C.; Prior, J.O. Peritoneal carcinomatosis in primary ovarian cancer staging: Comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin. Nucl. Med. 2015, 40, 371–377. [Google Scholar] [CrossRef]
- Lopez-Lopez, V.; Cascales-Campos, P.A.; Gil, J.; Frutos, L.; Andrade, R.J.; Fuster-Quiñonero, M.; Feliciangeli, E.; Gil, E.; Parrilla, P. Use of 18F-FDG PET/CT in the preoperative evaluation of patients diagnosed with peritoneal carcinomatosis of ovarian origin, candidates to cytoreduction and hipec. A pending issue. Eur. J. Radiol. 2016, 85, 1824–1828. [Google Scholar] [CrossRef]
- Lee, I.O.; Lee, J.-Y.; Kim, H.J.; Nam, E.J.; Kim, S.; Kim, S.W.; Lee, C.Y.; Kang, W.J.; Kim, Y.T. Prognostic significance of supradiaphragmatic lymph node metastasis detected by 18F-FDG PET/CT in advanced epithelial ovarian cancer. BMC Cancer 2018, 18, 1165. [Google Scholar] [CrossRef]
- Yuan, Y.; Gu, Z.-X.; Tao, X.-F.; Liu, S.-Y. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: A meta-analysis. Eur. J. Radiol. 2012, 81, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Wang, Y. Meta-analysis of the diagnostic value of 18F-FDG PET/CT in the recurrence of epithelial ovarian cancer. Front. Oncol. 2022, 12, 1003465. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Tsai, H.L.; Wang, H.; Crandall, J.; Javadi, M.S.; Wahl, R.L. Posttreatment FDG PET/CT in predicting survival of patients with ovarian carcinoma. EJNMMI Res. 2016, 6, 42. [Google Scholar] [CrossRef][Green Version]
- Hebel, C.B.; Behrendt, F.F.; Heinzel, A.; Krohn, T.; Mottaghy, F.M.; Bauerschlag, D.O.; Verburg, F.A. Negative 18F-2-fluorodeoxyglucose PET/CT predicts good cancer specific survival in patients with a suspicion of recurrent ovarian cancer. Eur. J. Radiol. 2014, 83, 463–467. [Google Scholar] [CrossRef]
- Hynninen, J.; Auranen, A.; Carpén, O.; Dean, K.; Seppänen, M.; Kemppainen, J.; Lavonius, M.; Lisinen, I.; Virtanen, J.; Grénman, S. FDG PET/CT in staging of advanced epithelial ovarian cancer: Frequency of supradiaphragmatic lymph node metastasis challenges the traditional pattern of disease spread. Gynecol. Oncol. 2012, 126, 64–68. [Google Scholar] [CrossRef]
- Anner, P.; Mayerhöfer, M.; Wadsak, W.; Geleff, S.; Dudczak, R.; Haug, A.; Hacker, M.; Karanikas, G. [18F]FDG-PET/CT and MRI for initial pelvic lymph node staging in patients with cervical carcinoma: The potential usefulness of [18F]FDG-PET/MRI. Oncol. Lett. 2018, 15, 3951–3956. [Google Scholar] [CrossRef] [PubMed]
- Cegla, P.; Urbanski, B.; Burchardt, E.; Roszak, A.; Cholewinski, W. Influence of 18F-FDG-PET/CT on staging of cervical cancer. Nucl. Med. 2019, 58, 17–22. [Google Scholar] [CrossRef]
- Driscoll, D.O.; Halpenny, D.; Johnston, C.; Sheehy, N.; Keogan, M. 18F-FDG-PET/CT is of limited value in primary staging of early stage cervical cancer. Abdom. Imaging 2015, 40, 127–133. [Google Scholar] [CrossRef]
- Lazzari, R.; Cecconi, A.; Jereczek-Fossa, B.A.; Travaini, L.L.; Dell’ Acqua, V.; Cattani, F.; Rizzo, S.; Fodor, C.; Landoni, F.; Orecchia, R. The role of [18F]FDG-PET/CT in staging and treatment planning for volumetric modulated Rapidarc radiotherapy in cervical cancer: Experience of the European Institute of Oncology, Milan, Italy. Ecancermedicalscience 2014, 8, 405. [Google Scholar]
- Grueneisen, J.; Schaarschmidt, B.M.; Heubner, M.; Aktas, B.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; Umutlu, L. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1814–1824. [Google Scholar] [CrossRef]
- Sarabhai, T.; Schaarschmidt, B.M.; Wetter, A.; Kirchner, J.; Aktas, B.; Forsting, M.; Ruhlmann, V.; Herrmann, K.; Umutlu, L.; Grueneisen, J. Comparison of 18 F-FDG PET/MRI and MRI for pre-therapeutic tumor staging of patients with primary cancer of the uterine cervix. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Singaram, N.K.; Hulikal, N.; Devi, B.V.; Manthri, R.; Chowhan, A.K. Utility of Whole Body 18F-FDG PET/CT in Comparison to Pelvic MRI in Evaluation of Local Staging of Early-Stage Carcinoma Cervix. Cureus 2022, 14, e32111. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Gupta, S.; Deodhar, K.; Chopra, S.; Rangarajan, V.; Purandare, N.; Mahantshetty, U. Preoperative imaging with positron emission tomography and computed tomography (18F-FDG PET/CT) or contrast-enhanced computed tomography (CECT) scan in operable cervical cancer: A prospective study. Eur. J. Gynaecol. Oncol. 2022, 1, 8. [Google Scholar]
- Eilsberger, F.; Noltenius, F.E.; Librizzi, D.; Wessendorf, J.; Luster, M.; Hoch, S.; Pfestroff, A. Real-Life Performance of F-18-FDG PET/CT in Patients with Cervical Lymph Node Metastasis of Unknown Primary Tumor. Biomedicines 2022, 10, 2095. [Google Scholar] [CrossRef]
- Tan, B.; Guo, J.; Wang, L.; Wang, L.; Chen, B. Application value of 18F-FDG PETCT imaging in the clinical initial diagnosis and follow-up of primary lesions of cervical cancer. Transl. Cancer Res. 2020, 9, 4005. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Han, F.; Sun, W.; Chen, Z. Evaluation of parametrial infiltration in cervical cancer with voxel-based segmentation of integrated 18F-FDG PET/MRI images: A preliminary study. Eur. J. Radiol. 2019, 118, 147–152. [Google Scholar] [CrossRef]
- Yu, L.; Jia, C.; Wang, X.; Lu, P.; Tian, M.; Wang, W.; Lou, G. Evaluation of ¹⁸F-FDG PET/CT in early-stage cervical carcinoma. Am. J. Med. Sci. 2011, 341, 96–100. [Google Scholar] [CrossRef]
- Loft, A.; Berthelsen, A.K.; Roed, H.; Ottosen, C.; Lundvall, L.; Knudsen, J.; Nedergaard, L.; Højgaard, L.; Engelholm, S.A. The diagnostic value of PET/CT scanning in patients with cervical cancer: A prospective study. Gynecol. Oncol. 2007, 106, 29–34. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, B.; Pei, X.; Liu, H.; Li, G. CT, MRI, and PET imaging features in cervical cancer staging and lymph node metastasis. Am. J. Transl. Res. 2021, 13, 10536–10544. [Google Scholar]
- Bashir, U.; Mallia, A.; Stirling, J.; Joemon, J.; MacKewn, J.; Charles-Edwards, G.; Goh, V.; Cook, G.J. PET/MRI in Oncological Imaging: State of the Art. Diagnostics 2015, 5, 333–357. [Google Scholar] [CrossRef]
- Koulis, T.A.; Doll, C.M.; Brown, D.; Traptow, L.; Bhayana, D.; Nelson, G.; Phan, T. Implementation and validation of a combined MRI-CT-based cervical cancer brachytherapy program using existing infrastructure. Brachytherapy 2016, 15, 319–326. [Google Scholar] [CrossRef]
- Vandecasteele, K.; Delrue, L.; Lambert, B.; Makar, A.; Lambein, K.; Denys, H.; Tummers, P.; Van den Broecke, R.; Villeirs, G.; De Meerleer, G. Value of magnetic resonance and 18FDG PET-CT in predicting tumor response and resectability of primary locally advanced cervical cancer after treatment with intensity-modulated arc therapy: A prospective pathology-matched study. Int. J. Gynecol. Cancer 2012, 22, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Gemer, O.; Eitan, R.; Gdalevich, M.; Mamanov, A.; Piura, B.; Rabinovich, A.; Levavi, H.; Saar-Ryss, B.; Halperin, R.; Finci, S.; et al. Integration of PET/CT into the preoperative evaluation of patients with early cervical cancer does not decrease the proportion of patients with positive lymph nodes found after surgery. Int. J. Gynecol. Cancer 2014, 24, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Scarsbrook, A.; Vaidyanathan, S.; Chowdhury, F.; Swift, S.; Cooper, R.; Patel, C. Efficacy of qualitative response assessment interpretation criteria at 18F-FDG PET-CT for predicting outcome in locally advanced cervical carcinoma treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 581–588. [Google Scholar] [CrossRef]
- Perrone, A.M.; Dondi, G.; Coe, M.; Ferioli, M.; Telo, S.; Galuppi, A.; De Crescenzo, E.; Tesei, M.; Castellucci, P.; Nanni, C.; et al. Predictive role of MRI and 18F FDG PET response to concurrent chemoradiation in T2b cervical cancer on clinical outcome: A retrospective single center study. Cancers 2020, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Rufini, V.; Collarino, A.; Calcagni, M.L.; Meduri, G.M.; Fuoco, V.; Pasciuto, T.; Testa, A.C.; Ferrandina, G.; Gambacorta, M.A.; Campitelli, M.; et al. The role of 18F-FDG-PET/CT in predicting the histopathological response in locally advanced cervical carcinoma treated by chemo-radiotherapy followed by radical surgery: A prospective study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1228–1238. [Google Scholar] [CrossRef]
- Su, T.-P.; Lin, G.; Huang, Y.-T.; Liu, F.-Y.; Wang, C.-C.; Chao, A.; Yen, T.C.; Lai, C.H. Comparison of positron emission tomography/computed tomography and magnetic resonance imaging for posttherapy evaluation in patients with advanced cervical cancer receiving definitive concurrent chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 727–734. [Google Scholar] [CrossRef]
- Draghini, L.; Costantini, S.; Vicenzi, L.; Italiani, M.; Loreti, F.; Trippa, F.; Arcidiacono, F.; Casale, M.; Mantello, G.; Maranzano, E. Positron emission tomography for staging locally advanced cervical cancer and assessing intensity modulated radiotherapy approach. Radiol. Med. 2019, 124, 819–825. [Google Scholar] [CrossRef]
- Hoon Chung, H.; Kim, J.W.; Kang, K.W.; Park, N.-H.; Song, Y.-S.; Chung, J.-K.; Kang, S.B. Predictive role of post-treatment [18F]FDG PET/CT in patients with uterine cervical cancer. Eur. J. Radiol. 2012, 81, e817–e822. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Schob, S.; Höhn, A.-K.; Bremicker, K.; Exner, M.; Stumpp, P.; Purz, S. Parameters of simultaneous 18F-FDG-PET/MRI predict tumor stage and several histopathological features in uterine cervical cancer. Oncotarget 2017, 8, 28285. [Google Scholar] [CrossRef][Green Version]
- Sanei Sistani, S.; Parooie, F.; Salarzaei, M. Diagnostic Accuracy of 18F-FDG-PET/CT and MRI in Predicting the Tumor Response in Locally Advanced Cervical Carcinoma Treated by Chemoradiotherapy: A Meta-Analysis. Contrast Media Mol. Imaging 2021, 2021, 8874990. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.J.; Jacobs, C.D.; Wong, T.Z.; Craciunescu, O.; Chino, J.P. Changes on Midchemoradiation Therapy Fluorodeoxyglucose Positron Emission Tomography for Cervical Cancer Are Associated with Prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Cetina, L.; Serrano, A.; Cantú-de-León, D.; Pérez-Montiel, D.; Estrada, E.; Coronel, J.; Hernández-Lucio, M.; Dueñas-González, A. F18-FDG-PET/CT in the evaluation of patients with suspected recurrent or persistent locally advanced cervical carcinoma. Rev. Investig. Clínica 2011, 63, 227–235. [Google Scholar]
- Chung, H.H.; Jo, H.; Kang, W.J.; Kim, J.W.; Park, N.-H.; Song, Y.-S.; Chung, J.K.; Kang, S.B.; Lee, H.P. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecol. Oncol. 2007, 104, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zheng, A.; Wang, F.; Lin, W.; Yang, X.; Han, L.; Chen, Y.; Bai, L. Diagnostic value of 18F-FDG-PET or PET-CT in recurrent cervical cancer: A systematic review and meta-analysis. Nucl. Med. Commun. 2014, 35, 144–150. [Google Scholar] [CrossRef]
- Sironi, S.; Buda, A.; Picchio, M.; Perego, P.; Moreni, R.; Pellegrino, A.; Colombo, M.; Mangioni, C.; Messa, C.; Fazio, F. Lymph node metastasis in patients with clinical early-stage cervical cancer: Detection with integrated FDG PET/CT. Radiology 2006, 238, 272–279. [Google Scholar] [CrossRef]
- Park, J.; Seo, S.; Kang, S.; Lim, S.; Lim, M.; Choi, H.; Kim, S.; Yoo, C.; Kim, J.; Park, S. The comparison of accuracy between PET and PET/CT for detecting lymph node metastasis in cervical cancer: Prospective surgicopathologic study. J. Clin. Oncol. 2007, 25, 5587. [Google Scholar] [CrossRef]
- Merlin, C.; Kelly, A.; Mestas, D.; Cachin, F.; Maublant, J. Prediction of lymph node status in uterine cervical cancer with 18FDG-PET/CT—Value of primary tumor uptake. Soc. Nucl. Med. 2008, 49, 248P. [Google Scholar]
- Yildirim, Y.; Sehirali, S.; Avci, M.; Yılmaz, C.; Ertopcu, K.; Tinar, S.; Duman, Y.; Sayhan, S. Integrated PET/CT for the evaluation of para-aortic nodal metastasis in locally advanced cervical cancer patients with negative conventional CT findings. Gynecol. Oncol. 2008, 108, 154–159. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, H.J.; Park, S.Y.; Lee, H.Y.; Seo, S.S.; Yoo, C.W.; Jung, D.C.; Kang, S.; Cho, K.S. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. Eur. J. Cancer 2009, 45, 2103–2109. [Google Scholar] [CrossRef]
- Bentivegna, E.; Uzan, C.; Gouy, S.; Leboulleux, S.; Duvillard, P.; Lumbroso, J.; Haie-Meder, C.; Schlumberger, M.; Morice, P. Correlation between [18F]Fluorodeoxyglucose Positron-Emission Tomography Scan and Histology of Pelvic Nodes in Early-Stage Cervical Cancer. Anticancer Res. 2010, 30, 1029–1032. [Google Scholar] [PubMed]
- Chung, H.H.; Kang, K.W.; Cho, J.Y.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kim, S.H.; Chung, J.K.; Kang, S.B. Role of magnetic resonance imaging and positron emission tomography/computed tomography in preoperative lymph node detection of uterine cervical cancer. Am. J. Obstet. Gynecol. 2010, 203, 156.e1–156.e5. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Deguchi, M.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Fusion of PET and MRI for staging of uterine cervical cancer: Comparison with contrast-enhanced 18F-FDG PET/CT and pelvic MRI. Clin. Imaging 2014, 38, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Guo, H.-M.; Lu, Y.-J.; Wu, Z.-X.; Zhang, K.; Han, J.-K. Role of 18F-FDG PET/CT in detecting pelvic lymph-node metastases in patients with early-stage uterine cervical cancer: Comparison with MRI findings. Nucl. Med. Commun. 2014, 35, 1204–1211. [Google Scholar] [CrossRef]
- Atri, M.; Zhang, Z.; Dehdashti, F.; Lee, S.I.; Ali, S.; Marques, H.; Koh, W.J.; Moore, K.; Landrum, L.; Kim, J.W.; et al. Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: Results of ACRIN6671/GOG0233 trial. Gynecol. Oncol. 2016, 142, 413–419. [Google Scholar] [CrossRef]
- Brunette, L.L.; Bonyadlou, S.; Ji, L.; Groshen, S.; Shuster, D.; Mehta, A.; Sposto, R.; Matsuo, K.; Lin, Y.G.; Roman, L.D. Predictive Value of FDG PET/CT to Detect Lymph Node Metastases in Cervical Cancer. Clin. Nucl. Med. 2018, 43, 793–801. [Google Scholar] [CrossRef]
- Lin, A.J.; Wright, J.D.; Dehdashti, F.; Siegel, B.A.; Markovina, S.; Schwarz, J.; Thaker, P.H.; Mutch, D.G.; Powell, M.A.; Grigsby, P.W. Impact of tumor histology on detection of pelvic and para-aortic nodal metastasis with 18F-fluorodeoxyglucose-positron emission tomography in stage IB cervical cancer. Int. J. Gynecol. Cancer 2019, 29, 1351–1354. [Google Scholar] [CrossRef]
- Reyneke, F.; Snyman, L.; Lawal, I.; Lengana, T.; Vorster, M.; Sathekge, M. Diagnostic value of sentinel lymph node scintigraphy and 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in the detection of metastatic lymph nodes in patients with early-stage cervical cancer. World J. Nucl. Med. 2020, 19, 240–245. [Google Scholar] [CrossRef]
- Gouy, S.; Seebacher, V.; Chargari, C.; Terroir, M.; Grimaldi, S.; Ilenko, A.; Maulard, A.; Genestie, C.; Leary, A.; Pautier, P.; et al. False negative rate at 18F-FDG PET/CT in para-aortic lymphnode involvement in patients with locally advanced cervical cancer: Impact of PET technology. BMC Cancer 2021, 21, 135. [Google Scholar] [CrossRef]
- Kaźmierczak, K.; Cholewiński, W.; Nowakowski, B. Comparison of positron emission tomography with computed tomography examination with histopathological assessment of pelvic lymph nodes in patients with cervical cancer treated surgically. Contemp. Oncol. 2021, 25, 160–167. [Google Scholar] [CrossRef]
- Vermolen, P.C.; van Trommel, N.E.; Vogel, W.V.; Adam, J.A.; van der Velden, J.; Mom, C.H. The issue of false positive lymph nodes on [18F] FDG-PET/CT for cervical carcinoma and consequences for treatment. Eur. J. Gynaecol. Oncol. 2021, 42, 943–950. [Google Scholar]
- Goyal, B.K.; Singh, H.; Kapur, K.; Duggal, B.S.; Jacob, M.J. Value of PET-CT in avoiding multimodality therapy in operable cervical cancer. Int. J. Gynecol. Cancer 2010, 20, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, W.; Ma, Y.; Liu, K.; Li, Y.; Wang, D. 18F-FDG PET/CT can correct the clinical stages and predict pathological parameters before operation in cervical cancer. Eur. J. Radiol. 2016, 85, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Dehdashti, F.; Herzog, T.J.; Mutch, D.G.; Huettner, P.C.; Rader, J.S.; Gibb, R.K.; Powell, M.A.; Gao, F.; Siegel, B.A.; et al. Preoperative lymph node staging of early-stage cervical carcinoma by [18F]-fluoro-2-deoxy-D-glucose–positron emission tomography. Cancer 2005, 104, 2484–2491. [Google Scholar] [CrossRef]
- Chung, H.H.; Park, N.H.; Kim, J.W.; Song, Y.S.; Chung, J.K.; Kang, S.B. Role of integrated PET-CT in pelvic lymph node staging of cervical cancer before radical hysterectomy. Gynecol. Obstet. Investig. 2009, 67, 61–66. [Google Scholar] [CrossRef]
- Signorelli, M.; Guerra, L.; Montanelli, L.; Crivellaro, C.; Buda, A.; Dell’Anna, T.; Picchio, M.; Milani, R.; Fruscio, R.; Messa, C. Preoperative staging of cervical cancer: Is 18-FDG-PET/CT really effective in patients with early stage disease? Gynecol. Oncol. 2011, 123, 236–240. [Google Scholar] [CrossRef]
- Adam, J.A.; van Diepen, P.R.; Mom, C.H.; Stoker, J.; van Eck-Smit, B.L.; Bipat, S. [18F] FDG-PET or PET/CT in the evaluation of pelvic and para-aortic lymph nodes in patients with locally advanced cervical cancer: A systematic review of the literature. Gynecol. Oncol. 2020, 159, 588–596. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef]
- Lee, J.; Lin, J.B.; Chang, C.L.; Sun, F.J.; Wu, M.H.; Jan, Y.T.; Chen, Y.J. Impact of para-aortic recurrence risk-guided intensity-modulated radiotherapy in locally advanced cervical cancer with positive pelvic lymph nodes. Gynecol. Oncol. 2018, 148, 291–298. [Google Scholar] [CrossRef]
- Fleming, S.; Cooper, R.; Swift, S.; Thygesen, H.; Chowdhury, F.; Scarsbrook, A.; Patel, C.N. Clinical impact of FDG PET-CT on the management of patients with locally advanced cervical carcinoma. Clin. Radiol. 2014, 69, 1235–1243. [Google Scholar] [CrossRef]
- Im, H.-J.; Yoon, H.-J.; Lee, E.S.; Kim, T.-S.; Kim, J.-Y.; Chung, J.-K.; Kim, S.K.; Park, S.Y. Prognostic implication of retrocrural lymph node involvement revealed by 18F-FDG PET/CT in patients with uterine cervical cancer. Nucl. Med. Commun. 2014, 35, 268–275. [Google Scholar] [CrossRef]
- Dag, Z.; Yilmaz, B.; Dogan, A.K.; Aksan, D.U.; Ozkurt, H.; Kızılkaya, H.O.; Arslan, D. Comparison of the prognostic value of F-18 FDG PET/CT metabolic parameters of primary tumors and MRI findings in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Brachytherapy 2019, 18, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ki, Y.; Kim, W.; Park, D.; Suh, D.; Kim, K.; Lee, J.; Jeon, H.; Nam, J. Positive pelvic lymph node on [18F]-FDG PET is a prognostic factor in early-stage high-risk cervical cancer treated by radical hysterectomy and adjuvant chemoradiotherapy. Eur. J. Gynaecol. Oncol. 2020, 41, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.V.; Loft, A.; Berthelsen, A.K.; Christensen, I.J.; Høgdall, C.; Engelholm, S.A. Survival outcomes in patients with cervical cancer after inclusion of PET/CT in staging procedures. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hou, X.; Hu, K.; Zhang, F. The Prognostic Value of Regional Lymph Node Metastasis in Cervical Cancer Patients with Stage IIIC Receiving PET/CT Examination. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e498. [Google Scholar] [CrossRef]
- Han, S.; Kim, H.; Kim, Y.J.; Suh, C.H.; Woo, S. Prognostic Value of Volume-Based Metabolic Parameters of 18F-FDG PET/CT in Uterine Cervical Cancer: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2018, 211, 1112–1121. [Google Scholar] [CrossRef]
- Mirpour, S.; Mhlanga, J.C.; Logeswaran, P.; Russo, G.; Mercier, G.; Subramaniam, R.M. The role of PET/CT in the management of cervical cancer. Am. J. Roentgenol. 2013, 201, W192–W205. [Google Scholar] [CrossRef]
- Rufini, V.; Garganese, G.; Ieria, F.P.; Pasciuto, T.; Fragomeni, S.M.; Gui, B.; Florit, A.; Inzani, F.; Zannoni, G.F.; Scambia, G.; et al. Diagnostic performance of preoperative [18F] FDG-PET/CT for lymph node staging in vulvar cancer: A large single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3303–3314. [Google Scholar] [CrossRef]
- Oldan, J.D.; Sullivan, S.A. Positron emission tomography-computed tomography for inguinal nodes in vulvar cancer. World J. Nucl. Med. 2018, 17, 139–144. [Google Scholar] [CrossRef]
- Crivellaro, C.; Guglielmo, P.; De Ponti, E.; Elisei, F.; Guerra, L.; Magni, S.; La Manna, M.; Di Martino, G.; Landoni, C.; Buda, A. 18F-FDG PET/CT in preoperative staging of vulvar cancer patients: Is it really effective? Medicine 2017, 96, e7943. [Google Scholar] [CrossRef]
- Collarino, A.; Garganese, G.; Olmos, R.A.V.; Stefanelli, A.; Perotti, G.; Mirk, P.; Fragomeni, S.M.; Ieria, F.P.; Scambia, G.; Giordano, A.; et al. Evaluation of dual-timepoint 18F-FDG PET/CT imaging for lymph node staging in vulvar cancer. J. Nucl. Med. 2017, 58, 1913–1918. [Google Scholar] [CrossRef]
- Dolanbay, M.; Ozcelik, B.; Abdulrezzak, U.; Serin, I.S.; Kutuk, M.S.; Uludag, S. F-18 fluoro-D-glucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in planning of surgery and sentinel lymph node screening in vulvar cancers. Arch. Gynecol. Obstet. 2016, 293, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, P.; Pinto, A.S.; Violante, L.; Nunes, S.; Teixeira, R.; Petiz, A.; Duarte, L.H. 18F-FDG PET/CT in Patients with Vulvar and Vaginal Cancer: A Preliminary Study of 20 Cases. Acta Médica Port. 2022, 35, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Peiró, V.; Chiva, L.; González, A.; Bratos, R.; Alonso, S.; Márquez, R.; Carballo, N.; Alonso-Farto, J.C. Utility of the PET/CT in vulvar cancer management. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, X.; Yang, X.; Li, Q. Diagnosis of primary clear cell carcinoma of the vagina by 18F-FDG PET/CT. Clin. Nucl. Med. 2019, 44, 332–333. [Google Scholar] [CrossRef]
- Triumbari, E.K.; de Koster, E.J.; Rufini, V.; Fragomeni, S.M.; Garganese, G.; Collarino, A. 18F-FDG PET and 18F-FDG PET/CT in vulvar cancer: A systematic review and meta-analysis. Clin. Nucl. Med. 2021, 46, 125–132. [Google Scholar] [CrossRef]
- Albano, D.; Bonacina, M.; Savelli, G.; Ferro, P.; Busnardo, E.; Gianolli, L.; Camoni, L.; Giubbini, R.; Bertagna, F. Clinical and prognostic 18F-FDG PET/CT role in recurrent vulvar cancer: A multicentric experience. Jpn. J. Radiol. 2022, 40, 66–74. [Google Scholar] [CrossRef]
- Christensen, D.S.; Petersen, L.K.; Dueholm, M.; Ipsen, P.; Marinovskij, E.; Christiansen, T.; Bouchelouche, K. Comparison of 18F-FDG PET/CT and MRI for detection of regional lymph node metastasis in vulvar cancer and vaginal cancer. Soc. Nucl. Med. 2019, 60, 554. [Google Scholar]
- Garganese, G.; Collarino, A.; Fragomeni, S.M.; Rufini, V.; Perotti, G.; Gentileschi, S.; Evangelista, M.T.; Ieria, F.P.; Zagaria, L.; Bove, S.; et al. Groin sentinel node biopsy and 18F-FDG PET/CT-supported preoperative lymph node assessment in cN0 patients with vulvar cancer currently unfit for minimally invasive inguinal surgery: The GroSNaPET study. Eur. J. Surg. Oncol. 2017, 43, 1776–1783. [Google Scholar] [CrossRef]
- Robertson, N.; Hricak, H.; Sonoda, Y.; Sosa, R.; Benz, M.; Lyons, G.; Abu-Rustum, N.R.; Sala, E.; Vargas, H.A. The impact of FDG-PET/CT in the management of patients with vulvar and vaginal cancer. Gynecol. Oncol. 2016, 140, 420–424. [Google Scholar] [CrossRef]
- Sponholtz, S.E.; Mogensen, O.; Hildebrandt, M.G.; Jensen, P.T. Clinical impact of pre-treatment FDG-PET/CT staging of primary ovarian, fallopian tube, and peritoneal cancers in women. Acta Obstet. Et Gynecol. Scand. 2020, 99, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Sakamoto, J.; Osaka, Y.; Shibata, T.; Fujita, S.; Sasagawa, T. Utility of 18F-fluorodeoxyglucose-positron emission tomography in the differential diagnosis of benign and malignant gynaecological tumours. J. Med. Imaging Radiat. Oncol. 2018; online ahead of print. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Oguri, H.; Yamada, R.; Maeda, N.; Kohsaki, S.; Fukaya, T. Preoperative evaluation of pelvic masses with combined 18F-fluorodeoxyglucose positron emission tomography and computed tomography. Int. J. Gynaecol. Obstet. 2008, 102, 124–127. [Google Scholar] [CrossRef]
- Nogami, Y.; Banno, K.; Irie, H.; Iida, M.; Kisu, I.; Masugi, Y.; Tanaka, K.; Tominaga, E.; Okuda, S.; Murakami, K.; et al. The efficacy of preoperative positron emission tomography-computed tomography (PET-CT) for detection of lymph node metastasis in cervical and endometrial cancer: Clinical and pathological factors influencing it. Jpn. J. Clin. Oncol. 2015, 45, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Grueneisen, J.; Beiderwellen, K.; Heusch, P.; Gratz, M.; Schulze-Hagen, A.; Heubner, M.; Kinner, S.; Forsting, M.; Lauenstein, T.; Ruhlmann, V.; et al. Simultaneous positron emission tomography/magnetic resonance imaging for whole-body staging in patients with recurrent gynecological malignancies of the pelvis: A comparison to whole-body magnetic resonance imaging alone. Investig. Radiol. 2014, 49, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.M.; Kirchner, J.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Schaarschmidt, B.M.; Forsting, M.; Herrmann, K.; Antoch, G.; Umutlu, L. Comparison of 18F-FDG PET/MRI and MRI alone for whole-body staging and potential impact on therapeutic management of women with suspected recurrent pelvic cancer: A follow-up study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 622–629. [Google Scholar] [CrossRef]
- Schwartz, M.; Gavane, S.C.; Bou-Ayache, J.; Kolev, V.; Zakashansky, K.; Prasad-Hayes, M.; Taouli, B.; Chuang, L.; Kostakoglu, L. Feasibility and diagnostic performance of hybrid PET/MRI compared with PET/CT for gynecological malignancies: A prospective pilot study. Abdom. Radiol. 2018, 43, 3462–3467. [Google Scholar] [CrossRef]
- Kirchner, J.; Sawicki, L.M.; Suntharalingam, S.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Deuschl, C.; Herrmann, K.; Antoch, G.; Forsting, M.; et al. Whole-body staging of female patients with recurrent pelvic malignancies: Ultra-fast 18F-FDG PET/MRI compared to 18F-FDG PET/CT and CT. PLoS ONE 2017, 12, e0172553. [Google Scholar] [CrossRef]
- Xin, J.; Ma, Q.; Guo, Q.; Sun, H.; Zhang, S.; Liu, C.; Zhai, W. PET/MRI with diagnostic MR sequences vs PET/CT in the detection of abdominal and pelvic cancer. Eur. J. Radiol. 2016, 85, 751–759. [Google Scholar] [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Makihara, N.; Ebina, Y.; Yamada, H.; Takahashi, S.; Sugimura, K. Value of fusion of PET and MRI in the detection of intra-pelvic recurrence of gynecological tumor: Comparison with 18F-FDG contrast-enhanced PET/CT and pelvic MRI. Ann. Nucl. Med. 2014, 28, 25–32. [Google Scholar] [CrossRef]
- Virarkar, M.; Devine, C.; Bassett, R.; Jr Javadi, S.; Faria, S.C.; Bhosale, P. Update on Diagnostic Performance of PET/MRI in Gynecological Malignancies: A Systematic Review and Meta-Analysis. J. Belg. Soc. Radiol. 2020, 104, 4. [Google Scholar] [CrossRef]
- Virarkar, M.; Ganeshan, D.; Devine, C.; Bassett, R.; Jr Kuchana, V.; Bhosale, P. Diagnostic value of PET/CT versus PET/MRI in gynecological malignancies of the pelvis: A meta-analysis. Clin. Imaging 2020, 60, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, D.; Hansen, N.L.; Loft, A. PET/CT Variants and Pitfalls in Gynecological Cancers. Semin. Nucl. Med. 2021, 51, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.S.; Atri, M.; Bandos, A.I.; Mannel, R.S.; Gold, M.A.; Lee, S.I. Identification of Distant Metastatic Disease in Uterine Cervical and Endometrial Cancers with FDG PET/CT: Analysis from the ACRIN 6671/GOG 0233 Multicenter Trial. Radiology 2018, 287, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, W.Y.; Liang, J.A.; Lu, Y.Y.; Wang, H.Y.; Tsai, S.C.; Kao, C.H. Opportunities for 2-[18F] fluoro-2-deoxy-D-glucose PET/CT in cervical-vaginal neuroendocrine carcinoma: Case series and literature review. Korean J. Radiol. 2012, 13, 760–770. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bentivegna, E.; Uzan, C.; Gouy, S.; Leboulleux, S.; Duvillard, P.; Lumbroso, J.; Haie-Meder, C.; Morice, P. The accuracy of FDG-PET/CT in early-stage cervical and vaginal cancers. Gynecol. Obstet. Fertil. 2011, 39, 193–197. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Ripoll, M.A.; Bergman, A.; Silins, I.; Poromaa, I.S.; Ahlström, H.; Stålberg, K. Validation of 18F-FDG PET/MRI and diffusion-weighted MRI for estimating the extent of peritoneal carcinomatosis in ovarian and endometrial cancer—A pilot study. Cancer Imaging 2021, 21, 34. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Gao, C.; Zeng, W. Comparison of 68Ga-FAPI and 18F-FDG PET/CT for the diagnosis of primary and metastatic lesions in abdominal and pelvic malignancies: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1093861. [Google Scholar] [CrossRef]
- Lakhani, A.; Khan, S.R.; Bharwani, N.; Stewart, V.; Rockall, A.G.; Khan, S.; Barwick, T.D. FDG PET/CT Pitfalls in Gynecologic and Genitourinary Oncologic Imaging. Radiographics 2017, 37, 577–594. [Google Scholar] [CrossRef]
- Tanizaki, Y.; Kobayashi, A.; Shiro, M.; Ota, N.; Takano, R.; Mabuchi, Y.; Yagi, S.; Minami, S.; Terada, M.; Ino, K. Diagnostic value of preoperative SUVmax on FDG-PET/CT for the detection of ovarian cancer. Int. J. Gynecol. Cancer 2014, 24, 454–460. [Google Scholar] [CrossRef]
- Liu, Y. Benign ovarian and endometrial uptake on FDG PET-CT: Patterns and pitfalls. Ann. Nucl. Med. 2009, 23, 107–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).