Understanding Chemistry and Unique NMR Characters of Novel Amide and Ester Leflunomide Analogues
Abstract
:1. Introduction
2. Results and Discussion
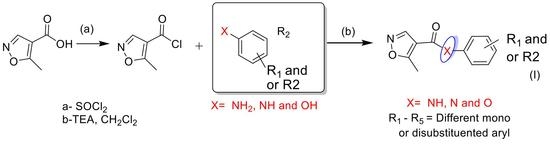
2.1. Synthetic Chemistry
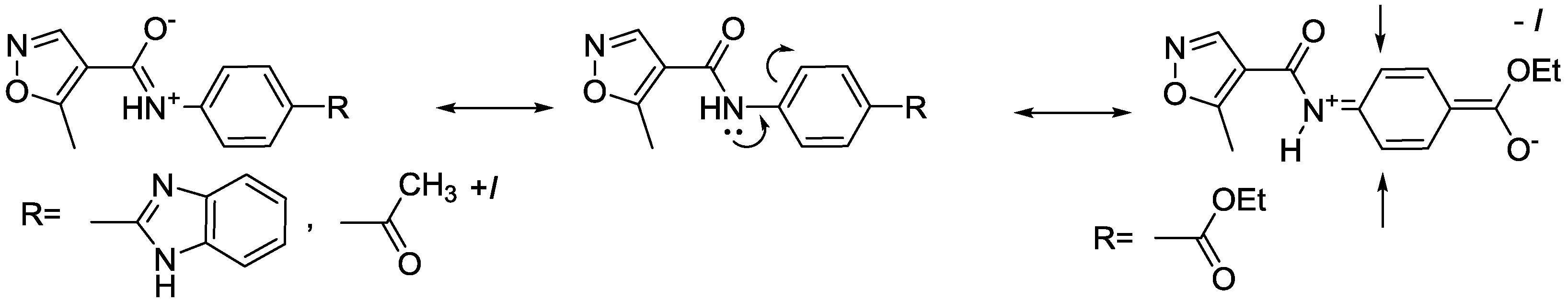
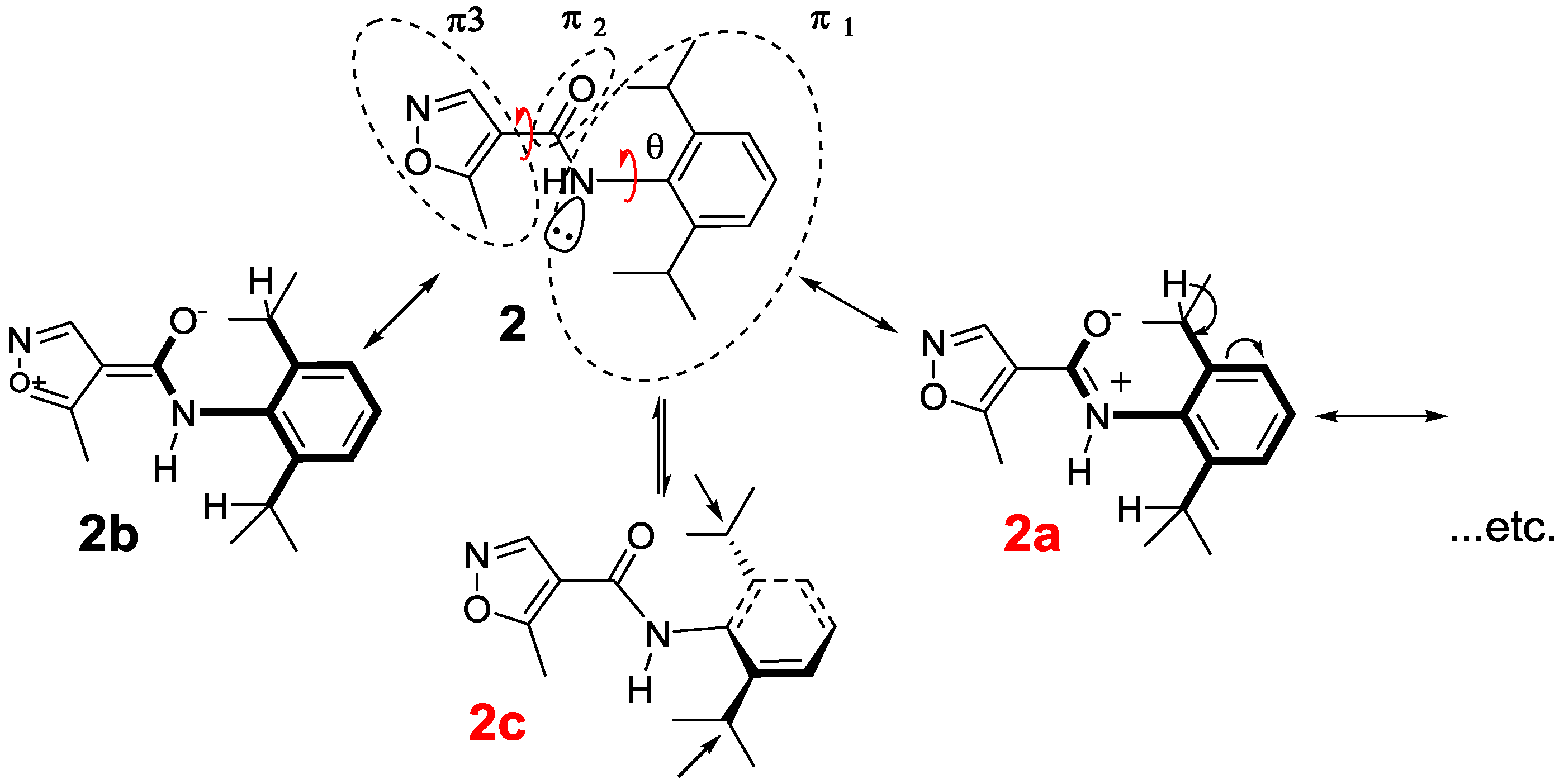
2.2. Spectroscopic Examination
2.3. Antioxidant Activity
- 900 µL of (ABTS/MnO2 mix) was transferred to cuvette of spectrophotometer (SPEKOL11) and the absorbance (Acontrol) was measured at 734 nm against blank (methanol/phosphate buffer (1:1); reading ca. 0.2.
- 900 µL of mix was transferred to 100 µL standard ascorbic acid in cuvette and the absorbance was measured against blank (methanol/phosphate buffer (1:1) + 100 µL of ascorbic acid).
- 900 µL of mix to 100 µL of sample was transferred in cuvette and the absorbance (Atest) was measured against blank (methanol/phosphate buffer (1:1) + 100 µL of sample).
- % Inhibition was calculated using the following equation; % Inhibition = ([Acontrol − Atest]/Acontrol) × 100.
- Changing the amide linkage with ester linkage decreased the antioxidant activity more than most of the amide Leflunomide analogues.
- The benzimidazole derivatives of Leflunomide 4 and 13 showed higher % of inhibition of radical production than Leflunomide. Benzimidazole derivatives are considered to be good chelating agents [27,28], therefore, our finding can pave the way for further in vivo studies of compounds 4 and 13. In addition, this also shows the linkage between antifibrotic and antioxidant activity which has been reported in literature [29].
3. Experimental Section
General
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, K.A.; Jayaroopa, P. Isoxazoles: Molecules with potential medicinal properties. Int. J. Pharm. Chem. Biol. Sci. 2013, 3, 294–304. [Google Scholar]
- I Keen, H.; Conaghan, P.G.; Tett, S.E. Safety evaluation of leflunomide in rheumatoid arthritis. Expert Opin. Drug Saf. 2013, 12, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, Y.; Lee, A.-R.; Huang, W.-H.; Chen, B.; Palfey, B.; Shaw, J. Comparison of Two Molecular Scaffolds, 5-Methylisoxazole-3-Carboxamide and 5-Methylisoxazole-4-Carboxamide. Curr. Pharm. Des. 2014, 20, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds; Wiley: Hoboken, NJ, USA, 1994; ISBN 0471016705. [Google Scholar]
- Cox, C.; Lectka, T. Solvent Effects on the Barrier to Rotation in Carbamates. J. Org. Chem. 1998, 63, 2426–2427. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K.; Becker, E.D.; De Menezes, S.M.C.; Granger, P.; Hoffman, R.E.; Zilm, K.W. International Union of Pure and Applied Chemistry Physical and Biophysical Chemistry Division Further Conventions for NMR Shielding and Chemical Shifts (IUPAC Recommendations 2008). Magn. Reson. Chem. 2008, 46, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.E.; Siddall, T.H. Nuclear magnetic resonance studies of amides. Chem. Rev. 1970, 70, 517–551. [Google Scholar] [CrossRef]
- Mandel, J.; Pan, X.; Hay, E.B.; Geib, S.J.; Wilcox, C.S.; Curran, D.P. Rotational Isomers of N-Methyl-N-arylacetamides and Their Derived Enolates: Implications for Asymmetric Hartwig Oxindole Cyclizations. J. Org. Chem. 2013, 78, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Roussel, C.; Berg, U. The Quantitative Analysis of Steric Effects in Heteroaromatics; Elsevier: Amsterdam, The Netherlands, 1988; pp. 173–299. [Google Scholar]
- Wolf, C. Dynamic Stereochemistry of Chiral Compounds; Royal Society of Chemistry: Cambridge, UK, 2007; ISBN 978-0-85404-246-3. [Google Scholar]
- LaPlante, S.R.; Fader, L.D.; Fandrick, K.R.; Fandrick, D.R.; Hucke, O.; Kemper, R.; Miller, S.P.F.; Edwards, P.J. Assessing Atropisomer Axial Chirality in Drug Discovery and Development. J. Med. Chem. 2011, 54, 7005–7022. [Google Scholar] [CrossRef] [PubMed]
- Bartels-Keith, J.R.; Cieciuch, R.F.W. Selective deshielding of aromatic protons in some ortho-substituted acetanilides. Can. J. Chem. 1968, 46, 2593–2600. [Google Scholar] [CrossRef]
- Burger, A. Medicinal Chemistry, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1970; p. 127. [Google Scholar]
- Hamdi, A.; Said, E.; Farahat, A.A.; El-Bialy, S.A.A.; Massoud, M.A.M. Synthesis and in vivo Antifibrotic Activity of Novel Leflunomide Analogues. Lett. Drug Des. Discov. 2016, 13, 912–920. [Google Scholar] [CrossRef]
- March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; Wiley: Hoboken, NJ, USA, 1992; ISBN 0471601802. [Google Scholar]
- Ayhan-Kilcigil, G.; Altanlar, N. Synthesis and antimicrobial activities of some new benzimidazole derivatives. Farmaco 2003, 58, 1345–1350. [Google Scholar] [CrossRef]
- Miklaszewski, B.; Niememtowski, S. Vergleichendes studium der drei isomeren (β)-amino-phenylbenzimidazole. Eur. J. Inorg. Chem. 1901, 34, 2953–2974. [Google Scholar]
- Klod, S.; Kleinpeter, E.; Kalder, L.; Koch, A.; Henning, D.; Kempter, G.; Benassi, R.; Taddei, F. Ab initio calculation of the anisotropy effect of multiple bonds and the ring current effect of arenes—Application in conformational and configurational analysis. J. Chem. Soc. Perkin Trans. 2 2001, 403, 1893–1898. [Google Scholar] [CrossRef]
- Faragher, R.J.; Motto, J.M.; Kaminski, M.A.; Schwan, A.L. A convenient synthesis of13C4-Leflunomide and its primary metabolite13C4-A77 1726. J. Label. Compd. Radiopharm. 2003, 46, 613–622. [Google Scholar] [CrossRef]
- Substituent R Alpha Shift Beta Shift. Available online: https://www.academia.edu/22461745/Substituent_R_Alpha_Shift_Beta_Shift (accessed on 4 December 2017).
- Gfeller, D.; Michielin, O.; Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Henen, M.A.; Belnou, M.; Cantrelle, F.X.; Kamah, A.; Qi, H.; Giustiniani, J.; Chambraud, B.; Baulieu, E.E.; Lippens, G.; et al. A β-turn motif in the steroid hormone receptor’s ligand-binding domains interacts with the peptidyl-prolyl isomerase (PPIase) catalytic site of the immunophilin FKBP52. Biochemistry 2016, 55, 5366–5376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Sergeyev, S.; Franck, P.; Orru, R.V.A.; Maes, B.U.W. Amine Activation: Synthesis of N-(Hetero)arylamides from Isothioureas and Carboxylic Acids. Org. Lett. 2016, 18, 4602–4605. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, A.; Brkic, D.; Martinovic, J.; Mijin, D.; Milcic, M.; Petrovic, S. Substituent effect on IR, 1H and 13C-NMR spectral data in N-(substituted phenyl)-2-cyanoacetamides: A correlation study. Chem. Ind. Chem. Eng. Q. 2013, 19, 67–78. [Google Scholar] [CrossRef]
- Takahata, Y.; Chong, D.P. Estimation of Hammett sigma constants of substituted benzenes through accurate density-functional calculation of core-electron binding energy shifts. Int. J. Quantum Chem. 2005, 103, 509–515. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Awad, E.A.; Badria, F.A. Synthesis, antimicrobial, antioxidant, anti-hemolytic and cytotoxic evaluation of new imidazole-based heterocycles. Eur. J. Med. Chem. 2011, 46, 1505. [Google Scholar] [CrossRef] [PubMed]
- Ayhan-Kılcıgil, G.; Kus, C.; Özdamar, E.D.; Can-Eke, B.; Iscan, M. Synthesis and Antioxidant Capacities of Some New Benzimidazole Derivatives. Arch. Pharm. 2007, 340, 607. [Google Scholar] [CrossRef] [PubMed]
- Maheswaran, N.; Saleshier, M.F.; Mahalakshmi, K.; Sureshkannan, V.; Parthiban, N.; Reddy, K.A. Synthesis and Characterization of 7-(1H-Benzimidazol-2-yl)-5-(Substituted Phenyl) Pyrido [2, 3-d] Pyrimidin-4-Amine for their Biological Activity. Int. J. Chem. Sci. 2012, 10, 43. [Google Scholar]
- Marian, A.J.; Senthil, V.; Chen, S.N.; Lombardi, R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J. Am. Coll. Cardiol. 2006, 47, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Shreenivas, M.T.; Kumara Swamy, B.E.; Manjunatha, J.G.; Chandra, U.; Srinivasa, G.R.; Sherigara, B.S. Synthesis, Antimicrobial Evaluation and Electrochemical Studies of some Novel Isoxazole Derivatives. Pharma Chem. 2011, 3, 224–234. [Google Scholar]
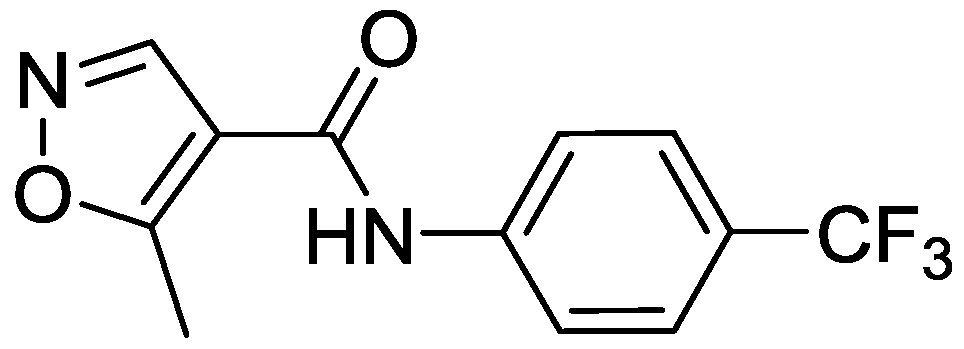

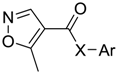
| Products | -X-Ar * | Yield (%) | Melting Points (°C) | Colour |
|---|---|---|---|---|
| 2 |  | 58 | 145–146 | Yellow |
| 3 |  | 68 | 130–131 | red |
| 4 | 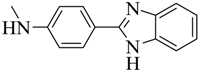 | 71 | 185–186 | gray |
| 5 | 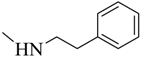 | 91 | 148–149 | white |
| 6 | 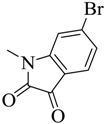 | 55 | 190–191 | red |
| 7 | 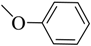 | 41 | 89–90 | yellow |
| 8 | 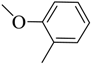 | 45 | 100–101 | yellow |
| 9 | 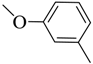 | 40 | 95–96 | yellow |
| 10 | 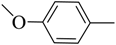 | 48 | 80–81 | white |
| 11 | 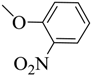 | 42 | 124–125 | white |
| 12 | 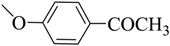 | 51 | 188–189 | Buff |
| 13 | 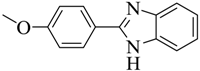 | 40 | 150–151 | White |
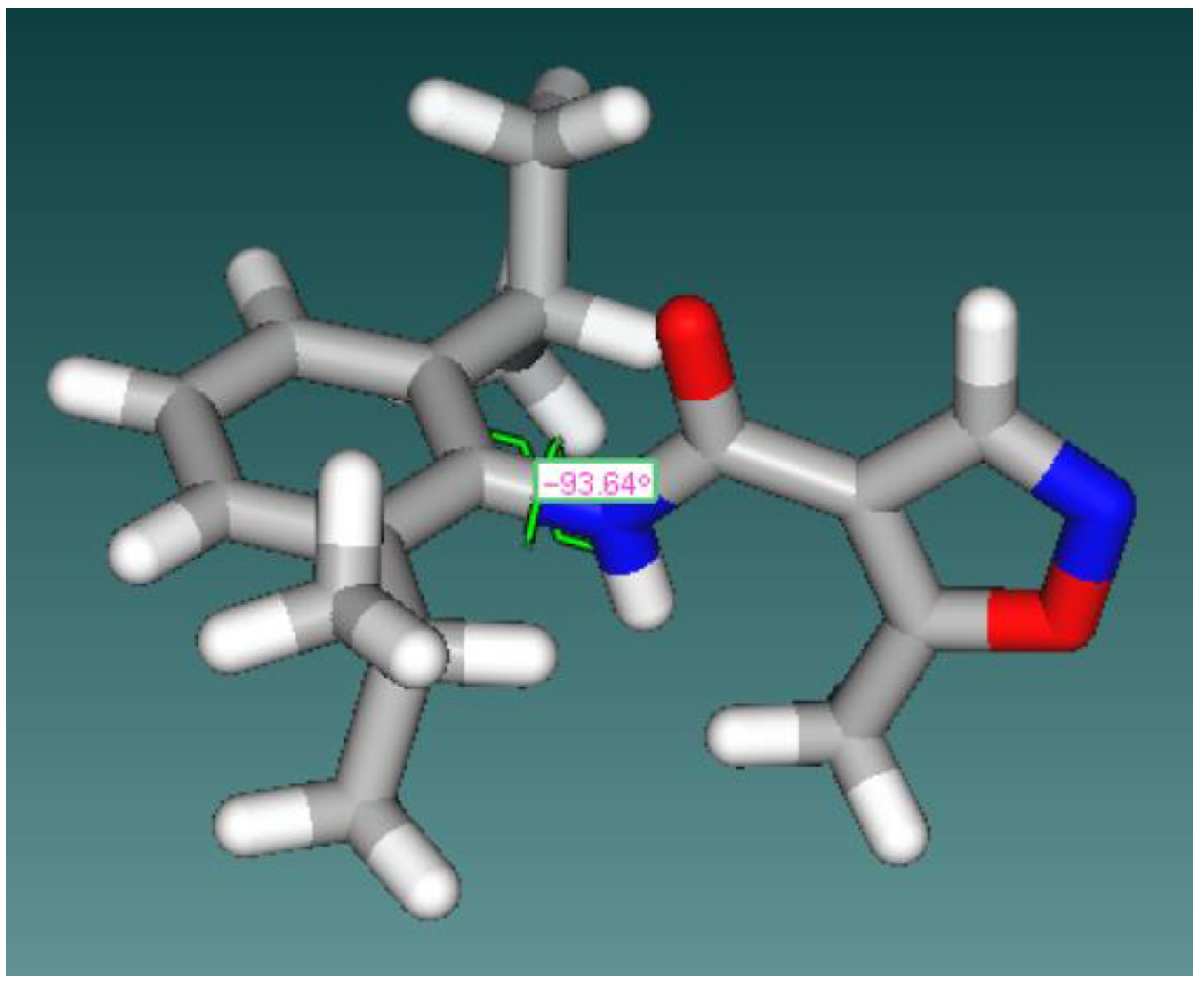
General Structure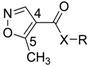 | Dihedral Angle between C=O and Phenyl Ring | Dihedral Angle between C–O and Isoxazolering |
|---|---|---|
Leflunomide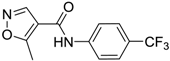 | −30.03° | −173.3° |
| 2 | 0.09° | 179.6° |
| 3 | 24.5° | 7.17° |
| 4 | 19.21° | 160.2° |
| 5 | −63.3° | 8.84° |
| 6 | 19.21° | 160.2° |
| 7 | 129.3° | −164.3° |
| 8 | −97.88° | 176° |
| 9 | −74° | 155.6 |
| 10 | −47° | 16.5° |
| 11 | −64° | 177.6° |
| 12 | −3.08° | −20.9° |
| 13 | 33.9° | 4.93° |
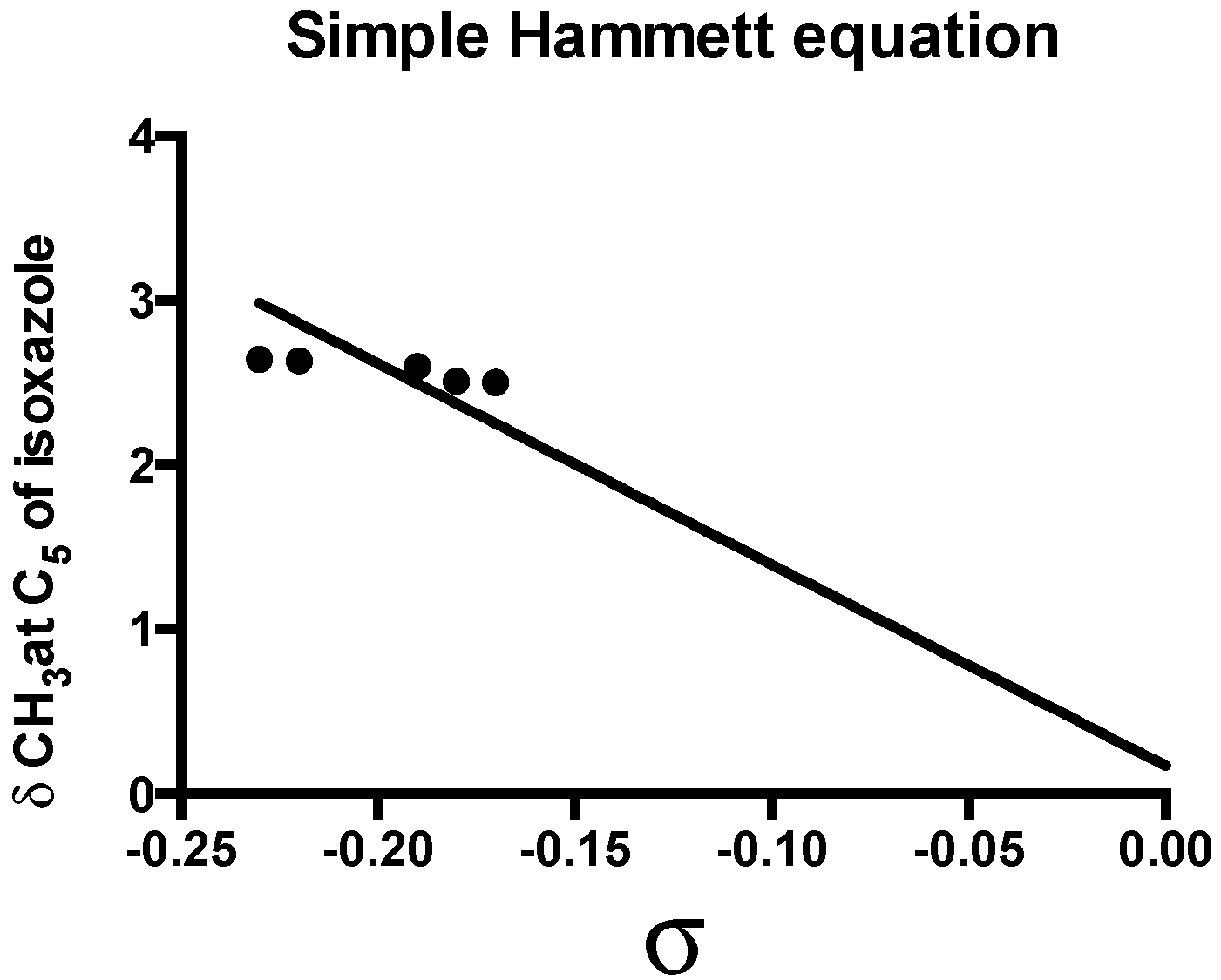
| Parameter | Value |
|---|---|
| ρ | −12.24 ± 1.419 |
| R | 0.9370 |
| SD | 1.419 |
| h | 0.169 |
| Sy.x | 0.266 |
| Compound | A | % Inhibition |
|---|---|---|
| ABTS Control | 0.480 | 0.00 |
| Ascorbic acid | 0.059 | 87.71 |
| Leflunomide | 0.315 | 34.38 |
| 2 | 0.360 | 25.00 |
| 3 | 0.331 | 31.04 |
| 4 | 0.252 | 47.50 |
| 5 | 0.351 | 26.88 |
| 6 | 0.355 | 26.04 |
| 7 | 0.372 | 22.50 |
| 8 | 0.385 | 19.79 |
| 9 | 0.397 | 17.29 |
| 10 | 0.406 | 15.42 |
| 11 | 0.332 | 30.83 |
| 12 | 0.355 | 26.04 |
| 13 | 0.239 | 50.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henen, M.A.; Hamdi, A.; Farahat, A.A.; Massoud, M.A.M. Understanding Chemistry and Unique NMR Characters of Novel Amide and Ester Leflunomide Analogues. Magnetochemistry 2017, 3, 41. https://doi.org/10.3390/magnetochemistry3040041
Henen MA, Hamdi A, Farahat AA, Massoud MAM. Understanding Chemistry and Unique NMR Characters of Novel Amide and Ester Leflunomide Analogues. Magnetochemistry. 2017; 3(4):41. https://doi.org/10.3390/magnetochemistry3040041
Chicago/Turabian StyleHenen, Morkos A., Abdelrahman Hamdi, Abdelbasset A. Farahat, and Mohammed A. M. Massoud. 2017. "Understanding Chemistry and Unique NMR Characters of Novel Amide and Ester Leflunomide Analogues" Magnetochemistry 3, no. 4: 41. https://doi.org/10.3390/magnetochemistry3040041
APA StyleHenen, M. A., Hamdi, A., Farahat, A. A., & Massoud, M. A. M. (2017). Understanding Chemistry and Unique NMR Characters of Novel Amide and Ester Leflunomide Analogues. Magnetochemistry, 3(4), 41. https://doi.org/10.3390/magnetochemistry3040041