1. Introduction
Electric vehicles (EVs) have become more and more popular in recent years, which can effectively face the global energy crisis [
1]. Lithium-ion batteries have been widely used for EV applications, given their long cycle life, high energy density, and environmental friendliness [
2,
3]. When the performance of the on-board batteries cannot meet the EV mileage and safety standards, many retired batteries need to be treated urgently [
4]. Second-use application is the optimal solution for retired EV batteries to effectively avoid energy waste and use the remaining value of retired batteries [
5]. However, the long-time performance tests increase the second-use application cost, and the regular classification basis (battery capacity or internal resistance) cannot guarantee the consistency of regrouped retired batteries, which will accelerate the battery performance degradation [
6,
7]. To enhance the rapid growth of the second-use application of retired batteries from EVs, the fast performance test strategy and multi-dimensional classification basis supply need to be well proposed. Fast providing the multi-dimensional classification basis for retired batteries can effectively improve the performance consistency of regrouped batteries, which promotes to release of the maximum available capacity and increases the economic value for second-use application.
In our previous study, we have proposed the fast capacity estimation method and the fast accelerated degradation fault diagnose under the larger rate constant-current charging condition [
6], and this study will focus on the identification of the micro-health parameters method under the same charging condition, which can improve the test efficiency and add the classification basis for retired batteries. The micro-health parameters stand for the performance of active material and electrolyte inside the battery, and the changes in the micro-health parameters can present the battery internal health state.
The complicated physico-chemical features of the battery have been depicted by a number of partial differential equations of the pseudo-two-dimensional (P2D) model, which has been frequently employed in research on lithium-ion battery performance [
8]. The influencing parameters in the P2D model intuitively reflects the performance of electrolyte and internal active material. The particular characteristics of the P2D model are the micro-health parameters to be determined, which will offer the multi-dimensional classification foundation for retired batteries [
9]. To effectively identify the micro-health parameters to analyze the internal health state by the P2D model, two problems need to be solved: (1) the simplification method of the complex P2D model; (2) the unambiguous identification method of the micro-health parameters.
For the first problem, since the P2D model is composed of multiple partial differential equations and the complicated calculation limits the practical application possibility, lots of researchers have carried out the P2D model simplification technology. Guo et al., first proposed the single-particle (SP) model of lithium-ion batteries. The SP model equates the porous structure of the electrode materials to a single particle and omits the calculation of the liquid-phase diffusion process, which greatly reduces computational complexity [
10,
11]. Since the SP model accuracy is low under the larger current charging or discharging condition, some researchers have subsequently added the control equations describing the liquid-phase diffusion process based on the SP model [
12,
13]. In addition, some other researchers have considered the problem of uneven current density distribution and developed the extended single-particle (ESP) model [
14,
15]. When the battery model structure is determined, the partial differential equations of the P2D model describing the electrochemical processes need to be simplified to realize the practical application. Among lots of studies, R.E. White and V.R. Subramanian have made outstanding contributions. In 2001, they first proposed the method of solving partial differential equations with boundary conditions in the P2D model. To further improve the calculation speed, they successively applied the separation variable [
16], polynomial fitting [
17], and orthogonal decomposition methods [
18,
19] to the control equation simplification of P2D model. Since the simplified model accuracy based on the above approximation methods is relatively low, the Páde approximation, fractional-order approximation, and Galerkin approximation methods have also been applied to the P2D model, which shows better accuracy simulating the battery charging and discharging behaviors [
20,
21,
22].
For the second problem, to effectively extract the battery micro-health parameters at different aging stages, researchers have carried out relevant studies on the specific parameter identification of the P2D model and analyzed the changing laws of different micro-health parameters during the aging process to study performance changes in active materials and electrolyte inside the battery. R.E. White research group also carried out related work earlier. They used the Levenberg-Marquardt algorithm to achieve the identification of solid-phase diffusion coefficients under 1/5 C, 1/2 C, 1 C, and 2 C constant-current discharging conditions [
23,
24]. A. Jokar et al., employed the genetic algorithm to recognize eight micro-health parameters including the initial lithium insertion of electrode and diffusion coefficients, etc., under 1/10 C, 1 C, 2 C, and 5 C constant-current discharging conditions [
25,
26]. After that, they tried to use the neural network method to realize the parameter identification including the solid-phase diffusion coefficient and electrochemical reaction coefficient [
27]. In addition, X. Han et al., identified the active material volume fraction related to battery capacity to analyze battery capacity degradation performance [
28]. R. Masoudi et al., used the homotopy optimization method to identify the number of lithium ions in the electrolyte, the solid-phase conductivity of the negative electrode, and the initial electrolyte concentration under the constant-current charging condition [
29]. Since the above methods depend on lots of known parameters, L. Zhang et al., designed dynamic working conditions for parameter identification of LiFePO
4/graphite and LiCoO
2/graphite batteries and identified more than 20 parameters of P2D model [
30,
31]. J. Li et al., first use the 1/50 C constant-current discharge condition to identify the initial lithium insertion rate of the positive and negative electrodes and parameters that vary with the battery aging, then the battery internal resistance is calibrated using the battery impedance test equipment. Finally, the parameters characterizing the electrochemical reaction polarization and electrolyte concentration polarization are achieved under the dynamic pulse condition [
31]. X. Li et al., investigated the effect of the change for characteristic parameters of the P2D model on the battery terminal voltage under constant-current charging condition. The parameters characterizing the solid-phase diffusion ability of positive and negative electrode under the high and low charging SOC interval, respectively. Parameters bound up with the ohmic resistance, and the liquid-phase diffusion ability are identified under the dynamic discharge conditions [
32].
Although the above model simplification methods and parameter identification methods can extract the micro-health parameters in the P2D model, these methods cannot be employed directly to the retired batteries with the two remarkable issues: (1) the coupling effects of non-target parameters on target parameters are difficult to be eliminated based on the above simplified models, (2) the designed multi-step test conditions for parameter identification increase the test cost of second-use application. In this study, the identification method of micro-health parameters characterizing the negative electrode active material and electrolyte performance for retired LiFePO4 batteries is proposed with two significant contributions. On one hand, the simplified P2D model with lumped parameters based on Padé approximation is developed. In the simplified model, the parameters characterizing negative electrode active material and electrolyte performance are linked to other parameters that are constant, not only reducing the quantity of parameters to be identified but also dispelling the coupling effects between various target parameters and other parameters. Hence, even the particular values of target parameters are not able to be directly obtained, their varying traces may still be observed effectively based on the lumped parameters. On the other hand, based on the sensitivity analysis results of target parameters on the charging voltage, the identification strategy for the micro-health parameters characterizing negative electrode active material and electrolyte performance under the larger rate constant-current charging condition is proposed. Combined with our previous work, the target micro-health parameters and battery capacity can be achieved under the same test condition, which can fast provide the multi-dimensional classification basis for retired batteries.
In line with these objectives, this study is organized as follows. In
Section 2, the simplified P2D model with lumped parameters based on Padé approximation is developed. In
Section 3, the identification strategy for the micro-health parameters characterizing negative electrode active material and electrolyte performance is proposed. In
Section 4, the identification results of micro-health parameters are discussed and analyzed, compared with the other two methods. Finally, conclusions are summarized in
Section 5.
2. The Reduced P2D Model Establishment with Lumped Micro-Health Parameters
In the P2D model, the active material particles of positive electrode and negative electrode are equivalent to spherical particles. This study assumes that all the reactions inside the electrode are distributed uniformly over all the particles. The direction from the positive/negative electrode to the negative/positive electrode through the electrolyte is defined as the
x direction, and the transport direction of lithium-ions in the active particle is defined as the
r direction, which is the radius direction of the active particle.
Figure 1 shows the P2D model schematic diagram, where
Ln is the thickness of negative electrode region;
Lp is the thickness of positive electrode region;
Ls is the thickness of separator region.
Although the physical meaning of micro-health parameters in the P2D model is clear, the mutual coupling between model parameters makes the identification of target parameters challenging. In this section, Padé approximation and parameter-lumped methods are employed to simplify the P2D model, ensuring that even through the particular values of target micro-health parameters may not be achieved, their varying traces may still be observed independently and effectively by the corresponding lumped parameters, and the undesired coupling effects between target parameters and other non-targeted parameters can also be eliminated.
2.1. Approximation of Solid-Phase Diffusion Process of P2D
In the P2D model, the solid-phase diffusion process of lithium-ions inside battery active particles can be described by Equation (1). The left side of Equation (1) stands for the variation in dynamic solid-phase concentration over time, while the right side represents the diffusion process of lithium-ion inside active particles based on Fick’s second law.
During the charging and discharging process of lithium-ion batteries, the solid-phase concentration gradient is generated inside the active particles of the electrode material. The concentration gradient at the center of the particle is zero, and the concentration gradient at the particle surface is determined by the lithium-ion solid-phase diffusion coefficient and the flow rate of the pore wall, thus, the boundary condition of the solid-phase diffusion process of the P2D model is given by Equation (2).
where
Cs,k is the solid-phase concentration of lithium-ion that changes with the charging or discharging time,
Ds,k is the solid-phase diffusion coefficient of lithium ions,
Jk is pore wall flow rate,
r is active particle radius,
r = 0 represents the active particle center,
r =
Rs represents the active particle surface,
Rs represents the particle radius,
p stands for the positive electrode,
n indicates the negative electrode, and
t is the charging or discharging time.
Taking the Laplace transform to the variable
t of Equation (1), the general solution for
Cs,k in Equation (1) is solved as:
where
D1 and
D2 are the coefficients that should be determined.
Substituting boundary conditions Equation (2) into Equation (3), the standard transfer function expression of
Cs,k can be obtained as:
The pore wall flow rate,
Jk, is usually calculated as:
where
A is the electrode surface area,
is the solid-phase volume fraction,
F is the Faraday’s constant,
IL is the load current.
Substituting Equation (5) into Equation (4), the standard transfer function expression of
Cs,k can be rewritten as:
In this study, Padé approximation is adopted to abate the solid-phase and liquid-phase diffusion processes of P2D model, which considers Padé approximation is more precise than the truncated Taylor series and typically still feasible when the Taylor series does not converge [
20]. Since the active particle surface concentration,
Csurf (at
r =
Rs position), is used to simulate the battery voltage behavior, the simplified expressions of
Csurf with distinct orders according to Padé approximation are summarized in
Table 1. More details involving Padé approximation re described in Ref. [
20].
2.2. Approximation of Liquid-Phase Diffusion Process of P2D
The liquid-phase diffusion process of P2D can be represented by Equation (7), which describes the diffusion process of lithium-ion between the positive and negative electrodes inside the battery. In Equation (6), the left side stands for the variation of dynamic concentration of electrolyte over time, and the right side represents the diffusion of lithium-ion in electrolyte based on Fick’s second law, and the electro-migration process with electrolyte transport number,
, and the flow rate of the pore wall,
Jk. Furthermore, the electrolyte continuity inside the battery is described based on the boundary conditions given by Equation (8):
where
Ce,dyn is the dynamic concentration of electrolyte that varies with electrode thickness, current density, and the charging or discharging time,
x = 0 represents the negative current collector,
x =
Lc represents the positive current collector,
Lc is the total thickness of the battery,
x indicates the direction along the electrode thickness,
De is the diffusion coefficient of electrolyte,
is the liquid-phase volume fraction.
Taking the Laplace transform to the variable
t of Equation (7), the general solution for
Ce,dyn in Equation (7) is solved as:
where
and
are the coefficients that should be determined, and
B is calculated as:
Substituting boundary conditions Equation (8) into Equation (9), the standard transfer function expression of
Ce,dyn can be obtained as:
It can be seen that the transfer function expression (10) of dynamic electrolyte concentration obtained based on boundary conditions Equation (8) does not contain the parameter x, which means Equation (11) cannot depict the dynamic concentration behavior of electrolyte along the direction of electrode thickness during battery charging and discharging.
To deal with the problem, our previous study proposed the novel boundary conditions shown in Equation (12), where
x =
Ln +
Ls stands for the positive electrode/separator boundary and
x =
Ln indicates the negative electrode/separator boundary.
According to the proposed electrode/separator boundary conditions, the standard transfer function expression of dynamic concentration of electrolyte at the positive electrode current collector,
, can be obtained as in Equation (13), and the standard transfer function expression of electrolyte concentration at the negative electrode current collector,
, can be obtained as in Equation (14).
Applying the Padé approximation to Equations (13) and (14), the reduced expressions of dynamic electrolyte concentration with various orders according to Padé approximation are listed in
Table 2.
2.3. Reduced P2D Model with Lumped Micro-Health Parameters
Based on the above reduced results of liquid-phase and solid-phase diffusion processes of the P2D model, 3-order reduced expression for Csurf and 2-order reduced expression for Ce,dyn are employed to simplify the terminal voltage of battery considering the model complexity and accuracy.
In the P2D model, the terminal voltage expression can be depicted as:
where
Uocv,p and
Uocv,n are the open circuit potential of the positive electrode and negative electrode, respectively;
SOCp,0 and
SOCn,0 are the initial lithium insertion of the negative electrode and positive electrode;
SOCn and
SOCp are the lithium insertion of the positive electrode and negative electrode, respectively;
and
are the liquid-phase potential of the positive electrode and negative electrode, respectively;
and
are the electrochemical reaction potential of the positive electrode and negative electrode, respectively;
RSEI is the solid/electrolyte interface impedance of active particle surface.
The expressions related to solid-phase diffusion can be further described as Equation (16); the expressions of
Uocv,p and
Uocv,n in Equation (14) can refer to Ref. [
33].
where,
Cmax,n is the maximum lithium insertion concentration of negative electrode.
2.3.1. Lumped Micro-Health Parameters Characterizing Electrolyte Performance
The liquid-phase potential difference between positive and negative electrodes can be calculated as follows:
where,
κe liquid-phase conductivity,
T is the test environment temperature, and
R is gas constant.
In the P2D model, the standard liquid-phase concentration overpotential is described as
ηcon, is written as:
where,
Ce is electrolyte concentration, which is the target parameter to be identified.
Substituting 2-order reduced expression for
Ce,dyn into liquid-phase concentration overpotential section in Equation (18), the transfer function of liquid-phase concentration overpotential can be obtained as:
In this study, the target parameters characterizing electrolyte performance include electrolyte concentration,
Ce, and diffusion coefficient,
De. In the transfer functions of Equation (18),
Ce and
De are linked to other parameters. Hence, the two object parameters is not possible to be identified unambiguously. To tackle this issue, Equation (19) is rewritten as Equation (20).
The parameters (except
De and
Ce) are not influenced by the decomposition of the electrolyte. Therefore, the rest parameters are constant. Ultimately, Equation (20) can be rewritten as Equation (19), which is a reduced overpotential model of electrolyte concentration with lumped micro-health parameters,
PDe and
PCe.
where,
Since the changes in and are only determined by De and Ce, and retain the physical significance of De and Ce. Consequently, the interference of non-target parameters on De and Ce can be dispelled.
2.3.2. Lumped Micro-Health Parameters Characterizing Electrolyte Performance
In P2D model, the micro-health parameters that can characterize negative electrode active material performance include negative electrode volume fraction, εn, and diffusion coefficient, Ds,n.
Since the change in the volume fraction of the active particles is directly related to the capacity loss of the electrode material, thus, the identification for
εn can be converted to the identification of negative electrode capacity,
Qn. According to the electrode capacity definition, the mathematical relationship between
Qn and
εn is described as Equation (23).
Thus, the transfer function of solid-phase surface concentration is rewritten as:
Based on Equation (16), Equation (24) can be rewritten as:
where the unit of
Qn is A⋅s in Equation (24), and the unit of
Qn is A⋅h in Equation (25).
Based on the above analysis, the identification for εn can be converted to the identification for Qn, which eliminates the coupling effect of parameters such as electrode plate area on the volume fraction of active particles.
For diffusion coefficient of the negative electrode,
Ds, since the continuous thickening of the SEI film lead to the solid-phase diffusion of lithium-ion weakened, and the thickened SEI film increases the active particle radius. The identification for
Ds,n can be converted to identification for
, and Equation (25) can be rewritten as Equation (26), where,
.
The changes in εn and Ds,n are only determined by Qn and . Thus, Qn and maintain the physical significance of εn and Ds. Consequently, the interference of non-target parameters on εn and Ds,n can be dispelled. In addition, according to the stable performance of positive electrode material of LiFePO4/graphite battery, Qp and can be set as the experience values.
2.3.3. Reduced P2D Model
Since this study considers the identification of micro-health parameters characterizing negative electrode material and electrolyte performance, the other physical and chemical processes in the P2D model will be uniformly approximated as the ohmic overpotential.
Linearizing the Bulter-Volmer equation of the electrochemical reaction process [
34]:
Thus, the electrochemical reaction overpotential,
ηdif, is described as:
The liquid-phase ohmic overpotential of Equation (17) is described as:
It can be seen that the model forms of Equations (28) and (29) can be written as the product of the invariant and the load current, and the lumped ohmic overpotential can be described as:
where
Pohm is the lumped ohmic resistance to be identified.
Based on the above lumped-parameter method, the reduced P2D model is described as:
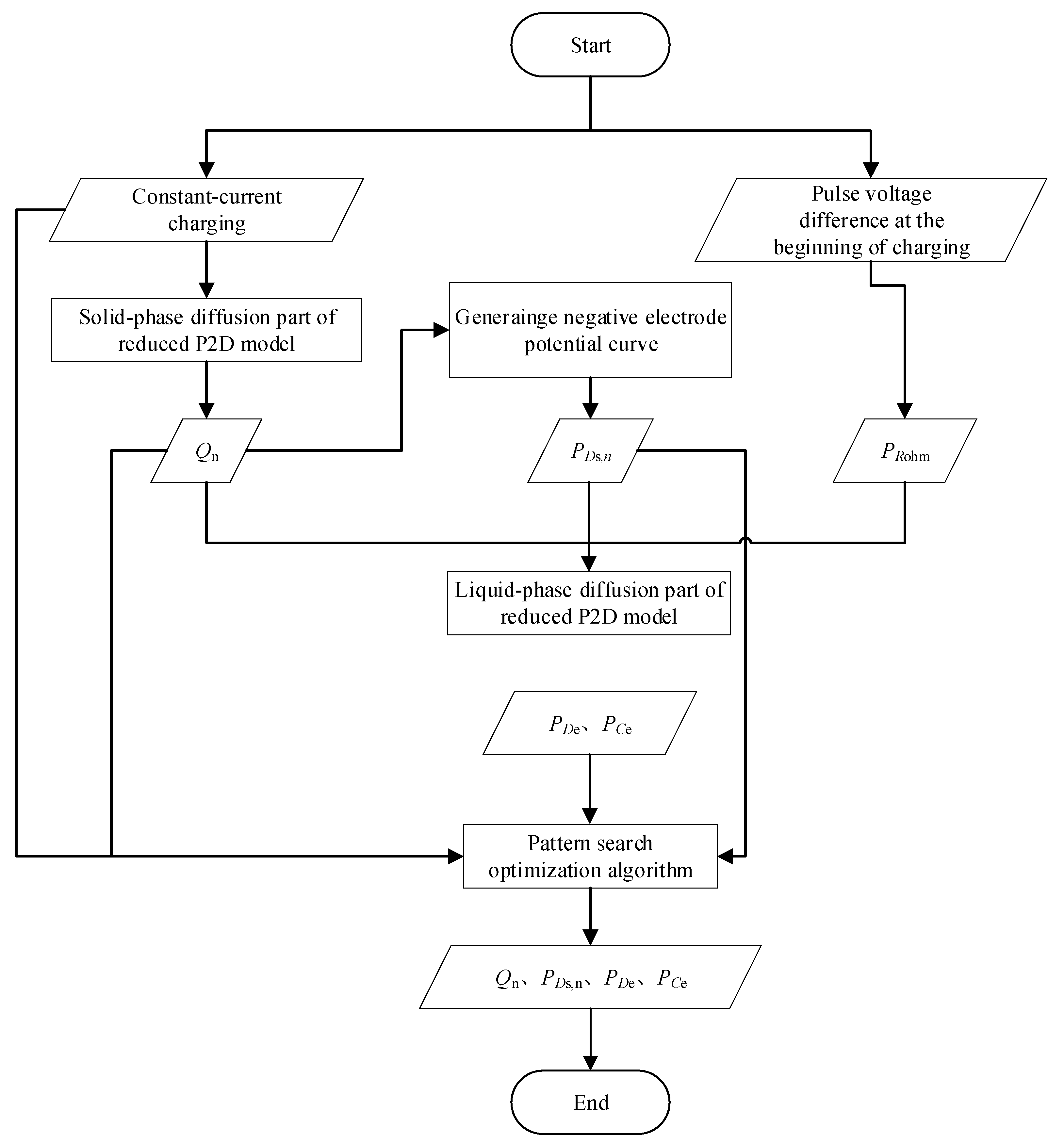
3. Fast Identification of the Target Micro-Health Parameters Based on the Reduced P2D Model
To fast identify the target micro-health parameters including Qn, PDs,n, PDe, and PCe, which characterize negative electrode active material (Qn and PDs,n) and electrolyte (PDe and PCe) performance, the constant-current charging condition which is the necessary test process for retired batteries, is employed in this study to shorten the test time of retired batteries. As the basis for accurate parameter identification, parameter sensitivity analysis under constant-current charging conditions is performed first in this section. Based on the parameter sensitivity analysis results, the fast identification strategy of the target micro-health parameters under constant-current charging conditions is proposed, subsequently.
3.1. Sensitivity Analysis of Target Micro-Health Parameters
Sensitivity analysis refers to the degree of dispersion of battery terminal voltage resulted from model parameters fluctuating within a certain range. The higher dispersion indicates that the terminal voltage is highly sensitive to the model parameters. Since the sensitivity of battery voltage to parameters
εn,
Ds,n,
Ce and
De is consistent with
Qn,
PDs,n,
PCe, and
PDe, herein,
Qn,
Ds,n,
Ce, and
De are divided into ten equal intervals within a certain range. Each value of
Qn,
Ds,n,
Ce, and
De within a certain range is simulated under constant-current charging conditions, and the effects on battery terminal voltage are analyzed and discussed. To observe the sensitivity of the battery terminal voltage to the parameter changes more clearly, the change ranges of target parameters are appropriately increased in this study.
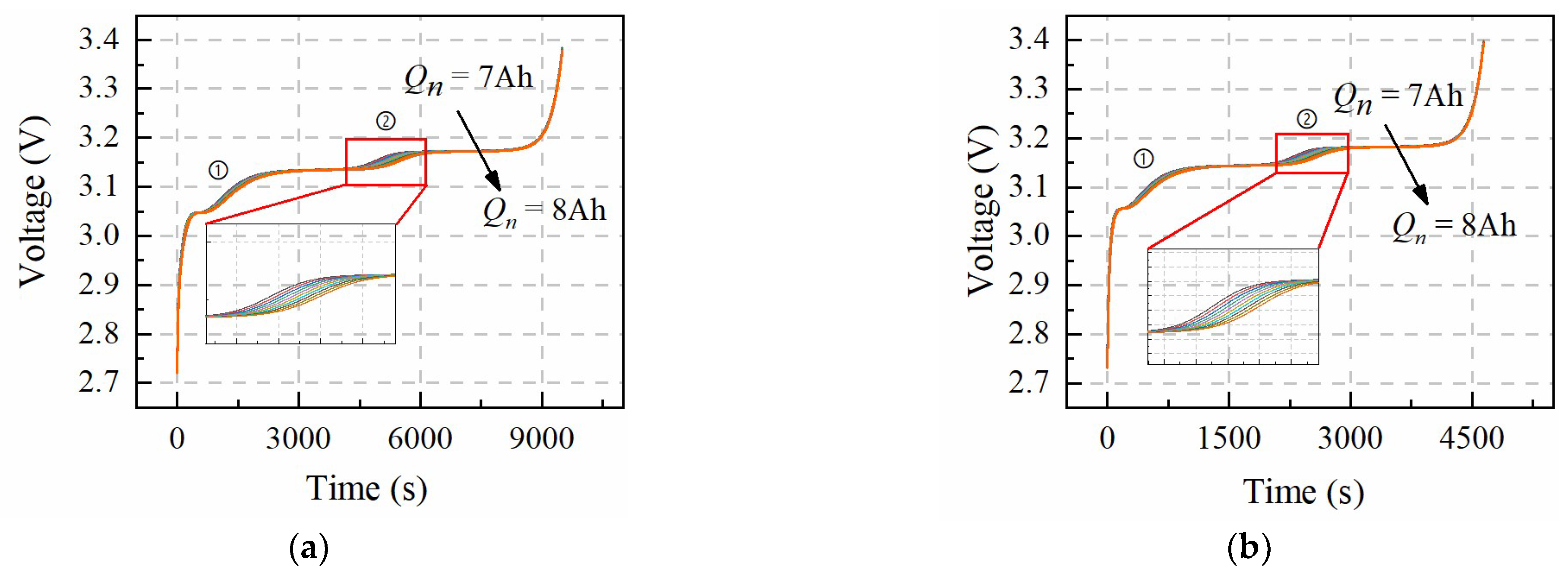
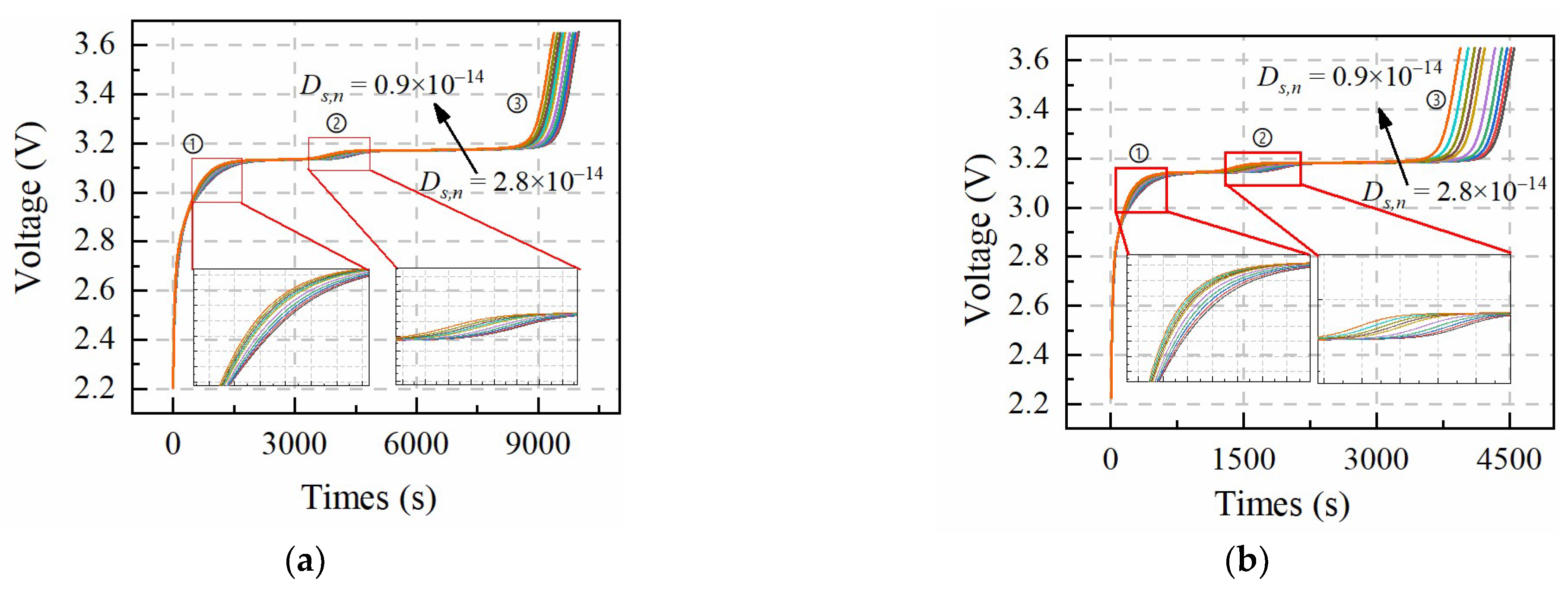
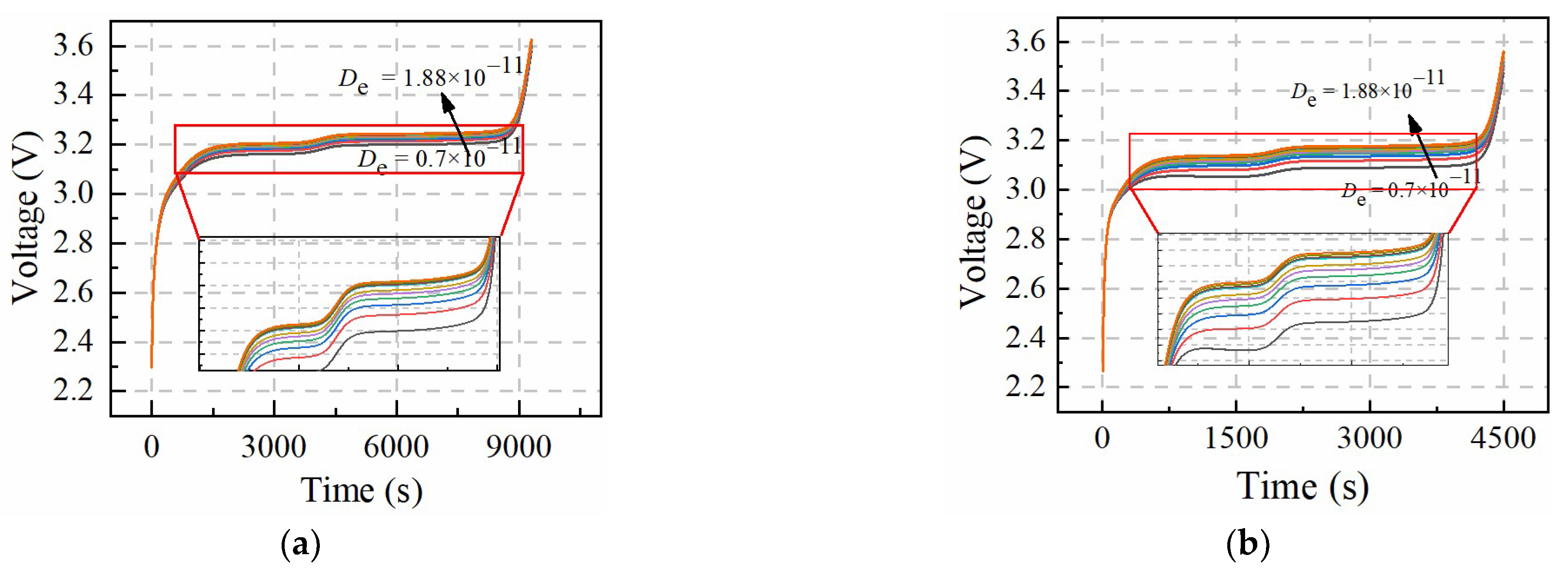
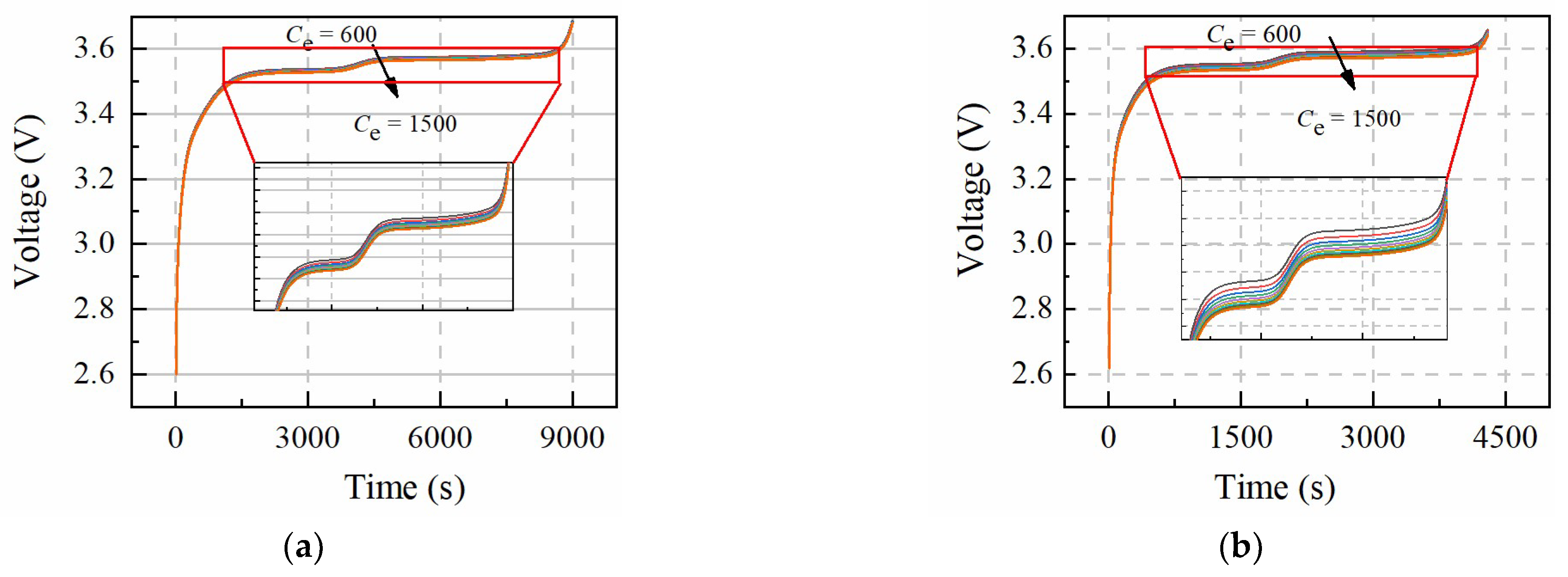
Table 3 shows the value change range of
Qn,
Ds,n,
Ce, and
De, and the sensitivity simulation results under 0.5 C and 1 C constant-current charging condition are plotted from
Figure 2,
Figure 3,
Figure 4 and
Figure 5.
Figure 2 shows that the influence of the changes in
Qn on the voltage curve is not consistent throughout the whole charging process with different charging rates. Number ➀ and ➁ positions of the voltage curve bundle present the discrete phenomenon, and the curve bundle at other positions during the charging process is almost overlapped. This phenomenon indicates that micro-health parameters,
Qn, can be identified at number ➀ and ➁ positions under the constant-current charging condition. In addition, the sensitivity of
Qn on the battery voltage bundle under 0.5 C and 1 C constant-current charging conditions exhibit the same results, which means the accurate identification of
Qn has nothing to do with the current rate and can be obtained under the larger rate constant-current charging condition.
In
Figure 3, the sensitivity of
Ds,n to the terminal voltage shows similar results to
Qn at number ➀ and ➁ positions. The difference from
Qn is at the number ➂ position of the constant-current charging curve bundle. Since the decrease of
Ds,n leads to the larger difference between the average and surface Li-ion concentration of active particles, the larger concentration difference will cause the battery to trigger the upper voltage limit in advance. In addition, the degree of dispersion of voltage curves at number ➀, ➁ and ➂ positions is obvious under the 1 C charging condition, which means
Ds,n is relatively easy to be identified under the larger charging rate.
Based on the sensitivity analysis results, Qn and PDs,n can be identified at number ➀ and ➁ positions of the larger constant-charging condition. The difference of number ➂ between Qn and PDs,n makes that PDs,n can be identified more accurately.
Figure 4 and
Figure 5 show the sensitivity of
De and
Ce to constant-current charging voltage, which presents similar results. The larger current rate causes the voltage curve dispersion to increase, which means
PDe and
PCe are easily identified under the larger rate constant-current charging condition. The sensitivity of
De and
Ce to the voltage curves is different from
Qn and
Ds,n, which means
PCe, and
PDe can be identified at the same constant-current charging condition.
3.2. Fast Identification Method of Micro-Health Parameters
Based on the above sensitivity analysis results, the sensitivity degrees of Qn, Ds,n, De, and Ce to battery terminal voltage are varying, especially under the large-rage constant-current charging condition, which means the identification of Qn, PDs,n, PDe, and PCe can be achieved under larger constant-current charging condition.
In this study,
Qn and
PDs,n are identified first according to the different sensitivity results with
PDe, and
PCe. To identify the micro-health parameters,
Qn and
PDs,n, the electrolyte concentration overpotential part of the terminal voltage expression is temporarily integrated into the ohmic internal resistance section,
, and Equation (15) is rewritten as Equation (32).
where,
.
Based on the Equations (26) and (32), the micro-health parameters
Qn and
PDs,n can be identified with the pattern search optimization algorithm, the detailed calculation procedures which are reported in our previous paper [
35].
To eliminate the impact of ohmic internal resistance on the identification results of micro-health parameters related to electrolyte performance, the ohmic internal resistance should be obtained at the moment of charging shown in Equation (33), which considers the response time of ohmic overpotential is shorter than concentration polarization overpotential.
where,
U1 is the moment before charging;
U2 is the start of charging.
After the micro-health parameters Qn and PDs,n, are confirmed, subsequently, PDe and PCe are extracted under constant-current charging condition with the pattern search optimization algorithm.
The flowchart of the proposed fast identification method for micro-health parameters (
Qn,
PDs,
PDe,
PCe) is shown in
Figure 6.
5. Conclusions
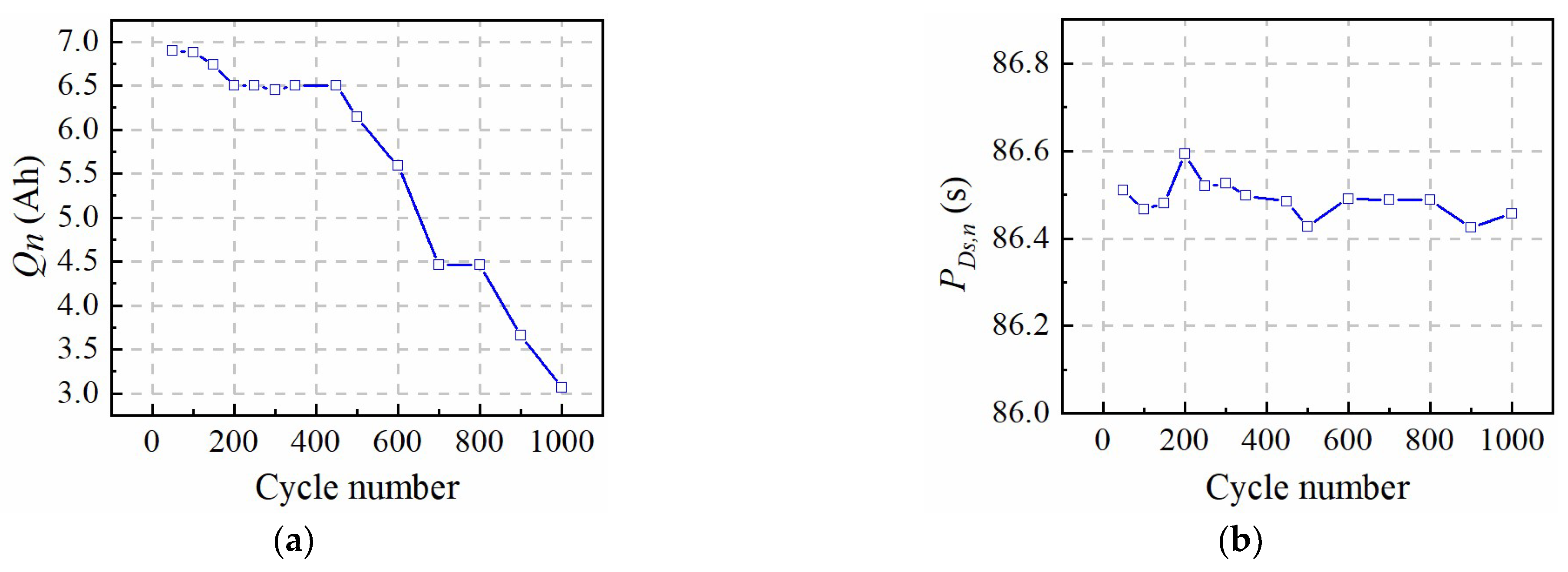
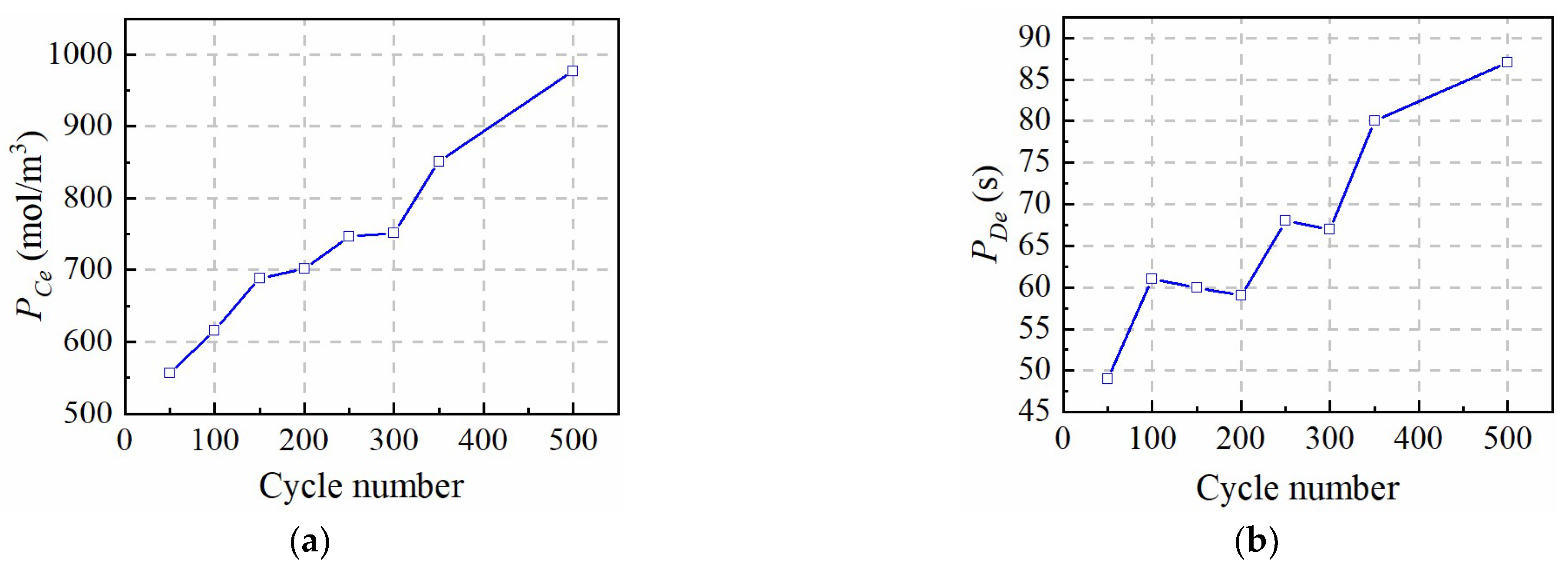
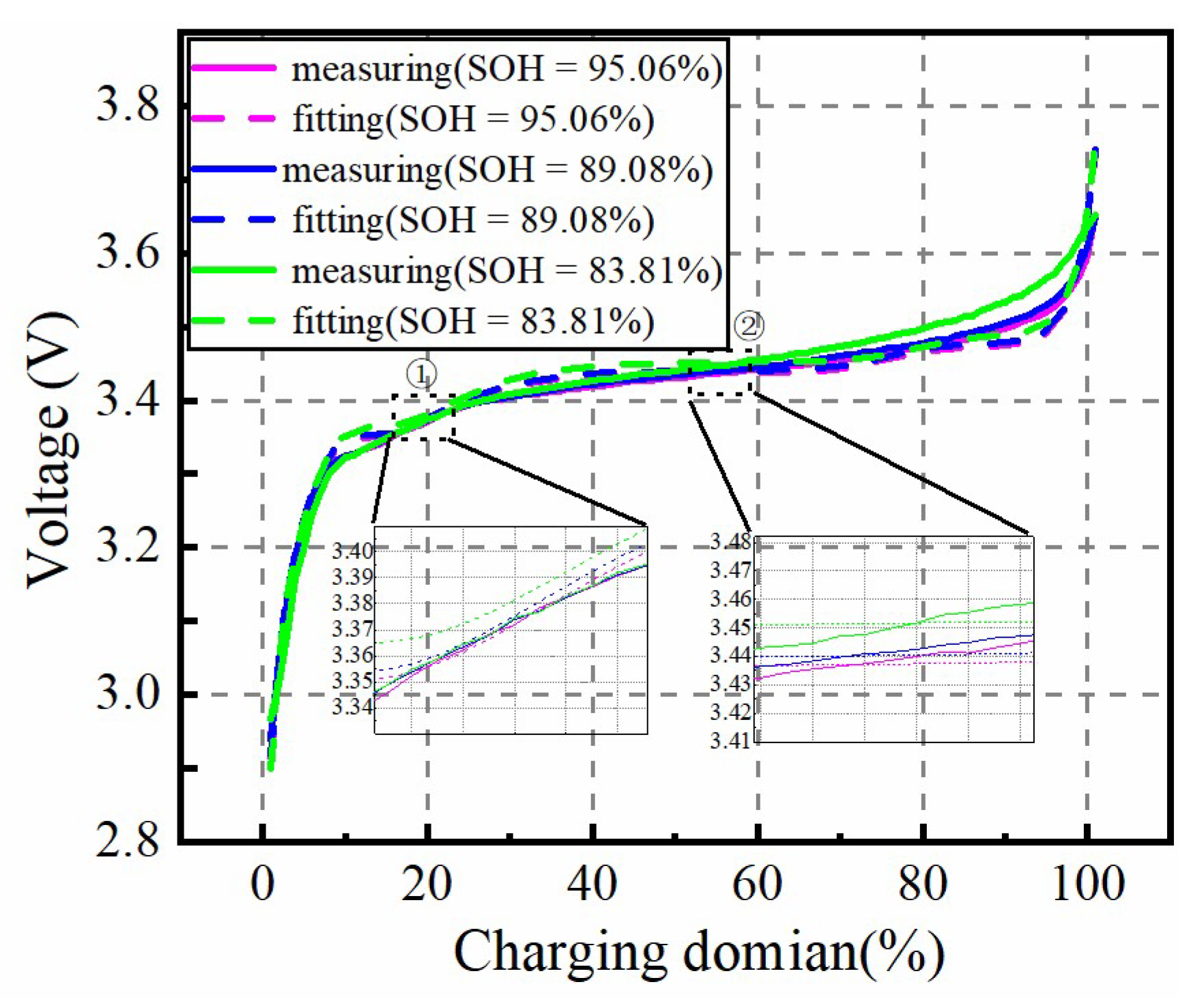
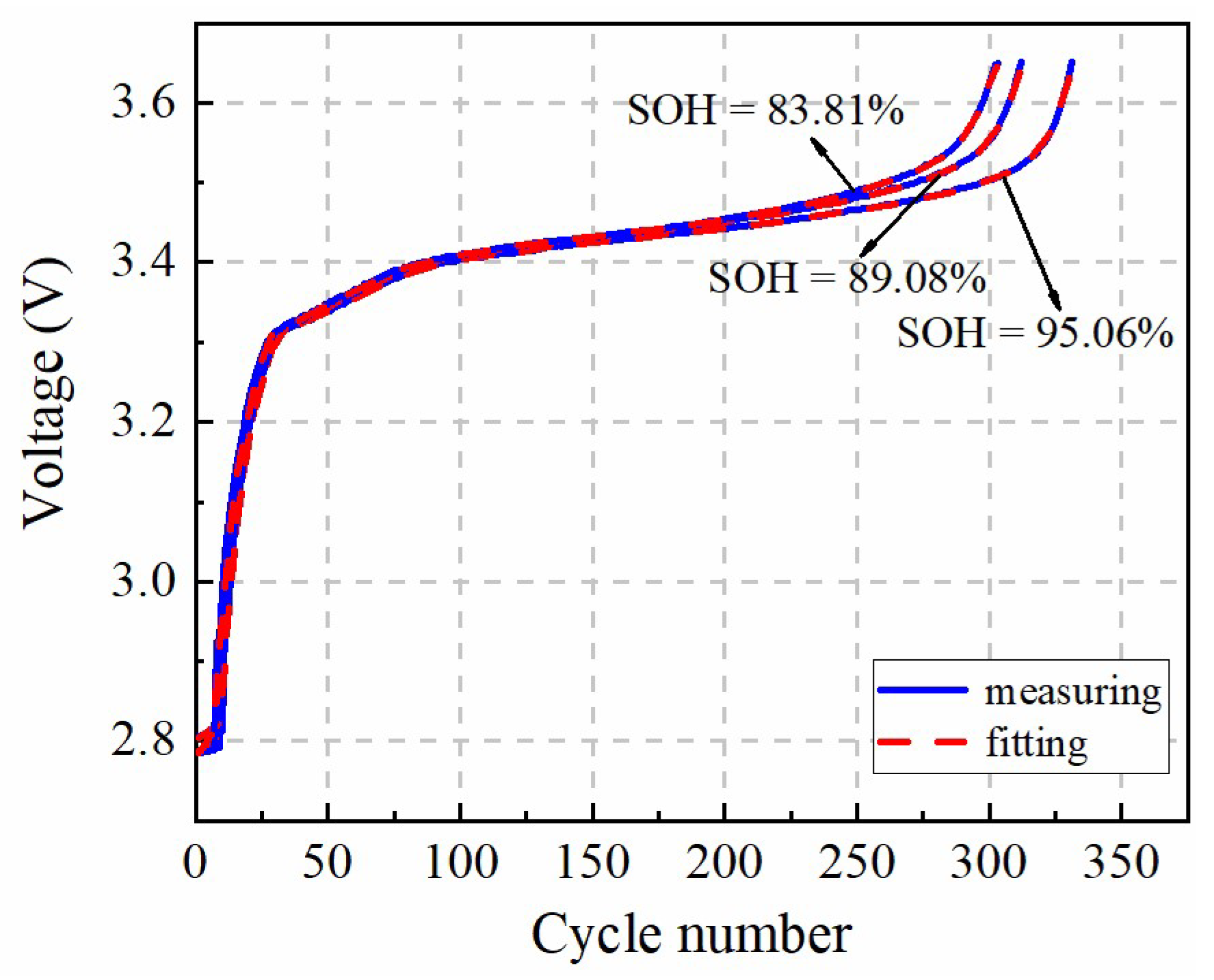
In this paper, a fast identification approach for micro-health parameters characterizing negative electrode material and electrolyte performance of LiFePO4 is proposed according to the reduced P2D model with Páde approximation. As the basis of micro-health parameter identification, diffusion processes of the liquid-phase and solid-phase of the P2D model are simplified with the Páde approximation method, respectively. Considering the analytical solution for the liquid-phase diffusion process cannot depict the dynamic performance of electrolyte concentration during charge or discharge condition, the improved boundary conditions for the P2D model are proposed to solve this problem. Based on the simplified solid-phase and liquid-phase diffusion expressions, a new battery terminal voltage model with lumped parameters for εn, Ds,n, De and Ce are developed. In this model, these target parameters and the other parameters of the P2D model, are lumped together, eliminating the coupling effects of non-target parameters on the coverage results of εn, Ds,n, De, and Ce. These lumped parameters are recognized based on the nonlinear optimization of pattern search, and the identification accuracy of the lumped parameters is verified under 1 C constant-current charging condition, which improves the parameter identification efficiency compared with other two identification methods. According to the above definite findings, the fast identification method for micro-health parameters characterizing negative electrode material and electrolyte performance can provide a multi-dimensional sorting basis for the second-use application of retired batteries from electric vehicles, and improve the performance consistency of regrouped retired batteries.
To be clear, for the high-rate charge or discharge condition, the uniform distribution of particle sizes need to be considered based on the proposed model in this study.