Abstract
To develop novel magnetic conductors exhibiting conducting/magnetic bifunctionalities and peculiar responses to applied magnetic fields, we synthesized new EDT-TTF (ethylenedithio-tetrathiafulvalene) donor containing a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical through a π-conjugated vinylene spacer 1 and examined its electronic and crystal structures, and physical properties. We also prepared its cation radical salts by an electrochemical oxidation method and successfully cleared the crystal structures and magnetic properties of the cation radical salts, 1·FeCl4 and 1·GaCl4. These salts have strongly dimerized one-dimensional arrays of the fully oxidized donor molecules, giving rise to the formation of spin-singlet state of the π cation radical spins in the dimer. On the other hand, the FeCl4− anion locates on the side of the dimers with very short S-Cl contacts and mediates very strong π-d interaction between the donor and anion moieties, resulting in the antiferromagnetic behavior of the Weiss temperature of θ = −3.9 K through its d-π-d interaction.
1. Introduction
In the field of molecular conductors, much interest has been focused on the development of magnetic conductors, which simultaneously exhibit conducting properties of organic layers of π-electron donors and magnetic properties of inorganic layers of magnetic transition metal anions. In such conductors, the conducting properties of organic layers can be controlled by the application of external magnetic fields and several peculiar physical phenomena such as field-induced superconductivities in the λ- and κ-type BETS salts with FeX4− anions (BETS = bis(ethylenedithio)tetraselenafulvalene; X = Cl and Br) [1,2,3] and anomalies of magnetoresistances corresponding to the spin-flop transitions of magnetic Fe3+ spins [4,5] have been yielded through the π-d interaction between the π-electrons of the organic layers and the magnetic d spins. On the other hand, the studies on molecular conductors using donor molecules substituted with stable organic radicals such as 2,2,6,6-tetramethylpiperidin-1-yloxyl (TEMPO) and nitronyl nitroxide radicals have been performed by several research groups [6,7,8,9] because cation radical salts of such stable radical-containing donors are expected to have strong intramolecular magnetic interactions between their π-cation radical spins and stable radicals and to show outstanding responses to the application of external magnetic fields [10]. Among them, we have also reported several tetrathiafulvalene (TTF)-based donor molecules containing stable organic radical parts such as TEMPO and 2,2,5,5-tetramethylpyrrolidin-1-yloxy (PROXYL) radicals [11,12,13], and discovered highly conducting cation radical salts using bis-fused TTF (TTP = 2,5-bis(1,3-dithiol-2-ylidene)-1,3,4,6-tetrathiapentalene) donors and a PROXYL radical [14,15,16]. Furthermore, we have developed new TTF and TTP donors containing a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical [17] because this radical part has an unsaturated 3-pyrroline ring with a C=C bond and smaller steric hindrance in comparison to the PROXYL radical having a saturated 3-pyrrolidine ring. However, conducting cation radical salts of these donors containing a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical could not be obtained probably due to the steric bulkiness of the radical part that is connected almost orthogonally to the ethylenedithio bridge of these donor molecules [17]. Therefore, to minimize the steric bulkiness of this radical part, we designed a new ethylenedithio-tetrathiafulvalene (EDT-TTF) molecule containing a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical through a π-conjugated vinylene spacer 1 because its π-conjugated hexatriene chain will ensure the coplanarity between the TTF and stable radical parts. In this paper, we will report the synthesis, electronic and crystal structures, and physical properties of new molecule 1. Furthermore, we will discuss the detail of X-ray single crystal structure analyses and magnetic properties of the FeCl4− and GaCl4− salts of molecule 1 prepared by an electrochemical oxidation method. Because the FeCl4− salt contains three kinds of spins, namely, the π-cation radical spins of the donor parts, the stable organic radicals and the Fe3+ d spins, the magnetic interactions between these spins are of special interest.
2. Results and Discussion
2.1. Synthesis of Donor 1
The EDT-TTF molecule containing a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical 1 was prepared as described in Scheme 1. The Wittig reagent of stable radical part 4 was prepared by the reported method from the corresponding bromomethyl derivative 3 [18], and was reacted successively with t-BuOK and formyl-substituted EDT-TTF 2 [19] in dry benzene at room temperature. After the column-chromatographic separation of the resultant mixture, donor molecule 1 having a vinylene spacer was obtained as air-stable orange microcrystals in 53% yield.
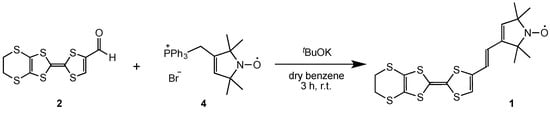
Scheme 1.
Synthesis of donor 1.
2.2. Electrochemical Properties and DFT Calculation of Donor 1
Electrochemical properties of 1 were investigated by cyclic voltammetry technique. Cyclic voltammograms were measured in benzonitrile at 25 °C using tetra-n-butylammonium hexafluorophosphate as a supporting electrolyte. The obtained redox potentials of donor 1 are summarized in Table 1 together with those of EDT-TTF and 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical derivative 5 [20] measured under the identical conditions. Molecule 1 showed three pairs of one-electron reversible redox waves (E1, E2 and E3). The E1 and E2 values (+0.50 V and +0.87 V vs. Ag/AgCl) are almost the same to those of EDT-TTF (+0.48 V and +0.88 V), suggesting that the first and second oxidations occur at the EDT-TTF part. Similarly, the third oxidation (E3) occurs at the stable radical part because the E3 value of 1 (+0.96 V) is almost the same as that of 5 (+0.99 V). These results suggest that the HOMO orbital of donor 1 mainly locates on the EDT-TTF part.

Table 1.
Redox potentials 1 of 1, ethylenedithio-tetrathiafulvalene (EDT-TTF) and stable organic radical 5.
A molecular orbital calculation of donor 1 was performed on the basis of the DFT theory at UB3LYP/6-31G(d, p) level using GAUSSIAN 09 package [21]. Figure 1 shows the molecular orbitals of donor 1 below the LUMO+2 level. The highest occupied two orbitals (120α and 119β: –4.76 eV) originate from the EDT-TTF part and the SOMO located under the HOMO orbitals (119α: –5.00 eV) localizes at the stable radical part. These results correspond to the above-mentioned electrochemical studies and indicate that the generated cation radical spin that exists on the EDT-TTF part and the localized stable radical spin can coexist in its cation radical salts prepared by an electrochemical oxidation method.
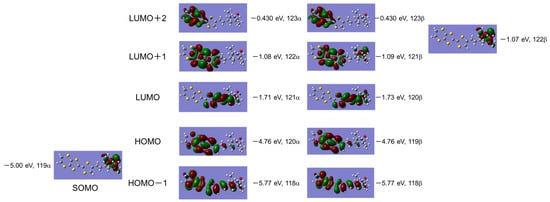
Figure 1.
Molecular orbitals of donor 1 calculated on the basis of the DFT method at UB3LYP/6-31G (d, p) level.
2.3. Crystal Structure Analysis and Physical Properties of Donor 1
X-ray crystal structure analysis was performed on an orange platelike single crystal of 1, which was obtained by recrystallization from dichloromethane/n-hexane. Figure 2 shows the ORTEP drawings of the molecular structure of 1. This crystal belongs to the monoclinic P21/c space group and one crystallographically independent molecule exists in the unit cell. As shown in Figure 2b, the TTF part adopts a boat-form conformation as is often observed in the neutral TTF derivatives. The vinylene spacer part has a trans-conformation and the vinylene spacer and pyrrolin-1-yloxyl parts show high planarity. This molecule has a slightly twisted molecular structure with a dihedral angle of 26° between the EDT-TTF moiety and radical moiety. As shown in Figure 3, the molecules are dimerized in a head-to-tail manner and form a so-called “κ type” molecular arrangement. The shortest S–S contacts are 3.61 Å in the dimer and 3.90 Å between the dimers. Because the shortest distances between the oxygen atoms of the stable radicals are 6.10, 6.17, and 6.43 Å, the intermolecular interaction between the radical parts seems to be very weak in the neutral crystal.
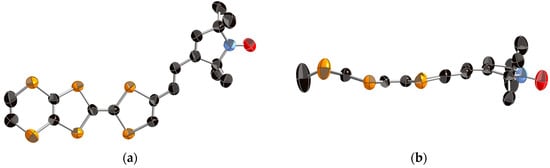
Figure 2.
ORTEP drawings of the molecular structure of neutral donor 1 (a) Top view and (b) side view. The hydrogen atoms are omitted for clarity.
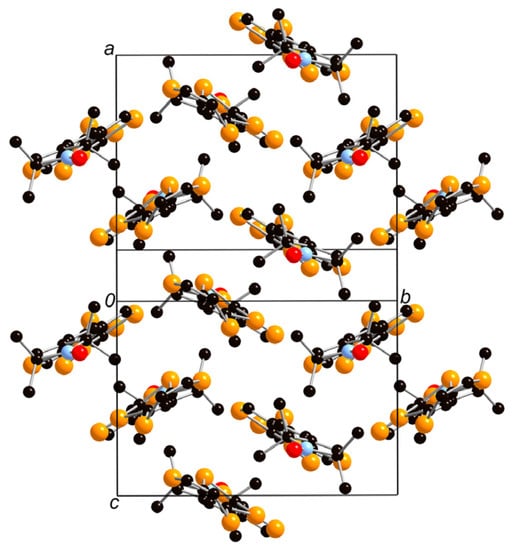
Figure 3.
Crystal structure of neutral donor 1. The hydrogen atoms are omitted for clarity.
Magnetic susceptibilities of neutral donor 1 were measured with a SQUID magnetometer (MPMS-XL, Quantum Design Inc., San Diego, CA, USA) under the applied field of 10 kOe in the temperature range of 2–300 K using a powder sample of 1. The temperature dependence of magnetic susceptibilities can be fitted by a Curie-Weiss law with a Curie constant of 0.365 emu·K·mol−1 that corresponds to the one S = 1/2 spin of the stable radical with a g-value of 2.00 per molecule and small Weiss temperature of θ = −1.5 K, suggesting the existence of very weak antiferromagnetic interaction between the stable radicals (See Figure S1a).
2.4. Crystal Structure Analyses and Magnetic Properties of the Cation Rarical Salts of Donor 1
X-ray crystal structure analyses were performed on needle-like single crystals of the FeCl4− and GaCl4− salts of donor 1, 1·FeCl4 and 1·GaCl4 obtained by galvanostatic oxidation in the mixture of dry 1,2-dichloroethane and dry ethanol (v/v = 1:9) using the tetraethylammonium salts of the counteranions as supporting electrolytes. Because these two crystals belong to the monoclinic P21/n space group and are completely isostructural to each other, only the structure of 1·FeCl4 will be discussed in detail in this section. In the unit cell, one donor 1 and one anion moiety are crystallographically independent, indicating that the donor:anion ratio of these crystals is 1:1 and each of the donor moieties is in the monocation radical state 1+·. The ORTEP drawings of the molecular structure and the crystal structures of 1·FeCl4 are shown in Figure 4 and Figure 5, respectively. As shown in Figure 4, the molecular structure of the donor moiety of 1·FeCl4 is similar to that of the neutral one (Figure 2), but is more planar with a dihedral angle of 15° between the EDT-TTF moiety and radical moiety than that of the neutral one (26°). Furthermore, the TTF framework has quite high planarity with a mean deviation from the least-squares plane of 0.027 Å due to its +1 oxidized state. As shown in the crystal structures (Figure 5), two donor molecules form a dimer in a head-to-tail manner with a very short interplanar distance of 3.41 Å in the dimer (interaction a1; the shortest S–S contact is 3.44 Å). The dimers construct a one-dimensional stacking structure along the a-axis with a longer interplanar distance of 3.63 Å between the dimers (interaction a2; the shortest S–S contact is 3.93 Å). Overlap integrals between the donor moieties along these stackings, which are calculated by the extended Hückel method [22], are a1 = 41.5 × 10−3 and a2 = 0.29 × 10−3, indicating its quite strongly dimerized one-dimensional electronic structure. On the other hand, the anion moiety locates on the side of the dimers with very short S–Cl contacts of 3.34 Å (interaction I) and 3.59 Å (interaction II), suggesting very strong π-d interaction between the donor and anion moieties. These anions form a uniform one-dimensional array with a relatively long Cl–Cl contact of 3.90 Å (interaction dd1) along the a-axis, suggesting weak direct intermolecular interaction between the anions. Furthermore, the oxygen atom of the stable radical part has a short O–S contact of 2.94 Å with the neighboring EDT-TTF moiety as shown in Figure 5a.
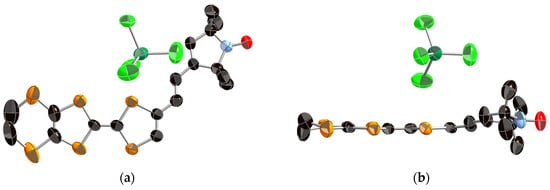
Figure 4.
ORTEP drawings of the molecular structure of 1·FeCl4 salt (a) Top view and (b) side view. The hydrogen atoms are omitted for clarity.
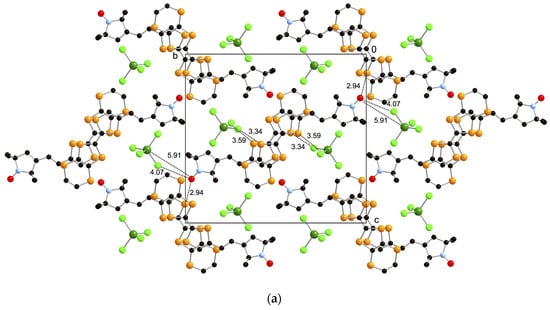
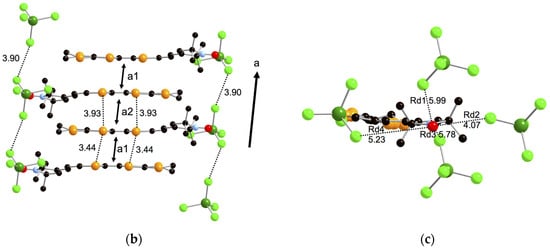
Figure 5.
(a) Crystal structure of 1·FeCl4 projected onto the bc-plane; (b) Stacking structure of 1·FeCl4 along the a-axis; (c) Interactions around the stable radical. The hydrogen atoms are omitted for clarity. Short intermolecular contacts (Å) are indicated as dotted lines. Overlap integrals: a1 = 41.5 × 10−3 and a2 = 0.29 × 10−3.
Paramagnetic susceptibilities (χp) of 1·FeCl4 and 1·GaCl4 were measured under the applied field of 10 kOe in the temperature range of 2–300 K. The temperature dependence of χp values of the GaCl4− salt obeyed a Curie law with a Curie constant of 0.371 emu·K·mol−1 that is very close to the calculated value for one S = 1/2 spin system per 1:1 salt (0.375 emu·K·mol−1) (See Figure S1c). This result suggests that two π cation radical spins on two EDT-TTF moieties in one dimer are paired to each other due to the strong intradimer interaction a1, resulting in the formation of a nonmagnetic spin-singlet state, and only the contribution from the stable radical part can be observed. On the other hand, the FeCl4− salt indicated a Curie-Weiss fitting of the temperature dependence of χp values with a Curie constant of 4.89 emu·K·mol−1 that corresponds to the sum of the calculated contributions from the high-spin Fe3+ spin (S = 5/2; 4.375 emu·K·mol−1) and the stable radical (S = 1/2; 0.375 emu·K·mol−1) (See Figure S1b). The obtained Weiss temperature of −3.9 K suggests the existence of antiferromagnetic interaction between these spins. Due to the strongly dimerized structure of fully oxidized EDT-TTF moieties, these two salts showed insulating conducting behaviors.
Magnetic exchange interactions (Jππ, Jdd, Jπd and JRd) between these paramagnetic moieties (the π cation radical on the EDT-TTF (π), the stable radical (R) and the magnetic FeCl4− anion (d)) are estimated from the overlap integrals calculated by the extended Hückel method [23,24]. The π-π interactions in the dimer (a1 = 41.5 × 10−3) and between the dimers (a2 = 0.29 × 10−3) correspond to Jππ1 = 3996 K and Jππ2 = 0.19 K, respectively, confirming the nonmagnetic state of the donor parts due to the spin-singlet formation caused by the strong dimerization. The calculated direct d-d interaction between the anions along the a-axis is a very small value of Jdd1 = 0.09 K due to its long Cl–Cl distance of 3.9 Å in comparison to the sum of the van der Waals radii of chlorine atoms (3.6 Å). On the other hand, the π-d interactions between the EDT-TTF moiety and the FeCl4− anion are very large values of JI = 7.7 K and JII = 19.6 K reflecting very short S–Cl contacts of 3.34 Å (interaction I) and 3.59 Å (interaction II) between them mentioned above, while the interactions between the stable radical R and the anion moiety are estimated to be small values of JRd1 = 0.30 K, JRd2 = 0.32 K, JRd3 = 0.03 K, and JRd4 = 0.77 K using the SOMO orbital localized on the stable radical (See Figure 5c). Such short S–Cl contacts can mediate the antiferromagnetic interaction between the donor and anion moieties as reported in (TTF)3 [(Cl)(Mo6Cl14)] complex in which a charge-enhanced S(δ+)–Cl(δ−) intermolecular interaction (3.229 Å) plays an important role to cause antiferromagnetic ordering of the complex [25]. Although the short O–S contact of 2.94 Å between the oxygen atom of the stable radical part and the neighboring EDT-TTF moiety might mediate the magnetic interaction between the stable radical parts, quite small Weiss temperature of 0.03 K of the GaCl4− salt suggests that the magnetic exchange interaction JRπ between the radical part and the donor part is negligible. The overall Jdd, Jπd and JRd values of this salt are obtained as the mean-field sum; Jdd = 2Jdd1 = 0.18 K, Jπd = JI + JII = 27.3 K and JRd = JRd1 + JRd2 + JRd3 + JRd4 = 1.42 K, respectively, suggesting that the π-d interaction Jπd seems to be dominant in the magnetic properties of this salt. Because the d-d interaction Jdd between the FeCl4− anion is a very small value of 0.18 K compared to the Weiss temperature of −3.9 K, the strong π-d interactions (I and II) and strong π-π interaction in the dimer (a1) will mediate an indirect antiferromagnetic d-d interaction of θ = −3.9 K through its d-π-d interaction in addition to the small contribution by the magnetic interaction between the stable radical and the anions, JRd = 1.39 K.
3. Materials and Methods
General Remarks: Benzene was distilled under nitrogen atmosphere over calcium hydride. 1, 2-Dichloroethane was distilled under nitrogen atmosphere over P2O5. Other chemical reagents were purchased and used without further purification. High-resolution mass spectra (HRMS) using FAB+ method was measured using a JEOL JMS-700 mass spectrometer (JEOL Ltd., Akishima, Tokyo, Japan). IR spectra were recorded on KBr pellets using a JASCO FT/IR-4100 spectrometer (JASCO Corp., Hachioji, Tokyo, Japan). Cyclic voltammograms were measured using a BAS Electrochemical Analyzer Model 612B (BAS Inc., Sumida-ku, Tokyo, Japan). ESR spectrum of the benzene solution of 1 was measured at room temperature with a JEOL JES-RE1X X-band ESR spectrometer (JEOL Ltd., Akishima, Tokyo, Japan).
Synthesis of 1: A solid mixture of t-BuOK (70 mg, 0. 62 mmol) and 4 [18] (180 mg, 0.37 mmol) was suspended in 30 mL of dry benzene under nitrogen atmosphere and stirring for 2 h at room temperature. Then, 2 [19] (100 mg, 0.31 mmol) was added and the reaction mixture was further stirred for 3 h. After the solvent was evaporated in vacuo, the crude mixture was purified by column-chromatography on silica gel with dichloromethane (Rf = 0.58) as an eluent. Further purification by recrystallization with dichloromethane/n-hexane gave orange microcrystals of 1 (76 mg, 0.17 mol, 53%). m.p. 198–200 °C (dec.); HRMS FAB+ (Matrix = 3-Nitrobenzyl alcohol) (C16H18NOS4): Found 368.0291; Calcd. 368.0271; IR (KBr) ν1151, 2359, 2972, 3747 cm−1; ESR (in benzene solution at r.t.) g = 2.0042, aN = 1.44 mT
Preparation of cation radical salts of 1: The FeCl4− and GaCl4− salts of 1 were prepared as black needle-like crystals by a galvanostatic (I = 0.4 µA) oxidation using a conventional H-type electrocrystallization cell in the presence of 1 (5.0 mg) and the corresponding tetraethylammonium salts of the anions (100 mg) as a supporting electrolyte under nitrogen atmosphere in the mixture of dry 1, 2-dichloroethane and dry ethanol (10 ml, v/v = 1:9) at 16 °C for a few weeks.
X-ray data collection and reduction for the single crystalline samples: X-ray diffraction data were collected for the single crystal of neutral donor 1 on a Rigaku AFC-7 Mercury CCD diffractometer (Rigaku Corp., Akishima, Tokyo, Japan) with a graphite monochromated Mo-Kα radiation (λ = 0.7107 Å) and for the single crystals of 1·FeCl4 and 1·GaCl4 on a Rigaku AFC-8 Mercury CCD diffractometer (Rigaku Corp., Akishima, Tokyo, Japan) with confocal X-ray mirror system [Mo-Kα radiation (λ = 0.71075 Å)] and a rotating anode generator (0.8 kW). Lorentz and polarization corrections were applied. The structures were solved by a direct method (SIR92) [26], expanded (DIRDIF94) [27] and refined on F with full-matrix least-squares analysis. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. All the calculations were performed using the CrystalStructure crystallographic software package of the Molecular Structure Corporation [28]. Crystal data and structure refinement parameters are given in Table 2. CCDC-1527054 (1), 1527055 (1·FeCl4) and 1527056 (1·GaCl4) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.

Table 2.
Crystallographic data for 1, 1·FeCl4 and 1·GaCl4.
Magnetic property measurements: Magnetic susceptibilities were measured in the temperatures range of 2–300 K under the applied field of 10 kOe with a SQUID magnetometer (MPMS-XL, Quantum Design Inc., San Diego, CA, USA). Paramagnetic susceptibilities (χp) were obtained by subtracting the diamagnetic contribution estimated using Pascal’s constants [29] from the observed magnetic susceptibilities.
4. Conclusions
We synthesized new EDT-TTF donor containing a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical through a π-conjugated vinylene spacer 1 and examined its electronic and crystal structures, and physical properties. We also prepared its cation radical salts by an electrochemical oxidation method and successfully cleared the crystal structures and magnetic properties of the cation radical salts, 1·FeCl4 and 1·GaCl4. These salts have the strongly dimerized one-dimensional arrays of the fully oxidized donor molecules, giving rise to the formation of spin-singlet state of the π cation radical spins in the dimer. On the other hand, the FeCl4− anion locates on the side of the dimers with very short S-Cl contacts and mediates very strong π-d interaction between the donor and anion moieties, resulting in the antiferromagnetic behavior of θ = −3.9 K through its d-π-d interaction. The new findings in this paper will lead to the future construction of organic magnetic conductors based on the stable radical-containing donors. The preparation of new cation radical salts of donor 1 and its analogues, especially partially oxidized conducting materials, are now in progress to realize magnetic-conducting bifunctional materials.
Supplementary Materials
The following are available online at www.mdpi.com/2312-7481/3/1/8/s1, Figure S1: The temperature dependences of magnetic susceptibilities of neutral donor 1 (a), 1·FeCl4 (b) and 1·GaCl4 (c) measured in the temperatures range of 2–300 K under the applied field of 10 kOe. Calcd. lines indicate Curie-Weiss fittings of the data.
Acknowledgments
This work was financially supported in part by Grants-in-Aid for Scientific Research (No. 15K05483) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This work was also supported by the Institute for Molecular Science, Okazaki, Japan for the X-ray diffraction measurements on a Rigaku AFC-8 Mercury CCD diffractometer.
Author Contributions
H.F. conceived and designed the experiments; K.H. performed the synthetic and magnetic susceptibility experiments; K.H. and H.F. analyzed the X-ray data; K.H. and H.F. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Uji, S.; Shinagawa, H.; Terashima, T.; Yakabe, T.; Terai, Y.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-field-induced superconductivity in a two-dimensional organic conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Fujiwara, E.; Nakazawa, Y.; Narymbetov, B.Z.; Kato, K.; Kobayashi, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. A novel antiferromagnetic organic superconductor κ-(BETS)2FeBr4 [where BETS = bis(ethylenedithio)tetraselenafulvalene]. J. Am. Chem. Soc. 2001, 123, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Cui, H.B.; Kobayashi, A. Organic metals and superconductors based on BETS (BETS = bis(ethylenedithio)tetraselenafulvalene). Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Xiao, X.; Fujiwara, H.; Sugimoto, T.; Nakazumi, H.; Noguchi, S.; Fujimoto, T.; Yasuzuka, S.; Yoshino, H.; Murata, K.; et al. A metallic (EDT-DSDTFVSDS)2FeBr4 salt: Antiferromagnetic ordering of d spins of FeBr4– ions and anomalous magnetoresistance due to preferential π-d interaction. J. Am. Chem. Soc. 2006, 128, 11746–11747. [Google Scholar] [CrossRef] [PubMed]
- Maesato, M.; Kawashima, T.; Furushima, Y.; Saito, G.; Kitagawa, H.; Shirahata, T.; Kibune, M.; Imakubo, T. Spin-Flop Switching and Memory in a Molecular Conductor. J. Am. Chem. Soc. 2012, 134, 17452–17455. [Google Scholar]
- Sugano, T.; Fukasawa, T.; Kinoshita, M. Magnetic interactions among unpaired electrons in charge-transfer complexes of organic donors having a neutral radical. Synth. Met. 1991, 43, 3281–3284. [Google Scholar] [CrossRef]
- Kumai, R.; Matsushita, M.M.; Izuoka, A.; Sugawara, T. Intramolecular Exchange Interaction in a Novel Cross-Conjugated Spin System Composed of π-Ion Radical and Nitronyl Nitroxide. J. Am. Chem. Soc. 1994, 116, 4523–4524. [Google Scholar] [CrossRef]
- Komatsu, H.; Matsushita, M.M.; Yamamura, S.; Sugawara, Y.; Suzuki, K.; Sugawara, T. Influence of Magnetic Field upon the Conductance of a Unicomponent Crystal of a Tetrathiafulvalene-Based Nitronyl Nitroxide. J. Am. Chem. Soc. 2010, 132, 4528–4529. [Google Scholar] [CrossRef] [PubMed]
- Souto, M.; Solano, M.V.; Jensen, M.; Bendixen, D.; Delchiaro, F.; Girlando, A.; Painelli, A.; Jeppesen, J.O.; Rovira, C.; Ratera, I.; et al. Self-Assembled Architectures with Segregated Donor and Acceptor Units of a Dyad Based on a Monopyrrolo-Annulated TTF-PTM Radical. Chem. Eur. J. 2015, 21, 8816–8825. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.M.; Kawakami, H.; Sugawara, T.; Ogata, M. Molecule-based system with coexisting conductivity and magnetism and without magnetic inorganic ions. Phys. Rev. B 2008, 77, 195208. [Google Scholar] [CrossRef]
- Fujiwara, H.; Kobayashi, H. New pi-extended organic donor containing a stable TEMPO radical as a candidate for conducting magnetic multifunctional materials. Chem. Commun. 1999, 2417–2418. [Google Scholar] [CrossRef]
- Fujiwara, H.; Fujiwara, E.; Kobayashi, H. Novel π-electron donors for magnetic conductors containing a PROXYL radical. Chem. Lett. 2002, 1048–1049. [Google Scholar] [CrossRef]
- Fujiwara, H.; Fujiwara, E.; Kobayashi, H. Synthesis, structure and physical properties of donors containing a PROXYL radical. Synth. Met. 2003, 135, 533–534. [Google Scholar] [CrossRef]
- Fujiwara, H.; Lee, H.J.; Kobayashi, H.; Fujiwara, E.; Kobayashi, A. A novel TTP donor containing a PROXYL radical for magnetic molecular conductors. Chem. Lett. 2003, 32, 482–483. [Google Scholar] [CrossRef]
- Fujiwara, H.; Lee, H.J.; Cui, H.B.; Kobayashi, H.; Fujiwara, E.; Kobayashi, A. Synthesis, structure, and physical properties of a new organic conductor based on a π-extended donor containing a stable 2,2,5,5-tetramethyl-1-pyrrolidinyloxy radical. Adv. Mater. 2004, 16, 1765–1769. [Google Scholar] [CrossRef]
- Otsubo, S.; Cui, H.B.; Lee, H.J.; Fujiwara, H.; Takahashi, K.; Okano, Y.; Kobayashi, H. A magnetic organic conductor based on a π donor with a stable radical and a magnetic anion—A step to magnetic organic metals with two kinds of localized spin systems. Chem. Lett. 2006, 35, 130–131. [Google Scholar] [CrossRef]
- Fujiwara, E.; Aonuma, S.; Fujiwara, H.; Sugimoto, T.; Misaki, Y. New π-electron donors with a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical designed for magnetic molecular conductors. Chem. Lett. 2008, 37, 84–85. [Google Scholar] [CrossRef]
- Krishna, M.C.; DeGraff, W.; Hankovszky, O.H.; Sár, C.P.; Kálai, T.; Jekő, J.; Russo, A.; Mitchell, J.B.; Hideg, K. Studies of Structure−Activity Relationship of Nitroxide Free Radicals and Their Precursors as Modifiers Against Oxidative Damage. J. Med. Chem. 1998, 41, 3477–3492. [Google Scholar] [CrossRef] [PubMed]
- Garin, J.; Orduna, J.; Uriel, S.; Moore, A.J.; Bryce, M.R.; Wegener, S.; Yufit, D.S.; Howard, J.A.K. Improved Syntheses of Carboxytetrathiafulvalene, Formyltetrathiafulvalene and (Hydroxymethyl)Tetrathiafulvalene —Versatile Building-Blocks for New Functionalized Tetrathiafulvalene Derivatives. Synthesis 1994, 1994, 489–493. [Google Scholar] [CrossRef]
- Kálai, T.; Balog, M.; Jekö, J.; Hideg, K. Synthesis and Reactions of a Symmetric Paramagnetic Pyrrolidine Diene. Synthesis 1999, 1999, 973–980. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The Intermolecular Interaction of Tetrathiafulvalene and Bis(ethylenedithio)tetrathiafulvalene in Organic Metals. Calculation of Orbital Overlaps and Models of Energy-band Structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Mori, T.; Katsuhara, M. Estimation of πd-Interactions in Organic Conductors Including Magnetic Anions. J. Phys. Soc. Jpn. 2002, 71, 826–844. [Google Scholar] [CrossRef]
- Mori, T.; Katsuhara, M.; Akutsu, H.; Kikuchi, K.; Yamada, J.; Fujiwara, H.; Matsumoto, T.; Sugimoto, T. Estimation of π d-interactions in magnetic molecular conductors. Polyhedron 2005, 24, 2315–2320. [Google Scholar] [CrossRef]
- Batail, P.; Livage, C.; Parkin, S.S.P.; Coulon, C.; Martin, J.D.; Canadell, E. Antiperovskite Structure with Ternary Tetrathiafulvalenium Salts: Construction, Distortion, and Antiferromagnetic Ordering. Angew. Chem. Int. Ed. Engl. 1991, 30, 1498–1500. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Crystallogr. 1994, 27, 435. [Google Scholar] [CrossRef]
- Beurskens, P.T.A.; Beurskens, G.; Bosman, W.P.; de Gelder, D.; Israel, R.; Smith, J.M.M. Technical Report of the Crystallography Laboratory; University of Nijmegen: Nijmegen, The Netherlands, 1994. [Google Scholar]
- CrystalStructure, 4.0; Crystal Structure Analysis Package; Rigaku Corp.: Akishima, Tokyo, Japan, 2000–2010.
- König, E. Landolt Bornstein, Group II: Atomic and Molecular Physics, Vol. 2, Magnetic Properties of Coordination and Organometallic Transition Metal Compounds; Spring: Berlin, Germany, 1966. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).