Abstract
Steelmaking processes inevitably generate large amounts of byproducts, including slags, specks of dust, etc., and their treatment has been a critical issue for the steelmaking industry. Kish graphite is a valuable substance existing in steelmaking byproducts, and the recovery of Kish graphite has attracted more attention in recent years. The purpose of this study was to use a multi-stage froth flotation process for the beneficiation of Kish graphite and to investigate the influence of flotation conditions on the mass distribution of graphite and impurities. The results showed that the dust D2 contained ~34 wt.% of graphite and thus had the highest potential for the recovery of Kish graphite. The dosages of frother (methyl isobutyl carbinol, MIBC) at 0.005 kg/t and collector (kerosene) at 1 kg/t were optimal for the flotation of Kish graphite. After three-stage froth flotation, the graphite content of the concentrate was progressively increased to 84.09 wt.%, and the entire recovery rate was 93.05%. During the multi-stage froth flotation process, most of the impurities were separated in stage I, but the Fe-containing impurities were mainly separated in stage II. Some Ca2+, Na+, and K+ were leached out, and there were barely any heavy metals in the liquid phases.
1. Introduction
Steel is the most widely used metal in engineering and construction, and thus, steelmaking has been an important industry in modern society. The crude steel production in the world has grown to 1885 million tons in 2022, and the average growth rate is 1.7% from 2017 to 2022 [1]. When producing steel, various byproducts (slag, sludge, dust, etc.) are generated simultaneously, depending on the steelmaking routes. The electric arc furnace (EAF) route generates around 200 kg of byproducts to produce one ton of crude steel, while the blast furnace (BF) with basic oxygen furnace (BOF) route generates around 400 kg of byproducts. The recycling of this enormous amount of steelmaking byproducts is very critical, and it is one of the key elements of a circular economy [2]. Fisher and Barron [3] indicated that steelmaking slags could be recycled for cement, concrete aggregate, fertilizer, roadbed materials, riverbanks, artificial reefs, etc. Das et al. [4] reported that the steelmaking dust and sludge are generally recycled through the sintering plant to recover iron oxide. However, most of these recycling applications are low value-added or even valueless in the market; in many cases, steel companies must pay treatment fees for the recycling of byproducts. In order to promote resource circulation and establish a sound market economy, it is necessary to enhance the recycling value of steelmaking byproducts.
Kish graphite is a unique substance existing in steelmaking byproducts, and the recovery of Kish graphite should create additional benefits. According to the phase diagram of the Fe–C system [5], the solubility of carbon decreases with decreasing temperature in the cooling of molten iron, and the carbon in excess of the solubility limit precipitates as graphite flakes, namely Kish graphite. Liu and Loper [6] indicated that Kish graphite accumulated at various locations in steelmaking facilities, such as the area of desulfurization operations, dust collection systems, and waste dump sites, and high contents of Kish graphite can be found in steelmaking slag and dust. In Taiwan, the uncollected Kish graphite was dispersed by wind several years ago and consequently caused environmental pollution in the nearby area of steelmaking works. In recent years, the recovery of Kish graphite has received more research attention due to the supply risk of natural graphite flake. The United States and the European Union suggest that natural graphite has been one of the most critical raw materials due to its high economic importance and high risk of supply disruptions [7,8]. In response to this issue, Kish graphite may be a viable alternative to natural graphite if the recovery is carried out. The U.S. Geological Survey [9] reported that it is technically feasible to recover high-quality graphite flakes from steelmaking Kish, but the recovery is currently not practiced because of the market price of graphite. Moreover, there is less information on the quantity and value of Kish graphite. In recent years, some investigations have studied the high-value applications of Kish graphite, e.g., cathode materials for aluminum-ion batteries and graphene nanoplatelets [10,11]. This thus provides an added incentive for the recovery of Kish graphite.
To achieve the recovery of Kish graphite, an economically feasible method must be developed. Conventionally, froth flotation is an effective method to remove impurities from natural graphite ore, and it is relatively low-cost and environmentally friendly compared to high-temperature purification and chemical treatments [12,13]. Natural graphite ore usually contains some impurities from gangue minerals, such as quartz, mica, feldspar, silicates, and carbonates [14,15], while Kish graphite normally includes iron oxides, lime, silica, and alumina that are from the raw materials of steelmaking [16]. Because the constituents of impurities are different between natural graphite and Kish graphite, the operating conditions of froth flotation for the beneficiation of Kish graphite should be studied further. Some studies also indicate that flotation reagents, i.e., depressants, collectors, and frothers, can significantly affect the surface properties and flotation performance of a mineral [14,17,18]. Hence, the purpose of this study was to use a multi-stage froth flotation for the beneficiation of Kish graphite and to investigate the appropriate operating conditions. The effects of frother and collector on graphite content and recovery rate were studied, and the mass distribution of elements in the multi-stage froth flotation was revealed.
2. Results and Discussion
2.1. Characterization of Steelmaking Byproducts
The previous study [6] reported that Kish graphite can be found in the slag from the desulfurization process and the dust collected. Accordingly, the desulfurization slags S1 and S2 and the dusts D1 and D2 were sampled and analyzed in this research. Table 1 presents the chemical compositions of S1, S2, D1, and D2. The slags S1 and S2 had high moisture contents (24.73 wt.% and 26.96 wt.%, respectively), which were significantly higher than those of the dusts D1 and D2. During the treatment process of desulfurization of slag in the integrated steel mill, slag cooling was performed by pouring a large amount of water into slag ladles. Du et al. [19] describe a similar wet process for the cooling of desulfurization slag, and such a process results in the high moisture contents of the slags S1 and S2. The slags and specks of dust had various values of loss on ignition (LOI) between 8.64 wt.% and 34.99 wt.%, which may be attributed to the decomposition of minerals and the oxidation of Kish graphite. In terms of elemental composition, Ca, Fe, Si, and Al were the major elements in the steelmaking byproducts, and Mg, Na, and K existed in small amounts of less than 3 wt.%. The largest amount of metal in the slags was Ca (29.90 wt.% in S1 and 31.41 wt.% in S2), while that in the dust was Fe (24.49 wt.% in D1 and 18.89 wt.% in D2). The slags S1 and S2 also contained 5.47 wt.% and 7.30 wt.% of Fe, respectively. In addition, the steelmaking byproducts had various contents of Al (1.81–5.76 wt.%) and similar contents of Si (3.30–3.80 wt.%). The above significant elements should be highly related to the subsequent beneficiation process of Kish graphite. Regarding heavy metals, the slags S1 and S2 contained small amounts of Mn, trace Cr, and Pb. Some studies also reported that Mn and Cr were the major heavy metals existing in desulfurization slag [20,21]. In addition to Mn, Cr, and Pb, the specks of dust D1 and D2 contained some Zn at 893 mg/kg and 640 mg/kg, respectively. The heavy metals may be leached into the liquid phase of froth flotation and, in practice, could affect wastewater treatments.

Table 1.
Chemical compositions of steelmaking slags and specks of dust.
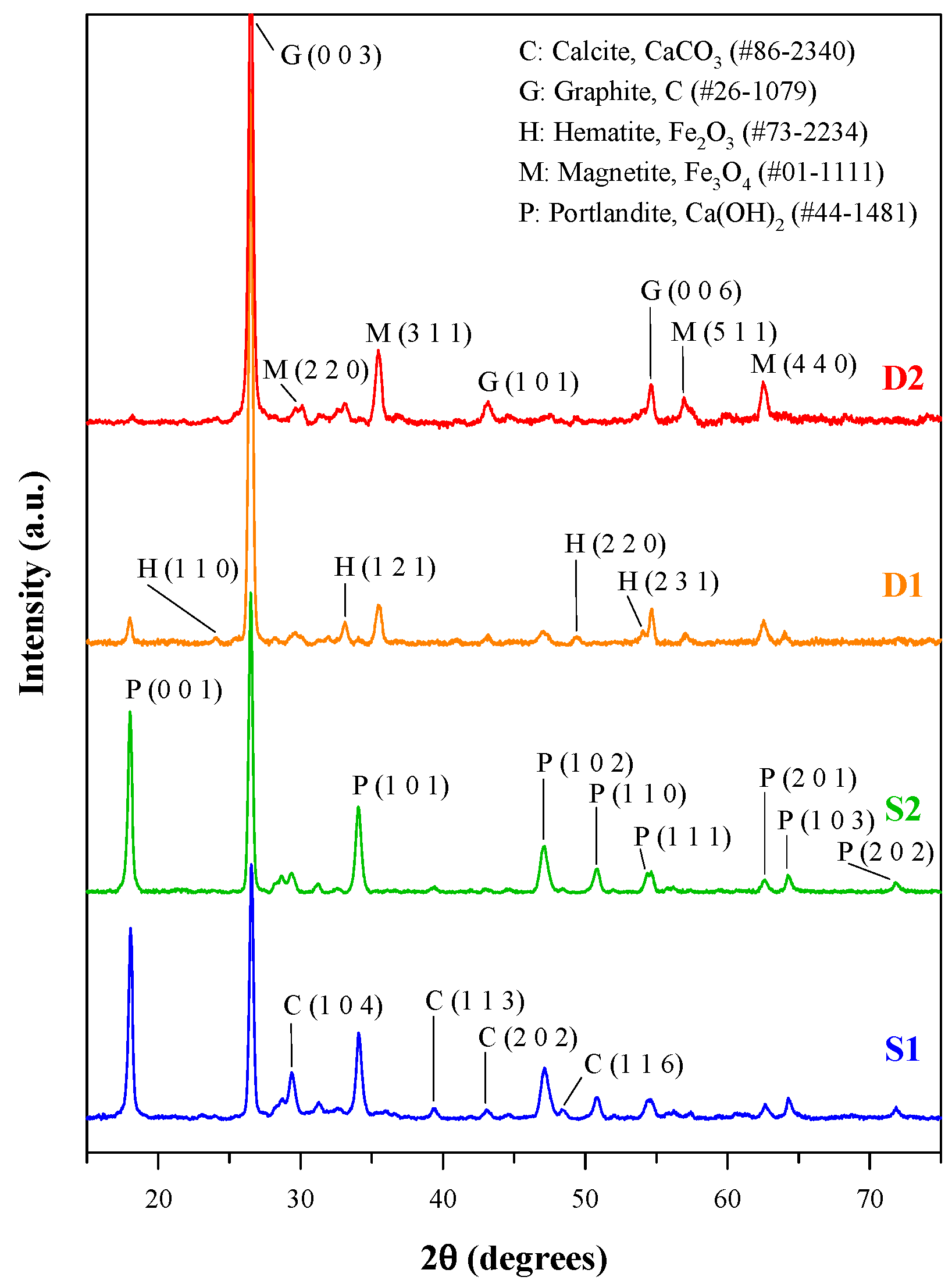
The X-ray diffraction (XRD) patterns of the slags and specks of dust are presented in Figure 1. It is obvious that graphite is the dominant crystalline phase in the steelmaking byproducts, and dust D2 has the highest diffraction intensity of graphite. Portlandite, Ca(OH)2, was found in all the slags and specks of dust, but its diffraction intensity was much lower in the XRD patterns of specks of dust. Moreover, the slags S1 and S2 contained calcite (CaCO3), whereas the specks of dust D1 and D2 contained some iron oxides, including hematite (Fe2O3) and magnetite (Fe3O4). These results of mineralogical composition are consistent with the elemental contents of the steelmaking byproducts [22].
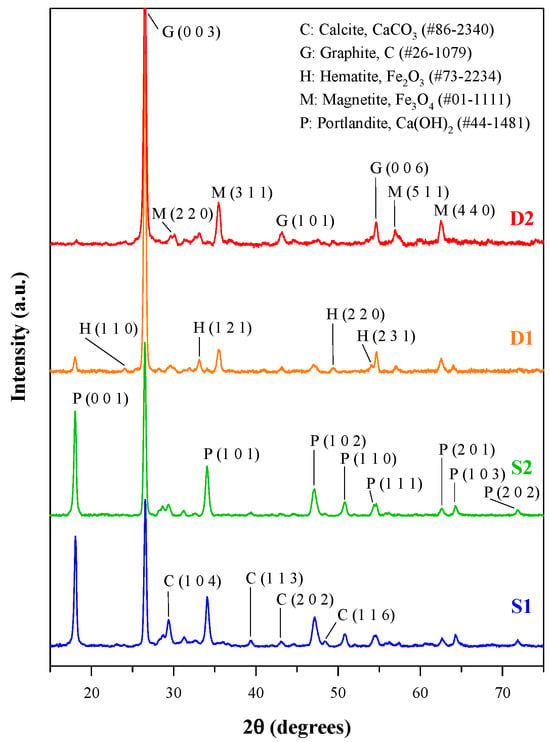
Figure 1.
XRD patterns of steelmaking slags and dusts.
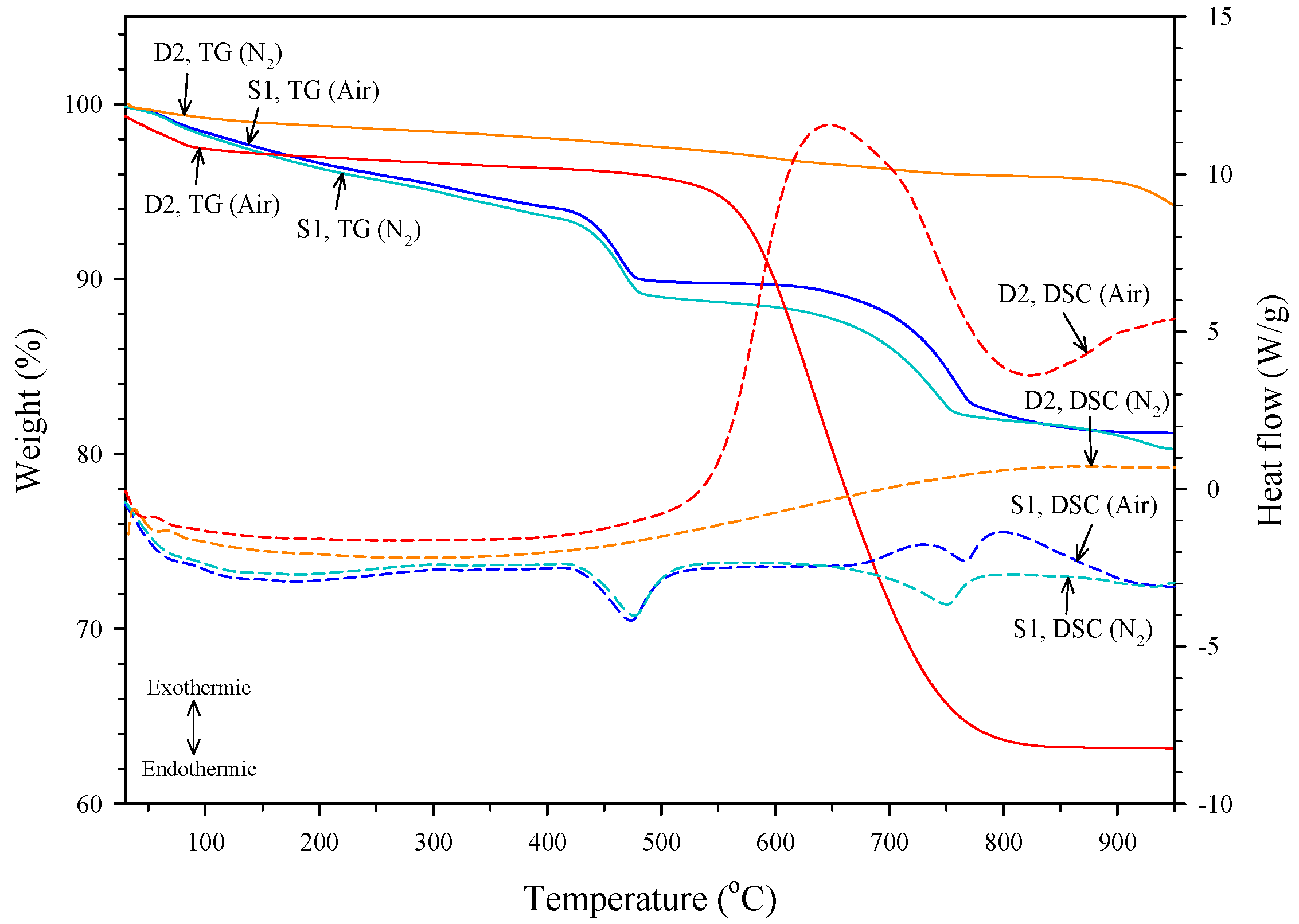
The graphite content was determined by using a simultaneous differential scanning calorimetry and thermogravimetric analyzer (DSC-TGA). Figure 2 shows the TG and DSC curves of the slag S1 and the dust D2 under nitrogen and air atmospheres. The dust D2 had a great weight loss between 520 °C and 830 °C under the air atmosphere but no significant weight loss under the nitrogen atmosphere. The DSC curve of dust D2 showed that the weight loss under an air atmosphere is related to an exothermic reaction due to the oxidation of graphite. Jiang et al. [23] provided similar TG curves about the oxidation of natural graphite in the air atmosphere. The slag S1 had two significant weight losses under both nitrogen and air atmospheres. The weight loss located at the temperature range of 410–530 °C is associated with the dehydration of Ca(OH)2, which is an endothermic reaction and results in the release of H2O. The DSC curve of the slag S1 under a nitrogen atmosphere showed that an endothermic reaction due to the decarbonation of CaCO3 occurred at 650–780 °C. Under air atmosphere, the DSC curve of the slag S1 presented not only the decarbonation of CaCO3 but also the oxidation of graphite occurring simultaneously at 650–900 °C. The observed reactions related to the oxidation of graphite and the decompositions of Ca(OH)2 and CaCO3 are consistent with the results of LOI and XRD analyses. In this study, the graphite content was obtained based on the results of the DSC-TGA analysis under nitrogen and air atmospheres. Under the air atmosphere, both the decarbonation of CaCO3 and the oxidation of graphite occur, but the oxidation of graphite is absent under the nitrogen atmosphere. The graphite content of a sample can thus be obtained by calculating the difference in weight loss at the related temperature range between the two atmospheres. The graphite contents of the slags and dust are also given in Table 1. It was noted that the graphite content of dust D2 was much higher than that of D1, S1, and S2, and this showed that recovering Kish graphite from dust D2 may be easier and more efficient.
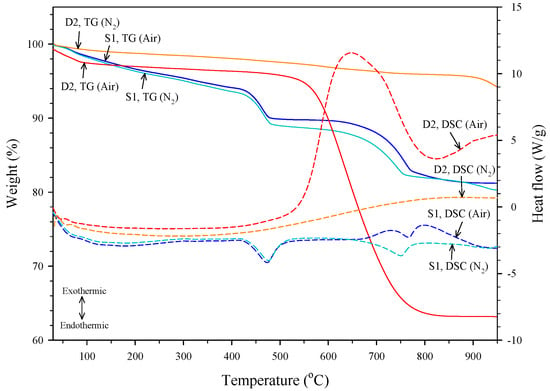
Figure 2.
TG and DSC curves of S1 and D2 under nitrogen and air atmospheres.
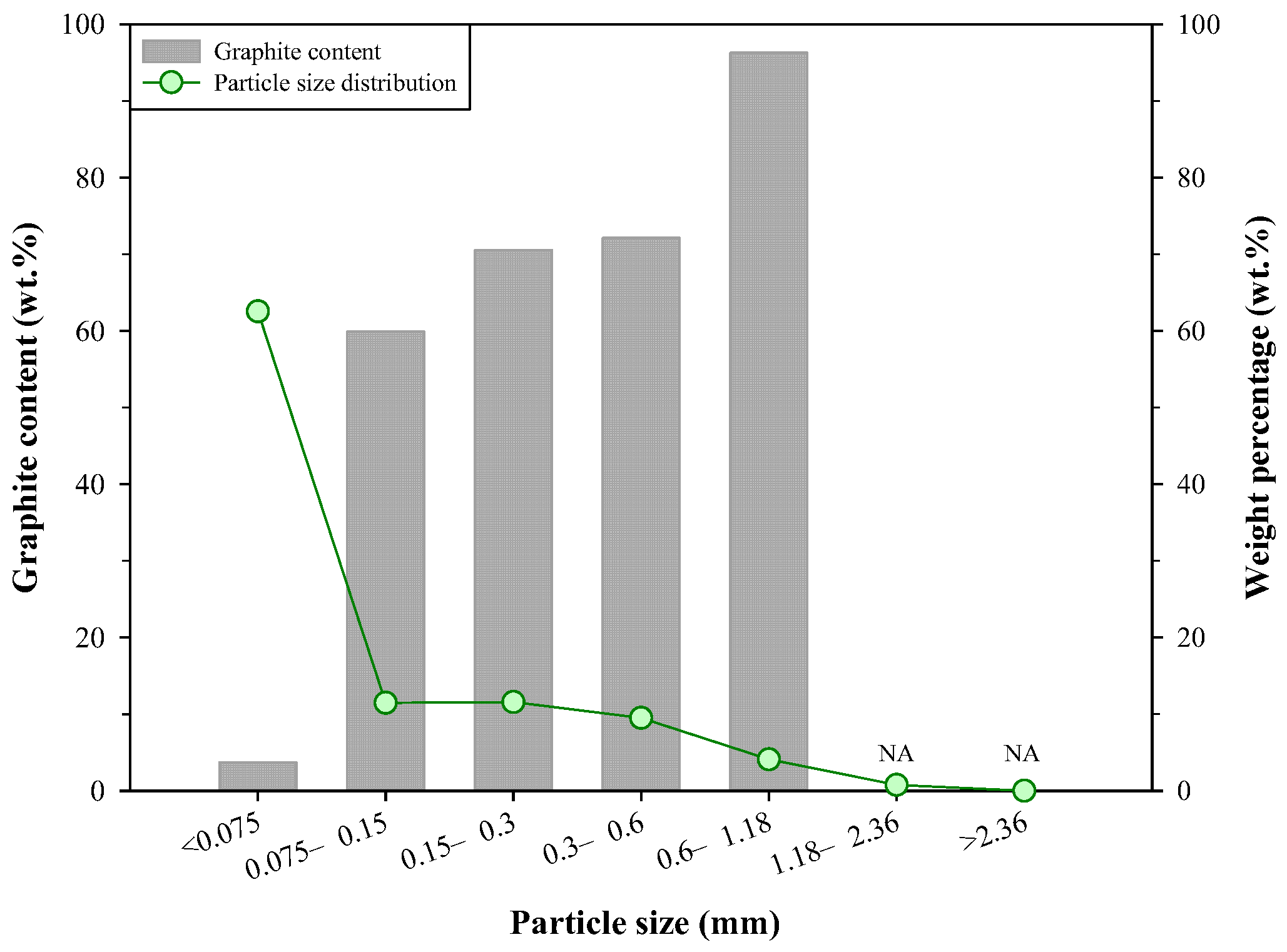
In summary, from the above characterization of the steelmaking byproducts, the dust D2 has the highest potential for the recovery of Kish graphite. Therefore, it was selected as a candidate for the froth flotation test. Further analyses for the distribution of particle size and graphite content of dust D2 were conducted, and the results are presented in Figure 3. The particle size less than 0.075 mm accounted for the largest amount in the dust D2 (62.6 wt.%), and the particle size ranges of 0.075–0.15 mm, 0.15–0.3 mm, and 0.3–0.6 mm had similar weight percentages (11.5 wt.%, 11.6 wt.%, and 9.5 wt.%, respectively). The particle size range of 0.6–1.18 mm accounted for 4.1 wt.%, and the weight percentage of particles larger than 1.18 mm was less than 1 wt.%. It was found that the graphite content was related to particle size; generally, particles with larger sizes had higher graphite content. Although the <0.075 mm particles accounted for the largest portion in the dust D2, the graphite content in this particle size range was only 3.71 wt.%. Machemer [24] investigated the particulate materials in iron and steel manufacturing facilities, and it was found that the airborne particulate materials mainly included graphite flakes (tens of micrometers to millimeters in size) and other small particles (e.g., iron oxides, submicrometers to tens of micrometers in size). As a result, most of the graphite was larger than 0.038 mm, and the fine-size fraction (<0.038 mm) contained higher concentrations of metals. The dust samples collected in this study were from an air pollution control system and thus had similar features in the distribution of graphite and impurities. The particles between 0.15 mm and 0.6 mm contained 59.39–72.12 wt.% of Kish graphite, and the graphite content of the 0.6–1.18 mm particles reached 96.28 wt.%, a level of purity that already fulfills the requirement of some commercial graphite products (≥90 wt.%), e.g., industrial refractories and castings [15]. The above results also indicated that the impurities, e.g., iron oxides, should be mainly distributed in the fine particles. The distribution of graphite and impurities can provide some useful information for the operation of froth flotation.
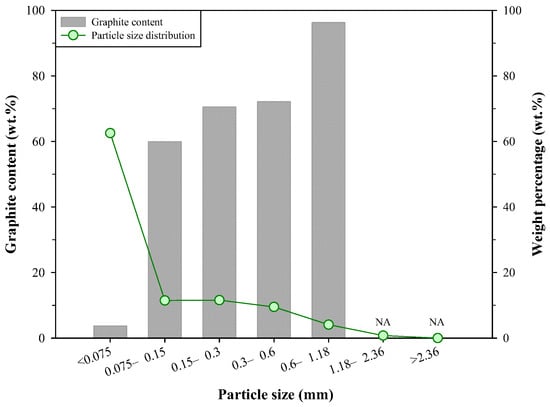
Figure 3.
Distribution of particle size and graphite content in D2.
2.2. Beneficiation of Kish Graphite with Froth Flotation
The dust D2 then proceeded to the beneficiation of Kish graphite with a froth flotation process. Because graphite is hydrophobic and has high natural floatability, it should be transferred to concentrate in froth flotation, leaving impurities in pulp or tailings. In the following sections, the graphite content in concentrate and the recovery rate were used to evaluate the performance of froth flotation. In an ideal situation, the graphite content in concentrate and the recovery rate are both supposed to be as high as possible, but the tendencies of these two variables are usually inconsistent. In reality, the balance between the graphite content in concentrate and the recovery rate should be found for the beneficiation of graphite with froth flotation. Equation (1) gives the calculation of the recovery rate after each stage of froth flotation:
where and are the weight percentages of concentrate and tailings, respectively; and are the graphite contents in concentrate and tailings, respectively. Table 2 shows the graphite content and recovery rate at different pulp densities in a single-stage froth flotation process. The dosage of methyl isobutyl carbinol (MIBC) was 0.175 kg/t, referring to a previous study [25]. It was found that the weight percentage and graphite content of concentrate significantly decreased with increasing pulp density, while those of tailings increased. At pulp densities of 20% and 30%, the graphite content in concentrate was lower than that in dust D2, and this showed that the beneficiation of Kish graphite failed. Furthermore, the recovery rate greatly decreased from 74.24% to 16.51% when the pulp density increased from 10% to 30%. Wills and Finch [26] have noted that the pulp density can be as low as 8% and as high as 55%, and commercial flotation works usually use pulp densities of 25–40%. The higher the pulp density, the smaller the flotation cell, and fewer reagents (frothers, collectors, etc.) are required; however, a high pulp density may cause overloading bubbles and the entrainment of mineralized bubbles in tailings, thus reducing the recovery rate. In this study, a pulp density of 10% was selected for the following experiments.

Table 2.
Graphite content and recovery rate at different pulp densities in single-stage froth flotation (MIBC = 0.175 kg/t).
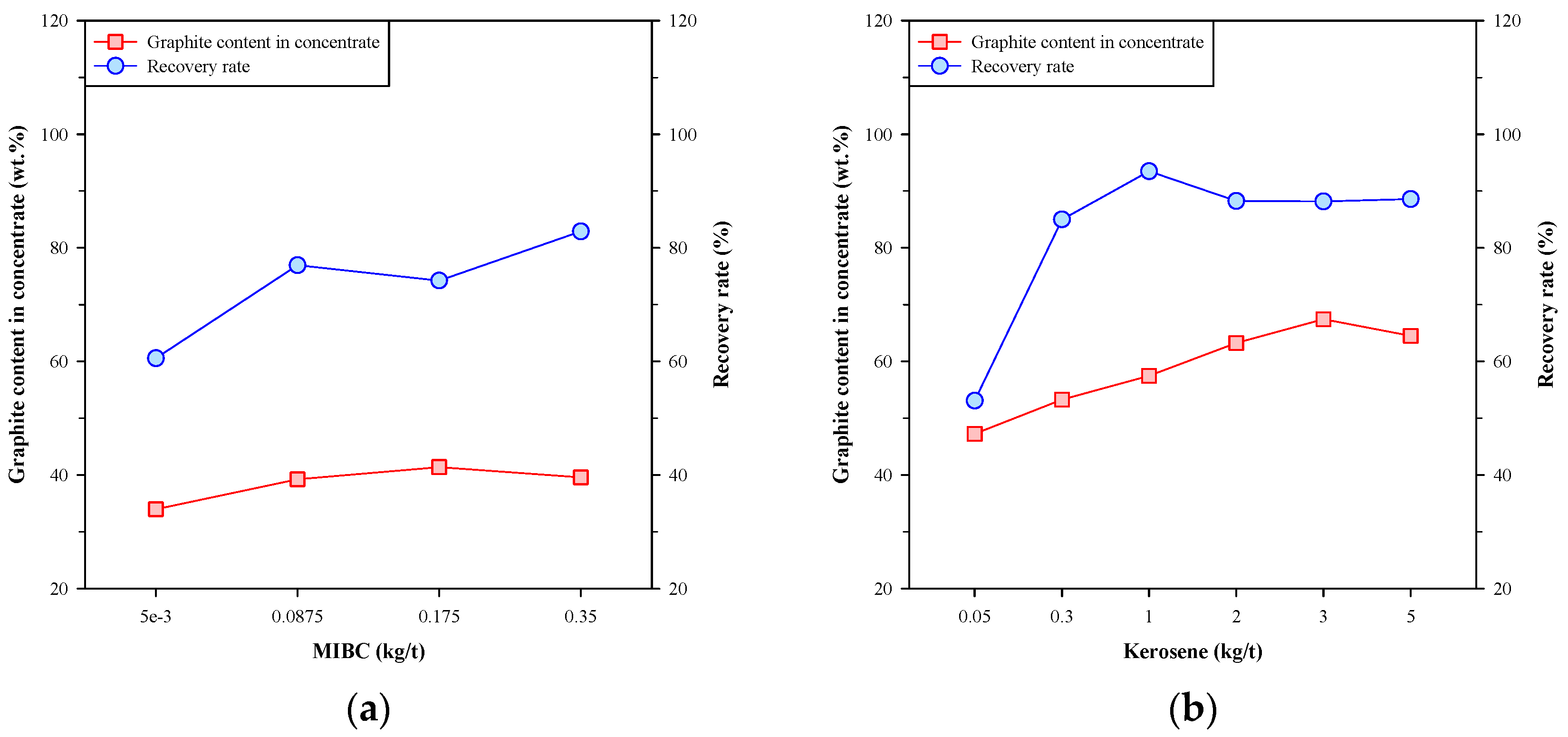
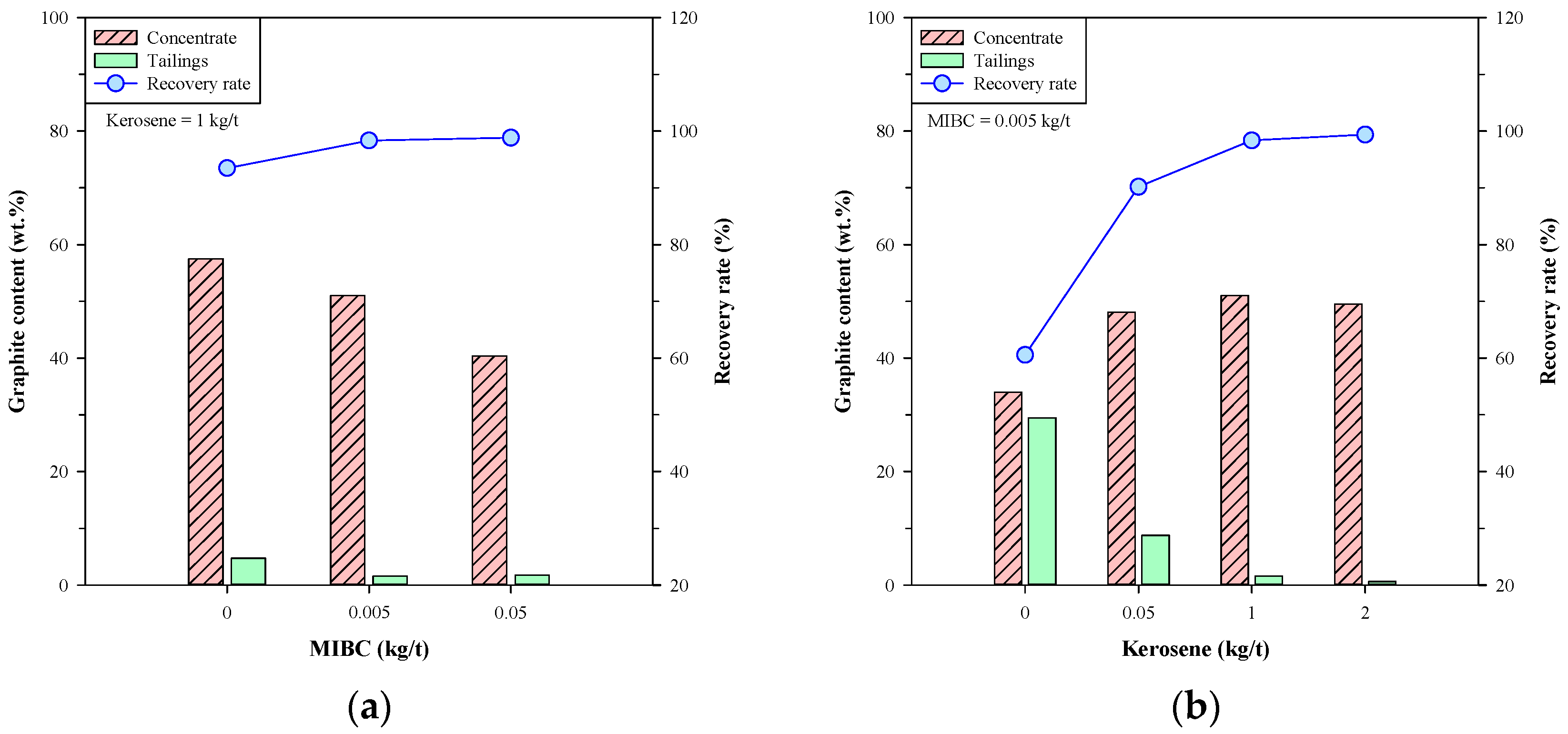
Figure 4 shows the graphite content in concentrate and the recovery rate of the single-stage froth flotation using MIBC and kerosene individually. In Figure 4a, the graphite content in concentrate was slightly increased with the dosage of MIBC, and the recovery rate was significantly increased from 60.54% to 82.89%. These results showed that MIBC had a major effect on the recovery rate of Kish graphite. On the other hand, Figure 4b shows that both the graphite content in concentrate and the recovery rate were increased to a maximum at a specific dosage of kerosene. The highest graphite content in concentrate was 67.44 wt.% at the kerosene dosage of 3 kg/t, and the maximum recovery rate (93.48%) was obtained by doping 1 kg/t of kerosene. Sun et al. [27] suggested that the kerosene droplets tend to coalesce together and undermine the collision probability between kerosene and graphite particles, thus affecting the efficiency of graphite flotation. The above findings suggest that using MIBC and kerosene individually has limitations on the beneficiation of Kish graphite, and the effect of kerosene is more significant than that of MIBC.
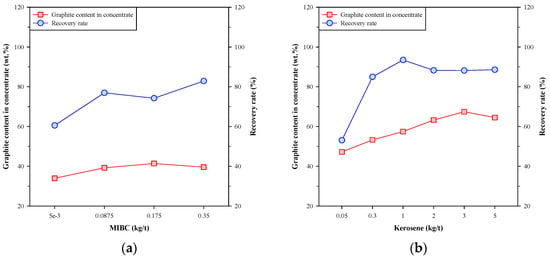
Figure 4.
Graphite content in concentrate and recovery rate of single-stage froth flotation using (a) MIBC or (b) kerosene.
Figure 5 presents the graphite content and recovery rate of single-stage froth flotation using MIBC and kerosene simultaneously. When doping 1 kg/t of kerosene (Figure 5a), the graphite contents in concentrate and tailings decreased with the dosage of MIBC, while the recovery rate attained over 98% at 0.005 kg/t of MIBC and above. In Figure 5b, the graphite content in concentrate increased with the dosage of kerosene, while that in tailings decreased to a very low level (<1.6 wt.% at 1 kg/t of kerosene). Moreover, the recovery rate greatly increased with the dosage of kerosene and reached >98% when doping 1 kg/t of kerosene or more. From these results, it was found that the improvements in graphite content in concentrate and the recovery rate were insignificant between 1 kg/t and 2 kg/t of kerosene, and the MIBC dosage of 0.005 kg/t seems sufficient for the froth flotation of Kish graphite. Arif Bhatti et al. [28] studied the beneficiation of low-grade graphite ore and indicated that low dosages of frother were enough for graphite flotation because of the natural floatability of graphite. Accordingly, 0.005 kg/t of MIBC and 1 kg/t of kerosene were concluded to be the appropriate dosages for the single-stage froth flotation of Kish graphite, and a concentrate with 51.00 wt.% graphite content and 98.36% recovery rate was obtained.
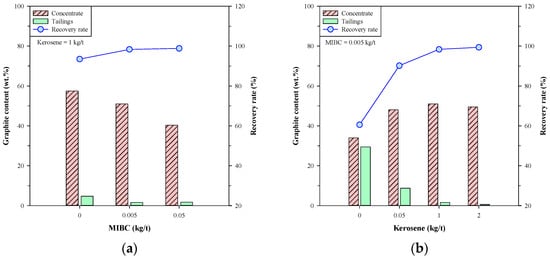
Figure 5.
Graphite content and recovery rate of single-stage froth flotation using both MIBC and kerosene; (a) various MIBC dosage with 1 kg/t of kerosene and (b) various kerosene dosages with 0.005 kg/t of MIBC.
Wills and Finch [26] indicated that the increase in impeller speed could recover more fine particles and increase the portion of concentrate, thus sometimes enhancing the recovery rate of a mineral. This study tried to increase the impeller speed from 1200 rpm to 2700 rpm, and the results are given in Table 3. Although the weight percentage of concentrate and recovery rate were slightly increased due to the increase in impeller speed, the graphite content in concentrate dropped from 51.00 wt.% to 47.13 wt.%. The improvement owing to increasing impeller speed seems insufficient, and higher impeller speed means more energy consumed for the operation of the froth flotation. Therefore, the lower impeller speed of 1200 rpm was retained for the multi-stage froth flotation process. The dosages of reagents and operating conditions of froth flotation used in stages I–III were the same, and the results are also presented in Table 3. From stage I to stage III, not only the weight percentage of concentrate but also its graphite content were enhanced progressively. The weight percentage of concentrate was increased from 65.24 wt.% to 86.68 wt.%, and the graphite content in concentrate was increased from 51.00 wt.% to 84.09 wt.% after the multi-stage froth flotation. The recovery rate of each stage was >95%, and the overall recovery rate was 93.05%. Robinson Jr. et al. [8] have reported that the product grade of flake graphite is 75–97 wt.%, and it is suggested that the concentrate of stage III already fulfills the requirement of commercial graphite products. However, the graphite content for industrial uses is 94–97 wt.%, and for electronic applications, it must be >99 wt.% [15]. For these applications, the Kish graphite beneficiated with multi-stage froth flotation needs to be purified further.

Table 3.
Graphite content and recovery rate of multi-stage froth flotation (MIBC = 0.005 kg/t and kerosene = 1 kg/t).
2.3. Mass Distributions of Elements in Multi-Stage Froth Flotation
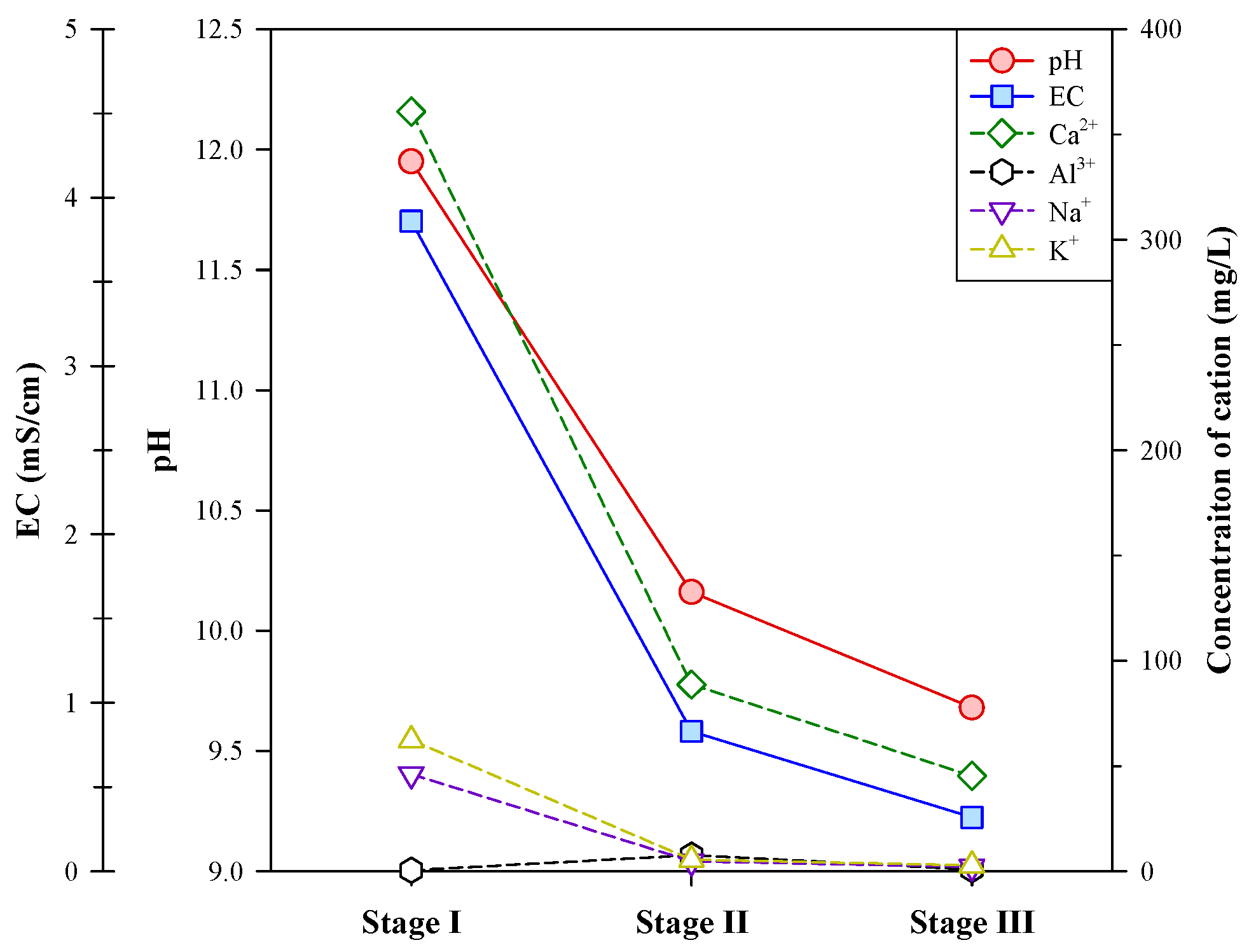
In addition to graphite, this study analyzed the contents of other elements (such as Ca, Si, Fe, Al, Na, K, etc.) in the concentrates and tailings of the multi-stage froth flotation and their ion concentrations in the liquid phase of each stage. Figure 6 shows the variations in the concentration of major cations (Ca2+, Al3+, Na+, and K+), pH, and electrical conductivity (EC) of liquid phases between flotation stages.
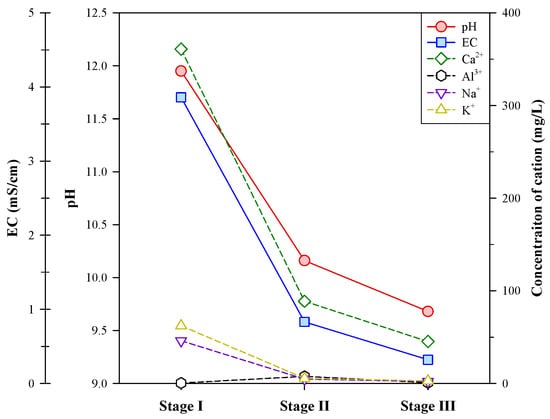
Figure 6.
Variations in pH, EC, and concentration of major cations in liquid phases between flotation stages.
In stage I, the liquid phase had high pH and EC values (11.95 and 3.86 mS/cm, respectively), and a high concentration of Ca2+ (360.9 mg/L) was detected. The high pH value should be mainly attributed to the dissolution of Ca(OH)2, which was accompanied by the leaching of Ca2+. Some alkali metal ions, Na+ (46.1 mg/L) and K+ (62.3 mg/L), were also analyzed in the liquid phase of stage I. Gray [29] has noted that the higher the ion concentration in a solution, the more conductive it would be, although the EC response is non-linear. The high concentrations of Ca2+, Na+, and K+ in the liquid phase of stage I were reflected in the high EC value. From stage I to stage II, the pH and EC values sharply dropped, and the concentrations of Ca2+, Na+, and K+ decreased significantly. A slight increase in the concentration of Al3+ was found in stage II, and this should be due to the decrease in pH. In stage III, the pH and EC values continued decreasing, but the decrease was more moderate. The concentration of Ca2+ decreased to 45.2 mg/L, and the other cations were below 3 mg/L. The concentrations of Si4+, Mg2+, Fe3+, and heavy metals (Cr3+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Cd2+, and Ba2+) in the liquid phases were also analyzed in this study, and most of them were not detected (<0.01 mg/L) except for Si4+, Mg2+, and Ba2+. The concentrations of Si4+ in the liquid phases were all below 0.5 mg/L, and Mg2+ was only detected in the liquid phase of stage III at 1.12 mg/L. A trace concentration of Ba2+ (0.14 mg/L) was found in the liquid phase of stage I. The above findings suggest that most of the soluble components were leached out in stage I, and the other impurities, e.g., iron oxides, mostly remained in the solid phases during the multi-stage froth flotation.
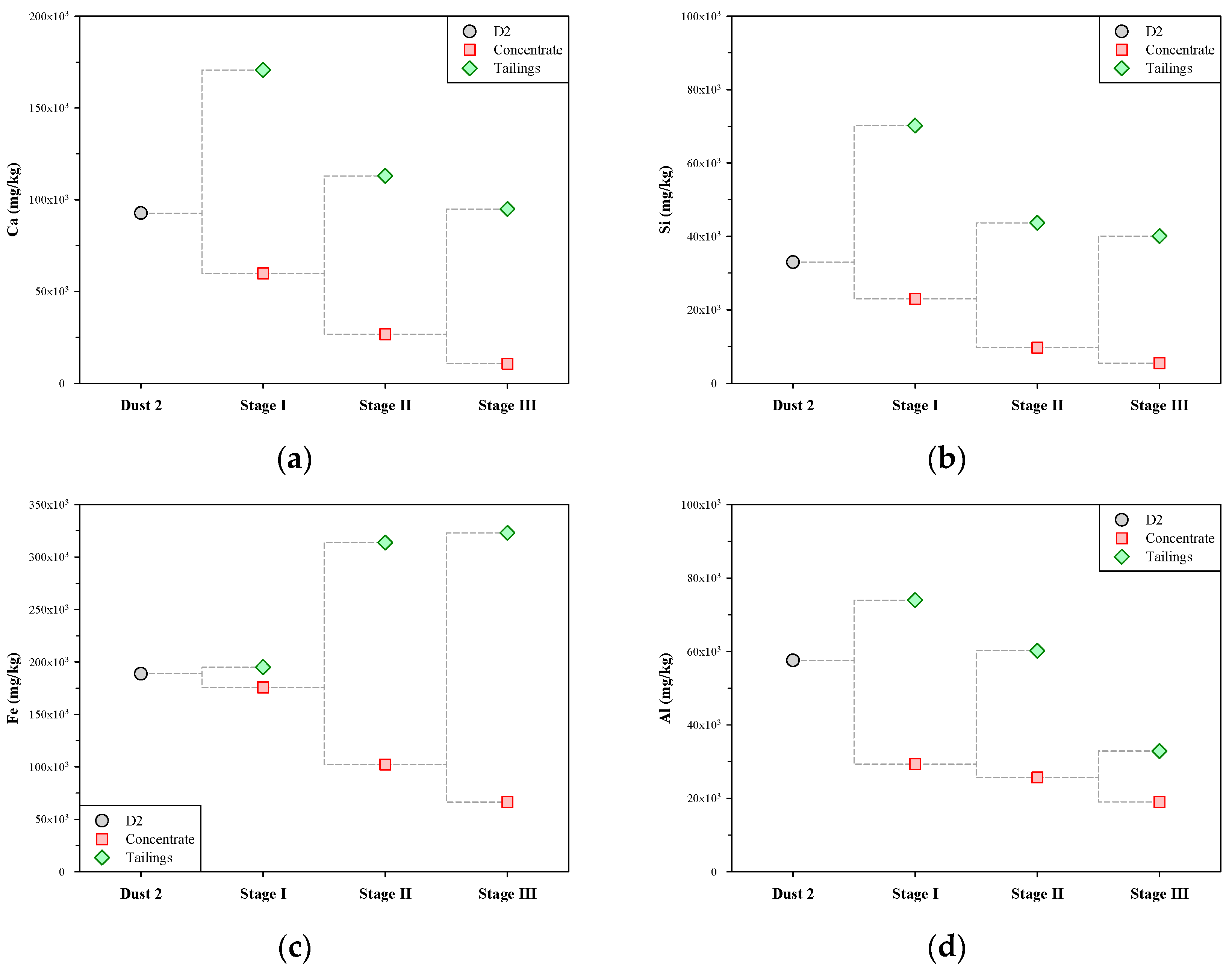
The contents of Ca, Si, Fe, and Al in the concentrates and tailings of the multi-stage froth flotation are shown in Figure 7. In terms of Ca (Figure 7a), the content of Ca in the tailings of each stage was significantly higher than that in the concentrate. This means that each stage of froth flotation was effective in transferring Ca-bearing impurities to tailings, thus continuously removing Ca from the concentrate. The content of Ca in the dust D2 was 92,700 mg/kg, and after the multi-stage froth flotation, that in the concentrate of stage III was decreased to 10,700 mg/kg. The variation in the content of Si during the multi-stage froth flotation was similar to that of Ca (see Figure 7b). There was a significant difference in the content of Si between the concentrate and tailings in the same stage of froth flotation. The content of Si in the dust D2 was 33,000 mg/kg, and it was reduced stage by stage to 5500 mg/kg after the multi-stage froth flotation.
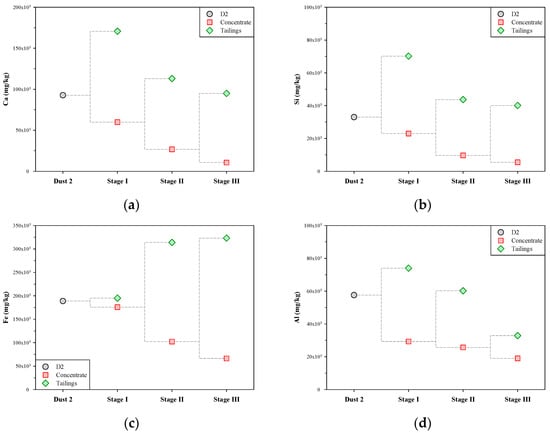
Figure 7.
Metal contents in concentrate and tailings of multi-stage froth flotation: (a) Ca, (b) Si, (c) Fe, and (d) Al.
Figure 7c shows that the variation in the content of Fe during the multi-stage froth flotation was very different from that of Ca, Si, and Al. In stage I, the concentrate and tailings had similar contents of Fe, which were close to that in the dust D2 (188,900 mg/kg). In stages II and III, the content of Fe in concentrate decreased while that in tailings greatly increased. The results show that the Fe-bearing impurities were almost not separated in stage I but significantly transferred to tailings in the subsequent stages. A possible explanation for these findings may lie in the pH variation in the liquid phases during the multi-stage froth flotation. Yu et al. [30] reported that the main isoelectric point (IEP) of iron oxides is about 8.4, and when the pH is above 8.4, the iron oxides charge negatively. Carlson and Kawatra [31] also noted that the Zata potential of iron oxides, including hematite and magnetite, decreases with increasing pH. In stage I, the pH of the liquid phase was 11.95, and this should make iron oxide particles disperse in the pulp and thus hard to remove. The pH of the liquid phase greatly decreased in stages II and III. The iron oxide particles should tend to aggregate and then remain in the tailings. In Figure 7d, Al varied in content as Ca and Si did during the multi-stage froth flotation. The content of Al was progressively reduced from 57,600 mg/kg (in the dust D2) to 19,000 mg/kg (in the concentrate of stage III). Overall, the above findings suggest that multi-stage froth flotation can successfully remove the impurities of Ca, Si, Fe, and Al from the dust D2 and thus obtain a concentrate containing lower levels of impurities.
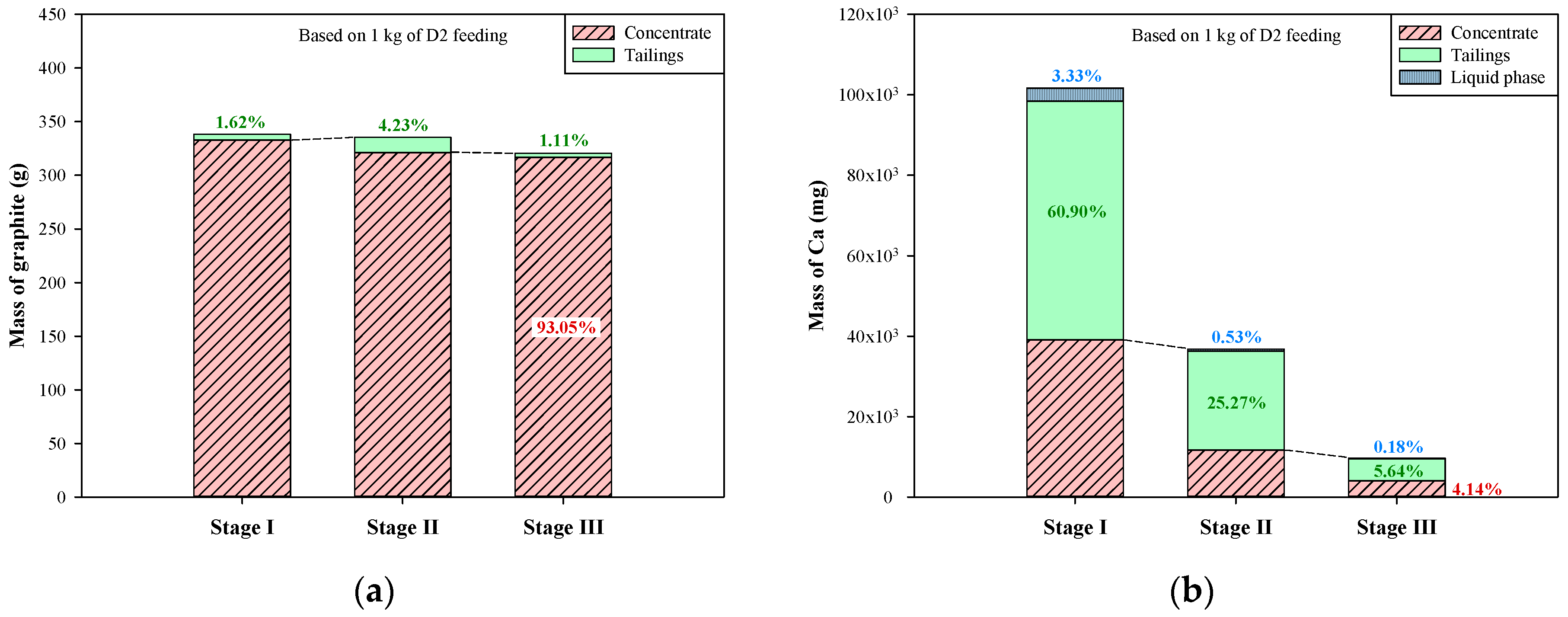
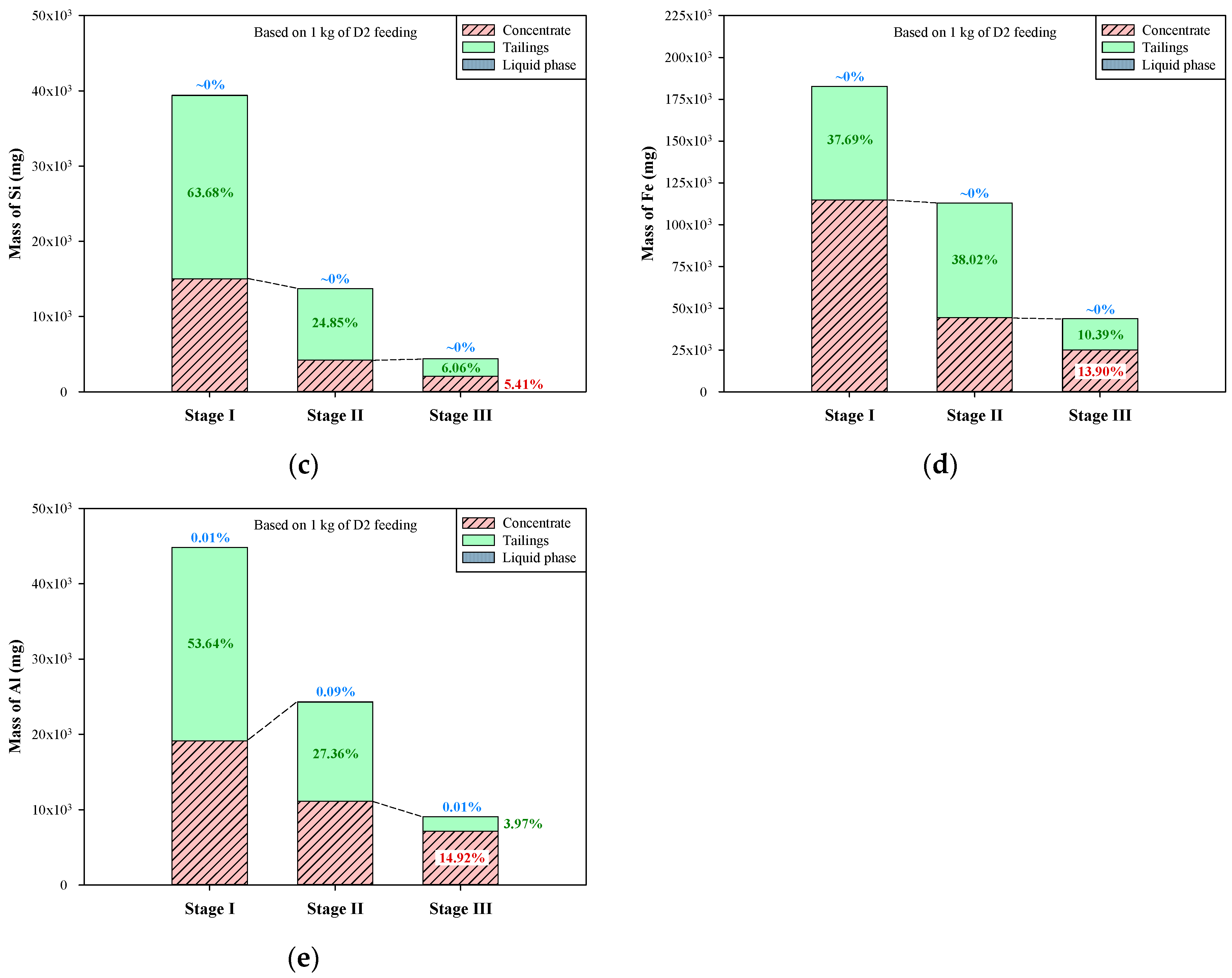
Based on the above compositions of liquid phases, concentrates, and tailings, the mass distribution of graphite and the other elements (Ca, Si, Fe, and Al) during the multi-stage froth flotation can be acquired by further considering the volumes of liquid phases and the weights of concentrates and tailings. The relevant results are presented in Figure 8 (based on one kg of dust D2). The mass distribution of graphite during the multi-stage froth flotation is shown in Figure 8a, and the graphite in liquid phases was not included due to the water-insolubility of graphite. It is clear that most of the graphite was retained in the concentrates. The leakages of graphite to tailings in stages I–III were only 1.62%, 4.23%, and 1.11%, respectively, and around 93% of graphite was recovered. Weng et al. [32] used a multi-stage grinding-flotation process to treat low-grade graphite ore, and the total recovery was only 81.16%. In terms of the other elements, the dissolution of impurities was taken into consideration because some minerals are soluble in water. In Figure 8b, the mass distribution of Ca showed that small parts of Ca were transferred to the liquid phases during the multi-stage froth flotation, especially in stage I (3.33%). Although the liquid phases contained high concentrations of Ca2+ between 45.2 and 360.9 mg/L, the sum of Ca distributed to liquid phases was only 4.04%. At each stage of froth flotation, Ca was effectively transferred to tailings, thus continuously reducing the amount of Ca in concentrates. The Ca remaining in the concentrate of stage III just accounted for 4.14% of the total amount of Ca in the dust D2. For the mass distribution of Si (Figure 8c), there was almost no Si dissolving in the liquid phases during the multi-stage froth flotation. However, the transfer of Si was very similar to that of Ca between concentrates and tailings. A large portion of Si was transferred to tailings at each stage, leaving a small amount of Si in concentrates. The concentrate of stage III contained only 5.41% of total Si from the dust D2.
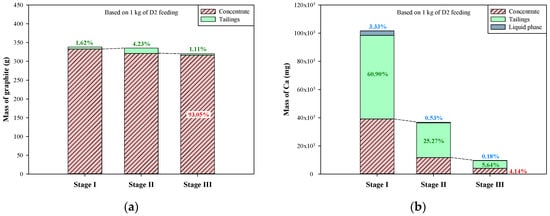
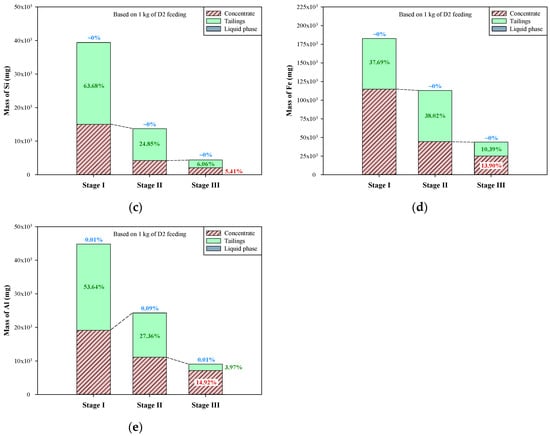
Figure 8.
Mass distribution of graphite and metals in multi-stage froth flotation: (a) graphite, (b) Ca, (c) Si, (d) Fe, and (e) Al.
In Figure 8d, the mass distribution of Fe presents a pattern that is quite different from those of Ca and Si. In alkali conditions, iron oxides are barely soluble in water; therefore, the portions of Fe in liquid phases were close to 0% (the pH values of liquid phases were >9.5). In stage I, the concentrate contained more Fe than the tailings, a finding that shows that the removal of Fe-bearing impurities was ineffective. The major transfer of Fe occurred in stage II, in which ~38% of Fe was transferred to tailings and then removed. At the end of stage III of froth flotation, the concentrate contained 13.9% of total Fe from the dust D2. In terms of the mass distribution of Al during the multi-stage froth flotation (Figure 8e), it was found that the portions of Al in the liquid phases of stages I–III were very low, between 0.01% and 0.09%. Al was effectively transferred to tailings in stages I and II, but the transfer was not effective in stage III. The transfer of Al in the first two stages was 81% in total, whereas that in stage III was only 3.97%. After the multi-stage froth flotation, there was still 14.92% of Al remaining in the concentrate of stage III. Generally, multi-stage froth flotation can successfully beneficiate graphite and remove most of the impurities from the dust D2. However, the major elements of impurities showed different patterns of transfer. Ca, Si, and Al were mostly transferred to tailings in stage I, but the major transfer of Fe occurred in stage II. Furthermore, over 95% of Ca and over 94% of Si were removed after the multi-stage froth flotation, leaving less than 6% of Ca and Si in the concentrate, while about 14% of Fe and 15% of Al were not removed. The remaining Fe and Al should be further treated with a purification process, depending on the final application of the graphite product.
3. Materials and Methods
3.1. Materials
In this study, the steelmaking byproducts were sampled in an integrated steel mill located in Kaohsiung, Taiwan. Two steelmaking slags (S1 and S2) were obtained in the desulfurization process, and two dusts (D1 and D2) were collected in the air pollution control systems at different cooling stations of torpedo cars. The slag and dust samples were brought back to the laboratory and then dried at 105 °C for 24 h to a constant weight. The dried sample was homogeneously mixed, and a subsample (~100 g) was obtained by using the quartering method and then ground to a fine powder less than 0.075 mm with a planetary ball mill (PM 100, Retsch, Haan, Germany) for the subsequent analyses.
3.2. Multi-Stage Froth Flotation
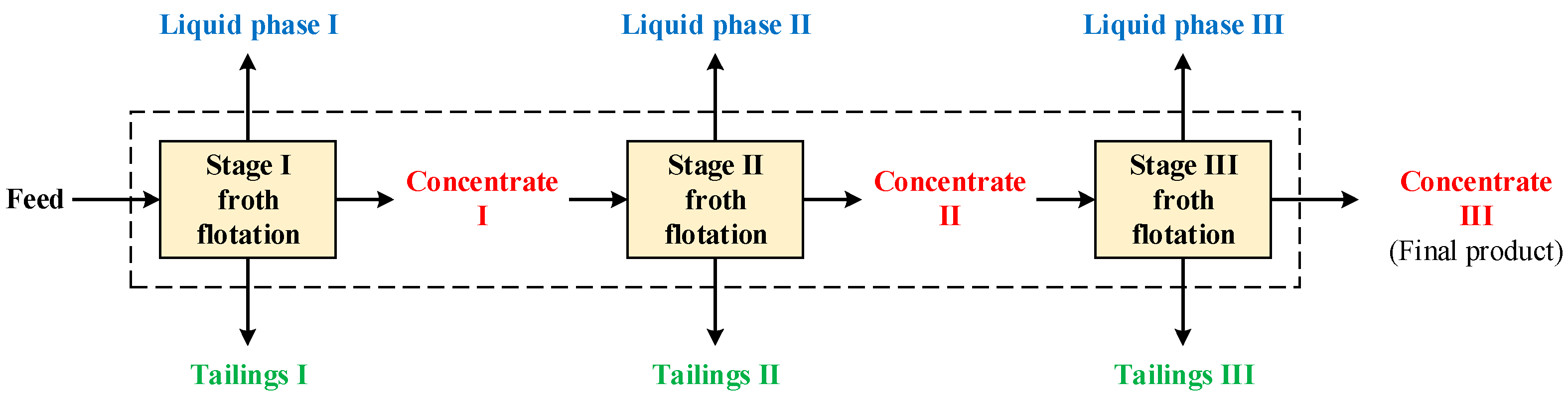
To conduct the experiment of froth flotation, a laboratory flotation machine (D12, Metso, Danville, PA, USA) was employed. The impeller was made of urethane, and the design speed range of the impeller was 800–3200 rpm. A 1000-g stainless steel flotation tank was used, and the pulp density was set at 10–30%. In terms of additives, MIBC (99%, Alfa Aesar, Ward Hill, MA, USA) was adopted as the frother, and kerosene (CPC Corporation, Taipei, Taiwan) was selected as the collector for the recovery of Kish graphite. Figure 9 depicts the flowsheet of the multi-stage froth flotation. The multi-stage froth flotation process included three stages, and each stage generated concentrate, tailings, and liquid phases. The concentrate of a stage was used for the feed of the next stage, and the tailings and liquid phase of each stage were collected for further analysis.
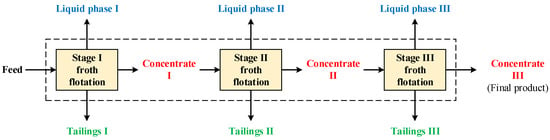
Figure 9.
Flowsheet for multi-stage froth flotation.
3.3. Analyses
The samples of steelmaking byproducts (S1, S2, D1, and D2) were dried at 105 °C to a constant weight for the determination of moisture. The dried samples were then calcined at 950 °C for 3 h to measure the LOI and ash content. In order to determine the chemical compositions of the steelmaking byproducts, the slag and dust samples were digested first with HNO3, HCl, and HBF4 at ~175 °C for 35 min by using a high-performance microwave digestion unit (START D, Milestone, Sorisole, Italy). The concentrations of elements in the digests were analyzed with an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 2000 DV, PerkinElmer, Waltham, MA, USA). To determine the graphite content of samples, a DSC-TGA (SDT 2960, TA Instrument, New Castle, DE, USA) was employed. Approximately 30 mg of powder sample was placed in an alumina crucible, and the temperature was increased from ambient temperature to 1000 °C at a heating rate of 10 °C/min under a dynamic nitrogen or air atmosphere (100 mL/min). An XRD system (D8 Advance, Bruker, Karlsruhe, Germany) with Cu Kα radiation was used to examine the mineralogical compositions of the slags and specks of dust. The XRD analysis was performed using step-scanning mode with a step size of 0.03°2θ and a step time of 2 s. The particle size analysis for the dust D2 was conducted using a test sieve shaker (AS 200 Basic, Retsch, Haan, Germany) with standard test sieves of 0.075 mm, 0.15 mm, 0.3 mm, 0.6 mm, 1.18 mm, and 2.36 mm. After the multi-stage froth flotation process, the concentrate and tailings of each stage were also dried and digested, the same as the procedure mentioned above. The concentrations of elements in the digests and the liquid phase of each stage were determined by the ICP-OES.
4. Conclusions
In the steelmaking byproducts, dust D2 had the highest potential for the recovery of Kish graphite due to its significant graphite content, nearly 34 wt.%. The major elements of impurities in the dust D2 included Fe, Ca, Al, and Si, and the trace heavy metals were Mn, Cr, and Zn. Magnetite, hematite, and portlandite were observed as impurities existing in the dust D2. The distribution of graphite and impurities was related to the particle size of the dust, D2. The course fractions (0.075–1.18 mm) had higher graphite contents, and the <0.075 mm fraction contained most of the impurities. In single-stage froth flotation, the graphite content of the concentrate and the recovery rate significantly decreased with increasing pulp density, and a pulp density of 10% was selected. In terms of the effects of the flotation reagents, the frother (MIBC) primarily increased the recovery rate, while the collector (kerosene) both increased the recovery rate and the graphite content of the concentrate. The optimal dosages of MIBC and kerosene were 0.005 kg/t and 1 kg/t, respectively. A concentrate with 84.09 wt.% of graphite content was obtained after three-stage froth flotation, and the entire recovery rate was 93.05%. During the multi-stage froth flotation, some cations (e.g., Ca2+, K+, and Na+) leached to the liquid phases, especially in stage I, and thus resulted in high pH and EC values. The impurities were separated at different stages of froth flotation. The separation of Ca, Si, and Al began in stage I, while Fe was mainly separated in stage II. Future work on the purification of graphite will be required if the final product is used for industrial and electronic applications.
Author Contributions
Conceptualization, Y.-L.C.; methodology, Y.-L.C.; validation, Y.-L.C.; formal analysis, W.-P.C. and P.-Y.H.; investigation, W.-P.C. and P.-Y.H.; data curation, W.-P.C. and P.-Y.H.; writing—original draft preparation, W.-P.C. and Y.-L.C.; writing—review and editing, Y.-L.C.; supervision, Y.-L.C.; project administration, Y.-L.C.; funding acquisition, Y.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Council (NSTC), Taiwan, for its financial support of this study (Contract No.: 112-2221-E-006-044).
Data Availability Statement
The data presented in this study are available on request. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors graciously thank Juu-En Chang for providing research suggestions and analysis instruments, such as DSC-TGA, ICP-OES, etc.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- World Steel Association. World Steel in Figures 2023. 2023. Available online: https://worldsteel.org/publications/bookshop/world-steel-in-figures-2023/ (accessed on 10 November 2023).
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and recycling of by-products in the steel sector: Recent achievements paving the way to circular economy and industrial symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Naraghi, R.; Selleby, M.; Ågren, J. Thermodynamics of stable and metastable structures in Fe–C system. Calphad 2014, 46, 148–158. [Google Scholar] [CrossRef]
- Liu, S.; Loper, C.R. Kish, a source of crystalline graphite. Carbon 1991, 29, 1119–1124. [Google Scholar] [CrossRef]
- Blengini, G.; El Latunussa, C.; Eynard, U.; Torres De Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials (2020)—Final Report; European Commission: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Robinson, G.R., Jr.; Hammarstrom, J.M.; Olson, D.W. Graphite. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; Professional Paper; U.S. Geological Survey: Reston, VA, USA, 2017; pp. J1–J24. [Google Scholar]
- U.S. Geological Survey. Graphite (Natural); U.S. Geological Survey: Reston, VA, USA, 2022.
- Wang, S.; Kravchyk, K.V.; Krumeich, F.; Kovalenko, M.V. Kish graphite flakes as a cathode material for an aluminum chloride-graphite battery. ACS Appl. Mater. Interfaces 2017, 9, 28478–28485. [Google Scholar] [CrossRef]
- An, J.-C.; Kim, H.J.; Hong, I. Preparation of Kish graphite-based graphene nanoplatelets by GIC (graphite intercalation compound) via process. J. Ind. Eng. Chem. 2015, 26, 55–60. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, T.; Peng, Y.; Xie, G.; Wu, E. Multi-stage flotation for the removal of ash from fine graphite using mechanical and centrifugal forces. Minerals 2018, 8, 15. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Numata, Y. Flotation of graphite. Miner. Eng. 1991, 4, 975–982. [Google Scholar] [CrossRef]
- Chehreh Chelgani, S.; Rudolph, M.; Kratzsch, R.; Sandmann, D.; Gutzmer, J. A review of graphite beneficiation techniques. Miner. Process. Extr. Metall. Rev. 2016, 37, 58–68. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Shin, S.-H.; Jung, S.-M.; Choi, H.-J.; Xu, J.; Baek, J.-B. One-pot purification and iodination of waste kish graphite into high-quality electrocatalyst. Part. Part. Syst. Charact. 2017, 34, 1600426. [Google Scholar] [CrossRef]
- Lin, S.; Chai, X.; Zhang, H.; Zhou, S.; Meng, X. The effect of calcium hypochlorite on the adsorption of diethyldithiocarbamate (DDTC) on the surface of molybdenite and bismuthinite. Colloids Surf. Physicochem. Eng. Asp. 2023, 676, 132270. [Google Scholar] [CrossRef]
- Lin, S.; He, J.; Liu, R.; Hu, Y.; Sun, W. Depression behavior and mechanism of pyrogallol on bismuthinite flotation. J. Clean. Prod. 2021, 281, 125322. [Google Scholar] [CrossRef]
- Du, Y.; Fu, C.; Gong, B.; Miao, E.; Zheng, X.; Xiong, Z.; Zhao, Y.; Zhang, J. Real-time investigation of the CO2 mineral carbonation reaction rate through direct aqueous route using semi-dry desulfurization slag. J. CO2 Util. 2021, 51, 101614. [Google Scholar] [CrossRef]
- Wang, C.-C. Modelling of the compressive strength development of cement mortar with furnace slag and desulfurization slag from the early strength. Constr. Build. Mater. 2016, 128, 108–117. [Google Scholar] [CrossRef]
- Ho, C.-L.; Huang, W.-L.; Wang, H.-Y. Study of the volume stability of slag cement mortar applied to desulfurization slag during high temperature operation. Constr. Build. Mater. 2017, 144, 147–157. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Manjón Fernández, Á.; Masaguer Torres, V. Hydrometallurgical processes for the recovery of metals from steel industry by-products: A critical review. J. Sustain. Metall. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Jiang, W.; Nadeau, G.; Zaghib, K.; Kinoshita, K. Thermal analysis of the oxidation of natural graphite—Effect of particle size. Thermochim. Acta 2000, 351, 85–93. [Google Scholar] [CrossRef]
- Machemer, S.D. Characterization of airborne and bulk particulate from iron and steel manufacturing facilities. Environ. Sci. Technol. 2004, 38, 381–389. [Google Scholar] [CrossRef]
- Öney, Ö.; Samanli, S. Determination of optimal flotation conditions of low-grade graphite ore. In Proceedings of the Mineral Engineering Conference 2016, Paper 01002, Swieradow-Zdroj, Poland, 25–28 September 2016. [Google Scholar]
- Wills, B.A.; Finch, J.A. Chapter 12—Froth Flotation. In Wills’ Mineral Processing Technology, 8th ed.; Wills, B.A., Finch, J.A., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 265–380. [Google Scholar]
- Sun, K.; Qiu, Y.; Zhang, L.; Liu, Q.; Mao, Z.; Qian, Y. Enhanced fine flake graphite flotation and reduced carbon emission by a novel water-in-oil kerosene emulsion. Colloids Surf. Physicochem. Eng. Asp. 2022, 650, 129603. [Google Scholar] [CrossRef]
- Arif Bhatti, M.; Kazmi, K.R.; Zahra, S.; Mehmood, A.; Mehmood, R. Beneficiation study on low-grade graphite ore of Shounter Valley, Azad Kashmir, Pakistan. J. Chem. Soc. Pak. 2020, 42, 1–9. [Google Scholar]
- Gray, J.R. Conductivity Analyzers and Their Application. In Environmental Instrumentation and Analysis Handbook; Wiley Online Library: Hoboken, NJ, USA, 2004; pp. 491–510. [Google Scholar]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 2017, 114, 26–36. [Google Scholar] [CrossRef]
- Carlson, J.J.; Kawatra, S.K. Factors affecting zeta potential of iron oxides. Miner. Process. Extr. Metall. Rev. 2013, 34, 269–303. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Song, S.; Liu, Y. Reducing the entrainment of gangue fines in low grade microcrystalline graphite ore flotation using multi-stage grinding-flotation process. Minerals 2017, 7, 38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).